Abstract
We examined the proliferative and cytokine-producing activities of CD4+ T cells from young mice of the senescence-accelerated mouse strain SAMP1, which had shown markedly low T-dependent antibody-producing responses. When splenic T cells were cultured with concanavalin A (Con A), the percentage of CD4+ cells decreased earlier in SAMP1 than in C3H/He mice. At 40 hr of culture, the percentage of BrdU-labelled proliferating CD4+ cells increased strongly in C3H/He, but only slightly in SAMP1. When purified CD4+ T cells were cultured with Con A, the percentage of 5-bromo-2′-deoxyuridine (BrdU)-labelled cells peaked at around 48 hr of culture in both strains, but decreased significantly at 64 hr in SAMP1. The production of interleukin (IL)-2 but not IL-4 or interferon-γ (IFN-γ) was significantly lower in SAMP1 than in C3H/He at 48 hr of culture. IL-2 production was also markedly low in SAMP1, even under the stimulation of anti-CD3 with anti-CD28 antibodies. The frequency of cells producing IL-2 was significantly lower in SAMP1 than in C3H/He at 6–24 hr of culture with Con A. The percentage of annexin-positive and propidium iodide (PI)-negative apoptotic cells was significantly higher in SAMP1 than in C3H/He at 96 hr of culture. Exogenous IL-2 prevented the decrease in BrdU-labelled cells and the increase in apoptotic cells in the SAMP1 cell culture. These results indicate that SAMP1 CD4+ T cells cannot produce IL-2 at levels sufficient to support cell proliferation and survival. This may account for the weak T-dependent antibody response in SAMP1 mice.
Introduction
Studies in a number of laboratories have indicated that many immune responses decline progressively with ageing. This phenomenon has been termed immunosenescence, and was mainly associated with T-cell responses,1,2 including an age-associated decline in helper activity in the antibody response, delayed-type hypersensitivity (DTH) responses, proliferative responses and interleukin (IL)-2 production.3–7 To clarify the mechanisms responsible for these declines, many researchers have examined age-related alterations in various factors such as cell-surface molecules, signal transducers and transcription factors in T cells.2,8 However, the mechanisms of these age-related changes remain obscure, and further progress in the study of immunosenescence may require a new experimental system.
Inbred strains of SAM mice, established by Takeda et al.,9,10 consist of short-lived, accelerated senescence-prone strains (SAMP) and long-lived, accelerated senescence-resistant strains (SAMR). SAMP1, one of the SAMP strains, shows a short life span of ≈ 10 months and early progressive changes with ageing, such as a loss of passivity and reactivity, coarse skin, hair loss, periophthalmic lesions and increased lordokyphosis of the spine, after normal development and maturation. SAMP1 mice also show an early, age-related functional decline in their immune system, especially a profound defect in their in vitro antibody response to T-dependent (TD) antigen, even at 2 months of age,11 and the SAMP1 strain of mice is considered to be a very useful animal model for research into immunosenescence. Our previous research demonstrated that these defects in SAMP1 mice can be ascribed to impaired T helper (Th) cell activity.12 However, what causes this functional impairment in the Th cells remains to be determined. The aim of this study was to investigate the crucial alterations or impairments in the Th-cell functions of SAMP1 mice.
CD4+ T cells stimulated by antigen-pulsed antigen-presenting cells (APCs),13 anti-CD3 and anti-CD28 antibodies,14 or lectins such as concanavalin A (Con A),15 produce a large amount of IL-2, which then drives the proliferation of the activated T cells and maintains their survival. Activated CD4+ T cells also produce other cytokines such as IL-4 and interferon-γ (IFN-γ). IL-4 promotes the differentiation of activated CD4+ T cells into effector cells, called Th2 cells, for the antibody response to TD antigen, whereas IFN-γ inhibits it.16 Therefore, we examined the ability of SAMP1 splenic CD4+ T cells to proliferate, survive and produce IL-2, IL-4 and IFN-γ in response to Con A and, in addition, studied the production of IL-2 under anti-CD3 antibody stimulation, in the presence of absence of anti-CD28 antibody.
Materials and methods
Mice
SAMP1 (H-2 k) or C3H/HeSlc (H-2 k) mice (2–3 months old) were used in this study, the latter as a major histocompatibility complex (MHC)-identical control strain. The C3H/He mice were originally purchased from Japan SLC Inc. (Hamamatsu, Japan). These mice were reared under conventional conditions at 24 ± 2° and 45 ± 10% humidity, and were maintained on a commercial diet (CE-2; Nihon CLEA, Tokyo, Japan) and tap water ad libitum. All animals were handled according to the Guidelines for Animal Experiments of Kyoto University.
Preparation of splenic T cells and CD4+ T cells
Whole spleen-cell suspensions were prepared in cold Eagle's minimal essential medium (MEM) (Nissui Seiyaku, Tokyo, Japan) adjusted to pH 7·2 with sodium bicarbonate, as previously described.11 Splenic T cells were enriched by nylon-wool adherence of spleen cells, according to the methods of Julius et al.17 The purity of the T cells in the suspension was > 70%, as determined by flow cytometry. CD4+ T cells were positively separated from whole spleen cells by using Dynabeads Mouse CD4 (L3T4) (Dynal, Oslo, Norway), and detachment from the Dynabeads was achieved by incubating the rosetted CD4+ T cells with DETACHaBEAD (Dynal), in accordance with the manufacturer's instructions. The purity of the purified CD4+ T cells was > 95%, as determined by flow cytometry.
