Abstract
NOD/LtSz-prkdcscid/prkdcscid (non-obese diabetic-severe combine immunodeficiency; NOD-scid) mice grafted with human peripheral blood lymphoid cells have been used as an in vivo humanized mouse model in various studies. However, cytotoxic human T cells are induced in this model during immune responses, which gives misleading results. To assist in grafting of human lymphocytes without the induction of cytotoxic human T cells, we investigated the effects of T helper type 1 (Th1) and Th2 cytokines on human lymphocyte grafting and migration, as well as the production of immunoglobulin deposited in glomeruli and human immunodeficiency virus-1 (HIV-1) infection using NOD-scid mice. Administration of interleukin-18 (IL-18) and IL-12 enhanced the grafting of human CD4+ and CD8+ T cells in the mice, whereas co-administration prevented grafting due to interferon-γ-dependent apoptosis. Immunoglobulin A (IgA) deposits were observed in mice treated with IL-18 alone, but not in those given phosphate-buffered saline, IL-12 alone, or IL-18 + IL-12. A high rate of HIV infection was also observed in the IL-18-treated group. Together, these results indicate that IL-18 may be effective for the grafting and migration of CD4+ and CD8+ T cells, except for the induction of apoptosis and regulation of class-switching IgA. IL-18-administered NOD-scid mice provide a useful small humanized model for the study of HIV infection and IgA nephropathy.
Introduction
In the past few years, NOD/LtSz-prkdcscid/prkdcscid (non-obese diabetic-severe combine immunodeficiency; NOD-scid) mice grafted with human peripheral blood lymphoid cells (PBLs) have been used as in vivo models for studying transplantation of haematolymphoid cells, human tumour biology, and human-specific infection agents such as human immunodeficiency virus (HIV).1–4 This mouse strain supports levels of human cell grafting that are five- to 10-fold greater than those obtained in C.B-17-scid mice.5 As a result, the human PBL–NOD-scid model is employed for long-term in vivo analysis of immunoregulatory interactions between human lymphocyte activation and pathogens such as HIV. However, it appears that graft-versus-host-disease reactivity is a central feature of human PBL–scid chimaerism,6 as cytotoxic human T cells are induced in these mice during immune responses. Specifically, T cells are not deleted, but rather are activated and then migrate, survive, and remain in all tissues. Thus, cytotoxic cells may give misleading results during the immune response by human lymphocytes in these mice.
A critical advance in cellular immunology has been the discovery of two functionally distinct T-cell subsets, T helper type 1 (Th1) and type 2 (Th2) cells, that are classified on the basis of their cytokine expression. Th1 cells produce cytokines that are associated with inflammation and the cell-mediated immune response such as interferon-γ (IFN-γ). In contrast, Th2 cells release cytokines that promote antibody-mediated immune responses and inhibit Th1 responses to cytokines such as interleukin-4 (IL-4), IL-5, IL-6 and IL-10.7,8 Recently, a novel cytokine, IL-18, was cloned and found to exhibit powerful Th1-promoting activities, as well as those of natural killer cells and CD8 effector T cells in synergy with IL-12.9–13 IL-18 also enhances the production of granulocyte–macrophage colony-stimulating factor and IL-2, and is thought to reduce the production of Th2 cytokines such as IL-10 via IFN-γ induction.14 In contrast, it was reported that IL-18 can induce Th2 cytokine and CD154 expression, as well as contribute to CD4+ T cell, IL-4, and signal transducers and activators of transcription (stat) 6-dependent immunoglobulin E (IgE) production.15,16 These results indicate a possibility that these cytokines are helpful for the regulation of human lymphocytes in the NOD-scid mouse system.
In the present study, we investigated the effects of Th1 and Th2 cytokines on grafting of human PBL in NOD-scid mice and found that IL-18 was effective in the grafting and activation of human CD4+ and CD8+ T cells in mouse tissues. Moreover, it was demonstrated that IL-18-dependent grafting may play a critical role in apoptosis during human CD4+ and CD8+ regulation in the model mice. An in vivo administration of IL-18 alone increased the rate of HIV infection, as well as deposits of IgA in serum and glomeruli, indicating that IL-18 affects the development of human immune responses in this humanized mouse system.
Materials and methods
Mice
Specific pathogen-free, 8-week-old female NOD-scid mice were generated and bred under specific pathogen-free conditions at the National Institute of Infectious Diseases Animal Care Unit (Tokyo, Japan). Females between 8 and 16 weeks of age were used in all of the experiments, which were performed in accordance with our institutional guidelines.
Antibodies
The following monoclonal antibodies (mAbs) were purchased from BD PharMingen (San Diego, CA): fluorescein isothiocyanate (FITC) and phycoerythrin (PE) -conjugated anti-human CD4 (clone RPA-T4), FITC-conjugated anti-human CD45 (H130), PE-conjugated anti-human CD8 (RPA-T8), FITC-conjugated anti-mouse CD45 (30-F11), FITC-conjugated annexin V (3E2), human IgM (G20-127), mouse IgM (R6-60.2), and unconjugated anti-human IFN-γ (clone 25723.11). FITC-conjugated human IgG and human IgA antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Biotin-conjugated human IgA1 (clone B3506B4) and IgA2 (clone A9604D2) were purchased from Southern Biotechnology Associates (Birmingham, AL). FITC-conjugated human C3 antibody was purchased from Antibodies Incorporated (Davis, CA). Alkalin phosphatase-conjugated goat anti-human IgG (γ), IgA (α), IgM (µ), and mouse IgG (H + L) were purchased from Zymed Laboratory (South San Francisco, CA).
Transplantation
PBLs were isolated from 400 ml of peripheral blood taken from a normal healthy volunteer by separation using Ficoll–Hypaque gradients (Immuno-Biological Laboratories, Gunma, Japan). The cells were washed three times in Hanks' balanced salt solution (HBSS; Gibco Laboratories, Life Technologies, Paisley, UK) and adjusted to a 3 × 107 concentration in HBSS. A suspension of human PBL was then administered intraperitoneally at 500 μl per mouse using the transplantation technique of Mosier et al.17 Three to five female mice from a single litter were grafted with PBLs from a donor and used as an experimental group.
