Abstract
The roles of histamine in the anaphylactic increase in vascular permeability and leucocyte infiltration were analysed in an air pouch-type allergic inflammation model in histidine decarboxylase-deficient (HDC−/−) mice and wild-type mice. In the immunized wild-type mice, histamine content in the pouch fluid and vascular permeability in the anaphylaxis phase were increased by injection of the antigen solution into the air pouch. However, in the immunized HDC−/− mice, the antigen challenge did not increase histamine content in the pouch fluid and vascular permeability in the anaphylaxis phase. Number of leucocytes (more than 83% are neutrophils) in the pouch fluid 4–24 hr after the antigen challenge in the HDC−/− mice was significantly higher than that in the wild-type mice. Simultaneous injection of histamine with the antigen solution into the air pouch of the immunized HDC−/− mice reduced the antigen-induced leucocyte infiltration at 4 hr. Simultaneous injection of the H2 antagonist cimetidine but not the H1 antagonist pyrilamine with the antigen solution into the air pouch of the immunized wild-type mice further increased leucocyte infiltration at 4 hr. The levels of macrophage inflammatory protein-2 at 2 hr and of tumour necrosis factor-α at 4 hr in the pouch fluid of the HDC−/− mice were significantly higher than those of the wild-type mice. These findings indicate that histamine plays significant roles not only in the anaphylactic increase in vascular permeability via H1 receptors but also in the negative regulation of neutrophil infiltration via H2 receptors in allergic inflammation.
Introduction
Histamine, a chemical mediator of allergy and inflammation, is synthesized from l-histidine by histidine decarboxylase (HDC), an enzyme exclusively responsible for histamine production in mammals. HDC is constitutively expressed in mast cells and basophils1 and histamine is stored in their granules. Histamine is released from these cells by the antigen stimulation within a few minutes. In contrast, in macrophages,2,3 T cells4 and neutrophils,5 HDC is induced by inflammatory stimuli, and histamine is produced and released continuously.6 Histamine is involved in the anaphylactic responses such as constriction of smooth muscles and increase in the vascular permeability via H1 receptors. In addition, histamine regulates functions of immune and inflammatory cells via H2 receptors.6,7 For example, histamine inhibits chemotaxis of neutrophils,8 production of cytokines such as interleukin-1 (IL-1),9 IL-2,10 IL-12,11,12 and tumour necrosis factor-α (TNF-α).13 On the other hand, the production of IL-1012 and IL-1114 is augmented by histamine. Although the action of histamine on the inflammatory cells has well been examined in in vitro systems, the role of endogenous histamine in the late phase of allergic inflammation has not been completely clarified.
Recently, we established the HDC-deficient (HDC−/−) mouse strain.15 In the present study, we have established an air pouch-type allergic inflammation model in mice, and using HDC−/− mice and wild-type mice, we have analysed the roles of histamine in allergic inflammation.
Materials and methods
Mice
The HDC−/− (ICR-129) mice and the wild-type control mice (F3-F4) used in the present study were obtained as described elsewhere.15 Mice were treated in accordance with the procedure approved by the Animal Ethics Committee of the Graduate School of Pharmaceutical Sciences, Tohoku University, Japan.
Induction of air pouch-type allergic inflammation
Mice were immunized by intradermal injection of 1 mg of the antigen azobenzenearsonate-conjugated acetyl bovine serum albumin16 as an emulsion with the aid of Freund's complete adjuvant (Difco Laboratories, Detroit, MI). Three days later, 4 ml of air was injected subcutaneously on the dorsum to make an oval-shaped air pouch. Six days after the injection of air, 2 ml of air was again injected into the pouch. Twenty-four hours later, 1 mg of the antigen dissolved in 2 ml of a sterile solution of 2% (w/v) sodium carboxymethylcellulose (Cellogen F3H, Daiichi Kogyo, Niigata, Japan) in saline supplemented with antibiotics (0·1 mg/ml penicillin G potassium and 0·1 mg/ml streptomycin sulphate, Meiji Seika, Co., Tokyo, Japan) was injected into the preformed air pouch to provoke allergic inflammation. A group of mice that had been injected intradermally with the emulsion containing no antigen, received the same volume of the antigen solution into the air pouch, and served as ‘non-immunized mice’.