Culture
The culture medium used was RPMI-1640 (Nissui Seiyaku) supplemented with 5% fetal calf serum (FCS), 2 mm l-glutamine and 5 × 10−5 m 2-mercaptoethanol, adjusted to pH 7·2 with sodium bicarbonate. Splenic T cells and purified CD4+ T cells were cultured at 2·5 × 106 and 2·5 × 105 cells per well in 24-well and 96-well culture plates (Becton-Dickinson, Franklin Lakes, NJ), respectively, with 2·5 µg/ml Con A (Sigma, St. Louis, MO) in a humidified atmosphere of 5% CO2 in air at 37°. In some cultures of purified CD4+ T cells, 20 ng/ml IL-2, IL-4, or IFN-γ (BD Pharmingen, San Diego, CA) was added at 48 hr of culture. Purified CD4+ T cells were also cultured under stimulation with 5 µg/ml immobilized anti-CD3 antibody (145-2C11; Cedarlane, Hornby, Ontario, Canada), with or without 5 µg/ml soluble anti-CD28 antibody (37·51; BD Pharmingen) instead of stimulation with Con A. The cells or culture supernatants were then harvested at several time-points and assayed.
Staining and analysis of splenic T cells
Splenic T cells were harvested after 0, 20 or 40 hr of culture and stained with a fluorescein isothiocyanate (FITC)-labelled anti-CD4 monoclonal antibody (mAb) (CT-CD4) or anti-CD8 mAb (CT-CD8a; both from Caltag Laboratories, Burlingame, CA) on ice in the dark for 20 min. The immunostained cells were analysed using a FACscan flow cytometer and CELLQuest software (both from Becton-Dickinson) with scatter gates set to include all viable cells.
Measurement of proliferating cells
A 5-bromo-2′-deoxyuridine (BrdU) Labelling and Detection KitI (Roche Diagnostics, Mannheim, Germany) was used to assay the proliferating cells in a culture of splenic T cells or purified CD4+ T cells. BrdU was added to the culture at a concentration of 10 µg/ml 30 min prior to harvest. The harvested cells were stained with the antibodies included in the kit in accordance with the manufacturer's instructions. For splenic T cells, the cells were immunostained with phycoerythrin (PE)-labelled anti-CD4 or anti-CD8 mAbs prior to staining with anti-BrdU. The BrdU incorporation and the cellularity were analysed by flow cytometry, and the proliferating CD4+ cells were distinguished from the proliferating CD8+ cells. For purified CD4+ T cells, the cells were finally resuspended in 50 µg/ml propidium iodide (PI). The BrdU incorporation and DNA content were analysed by using flow cytometry, and those cells incorporating BrdU at the S-phase were defined as proliferating cells.
Enzyme-linked immunosorbent assay (ELISA)
The supernatants from cultures of purified CD4+ T cells were harvested at 24, 48, 72 or 96 hr of culture. The amount of IL-2, IL-4 and IFN-γ in the supernatant was assayed by using a Quantikine M ELISA kit (R & D Systems, Minneapolis, MN) in accordance with the manufacturer's instructions. Finally, the absorbance at 450 and 570 nm in each well was measured using a Corona Microplate reader MTP100 (Corona Electric, Ibaragi, Japan).
ELISPOT assay
A Murine IL-2 Eli-spot (Diaclone, Besançon, France) was used to assay the frequency of cells producing IL-2 in the culture of purified CD4+ T cells. Cells were harvested after 6, 12 or 24 hr of culture with Con A and incubated for 12 hr in polyvinylidene difluoride (PVDF)-bottomed-well plates (Millipore, Bedford, MA), which were precoated with the primary anti-IL-2 antibody. In some cultures (to assay the frequency of cells producing IL-2 immediately after stimulation), cells were cultured directly in the antibody-coated plates with Con A for 6 hr. Cells were removed from the wells by lysis and washing with phosphate-buffered saline (PBS) containing 0·1% Tween, and the plates were incubated with the secondary biotinylated antibody, followed by incubation with streptavidin conjugated to alkaline phosphatase. The plates were then incubated with 5-bromo-4-chloro-3-indolyl-phosphate and nitrobluetetrazolium (BCIP/NBT) substrate, rinsed with distilled water and dried overnight. The number of spots per well was counted under a stereoscopic microscope.
Cell death
Purified CD4+ T cells were harvested at 24, 48, 72, 96 or 120 hr of culture and assayed for cell death according to two methods. In method one, the harvested cells were fixed with cold 70% ethanol. Then, fixed cells were washed twice with PBS containing 1% FCS and stained with 50 µg/ml PI. Cells with a lower DNA content than the diploid cells (which are termed ‘low DNA-content cells’ in the present report) were counted (using flow cytometry) as cells undergoing apoptotic cell death. In the second method, an Annexin-V-FLUOS Staining Kit (Roche) was used to assay apoptotic cells, showing the translocation of phosphatidylserine (PS) from the inner side of the plasma membrane to the outer layer. Results of the PI exclusion test were examined simultaneously to discriminate necrotic cells from the annexin V positively stained apoptotic cells. The cells were stained and analysed by flow cytometry.