Injection of cytokines
The experimental design is described in Fig. 1. One day after experimental and control groups of NOD-scid mice were transplanted with human PBLs, the experimental group animals were administered 62·5, 125, or 250 ng of a recombinant cytokine intraperitoneally [human IL-18 (Medical & Biological Laboratories, Code Bool), human IL-12, human IL-4 (R & D Systems, Minneapolis, MN), human IL-6 (BIOSOURCE, Camarillo Calfornia, CA), or human IFN-γ (Pepro Teck, Rocky Hill, NJ)] in 300 μl of phosphate-buffered saline (PBS), pH 7·4. A combination of IL-18 at 250 ng and IL-12 at 250 ng (IL-18 + IL-12) was also administered to a group of human PBL–NOD-scid mice. Control mice were injected intraperitoneally with 200 μl of PBS only. In each experimental set, PBS was administered as a control to compare with the single or mixed cytokines in mice grafted with lymphocytes from the donor. Intraperitoneal injections were given once per week (three times in total) in both the experimental and control groups. Three weeks after the last injection, serum, spleen, kidney, liver and peritoneal cells were taken for the following experiments. Each experiment was performed independently three or four times.
Figure 1.
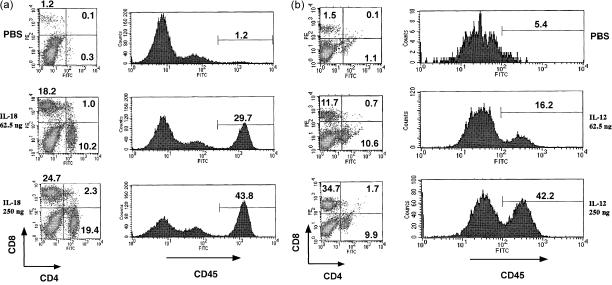
Flow cytometric analysis of human CD45, CD8 and CD4 T-cell populations present in the spleen from grafted NOD-scid mice treated with human IL-18 (a) and human IL-12 (b). Three NOD-scid mice transplanted with human PBL were given the control (PBS), IL-18 (62·5 ng), and IL-18 (250 ng), or control, IL-12 (62·5 ng) and IL-12 (250 ng/mouse). Human PBL in spleen cells were analysed 3 weeks after the last administration for human CD45+ single and CD4+ CD8+ double staining. The analyses were performed on gated lymphocytes with FSC/SSC characteristics. Lymphocytes can be distinguished in the quadrant analysis. The number in the upper left and lower right quadrants are the percentages of CD8+ CD4− T cells and CD4+ CD8− T cells, respectively. The histograms show CD45 single-stained lymphocytes, while the numbers in each histogram indicate the per cent of gated cells. The vertical axis varied from maxima of 10 to 200 for the analysis of CD45+ cells, because there was interexperimental variation in compatibility of human lymphocytes from a donor to NOD-scid mice from a single litter. Data shown are representative of four independent experiments.
Flow cytometric analysis
Single cell suspensions of spleen cells were prepared by gently homogenizing the cells with ice-cold HBSS. Single cell suspensions of peritoneal cells were collected by washing the peritoneal cavity with HBSS. All cell suspensions were washed once in ice-cold HBSS as described below. Spleen or peritoneal cells were stained with FITC- or PE-conjugated antihuman marker mAbs in PBS-1% BSA and washed with HBSS medium. At least 104 to 105 live spleen cells, including mouse and human lymphoid cells, were acquired in each run. For each mouse analysed, cells were also stained with mouse IgG conjugated to FITC and PE as an isotype control. Spleen or peritoneal cells from a non-transplanted NOD-scid mouse were stained in parallel as an additional negative control. Fluorescence levels that excluded greater than 98% of the cells in the negative controls were considered to be positive and specific for human staining. The cells were fixed in a 3% formalin/HBSS solution and stored at 4° until flow cytometric analysis. Samples gated on the forward light scatter (FSC) and side light scatter (SSC) were used to identify viable lymphocytes. Proportions of the major subsets were determined by single and quadrant analysis. The percentage of FITC- and PE-positive cells was measured by a FACSscan using the CELLquest program (Beckton Dickinson, San Jose, CA).
Apoptosis assay
Single cell suspensions of spleen cells prepared from hu-PBL-NOD-scid mice injected with cytokines were washed once in ice-cold HBSS and stained with PE-conjugated anti-human CD4 or CD8 antibodies. One hour later, the cells were washed three times in ice-cold HBSS, and then stained with annexin V-FITC and propidium iodide (PI) according to the manufacturer's instructions. Apoptotic cells were analysed by a FACSscan using the CELLquest program.
Immunohistochemistry
Tissue samples were placed in Tissue-Tek OCT compound (Sakura Finetek Europe BU, Zoeterwoude, the Netherlands) and stored at −80°. Five-micrometre cryostat sections were prepared and placed on gelatin-coated slides (Matsunami Glass, Tokyo, Japan). After air-drying, sections were fixed in cold acetone for 10 min, rinsed in PBS, and then incubated with blocking solution (Vector Laboratories, Burlingame, CA) for 20 min. This was followed by incubation with FITC-conjugated mAbs (human IgM, IgG, IgA, mouse IgM, and C3) and biotin-conjugated IgA1 and IgA2 at room temperature for 1 hr. After treatment with the biotin-conjugated antibody, streptoavidin-FITC was added to the sections. The sections were then washed three times with cold PBS for 30 min and analysed using a microscope. As a control, the mAbs were replaced by FITC-conjugated mouse IgM mAb, which consistently yielded negative results.