Measurements of vascular permeability and leucocyte infiltration
The mice were injected intravenously with 0·1 ml of 0·5% (w/v) Evans blue (Wako Chemical Co., Osaka, Japan) in saline just before the antigen challenge. Thirty minutes after the antigen challenge, the mice were killed by cutting the carotid artery under diethylether anaesthesia. The whole fluid in the pouch was collected, diluted twofold with ice-cold saline and centrifuged at 10 000 g at 4° for 15 min. The content of Evans blue in 200 μl of the supernatant fraction of the pouch fluid was determined by measuring absorbance at 620 nm. The total amount of Evans blue leaked into the pouch fluid during the 30-min period was calculated and expressed as per cent of the amount of Evans blue injected, and served as an index of vascular permeability.
The number of leucocytes in the pouch fluid was counted using a haemocytometer. Leucocytes in the pouch fluid were smeared on to glass slides, stained using May–Grünwald–Giemsa solution, and the population of leucocytes was determined.
Measurements of histamine, macrophage inflammatory protein (MIP)-2 and TNF-α in the pouch fluid
The pouch fluid was diluted twofold with ice-cold saline and centrifuged at 10 000 g at 4° for 10 min. Histamine in the supernatant was measured according to the method described by Shore et al.17 MIP-2 and TNF-α levels in the supernatant were determined using a MIP-2 enzyme-linked immunosorbent assay (ELISA) kit developed in our laboratory18 and a TNF-α ELISA kit (obtained from BioSource International, Camarillo, CA), respectively.
Drug treatments
Pyrilamine maleate, cimetidine, or histamine (Sigma Chemical Co., St Louis, MO) was dissolved in saline and added to the antigen solution, and 2 ml of the antigen solution was injected into the air pouch.
Statistical analysis
Results were analysed for statistical significance by Student's t-test for unpaired observations.
Results
The role of histamine in anaphylactic increase in vascular permeability
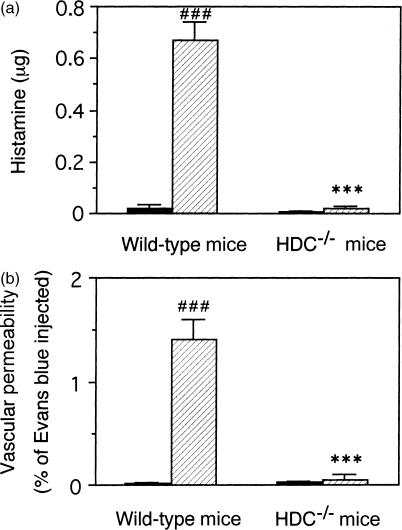
Histological observations revealed that mast cells in the subcutaneous tissue of the dorsum both in the immunized HDC−/− mice and in the wild-type mice were degranulated 30 min after the injection of the antigen solution into the air pouch, indicating that the mast cells in both the HDC−/− mice and the wild-type mice were equally activated by the antigen challenge (data not shown). The histamine content in the pouch fluid collected 30 min after the antigen challenge was clearly increased in the immunized wild-type mice compared to the non-immunized wild-type mice (Fig. 1a). However, in the immunized HDC−/− mice, histamine content in the pouch fluid at 30 min was not increased by the antigen challenge, and the level was almost the same as that of the non-immunized wild-type mice (Fig. 1a). Vascular permeability during the first 30 min after antigen challenge was also prominently increased in the immunized wild-type mice, but almost no response was observed in the immunized HDC−/− mice (Fig. 1b).
Figure 1.