Statistical analysis
Significant differences between the SAMP1 and C3H/He mouse cells were determined by repeated-measures analysis of variance (anova), except the results of the IL-2 ELISPOT assay, which were analysed by standard anova. The significance of the effect of exogenous cytokines on the proliferation of CD4+ T cells was determined by anova with a Student-Newman-Keuls posthoc test. The statistical analysis was performed by StatView 5·0 J (SAS Institute, Cary, NC) software, referring to the publication Statistical Principles in Experimental Design.18
Results
Changes in the cellularity of splenic T cells cultured with Con A
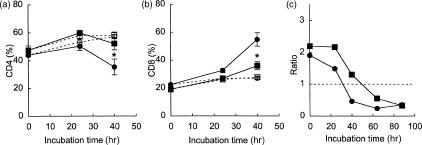
Splenic T cells (enriched by nylon-wool adherence) were cultured with Con A, and changes in the cellularity of the cultures were examined (Fig. 1). In the SAMP1 cells, the percentage of CD4+ cells decreased and was significantly lower than in the C3H/He cells at 40 hr of culture (Fig. 1a). Conversely, the percentage of CD8+ cells increased to a significantly higher level in the SAMP1 cells than in the C3H/He cells at the same time-point (Fig. 1b). The ratio of CD4+ to CD8+ cells decreased earlier in the SAMP1 cells than in the C3H/He cells, but the ratio at 88 hr of culture in the C3H/He cells reached the same level as the SAMP1 cells at 40 hr of culture (Fig. 1c). These results indicate that changes in the cellularity of CD4+ and CD8+ cells occurred earlier in the SAMP1 cells than in the C3H/He cells during culture.
Figure 1.
Changes in the percentage of CD4+ (a) and CD8+ cells (b) in splenic T-cell cultures. Splenic T cells from SAMP1 (circles) or C3H/He (squares) mice were cultured with (closed symbols) or without (open symbols) concanavalin A (Con A). The data represent the mean value ± SE from three experiments. In one experiment, the cells were cultured for longer (c). The data from this experiment are shown as a ratio of the CD4+ cells to the CD8+ cells at each time-point. Statistically significant differences between the SAMP1 and C3H/He cells are shown by an asterisk (*P < 0·05).
Proliferation of CD4+ and CD8+ cells in the T-cell culture
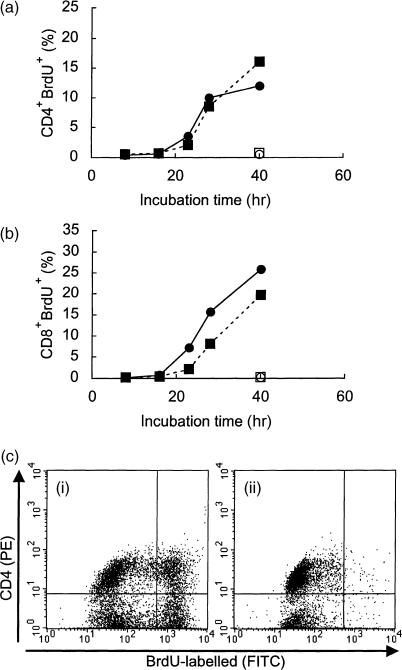
To investigate whether the early decrease in the percentage of CD4+ cells in the SAMP1 T-cell culture could be attributable to some impairment of the proliferative ability of the CD4+ cells, splenic T cells were stimulated with Con A and the proliferation of CD4+ and CD8+ cells was compared between SAMP1 and C3H/He cells (Fig. 2). In the C3H/He cells, the percentages of proliferating CD4+ and CD8+ cells increased strongly at 40 hr of culture. In the SAMP1 cells, however, the percentage of proliferating CD4+ cells was increased only slightly at 40 hr, although the proliferating CD8+ cells increased strongly. This early slowdown in the proliferation of CD4+ cells from SAMP1 mice was also observed when similar comparisons were made with B10.BR cells (data not shown). These results suggest that this low proliferative activity may be related to the early decrease in CD4+ cells in the culture of SAMP1 splenic T cells.
Figure 2.
Kinetics of proliferating CD4+ (a) and CD8+ cells (b) in splenic T-cell cultures. Splenic T cells from SAMP1 (circles) or C3H/He (squares) mice were cultured with (closed symbols) or without (open symbols) concanavalin A (Con A). At each time-point, the cells were labelled with 5-bromo-2′-deoxyuridine (BrdU) for the last 30 min of the culture period and the percentages of BrdU-labelled CD4+ and CD8+ cells were analysed using flow cytometry. Figure 2(c) shows representative dot-plot data from SAMP1 cells cultured with (i) or without (ii) Con A for 40 hr. The vertical and horizontal axes show the intensity of CD4 expression and the BrdU incorporation, respectively. Dot-plots of proliferating CD4+ cells are shown in the right upper quadrant. The percentage of proliferating CD4+ cells was calculated by dot-plot analysis. The percentage of proliferating CD8+ cells was defined likewise. A representative plot is shown.