Cytokine and human antibodies enzyme-linked immunosorbent assay (ELISA)
The concentrations of human cytokines (IFN-γ and IL-4) in sera from NOD-scid mice transplanted with human PBL were determined by a two-site sandwich ELISA kit (Biosource International, Camarillo, CA). A standard curve was generated using the recombinant cytokines. The manufacturer reported the sensitivity threshold to be 1 pg/ml for IL-4 and IFN-γ. All samples were assayed in triplicate. Serum human antibodies were determined by an ELISA technique as previously described.18 Ninety-six-well microtitre H-plates (Sumitomo Bekelite, Tokyo, Japan) were coated overnight at 4° with 100 μl of 1 : 1000 dilution sheep anti-human immunoglobulin (Cappel, West Chester, PA) in 50 mm of carbonate buffer (pH 9·6). The plates were washed with PBS containing 0·05% Tween-20 (PBST) and blocked with 1% (wt/vol) skimmed milk in PBST. This was followed by the addition of 100 μl of a twofold serial dilution of human PBL–NOD-scid mice sera to the wells and incubation for 1 hr at 37°. The wells were then washed five times with PBST and further incubated for 1 hr at 37° with 100 μl of alkaline phosphatase-conjugated goat anti-human IgA(α), anti-human IgA1 (α1), anti-human IgA2 (α2), anti-human IgM (μ), and anti-human IgG (γ), each diluted 1 : 1000 in PBST. After five washes with PBST, bound antibodies were detected after the addition of 100 μl of 1 mg/ml paranitrophenyl phosphate (Sigma Chemical Co., St Louis, MO) as a substrate and incubation for 1 hr at 37°. Absorbance at 405 nm (A405) was measured with a microplate reader (Multiscan Bichromatic: Lobsystems, Helsinki, Finland).
HIV-1 RNA analysis
HIV-1MN and HIV-1Th22PF6 stock viruses were prepared by incubation with 100 tissue culture infectious dose 50 (TCID50) and normal human PBL activated with 5 μg of phytohaemagglutinin per ml for 24 hr, then cultured for 7 days.19 The cell-free supernatants were stored at −130° until use as a virus source. All procedures for infection and maintenance of human PBL–NOD-scid mice were performed in a biosafety level 3 facility in the National Institute of Infectious Diseases, Tokyo, Japan, under standard caging conditions. One week after the last administration of human IL-18, 105 TCID50 HIV-1MN and HIV-1Th22PF6 in 0·1 ml PBS were inoculated peritoneally into the human PBL–NOD-scid mice. One week later, the same dose of HIV-1MN was again inoculated. One week after the last inoculation, the mice were killed and examined for HIV RNA copy numbers in their sera. HIV RNA levels were determined from sera of the human PBL–NOD-scid mice given IL-18 and infected with HIV-1 using quantitative polymerase chain reaction amplification (Amplicor HIV-1 MonitorTM Test, Roche Diagnostics, Branchburg, NJ). HIV-1 RNA copy numbers were assessed using the manufacturer's reference standards. The HIV-1 assays differed in their lower level of sensitivity at 400 RNA copies/ml.
Statistical analysis
Statistical analysis was performed by anova. P-values of 0·05 or less were considered to indicate statistical significance.
Results
Effects of human IL-18, IL-12, IL-4 and IL-6 with human CD45-, CD4- and CD8-positive cells in NOD-scid mice
To investigate the effects of IL-18, IL-12, IL-4 and IL-6 with CD45+, CD4+ and CD8+ cells in human PBL–NOD-scid mice, spleen cells after administration with the cytokines were stained with mAbs to human CD45, CD4 and CD8, and examined by FACS. Even the addition of low doses of IL-18 or IL-12 (62·5 ng/mouse) induced substantial increases in CD45+ (29·7% or 16·2%), CD4+ CD8− (10·2% or 10·6%), and CD4− CD8+ cells (18·2% or 11·7%), in comparison with PBS (Fig. 1), while CD45+, CD4+ CD8−, and CD4− CD8+ cells were stimulated with higher doses of IL-18 (250 ng/mouse). Moreover, IL-18 and IL-12 stimulated these cells to a greater degree than IL-4 and IL-6 (data not shown). Thus, IL-18 and IL-12 were considered to be potential stimulators for the grafting of human PBL in the NOD-scid system. However, the relative ratio of CD4+ CD8− and CD4− CD8+ cells was fewer in the mice stimulated with higher doses of IL-12 (250 ng/mouse) than IL-18 (Fig. 2a). As an advanced experiment, we examined whether the cells were stimulated by mixtures of IL-18 and IL-12. As shown in Fig. 2(a,b), CD45+, CD4+ CD8−, and CD4− CD8+ cell proportions increased significantly, though the populations decreased, in the spleens of human PBL–NOD-scid mice after co-administration of IL-18 and IL-12, in comparison with PBS. Further, stimulation by IL-18 or IL-12 alone revealed a significant increase of the absolute numbers of CD45+, CD4+ CD8−, and CD4− CD8+ cells (Fig. 2b).
Figure 2.
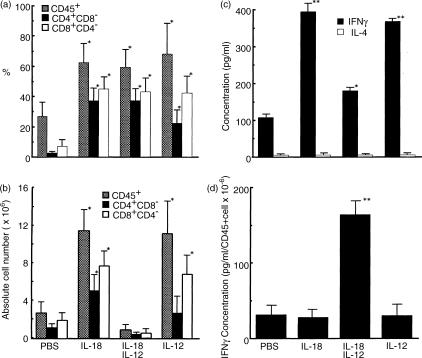
Effects of human IL-12 alone, human IL-18 alone, or IL-18 + IL-12 on T-cell proportion and population in grafted mice. Determinations of the proportion (a) and population (b) of human CD45+ cells, and human CD4+ CD8− and human CD4− CD8+ T cells from the spleens of grafted mice administrated with PBS and cytokines (250 ng/mouse) are shown. Human lymphocytes in the spleen cells were analysed 3 weeks after the last administration. Human CD45+ cells, and human CD4+ CD8− and human CD4− CD8+ T-cell populations were calculated from total spleen cell numbers of the mice using the proportions (%) of these cells. Analyses were performed on gated lymphocytes with FSC/SSC characteristics. Results are expressed as means ±SEM for four independent assays. (c) Serum IFN-γ and IL-4 concentration levels as determined by ELISA. Serum was collected 3 weeks after the last administration. (d) Ratio of IFN-γ concentration to human CD45+ cells in the spleen. Each value represents the mean ±SD of triplicate samples. Asterisks denote significant differences vs. PBS (*P < 0·05, **P < 0·01).