Decrease in the anaphylactic response in the HDC−/− mice. The immunized (hatched columns) or the non-immunized (closed columns) mice were injected intravenously with 0·l ml of 1% (w/v) Evans blue solution just before the injection of 2 ml of a 2% (w/v) solution of sodium carboxymethylcellulose in saline containing the antigen into the air pouch. Thirty minutes later, the pouch fluid was collected and the histamine (a) and Evans blue (b) contents were determined. The pouch-fluid volumes of the immunized and the non-immunized wild-type mice were 2·23 ± 0·03 ml and 2·20 ± 0·02 ml, respectively. Those in the HDC−/− mice were 2·10 ± 0·05 ml and 2·02 ± 0·05 ml, respectively. Vertical bars represent SEM from seven to eight mice. Statistical significance; ***P < 0·001 versus the corresponding wild-type mice, and ###P < 0·001 versus the corresponding non-immunized mice.
The role of histamine in leucocyte infiltration
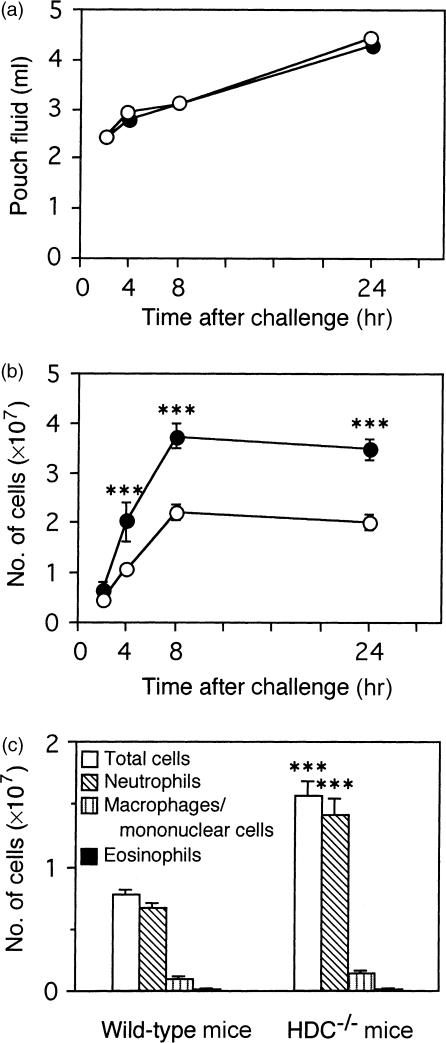
The number of leucocytes in the pouch fluid was determined in the immunized wild-type and HDC−/− mice after the antigen challenge. In both strains of mice, the number of infiltrating leucocytes was increased time dependently and reached a maximum at 8 hr. The number of leucocytes from 4 to 24 hr after the antigen challenge in the HDC−/− mice was significantly higher than those in the wild-type mice (Fig. 2b), although the pouch-fluid volume was not different in the two groups of mice (Fig. 2a). Differential counting of the infiltrating leucocytes revealed that the number of neutrophils was significantly higher in the HDC−/− mice than in the wild-type mice (Fig. 2c). In this model, infiltration of eosinophils at 4 hr was very low in both groups (Fig. 2c). The population of each type of leucocyte infiltrating the pouch fluid did not change significantly from 4 to 24 hr in either mouse strain (data not shown).
Figure 2.
Time changes of pouch-fluid volume and leucocyte infiltration in the immunized HDC−/− and wild-type mice. Two millilitres of the antigen solution was injected into the air pouch of the immunized HDC−/− (•) and wild-type (○) mice and the pouch fluid was collected at the indicated time. Pouch-fluid volume (a), number of leucocytes infiltrating the pouch fluid (b), and the cell population of the infiltrating leucocytes in the pouch fluid 4 hr after the antigen challenge (c) were determined. Vertical bars represent SEM from seven to eight mice. Statistical significance; ***P < 0·001 versus the corresponding wild-type mice.