Proliferative response of purified CD4+ T cells
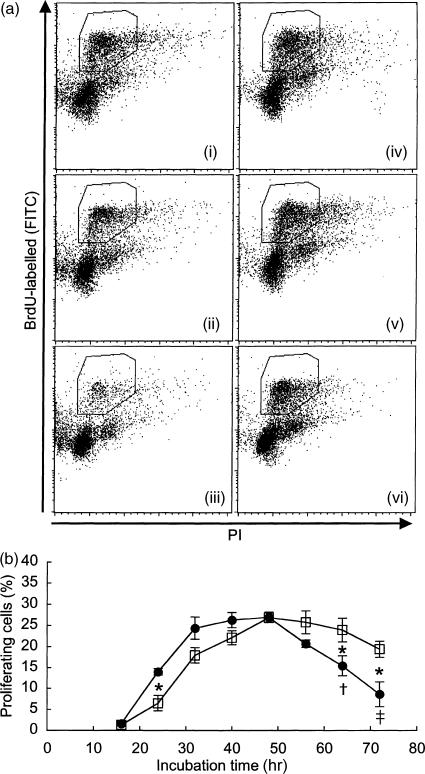
To confirm impairment in the proliferation of SAMP1 CD4+ T cells, and to determine whether the cause of this impairment exists in the CD4+ T cells themselves or in other cells, CD4+ T cells purified from whole spleen cells were stimulated with Con A, and the changes in proliferative response over the time course of culture were compared between SAMP1 and C3H/He cells (Fig. 3). Although the percentage of proliferating cells began to increase slightly earlier in the SAMP1 cells than in the C3H/He cells, this increase in proliferating cells approached a plateau at around 48 hr of culture in both strains. However, in the SAMP1 cells, the percentage of proliferating cells had decreased significantly at 64 hr of culture, being significantly lower than in the C3H/He cells at this time-point. In contrast, in the C3H/He cells, the percentage of proliferating cells decreased slightly at 72 hr of culture, but the difference between 48 hr and 72 hr of culture was not significant. These results indicate that CD4+ T cells from SAMP1 mice cannot continue to proliferate for a long time, and that this impaired proliferation is intrinsic to the CD4+ T cells, but not to other cells.
Figure 3.
Kinetics of proliferating cells in a purified CD4+ T cell culture stimulated with concanavalin A (Con A). At each time-point, the cells were labelled with 5-bromo-2′-deoxyuridine (BrdU) for the last 30 min of the culture period, and the percentage of proliferating cells was analysed by flow cytometry. Figure 3(a) shows representative dot-plot data from cells harvested at 48 hr (i and iv), 64 hr (ii and v) and 72 hr (iii and vi) of culture for SAMP1 (i–iii) and C3H/He (iv–vi) mice. The vertical and horizontal axes indicate the BrdU incorporation and the DNA content, respectively. Dot-plots of the proliferating cells are shown in the region surrounded by the solid line. Figure 3(b) represents summarized data from both SAMP1 (closed symbols) and C3H/He (open symbols) cells. The data represent the mean value ± SE from eight cell preparations, each of which was prepared separately from eight mice in four experiments. Statistically significant differences between the two strains (*P < 0·05, **P < 0·01) and between 48 and 64 or 72 hr of culture within each strain (†P < 0·05, ‡P < 0·01) are shown.
Production of IL-2, IL-4 and IFN-γ by purified CD4+ T cells in culture
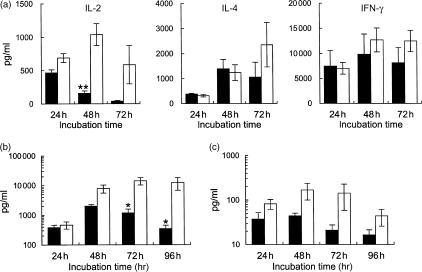
We observed that the early decrease in the percentage of CD4+ cells among the SAMP1 T cells, as shown in Fig. 1, was attenuated by mixing C3H/He T cells into the culture in a dose-dependent manner, but the addition of SAMP1 T cells did not affect the cellularity of the C3H/He T-cell culture (data not shown). This observation suggests the possibility of reduced production of certain humoral factors in the SAMP1 T-cell culture. Therefore, to investigate whether the CD4+ T cells from SAMP1 mice can produce cytokines to support their proliferation and survival, and to influence their development into effector cells with Th2 cell activity, we compared the amounts of IL-2, IL-4 and IFN-γ produced by Con A-stimulated CD4+ T cells between SAMP1 and C3H/He mice (Fig. 4a). CD4+ T cells from SAMP1 mice produced IL-2 in substantial amounts, similar to the levels produced by C3H/He cells at 24 hr of culture. However, IL-2 production in the SAMP1 cells then decreased markedly as the culture time extended, while the C3H/He cells increased or maintained their IL-2 levels. At 48 hr of culture, the IL-2 production was significantly lower in the SAMP1 cell cultures than in the C3H/He cell cultures. On the other hand, the production of IL-4 and IFN-γ was not significantly different between SAMP1 and C3H/He cells. Moreover, in the culture stimulated with immobilized anti-CD3 and soluble anti-CD28 antibodies, the SAMP1 cells also showed a significantly lower production of IL-2 than the C3H/He cells at 72 and 96 hr of culture (Fig. 4b), and a decrease in IL-2 production was also observed in the SAMP1 cell culture stimulated with anti-CD3 antibody alone (Fig. 4c). These results indicate that the SAMP1 CD4+ T cells cannot produce sufficient amounts of IL-2, although their production of IL-4 and IFN-γ are normal, and that their production of IL-2 is low even under stimulation with anti-CD3 and anti-CD28 antibodies.