Human IFN-γ induction by human IL-18 and human IL-12 in NOD-scid mice
IL-18 has been shown to be a strong co-factor along with IL-12 for type 1 cytokine (IFN-γ) induction.12,14,20–22 We examined the effects of IL-18 and IL-12 alone, as well as those of IL-18 + IL-12 on human IFN-γ and IL-4 production in NOD-scid mice. As shown in Fig. 2(c), we found that IL-18 or IL-12 alone dramatically enhanced human IFN-γ production in comparison with the control (PBS) in sera from human PBL–NOD-scid mice. Furthermore, a combined IL-18 + IL-12 administration showed lower increases of human IFN-γ production (Fig. 2c) and higher increases of human IFN-γ production per number of human CD45 spleen cells (Fig. 2d). However, IL-18 or IL-12 alone did not affect human IFN-γ production per number of human CD45 cells in comparison with the control (Fig. 2d). Human IL-4 levels were tested as an undetectable concentration (5 pg/ml) for the sake of convenience in all mice (Fig. 2c).
Apoptotic cell induction by human IL-18 and IL-12 in NOD-scid mice
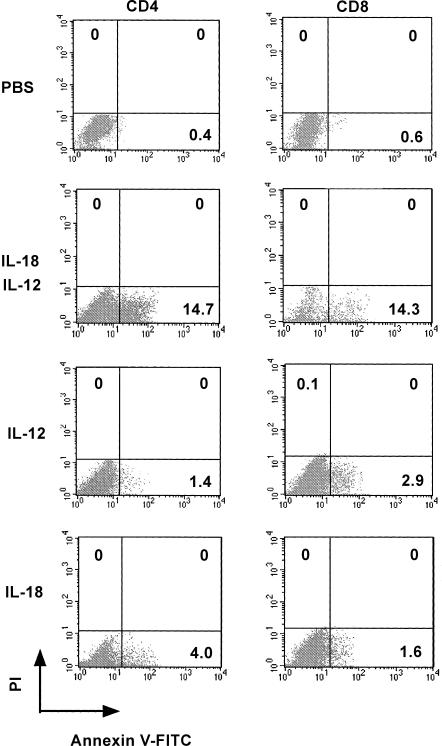
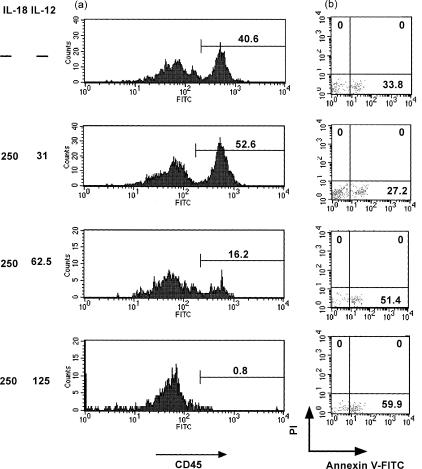
As shown in Fig. 2, circulating human lymphocytes treated with IL-18 + IL-12 were reproducibly characterized by a decrease in the number of human CD45+ cells, as well as human CD4+ CD8− and human CD4− CD8+ T cells in the spleen of human PBL–NOD-scid mice. We then evaluated the percentage of annexin V+ cells 21 days after the last cytokine administration. Lymphocyte migration into the spleen generated by the stimulation of IL-18 + IL-12 exhibited high levels of annexin V+ CD4+ (14·7%) and CD8+ T (14·3%) cells, as compared with the control and IL-18 or IL-12 alone (Fig. 3). We also examined the direct effects of IL-18 + IL-12 on human lymphocytes for the induction of apoptotic cells in the peritoneal cavity and found that IL-12 dramatically enhanced IL-18-dependent annexin V+ CD8+ T-cell induction in a dose-dependent manner (Fig. 4). In contrast, high doses of the combined cytokines notably reduced hu-CD45+ cell populations in the peritoneal cavity (Fig. 4).
Figure 3.
Induction of apoptosis in spleen human CD4+ and human CD8+ T cells from human PBL–NOD-scid mice treated with PBS, human IL-18 alone, human IL-12 alone, or IL-18 + IL-12. Flow cytometric analyses of annexin V+ human CD4+ and CD8+ T cells are presented. Human PBL–NOD-scid mice were given PBS and cytokines (250 ng/mouse). Analyses were performed on gated lymphocytes with FSC/SSC characteristics. Furthermore, PI− CD4+ and PI− CD8+ T cells were also gated on the quadrants. The lymphocytes (dots) can be distinguished in the quadrant analyses of PI and annexin V. The number in each quadrant indicates differentiation markers. Data are representative of three independent experiments with similar results obtained in each experiment.
Figure 4.
Effects of human IL-12 on human IL-18-dependent up-regulation of human CD4+ and CD8+ T cells from the peritoneal cavity in human PBL–NOD-scid mice. The histogram shows CD45 single-stained lymphocytes. The number in each histogram indicates the per cent of gated cells. Analyses were performed on gated lymphocytes with FSC/SCC characteristics. Flow cytometric analyses of annexin V+ human CD8+ T cells. Analyses were performed on gated PI− human CD8+ T cells. The lymphocytes can be distinguished in the lower right analysis quadrants of PI and annexin V. Numbers in the quadrants indicate the per cent of gated cells expressing the indicated markers. Data are representative of three independent experiments with similar results obtained in each.