The effects of histamine antagonists and histamine on the leucocyte infiltration
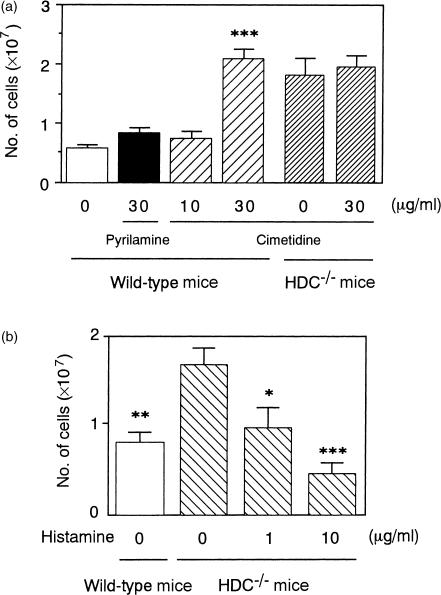
To clarify whether the difference in the number of infiltrating leucocytes in the pouch fluid between the immunized HDC−/− mice and the wild-type mice is a result of the difference in the amount of released histamine, effects of the intrapouch injection of histamine antagonists or histamine on the number of leucocytes infiltrating the pouch fluid 4 hr after the antigen challenge were examined. In the immunized wild-type mice, treatment with the H2 antagonist cimetidine but not the H1 antagonist pyrilamine increased leucocyte infiltration, but in the immunized HDC−/− mice, cimetidine did not affect leucocyte infiltration (Fig. 3a). Furthermore, in the immunized HDC−/− mice, treatment with histamine reduced the leucocyte infiltration in a dose-dependent manner (Fig. 3b).
Figure 3.
Effects of cimetidine, pyrilamine and histamine on leucocyte infiltration in the immunized HDC−/− and wild-type mice. Two millilitres of the antigen solution containing cimetidine, pyrilamine, or histamine at the concentrations indicated was injected into the air pouch of the immunized mice. Number of leucocytes in the pouch fluid 4 hr after the injection was determined. Vertical bars represent SEM from seven mice. Statistical significance; ***P < 0·001 versus the control wild-type mice (a), *P < 0·05, **P < 0·01, ***P < 0·001 versus the control HDC−/− mice.
The levels of MIP-2 and TNF-α in the pouch fluid
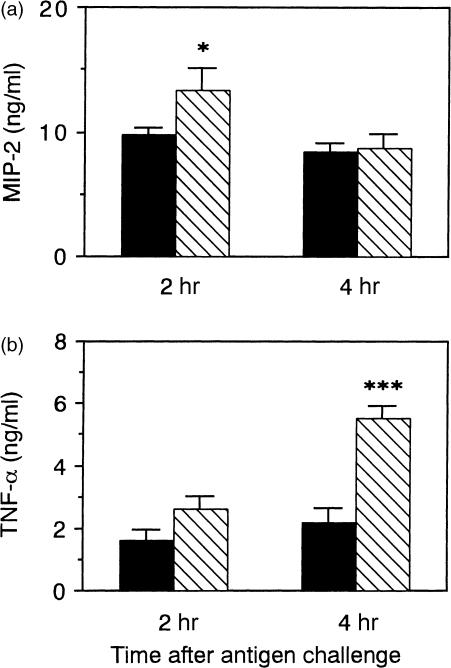
The levels of MIP-2 at 2 hr and of TNF-α at 4 hr in the pouch fluid were significantly higher in the immunized HDC−/− mice than in the immunized wild-type mice (Fig. 4), and there was no significant difference in the pouch-fluid volume between the two strains at 2 and 4 hr (Fig. 2a).
Figure 4.
The levels of MIP-2 and TNF-α in the pouch fluid of the immunized HDC−/− and wild-type mice. Two millilitres of the antigen solution was injected into the air pouch of the immunized wild-type mice (closed columns) and HDC−/− mice (hatched columns), and the pouch fluid was collected 2 and 4 hr after the antigen challenge. The levels of MIP-2 (a) and TNF-α (b) in the supernatant fraction of the pouch fluid were determined. Vertical bars represents SEM from seven mice. Statistical significance; *P < 0·05, ***P < 0·001 versus the corresponding control.