Figure 4.
Production of interleukin (IL)-2, IL-4 and interferon-γ (IFN-γ) by purified CD4+ T cells. Cells from SAMP1 (closed column) or C3H/He (open column) mice were cultured with concanavalin A (Con A), and the supernatants were assayed for IL-2, IL-4 and IFN-γ at 24– 72 hr of culture (a). The data represent the mean value ± SE from eight cell preparations, each of which was prepared separately from eight mice in four experiments. In other experiments, cells were cultured under the stimulation of immobilized anti-CD3 antibody together with (b) or without (c) soluble anti-CD28 antibody, and assayed for IL-2 production at 24–96 hr of culture. The data represent the mean value ± SE of four experiments. Statistically significant differences between strains are shown (**P < 0·01, *P < 0·05).
The frequency of cells producing IL-2
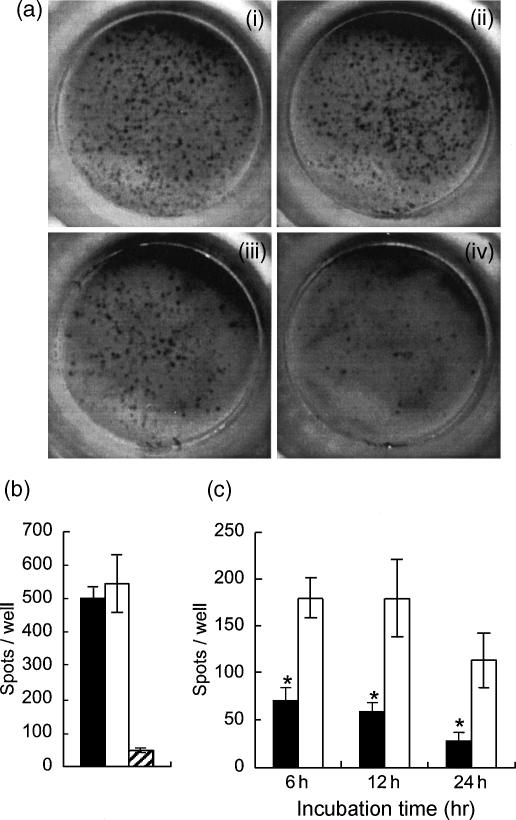
To investigate IL-2 production of the SAMP1 CD4+ T cells at each time-point of culture, we examined the frequency of cells producing IL-2 by ELISPOT assay (Fig. 5). Purified CD4+ T cells were cultured with Con A, harvested and washed at 6–24 hr of culture, and incubated in the anti-IL-2 antibody-coated plates for 12 hr. The number of spots per well was significantly lower in the SAMP1 cell cultures than in the C3H/He cell cultures at every time-point (Fig. 5c). The number of spots in the assay of cells harvested at 48 hr of culture was decreased to background levels in both strains (data not shown). In addition, to investigate the production of IL-2 immediately after stimulation, purified CD4+ T cells were stimulated with Con A directly in the antibody-coated plates for 6 hr. Unlike the results shown in Fig. 5(c), the number of spots per well in the SAMP1 cells was as high as that in the C3H/He cells (Fig. 5b). These results indicate that the SAMP1 CD4+ T cells are able to produce IL-2 immediately after stimulation with Con A, but thereafter cannot maintain the production of IL-2.
Figure 5.
Cells producing interleukin (IL)-2 in the culture of purified CD4+ T cells. Cells from SAMP1 (closed column) or C3H/He (open column) mice were cultured with concanavalin A (Con A), and assayed for cells producing IL-2 at 6–24 hr of culture by ELISPOT assay (c). In some of the cultures, cells were stimulated with Con A directly in the assay plates for 6 hr (b). The hatched column is the C3H/He cell culture without Con A. Representative spots of the SAMP1 cells (ii and iv) or the C3H/He cells (i and iii) stimulated directly in the assay plates (i and ii) or harvested and assayed at 6 hr after stimulation (iii and iv) are shown in (a). The data represent the mean value ± SE from three experiments. Statistically significant differences between strains are shown (*P < 0·05)
Effect of exogenous cytokines on the proliferative response of purified CD4+ T cells
To investigate whether early cessation of the proliferation of SAMP1 CD4+ T cells was caused by insufficient IL-2 production, we examined the effect of exogenous cytokines on the impaired proliferative response of the SAMP1 cells. Purified CD4+ T cells were stimulated with Con A alone at the start of culture, IL-2, IL-4 or IFN-γ was added exogenously at 48 hr of culture, and the proliferative response at 72 hr of culture was assayed (Fig. 6). Exogenous IL-2 prevented the significant decrease previously observed in proliferating cells at 72 hr in the SAMP1 cell culture; the proliferative response was significantly higher in the culture supplied with exogenous IL-2 than in the medium alone and in control cells cultured without exogenous IL-2. Exogenous IL-2 also prevented the previously observed slight decrease in proliferation at 72 hr in the C3H/He cell culture. IL-4, but not IFN-γ, partially prevented the previously observed decrease at 72 hr in the SAMP1 cell culture. These results prove that insufficient IL-2 production in the CD4+ T cells leads to an early cessation of cell proliferation in the SAMP1 cells.