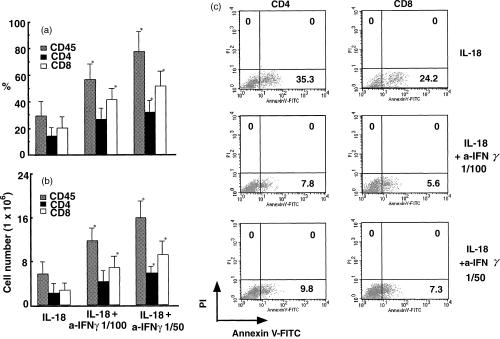
Effects of human IL-18 and IFN-γ on the migration of lymphocytes and apoptotic cell induction
We next focused on the mechanism of lymphocyte reduction in NOD-scid mice stimulated with IL-18 in combination with IL-12. Increasing doses of IFN-γ, known to induce cell cycle arrest and apoptosis,23,24 in serum correlated very well with the levels of apoptotic human CD4+ and CD8+ T cells (Figs 2 and 3). To confirm the effects of IFN-γ on the induction of apoptotic cells, the anti-IFN-γ neutralizing antibody was administered to human PBL–NOD-scid mice in combination with IL-18. As seen in Fig. 5(a,b), the proportions and populations of IL-18-dependent human CD4+ CD8− and CD8+ CD4− T cells were significantly up-regulated by the neutralizing anti-IFN-γ mAb. In contrast, the IL-18-dependent annexin V+ CD4+ and CD8+ T-cell populations were decreased by the anti-IFN-γ neutralizing antibody.
Figure 5.
Effects of anti-human IFN-γ mAb on human IL-18-dependent up-regulation and apoptotic cell induction of human CD45+ cells, and CD4+, and CD8+ T cells. Flow cytometric analyses of these cells present in the spleen from grafted NOD-scid mice treated with PBS, IL-18 (250 ng) + anti-IFN-γ mAb (1/100 dilution), and IL-18 (250 ng) + anti-IFN-γ mAb (1/50 dilution) were performed. The proportion (a) and population (b) of the cells were analysed on gated lymphocytes with FSC/SCC characteristics. Results are expressed as means ±SEM of three independent assays. (c) Annexin V+ hu-CD4+ or CD8+ T cells as determined by FACS analyses performed on gated PI− human CD4+ or CD8+ T cells. Lymphocytes can be distinguished in the quadrants of PI and annexin V. The number in the lower right quadrant indicates the per cent of gated cells expressing the indicated markers. Asterisks denote significant differences vs. IL-18 (*P < 0·05). Data are representative of three independent experiments with similar results obtained in each.
Human immunoglobulin levels in the sera of human PBL–NOD-scid mice following treatment with human IL-12 and IL-18
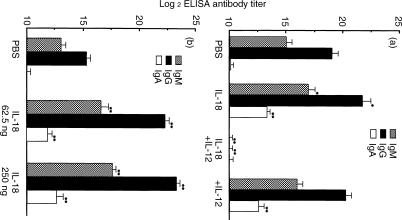
Cytokine expression strongly influences the production of specific immunoglobulin isotypes, as for example, IFN-γ induces class switching to the IgG2a isotype.25,26 To investigate a possible connection between stimulation with IL-18 and IL-12 and human immunoglobulin class switching in sera taken from human PBL–NOD-scid mice injected with PBS, IL-18 alone, IL-12 alone, or IL-18 + IL-12, the human IgM, IgG and IgA titres were measured. As shown in Fig. 6(a), titres of human IgM, IgG and IgA1 in PBL–NOD-scid mice given IL-18 or IL-12 alone were higher than those observed in PBS or IL-12 + IL-18 administered mice. In addition, administrations of IL-18 at both 62·5 and 250 ng/mouse induced significantly higher (P < 0·01) serum IgM, IgG and IgA1 titre levels as compared to the control (PBS group) (Fig. 6b). In contrast, IL-12 + IL-18 administration significantly reduced (P < 0·01) serum human IgM and IgG levels. IgA2 was not detected in serum from any of the mice (data not shown). Furthermore, the anti-IFN-γ antibody was effective for IL-18-dependent grafting of human lymphocytes, but did not cause an IL-18-dependent immunoglobulin titre increase in serum (data not shown). To evaluate the polyclonal antibody induction to antigens by these cytokines, deposits of IgM, IgG and IgA1 were measured in glomeruli from the mice. As shown in Fig. 7, IgG and IgM were observed in glomeruli from all groups, while deposits of IgA were also observed in those administered IL-18 alone, but not with PBS, IL-12 alone, or IL-12 + IL-18. Further, C3 was localized in the glomeruli of all treated mice.
Figure 6.
Effects of human IL-18 and human IL-12 on immunoglobulin production in sera from human PBL–NOD-scid mice. Sera were collected 3 weeks after the last administration of cytokines. Human serum IgM, IgG and IgA1 titres were analysed by ELISA. The human PBL–NOD-scid mice were given (a) PBS, IL-18 (250 ng), IL-18 (250 ng) + IL-12 (250 ng), and IL-12 (250 ng), or (b) PBS, IL-18 (62·5 ng), and IL-18 (250 ng). Results are expressed as the mean ±SD of log2 of the reciprocal titres of the lowest dilution that resulted in an absorbance of 0·1 above the conjugated control (no coat) in triplicate assays. The experiments were performed on three lots of serum antibodies from the administrated human PBL–NOD-scid mice, and similar results were obtained in each experiment. Asterisks denote significant differences vs. PBS (*P < 0·05, **P < 0·01).
Figure 7.
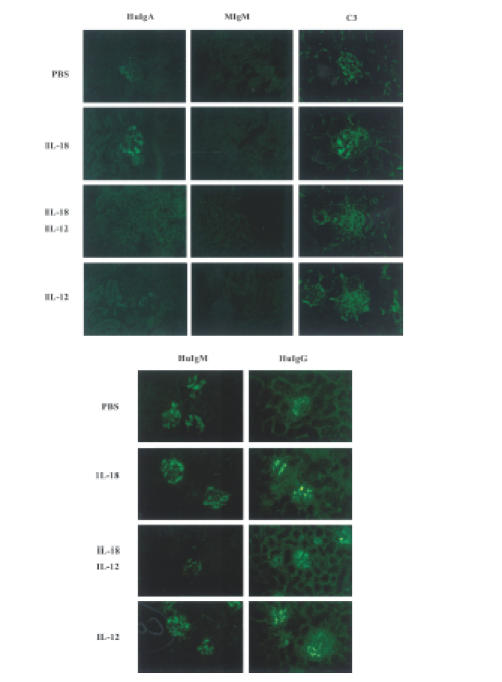
Immune fluorescence findings in kidneys from mice presented in Fig. 6(a). The kidney sections were stained by FITC-conjugated mAbs (human IgM, IgG, IgA1, mouse IgM, and C3). Magnification × 400. Results are representative of three independent experiments with similar results obtained in each.