Discussion
Destruction of the HDC gene results in a marked reduction of the tissue histamine content.15 The present study also indicated that histamine in mast cells is almost completely depleted in HDC−/− mice because the antigen challenge did not induce an increase in histamine levels in the pouch fluid of actively immunized HDC−/− mice (Fig. 1a). Using the HDC−/− mice, we confirmed that histamine plays a significant role not only in the anaphylactic increase in vascular permeability but also in the negative regulation of neutrophil infiltration, as previously reported in the rat allergic inflammation model.19
The critical involvement of mast cells in the anaphylactic increase in vascular permeability was demonstrated in mast cell-deficient mice such as W/Wv mice.20 Because mast cells release various vasoactive mediators such as serotonin,21 it is difficult to clarify which vasoactive mediators are responsible for the anaphylactic increase in vascular permeability using the mast cell-deficient mice. In the present study, using HDC−/− mice, we have shown that histamine released from mast cells is exclusively responsible for the vascular permeability increase during the anaphylactic phase in mice. It was also indicated that the involvement of serotonin in the anaphylactic increase in vascular permeability is negligible, because no significant increase in vascular permeability in the anaphylaxis phase was induced in the HDC−/− mice (Fig. 1b).
Although the HDC−/− mice did not express an anaphylactic increase in vascular permeability, there was no difference in the pouch-fluid volume between the HDC−/− mice and the wild-type mice at 4–24 hr after the antigen challenge. In this model, the transient increase in vascular permeability during the first 30 min did not affect the following pouch-fluid accumulation. In addition, because the intrapouch injection of the cyclo-oxygenase inhibitor indomethacin (20 μg/pouch) reduced the pouch-fluid volume 8 hr after the antigen challenge in both mice [for the wild-type mice: control mice 3·29 ± 0·03, indomethacin-injected mice 2·93 ± 0·05 (P < 0·01); for the HDC−/− mice: control mice 3·18 ± 0·03, indomethacin-injected mice 2·87 ± 0·05 (P < 0·01); means ±SEM from five mice], it is likely that cyclo-oxygenase products play a role in the pouch-fluid accumulation as previously described in allergic air pouch-type inflammation in rats.22
The antigen-induced neutrophil infiltration in the late phase in the HDC−/− mice was also found to be significantly higher than that in the wild-type mice (Fig. 2). The enhanced infiltration of neutrophils in the HDC−/− mice might be a result of the lack of histamine because it was reversed by the intrapouch injection of histamine(Fig. 3b). In addition, the leucocyte infiltration induced by the antigen challenge in the wild-type mice was enhanced by cimetidine but not by pyrilamine (Fig. 3a), indicating that the endogenous histamine down-regulates the antigen-induced neutrophil infiltration via H2 receptors. In this model, the number of eosinophils infiltrating the pouch fluid was very low in both strains. Therefore, this model is not suitable for the analysis of histamine's role in eosinophil infiltration.
Histamine H2 receptors are expressed on various cells including neutrophils, macrophages and lymphocytes, and histamine regulates the functions of these cells via H2 receptors.6,7 In addition, it is reported that histamine inhibits neutrophil chemotaxis in vitro,8 neutrophil infiltration in vivo,19,23 and cytokine production in vitro.9,13 Our findings suggested that endogenous histamine down-regulates the production of MIP-2 and TNF-α (Fig. 4). However, the possibility remains that the higher levels of MIP-2 and TNF-α in the pouch fluid of the immunized HDC−/− mice are a result of the increased number of infiltrating leucocytes that produce MIP-2 and TNF-α. We reported that the chemokine responsible for neutrophil infiltration in the air pouch-type allergic inflammation in rats is MIP-2.24 MIP-2 also participates in neutrophil infiltration in several inflammatory models in mice.25,26 Therefore, the higher level of MIP-2 in the pouch fluid of the immunized HDC−/− mice at 2 hr (Fig. 4a) might partly participate in neutrophil infiltration thereafter.