Figure 6.
Effects of exogenous cytokines on the proliferative response of purified CD4+ T cells. Cells from SAMP1 or C3H/He mice were stimulated with concanavalin A (Con A) and supplemented with interleukin (IL)-2, IL-4, interferon-γ (IFN-γ) or medium alone at 48 hr of culture. The percentage of proliferating cells was then analysed at 48 hr (control) and at 72 hr of culture, and the ratio of the percentage at 72 hr to that at 48 hr was calculated in each experiment. The data represent the mean value ± SE from three experiments. Statistically significant differences between the control (72 hr None) and medium alone or cytokine cultures is shown (*P < 0·05, **P < 0·01).
Apoptosis of purified CD4+ T cells in the culture
Our preliminary flow cytometric tests with forward scatter and side scatter showed an early increase in the percentage of dead cells in the culture of SAMP1 CD4+ T cells (data not shown). Therefore, we investigated whether insufficient IL-2 production might result in the early apoptosis of CD4+ T cells from SAMP1 mice and reduce their survival. First, we evaluated (by using flow cytometric analysis of low DNA-content cells) the cells undergoing apoptotic cell death (Fig. 7a,b). The percentage of low DNA-content cells increased earlier in the SAMP1 cell cultures than in the C3H/He cell cultures, with significant differences observed at 72 and 96 hr of culture. However, the addition of exogenous IL-2 at 48 hr of culture prevented this increase in the low DNA-content cells and eliminated the differences between the SAMP1 and C3H/He cells. Moreover, to confirm the early apoptosis in the SAMP1 CD4+ T-cell culture, cells were assayed by staining with annexin V for the translocation of PS, which is exposed on the surface of apoptotic lymphocytes and recognized by phagocytes which remove them19,20 (Fig. 7c,d). Although the percentage of annexin-positive and PI-negative apoptotic cells gradually increased until 72 hr of culture in both strains, thereafter it increased more in the SAMP1 cells than in the C3H/He cells with significant differences observed at 96 hr and 120 hr of culture. The addition of exogenous IL-2 to the culture retarded the increase in apoptotic cells and eliminated the difference between the SAMP1 and C3H/He cells at 96 hr of culture. These results indicate that purified CD4+ T cells from SAMP1 mice undergo apoptosis early in culture because of insufficient IL-2 production.
Figure 7.
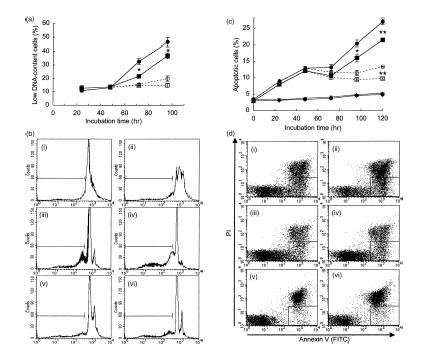
Apoptotic cells in a culture of purified CD4+ T cells. Cells from SAMP1 (circles) or C3H/He (squares) mice were stimulated with concanavalin A (Con A) and supplemented with interleukin (IL)-2 in some cultures (open symbols) but not in others (closed symbols) at 48 hr of culture. The percentage of low DNA-content cells was analysed by staining with propidium iodide (PI) and flow cytometry (a). Figure 7(b) shows representative data from three experiments. Histograms of cells at 24 hr (i), 48 hr (ii), 72 hr (iii and v) and 96 hr (iv and vi) of culture with (v and vi) or without (iii and iv) the addition of IL-2 at 48 hr in SAMP1 (closed histogram) or C3H/He (open) cells are shown. Histograms of the low DNA-content cells are shown within the marker. In addition, the percentage of annexin-positive and PI-negative cells, representing apoptotic cells, was analysed by using flow cytometry (c). Figure 7(d) shows representative data from three experiments. Dot-plots of cells at 96 hr of culture with (i–iv) or without (v and vi) Con A, and with (iii and iv) or without (i and ii) the addition of exogenous IL-2 at 48 hr of culture in SAMP1 (ii, iv and vi) or C3H/He (i, iii and v) cells are shown. Dot-plots from apoptotic cells are shown in the region surrounded by the solid line. The data represent the mean value ± SE from three experiments. Statistically significant differences between the two strains under the same culture conditions are shown (*P < 0·05, **P < 0·01).
Discussion
Based on the results of this study, we found that splenic CD4+ T cells from SAMP1 mice show abnormally short-lasting production of IL-2 in response to stimulation with Con A, leading to the impaired proliferation and survival of these cells, and that they show markedly low production of IL-2, even under stimulation with anti-CD3 and anti-CD28 antibodies. Such impairments caused by the insufficient production of IL-2 may account for the impaired antibody response and natural killer (NK) activity of spleen cells from SAMP1 mice observed in our previous studies.11,12,21,22 This idea is supported by the other studies showing an improvement in the in vitro immune responses of the cells from aged mice following the addition of exogenous IL-2 to the culture.6,13
SAMP1 mice have been used as an animal model of immunosenescence because we previously demonstrated that SAMP1 mice show not only senescence acceleration but also a decrease in several immune functions even at a young age, most notably a reduced in vitro T-dependent antibody response owing to the impaired activity of Th cells.11,12 However, some researchers might think that there are some difficulties of using SAMP1 mice as an animal model for immunosenescence because whether SAMP1 Th cells show a decrease in fundamental functions, including proliferative response, cytokine production and survival ability, as do the cells from aged mice, remains to be examined. Thus, our present report is the first demonstration that young animals, from a unique mouse strain, which have a short life span and senescence acceleration, show impaired proliferation and survival of splenic CD4+ T cells resulting from abnormally low IL-2 production. In addition, this experimental system, using the splenic CD4+ T cells from SAMP1, mice is a potentially useful model for using to further investigate the mechanisms of immunosenescence. In this model, cells demonstrating several functional decreases, as observed in aged animals, are available at a younger age, and genomic research regarding the low production of IL-2 is possible.