Quantification of HIV-1 RNA in the human PBL–NOD-scid mice infected with HIV-1
To evaluate the effect of HIV infection on the activity, IL-18-dependent human T cells grafted into NOD-scid mice, HIV-1MN and HIV-1Th22PF6 were inoculated and quantification of HIV-1 RNA copies in the sera was performed. HIV-1 RNA was detected in the controls (PBS-administrated human PBL–NOD-scid mice). In contrast, greater than two- and 30-fold increases in HIV-1MN and HIV-1Th22PF6 RNA copy numbers, respectively, were detected (Table 1).
Table 1.
HIV-1 copy number in plasma from human PBL–NOD-scid mice inoculated peritoneally with HIV-1
| Stimulation | HIV-1 | Copy number (per ml) | |
|---|---|---|---|
| Exp. 1 | PBS | MN | 6842 |
| Exp. 2 | IL-18 | MN | 14604 |
| Exp. 3 | IL-18 | MN | 14155 |
| Exp. 4 | PBS | Th22PF6 | 15928 |
| Exp. 5 | PBS | Th22PF6 | 16728 |
| Exp. 6 | IL-18 | Th22PF6 | 541670 |
| Exp. 7 | IL-18 | Th22PF6 | 619650 |
| Exp. 8 | IL-18 | none | nd |
None, HIV-1 was not administered into human PBL-NOD-scid mice; nd, not detected.
Discussion
We examined the biological activities of human IL-18, IL-12, IL-4 and IL-6 on the grafting and migration of human lymphocytes, and found that IL-18 and IL-12 separately were more effective for grafting and activation of human lymphocytes in NOD-scid mice. In particular, IL-18 was not only a stimulator for CD8+ T cells but also CD4+ T cells in the mice. In addition, IL-18 and IL-12 stimulated migration of human lymphocytes in the spleen, because total spleen cell numbers of human PBL–NOD-scid mice increased in the injections of the two cytokines. However, a combination of IL-18 and IL-12 caused a remarkable down-regulation. IL-18 has been cloned as an IFN-γ-inducing factor,9,12 and in combination with IL-12 is known to activate IFN-γ production synergistically in T, B, and natural killer cells.22,27–29 We also found that IL-18 acted synergistically with IL-12 for IFN-γ production in the human PBL–NOD-scid mice. In T and B cells, up-regulation of the IL-18 receptor by IL-12 stimulation is one prevailing reason for the synergistic effect of IL-12 and IL-18 toward IFN-γ induction.29,30 IFN-γ increases were inversely correlated to the down-regulation of CD4+ and CD8+ T cells following the administration of a combination of IL-18 and IL-12. The IFN-γ thus produced, in general, has two necessary roles; one that involves the development of CD8+ cells into cytolytic effector cells and another that directly inhibits the growth of tumour cells.31 IFN-γ markedly increases the expression of both soluble and membrane-bound CD95 (Fas) in a dose-dependent manner,32 and has also been shown to induce cell cycle arrest and apoptosis in a B-cell line.23,24
IL-18 and IL-12 co-administration induced CD4+ and CD8+ T-cell apoptosis as well as IFN-γ production in the spleen and peritoneal cavity of human PBL–NOD-scid mice. The IL-18-dependent increase was also up-regulated, whereas IL-18-dependent apoptotic T-cell induction was inhibited by neutralizing with the anti-human IFN-γ mAb. In addition, exogenous IFN-γ also inhibited increases in the number of IL-18-dependent CD45+, CD4+ CD8−, and CD4− CD8+ T cells in NOD-scid spleens (data not shown). These findings suggest that IFN-γ might be essential for the regulation of human T cells during IL-12 and IL-18 administration in this mouse system.
Cytotoxic human T cells induced in the scid mice were accompanied by an increased sensitivity to apoptosis, which is known to regulate proliferation and maintain lymphocyte homeostasis.33,34 Nevertheless, a small proportion of the activated cytotoxic T cells survived and formed the memory population. Furthermore, the activation-induced cell death of cytotoxic T cells may occur at some sites in the body during immune stimulation.33 Since the cytotoxic T cells are not deleted, they survive and remain in NOD-scid mice tissues. In contrast, apoptotic human T-cell appearance is dependent on cytotoxicity against mouse tissues and is up-regulated during infiltration in a long-term NOD-scid culture. The infiltration of a large number of CD8+ T cells was also confirmed in several of the NOD-scid tissues such as those from the kidney, liver, gingival tissues and bone marrow (data not shown) Moreover, cytotoxic effects were demonstrated indirectly in mice given IL-18 + IL-12 by weight loss and an exhausted appearance.