It is reported that histamine increases IL-10 production but inhibits IL-12 production in peripheral lymphocytes.11,12 These reports suggest that histamine changes the T helper 1 (Th1)/Th2 balance to be Th2 dominant. In the Th2-dominant allergy, histamine is released from mast cells by the antigen challenge, and the infiltration of neutrophils is less than that observed in Th1-type allergic inflammation, but infiltration of eosinophils is prominent. The finding that histamine reduces neutrophil infiltration might explain the lower infiltration of neutrophils in Th2-type allergy. Further study will be required to prove this possibility. HDC−/− mice might be the most useful experimental animals with which to clarify such novel roles of histamine in allergic inflammation.
In conclusion, by using the HDC−/− mice and the wild-type mice, we demonstrated that histamine is exclusively responsible for the anaphylactic increase in vascular permeability, and down-regulates neutrophil infiltration via H2 receptors in allergic inflammation. One possible mechanism for the down-regulation of neutrophil infiltration by histamine is the reduction of MIP-2 production.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Exploratory Research (11877381) and a Grant-in-Aid for Specific Research on Priority Areas (12139202) from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- HDC
histidine decarboxylase
- MIP-2
macrophage inflammatory protein-2
- TNF-α
tumour necrosis factor-α
References
- 1.Kuramasu A, Saito H, Suzuki S, Watanabe T, Ohtsu H. Mast cell-/basophil-specific transcriptional regulation of human l-histidine decarboxylase gene by CpG methylation in the promoter region. J Biol Chem. 1998;273:31607–14. doi: 10.1074/jbc.273.47.31607. [DOI] [PubMed] [Google Scholar]
- 2.Takamatsu S, Nakashima I, Nakano K. Modulation of endotoxin-induced histamine synthesis by cytokine in mouse bone marrow-derived macrophages. J Immunol. 1996;156:778–85. [PubMed] [Google Scholar]
- 3.Shiraishi M, Hirasawa N, Kobayashi Y, Oikawa S, Murakami A, Ohuchi K. Participation of mitogen-activated protein kinase in thapsigargin- and TPA-induced histamine production in murine macrophage RAW 264.7 cells. Br J Pharmacol. 2000;129:515–24. doi: 10.1038/sj.bjp.0703085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoi R, Nakashima I, Kitamura Y, Asai H, Nakano K. Histamine synthesis by mouse T lymphocytes through induced histidine decarboxylase. Immunology. 1989;66:219–23. [PMC free article] [PubMed] [Google Scholar]
- 5.Shiraishi M, Hirasawa N, Oikawa S, Kobayashi Y, Ohuchi K. Analysis of histamine-producing cells at the late phase of allergic inflammation in rats. Immunology. 2000;99:600–6. doi: 10.1046/j.1365-2567.2000.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer DJ, Matloff SM, Rocklin RE. The influence of histamine on immune and inflammatory responses. Adv Immunol. 1984;35:209–68. doi: 10.1016/s0065-2776(08)60577-5. [DOI] [PubMed] [Google Scholar]
- 7.Falus A, Meretey K. Histamine: an early messenger in inflammatory and immune reactions. Immunol Today. 1992;13:154–6. doi: 10.1016/0167-5699(92)90117-p. [DOI] [PubMed] [Google Scholar]
- 8.Radermecker M, Maldague MP. Depression of neutrophil chemotaxis in atopic individuals. An H2 histamine receptor response. Int Arch Allergy Appl Immunol. 1981;65:144–52. doi: 10.1159/000232750. [DOI] [PubMed] [Google Scholar]
- 9.Dohlsten M, Kalland T, Sjogren HO, Carlsson R. Histamine inhibits interleukin 1 production by lipopolysaccharide-stimulated human peripheral blood monocytes. Scand J Immunol. 1988;27:527–32. doi: 10.1111/j.1365-3083.1988.tb02379.x. [DOI] [PubMed] [Google Scholar]