Although the CD4+ T cells from SAMP1 mice show remarkable impairments in their production of IL-2, other Con A-induced responses were normal. The restoration of impaired proliferation and the prolonged survival of CD4+ T cells following exogenous IL-2 addition to the SAMP1 cultures indicate that the central machinery of proliferation and survival is normal. This is also supported by the observation that proliferation was normal and strong during the early stages of culture in the SAMP1 cells. The restoration of the impaired functions by the addition of IL-2 indirectly indicates that high-affinity IL-2 receptor function is normal in the activated SAMP1 CD4+ T cells. Furthermore, the production of IL-4 and IFN-γ in SAMP1 was not different from that in the C3H/He cells, despite the markedly impaired production of IL-2 in the SAMP1 cells. These findings indicate that the common intracellular events required to produce these cytokines and the machinery for the secretion of humoral factors are normal in CD4+ T cells from SAMP1 mice. Therefore, it is clear that CD4+ T cells from SAMP1 mice have some defect in the intracellular events required to produce a sufficient amount of IL-2.
CD4+ T cells produce a large amount of IL-2 in the presence of costimulation through the CD28 molecules on their surface.23,24 Other research has previously shown that T lymphocytes derived from CD28−/− mutant mice have impaired responses to lectins, where lectin stimulation did not trigger IL-2 production, and IL-2 receptor alpha expression was significantly decreased.15 Therefore, the question arises of whether the low IL-2 production in the SAMP1 CD4+ T cells might be caused by a decrease in the expression and function of CD28 or in its downstream signal transduction. In fact, the production of IL-2 by CD4+ T cells was markedly low under stimulation with anti-CD3 and anti-CD28 antibodies in SAMP1. However, a similarly low production of IL-2 was also observed, even under stimulation with anti-CD3 antibody alone. As discussed above, our results indicate indirectly that the IL-2 receptor functions normally on the surface of SAMP1 CD4+ T cells after activation. Moreover, flow cytometric analysis confirmed that the expression of CD28 on the surface of SAMP1 CD4+ T cells was only slightly lower, at a level of about 80% that of the C3H/He cells (data not shown). These results indicate that the mechanism of low IL-2 production in the SAMP1 CD4+ T cells is not directly related to a decrease in the expression and function of CD28 or its signal transduction downstream.
It is of interest that the SAMP1 CD4+ T cells produce normal levels of IL-2 immediately after stimulation, but cannot thereafter maintain the production of IL-2 at levels sufficient to support proliferation and survival. For continuous IL-2 production in stimulated CD4+ T cells, the consecutive transcription of IL-2 mRNA and the stability of the mRNA produced are important.25 The stability and degradation of the mRNAs of several cytokines, including IL-2, the mechanism of which is largely unknown, is thought to be regulated by trans-acting factors, including AUF-1, ELAV family members and tristetraprolin (TTP), which bind AU-rich elements (AREs) in the 3′ untranslated region of the mRNA.26–28 Recently, Pucci and colleagues reported that when splenic CD4+ T cells were stimulated by anti-CD3 and anti-CD28 mAbs, the IL-2 concentration in the culture supernatants increased progressively in proportion to the time after stimulation in young mice, whereas in old mice, it increased at first but decreased soon thereafter.14 This observation is similar to ours in that the IL-2 production of C3H/He cells increased over a much longer period of time than that of the SAMP1 cells. Interestingly, they observed increased instability in the IL-2 mRNA from the CD4+ T cells of older mice at a later time-point of culture. They also observed that in the presence of cycloheximide, the CD4+ T cells from the older mice did not show a decrease in the binding activity of Nil2-a, which is a zinc finger protein that binds to a negative regulatory element to down-regulate the transcription of IL-2 mRNA, as compared to CD4+ T cells from younger mice. Therefore, we suspect the possibility that some inadequate regulation in the transcription of IL-2 mRNA and the instability of the mRNA produced may be involved in the impaired IL-2 production by the CD4+ T cells from SAMP1 mice.
We cannot deny the possibility of additional defects besides the low IL-2 production in SAMP1 CD4+ T cells, because our previous studies using splenocytes have indicated that Th2 cell activity, but not Th1 activity, was impaired in young SAMP1 mice.11,12 However, IL-2 is an essential factor for all T-cell responses. Thus, in further studies, we will explore why CD4+ T cells from SAMP1 mice cannot maintain IL-2 production and examine whether this insufficient production of IL-2 is the primary cause of the impaired antibody response in SAMP1 mice. We believe that further studies to solve these questions in SAMP1 mice will contribute to the elucidation of the mechanisms of immunosenescence.