Recently, Yoshimoto et al. reported that caspase-1 and IL-18 may be critical for the regulation of IgE production in vivo, providing potential therapeutic targets for allergic disorders.16 They also suggested that IL-18 may be a potential target of study in the effort to provide new insight into the potential mechanisms through which Th2 responses become dominant in an individual, and noted that there may be circumstances in which IL-18 is secreted independently of IL-12. Immunoglobulin class switching differentiation probably follows a tightly regulated programme that is activated by CD40 ligand, IL-4 and IL-10.35 Furthermore, distinct differences have been revealed in IgA and IgE isotype induction.36 To investigate the possible effect of human IL-18 in human IgA class switching as well as IgE, we used the human PBL–NOD-scid model system, in which specific antibodies were evaluated by the rate of human immunoglobulin deposits in mice glomeruli. Since serum from a human PBL–NOD-scid mouse influences the original PBL before grafting can produce a distinct concentration of human immunoglobulin, the effects of cytokines in serum human immunoglobulin cannot be evaluated correctly. The presence of immunoglobulin deposits revealed that a complex had formed between human immunoglobulin and mouse antigen during human immune reactions after grafting. IL-18 induced higher human IgA1 deposits in the glomeruli as compared with the PBS controls, whereas a combination of human IL-18 and human IL-12 reduced human IgM, IgG and IgA1 production in both serum and deposits in the glomeruli. It was previously reported that a combination of IL-12 and IL-18 induced anti-CD40-activated B cells to produce IFN-γ, inhibiting IL-4-dependent IgE and IL-G production,27 which is in agreement with our results. We also demonstrated that human IL-18 alone has human IL-12-independent effects in human PBL–NOD-scid mice, which showed that it may be effective for Th2 T-cell-dependent immunoglobulin-class switching in a long-time culture system.
Human PBL–NOD-scid mice have also been used for studying disorders other than HIV infection.2 Therefore, because of the higher levels of human lymphocyte grafting demonstrated by human IL-18, we also examined HIV-1 infection with human IL-18 and controls (PBS-administered human PBL–NOD-scid mice). An HIV-1 RNA assay showed higher rates of both HIV-1MN and HIV-1Th22PF6 infection in mice given human IL-18 than in the controls (Table 1), which indicated that IL-18 may support HIV infection to human lymphocytes in this mouse system. Many AIDS patients are characterized by IgA deposits in tissues such as the glomerular mesangium, hepatic sinusoids, and/or the skin.37 Human IL-18 also up-regulated human IgA1 production in serum and deposits in the glomeruli (Figs 6, 7), showing that a human IL-18-administered mouse model may be suitable for the study of HIV infection.
The pathogenesis of IgA nephropathy, the most prevalent form of glomerulonephritis world-wide, involves circulating macromolecular IgA1 complexes. However, the molecular mechanism(s) of the disease remain poorly understood and there is no good animal model for the study of mesangial IgA1 deposits, though many investigators have reported these mechanisms using rat or mouse models.38–40 Early studies that indicated that mice and rats do not have a subtype of IgA such as IgA1 may have been in error, as the present results suggest that IL-18 might induce Th cells to form an IgA1 immunocomplex. Chintalacharuvu et al. suggested that the increased production of IL-4 and IL-5 by PBL from IgA nephropathy patients could result in the production of abnormally glycosylated IgA.41 Therefore, cytokines related to Th2 responses may be the keys in the development of IgA nephropathy. IL-18 administered human PBL–NOD-scid mice with mesangial IgA1 deposits may provide a useful in vivo model for the development of a new mechanism. This is the first known report to document a humanized model responsible for the generation of IgA nephropathy.
In conclusion, it was demonstrated that IL-18 up-regulated CD4+ and CD8+ T-cell grafting and migration in several tissues from NOD-scid mice, as well as regulated class-switching immunoglobulin. IFN-γ production might be the key for matured human T-cell increases or decreases in mouse systems. Therefore, we indicated that IL-18-administered human PBL-NOD-scid mice were characterized as an effective humanized model of the human immune system, and useful for investigation of both HIV infection and IgAN in long-term cultures. Furthermore, this mouse system provides an appropriate environment for the study of cells unique to the human immune system.
Acknowledgments
The authors thank Dr Kenichi Shikata for his helpful discussions and advice. This work was supported in part by Grants-in-Aid for Development Scientific Research (11771171 and 12557158) from the Ministry of Education, Science, and Culture of Japan, and by a grant from the Japan Health Science Foundation to H.S. This study was also funded by a part of ‘Ground Research for Space Utilization’ promoted by NASDA and the Japan Space Forum.
References
- 1.Greiner DL, Shultz LD, Yates J, et al. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice. Am J Pathol. 1995;146:888–902. [PMC free article] [PubMed] [Google Scholar]
- 2.Hesselton RM, Greiner DL, Mordes JP, Rajan TV, Sullivan JL, Shultz LD. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type I infection in NOD/ LtSz-scid/ scid mice. J Infect Dis. 1995;172:974–82. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- 3.Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–91. [PubMed] [Google Scholar]
- 4.Shimoni A, Marcus H, Canaan A, Ergas D, Berrebi DM, Reisner Y. A model for human B-chronic lymphocytic leukemia in human/mouse radiation chimera: evidence for tumor-mediated suppression of antibody production in low-stage disease. Blood. 1997;89:2210–18. [PubMed] [Google Scholar]
- 5.Greiner DL, Shultz LD. Use of NOD/ lt Sz-scid/scid mice in biomedical research. In: Leiter EH, Aktinson MA, editors. NOD Mice and Related Strains: Research Applications in Diabetes, AIDS, Cancer and Other Diseases. Austin: R. G. Landes Co.; 1998. pp. 173–203. [Google Scholar]
- 6.Tary-Lehmann M, Saxon A, Lehmann PV. The human immune system in hu-PBL-scid mice. Immunol Today. 1995;16:529–33. doi: 10.1016/0167-5699(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 7.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 8.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 9.Ushio S, Namba M, Okura T, et al. Cloning of the cDNA for human IFN-γ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–9. [PubMed] [Google Scholar]