- 10.Dohlsten M, Sjogren HO, Carlsson R. Histamine inhibits interferon-γ production via suppression of interleukin 2 synthesis. Cell Immunol. 1986;101:493–501. doi: 10.1016/0008-8749(86)90160-7. [DOI] [PubMed] [Google Scholar]
- 11.van Den Pouwkraan TCTM, Snijders A, Boeije LCM, et al. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J Clin Invest. 1998;102:1866–73. doi: 10.1172/JCI3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–93. [PubMed] [Google Scholar]
- 13.Vannier E, Miller LC, Dinarello CA. Histamine suppresses gene expression and synthesis of tumor necrosis factor α via histamine H2 receptors. J Exp Med. 1991;174:281–4. doi: 10.1084/jem.174.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng T, Nathanson MH, Elias JA. Histamine augments cytokine-stimulated IL-11 production by human lung fibroblasts. J Immunol. 1994;153:4742–52. [PubMed] [Google Scholar]
- 15.Ohtsu H, Tanaka S, Terui T, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–6. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 16.Ohuchi K, Yoshino S, Kurihara A, et al. Delayed-type hypersensitivity as revealed on the footpads of mice to azobenzenearsonate-acetyl bovine serum albumin. Int Arch Allergy Appl Immunol. 1981;66:391–403. doi: 10.1159/000232848. [DOI] [PubMed] [Google Scholar]
- 17.Shore PA, Burkhalter A, Cohn VH. A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959;127:182–6. [PubMed] [Google Scholar]
- 18.Xiao Y-Q, Someya K, Morita H, Takahashi K, Ohuchi K. Involvement of p38 MAPK and ERK/MAPK pathways in staurosporine-induced production of macrophage inflammatory protein-2 in rat peritoneal neutrophils. Biochim Biophys Acta. 1999;1450:155–63. doi: 10.1016/s0167-4889(99)00042-7. [DOI] [PubMed] [Google Scholar]
- 19.Hirasawa N, Ohuchi K, Watanabe M, Tsurufuji S. Role of endogenous histamine in postanaphylactic phase of allergic inflammation in rats. J Pharmacol Exp Ther. 1987;241:967–73. [PubMed] [Google Scholar]
- 20.Wershil BK, Mekori YA, Murakami T, Galli SJ. 125I-Fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol. 1987;139:2605–14. [PubMed] [Google Scholar]
- 21.Weitzman G, Galli SJ, Dvorak AM, Hammel I. Cloned mouse mast cells and normal mouse peritoneal mast cells. Determination of serotonin content and ability to synthesize serotonin in vivo. Int Arch Allergy Appl Immunol. 1985;77:189–91. doi: 10.1159/000233782. [DOI] [PubMed] [Google Scholar]
- 22.Hirasawa N, Ohuchi K, Sugio K, Tsurufuji S, Watanabe M, Yoshino S. Vascular permeability responses and the role of prostaglandin E2 in an experimental allergic inflammation of air pouch type in rats. Br J Pharmacol. 1986;87:751–6. doi: 10.1111/j.1476-5381.1986.tb14593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kheiferts J, Thieme T, Mirkovich A, Ackerman N. The effects of histamine and serotonin on polymorphonuclear leukocytes accumulation in the rat. Eur J Pharmacol. 1986;128:179–86. doi: 10.1016/0014-2999(86)90764-8. [DOI] [PubMed] [Google Scholar]
- 24.Xiao Y-Q, Tanabe J, Edamatsu T, Hirasawa N, Mue S, Ohuchi K. Possible participation of macrophage inflammatory protein 2 in neutrophil infiltration in allergic inflammation in rats. Biochim Biophys Acta. 1997;1361:138–46. doi: 10.1016/s0925-4439(97)00034-3. [DOI] [PubMed] [Google Scholar]
- 25.Seebach J, Bartholdi D, Frei K, et al. Experimental Listeria meningoencephalitis. Macrophage inflammatory protein-1α and -2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J Immunol. 1995;155:4367–73. [PubMed] [Google Scholar]
- 26.Biedermann T, Kneilling M, Mailhammer R, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hyper-sensitivity reactions through tumor necrosis facor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–51. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]