Acknowledgments
We would like to thank Dr Akira Ohta, PhD, from the Institute for Frontier Medical Sciences, Kyoto University, for his helpful suggestions regarding the statistical analyses, and T. Matsushita, E. Deguchi, T. Watanabe, S. Matsuda and Y. Ikeda from the Institute for Frontier Medical Sciences, Kyoto University, for the maintenance of the mice. This research was partially supported by a budget and grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a Research Grant for Longevity Science (11C-04) from the Ministry of Health, Labour and Welfare of Japan.
Abbreviations
- APCs
antigen-presenting cells
- BCIP/NBT
5-bromo-4-chloro-3-indolyl-phosphate and nitrobluetetrazolium
- BrdU
5-bromo-2′-deoxyuridine
- Con A
concanavalin A
- ELISA
enzyme-linked immunosorbent assay
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- PI
propidium iodide
- PS
phosphatidylserine
- PVDF
polyvinylidene fluoride
- TD
T-dependent
- Th2
T helper 2
References
- 1.Makinodan T, Kay MM. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- 2.Pawelec G, Effros RB, Caruso C, Remarque E, Barnett Y, Solana R. T cells and aging (update February 1999) Front Biosci. 1999;4:D216–69. doi: 10.2741/pawelec. [DOI] [PubMed] [Google Scholar]
- 3.Hirokawa K, Utsuyama M, Kasai M, Kurashima C, Ishijima S, Zeng YX. Understanding the mechanism of the age-change of thymic function to promote T cell differentiation. Immunol Lett. 1994;40:269–77. doi: 10.1016/0165-2478(94)00065-4. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner WA, Makinodan T, Blahd WH. In vivo evaluation of age-associated changes in delayed-type hypersensitivity. Mech Ageing Dev. 1980;12:261–8. doi: 10.1016/0047-6374(80)90049-4. [DOI] [PubMed] [Google Scholar]
- 5.Hirokawa K, Utsuyama M, Goto H, Kuramoto K. Differential rate of age-related decline in immune functions in genetically defined mice with different tumor incidence and life span. Gerontology. 1984;30:223–33. doi: 10.1159/000212636. [DOI] [PubMed] [Google Scholar]
- 6.Thoman ML, Weigle WO. Lymphokines and aging: interleukin-2 production and activity in aged animals. J Immunol. 1981;127:2102–6. [PubMed] [Google Scholar]
- 7.Wakikawa A, Utsuyama M, Wakabayashi A, Kitagawa M, Hirokawa K. Age-related alteration of cytokine production profile by T cell subsets in mice: a flow cytometric study. Exp Gerontol. 1999;34:231–42. doi: 10.1016/s0531-5565(98)00062-x. [DOI] [PubMed] [Google Scholar]
- 8.Hirokawa K. Age-related changes of signal transduction in T cells. Exp Gerontol. 1999;34:7–18. doi: 10.1016/s0531-5565(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 9.Takeda T, Hosokawa M, Takeshita S, et al. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–94. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 10.Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of accelerated senescence. J Am Geriatr Soc. 1991;39:911–9. doi: 10.1111/j.1532-5415.1991.tb04460.x. [DOI] [PubMed] [Google Scholar]
- 11.Hosokawa T, Hosono M, Higuchi K, Aoike A, Kawai K, Takeda T. Immune responses in newly developed short-lived SAM mice. I. Age-associated early decline in immune activities of cultured spleen cells. Immunology. 1987;62:419–23. [PMC free article] [PubMed] [Google Scholar]
- 12.Hosokawa T, Hosono M, Hanada K, Aoike A, Kawai K, Takeda T. Immune responses in newly developed short-lived SAM mice. II. Selectively impaired T-helper cell activity in in vitro antibody response. Immunology. 1987;62:425–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–24. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pucci S, Doria G, Barile S, Pioli C, Frasca D. Inhibition of IL-2 production by Nil-2-a in murine T cells. Int Immunol. 1998;10:1435–40. doi: 10.1093/intimm/10.10.1435. [DOI] [PubMed] [Google Scholar]
- 15.Shahinian A, Pfeffer K, Lee KP, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–12. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 17.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–9. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 18.Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. 3. New York: McGraw-Hill, Inc.; 1991. [Google Scholar]
- 19.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 20.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 21.Hanada K, Hosono M, Hosokawa T, Chen WE, Tsuboyama T, Takeda T. Immune responses in newly developed short-lived SAM mice. III. Genetic control of defective helper T-cell activity in in vitro primary antibody response. Immunology. 1989;68:540–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Hanada K, Katoh H, Hosokawa T, Hosono M, Takeda T. Immune responses in newly developed short-lived SAM mice. IV. Chromosomal location of a gene controlling defective helper T-cell activity. Immunology. 1991;74:160–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–30. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–6. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 25.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–42. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson A, Peltz SW. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- 27.Chen CY, Shyu AB. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–70. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 28.Raghavan A, Robison RL, McNabb J, Miller CR, Williams DA, Bohjanen PR. HuA and tristetraprolin are induced following T cell activation and display distinct but overlapping RNA binding specificities. J Biol Chem. 2001;276:47958–65. doi: 10.1074/jbc.M109511200. [DOI] [PubMed] [Google Scholar]