- 10.Matsui K, Yoshimoto T, Tsutsui H, et al. Propionibacterium acnes treatment diminishes CD4+NK1.1+ T cells but induces type 1 T cells in the liver by induction of IL-12 and IL-18 production from kupffer cells. J Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- 11.Kohno K, Kataoka J, Ohtsuki T, et al. IFN-γ-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–50. [PubMed] [Google Scholar]
- 12.Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces IFNγ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto I, Kohno K, Tanimoto T, Ikegami H, Kunimoto M. Development of CD8 effector T cells is differentially regulated by IL-18 and IL-12. J Immunol. 1999;162:3202–11. [PubMed] [Google Scholar]
- 14.Micallef MJ, Ohtsuki T, Kohno K, et al. Interferon-γ-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647–51. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino T, Yagita H, Ortaldo JRR, Wiltrout H, Young HA. In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur J Immunol. 2000;30:1998–2006. doi: 10.1002/1521-4141(200007)30:7<1998::AID-IMMU1998>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimoto T, Mizutani H, Tsutsui H, et al. IL-18 induction of IgE: dependence on CD4+T cells, IL-4 and Stat 6. Nature Immunol. 2000;1:132–7. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 17.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;333:256–9. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 18.Senpuku H, Iizima T, Koga T, Nisizawa T. Identification of hu-antigenic epitopes in an alanine-rich repeating region using sera from hu-PBL-SCID mice immunized with a surface protein antigen of Streptococcus mutans. Oral Microbiol Immunol. 1996;5:343–9. doi: 10.1111/j.1399-302x.1996.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 19.Honda M, Matsuo K, Nakasone T, et al. Protective immune responses induced by secretion of a chimeric soluble protein from a recombinant Mycobacterium bovis bacillus Calmette-Guerin vector candidate vaccine for human immunodeficiency virus type 1 in small animals. Proc Nat Acad Sci USA. 1995;92:10693–7. doi: 10.1073/pnas.92.23.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon γ production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–64. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 21.Dinarello CA, Novick D, Puren AJ, et al. Overview of interleukin-18: more than an interferon-γ inducing factor. J Leukoc Biol. 1998;63:658–64. [PubMed] [Google Scholar]
- 22.Robinson D, Shibuya K, Mui A, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NF-kB. Immunity. 1997;7:571–81. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 23.Bromberg JF, Horvath CM, Wen ZR, Schreiber D, Darnell JE. Transcriptionally active stat1 is required for the antiproliferative effects of both interferon α and interferon γ. Proc Natl Acad Sci USA. 1996;93:7673–8. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trubiani O, Bosco D, DiPrimio R. Interferon-γ (IFN-γ) induces programmed cell death in differentiated human leukemic B cell lines. Exp Cell Res. 1994;215:23–7. doi: 10.1006/excr.1994.1309. [DOI] [PubMed] [Google Scholar]
- 25.Snapper C, Paul WE. Interferon γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 26.Berton MT, Uhr JW, Vitetta ES. Synthesis of germ-line γ1 immunoglobulin heavy-chain transcripts in resting B cell: induction by interleukin 4 and inhibition by interferon γ. Proc Natl Acad Sci USA. 1989;86:2829–33. doi: 10.1073/pnas.86.8.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimoto T, Okamura H, Tagawa Y, Iwakura Y, Nakanishi K. Interleukin 18 together with interleukin 12 inhibit IgE production by induction of interferon-γ production from activated B cells. Proc Natl Acad Sci USA. 1997;94:3948–53. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda K, Tsutsui H, Yoshimoto T, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimoto T, Takeda K, Tanaka T, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells synergism with IL-18 for IFN-γ production. J Immunol. 1998;161:3400–7. [PubMed] [Google Scholar]
- 30.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the haemostatic regulation of immune responses. Immunol Today. 1995;16:569–74. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 31.Farrar JD, Katz KH, Windsor J, Thrush G, Scheuermann EH, et al. Cancer dormancy. VII. A regulatory role for CD8+ T cells and IFN-γ in establishing and maintaining the tumor-dormant state. J Immunol. 1999;162:2842–9. [PubMed] [Google Scholar]
- 32.Riccoli A, Starace D, D'Alessio A, et al. TNF-α and IFNγ regulate expression and function of the Fas system in the seminiferous epithelium. J Immunol. 2000;165:743–9. doi: 10.4049/jimmunol.165.2.743. [DOI] [PubMed] [Google Scholar]
- 33.Akbar AN, Salmon M. Cellular environment and apoptosis tissue microenvironments control activated T-cell death. Immunol Today. 1997;18:72–6. doi: 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed R, Gray D. Immunological memory and protective immunity understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 35.Cerutti A, Zan H, Schaffer A, et al. CD40 ligand and appropriate cytokines induce switching to IgG, IgA and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol. 1998;160:2145–57. [PMC free article] [PubMed] [Google Scholar]
- 36.Zelazowski P, Carratsco D, Rosas FR, Moorman MA, Bravo R, Anapper CM. B cells genetically deficient in the c-Rel transactivation domain home selective defects in germline CH transcription and Ig class switching. J Immunol. 1997;159:3133–9. [PubMed] [Google Scholar]
- 37.Lightfoote MM, Folks TM, Redfield R, Gold J, Sell KW. Circulating IgA immune complexes in AIDS. Immunol Invest. 1985;14:341–5. doi: 10.3109/08820138509022669. [DOI] [PubMed] [Google Scholar]
- 38.Launay P, Grossetete B, Arcos-Fajardo M, et al. Fc alpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Bergeris disease) Evidence for pathogenic soluble receptor-Igα complexes in patients and CD89 transgenic mice. J Exp Med. 2000;191:1999–2009. doi: 10.1084/jem.191.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamata T, Nogaki F, Fagarason S, et al. Increased frequency of surface IgA-positive plasma cells in the intestinal lamina propria and decreased IgA excretion in hyper IgA (HIGA) mice. A murine model of IgA nephropathy with hyperserum IgA. J Immunol. 2000;165:1387–94. doi: 10.4049/jimmunol.165.3.1387. [DOI] [PubMed] [Google Scholar]
- 40.van Dixhoorn MG, Sato T, Muizert Y, et al. Combined glomerular deposition of polymeric rat IgA and IgG aggravates renal inflammation. Kidney Int. 2000;58:90–9. doi: 10.1046/j.1523-1755.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 41.Chintalacharuvu SR, Emancipator SN. The glycosylation of IgA produced by murine B cells is altered by Th2 cytokines. J Immunol. 1997;159:2327–33. [PubMed] [Google Scholar]