Abstract
It has been reported that serum immunoglobulin E (IgE) from certain atopic patients can sensitize basophils to release histamine in response to IgE-dependent histamine-releasing factors (HRFs). It has also been shown that patients suffering from severe forms of atopy may contain IgE autoantibodies. It was investigated whether HRF-responsive sera contained IgE autoantibodies and if there was an association between IgE autoreactivity and IgE-dependent responsiveness to HRF. The presence of HRF-responsive IgE (IgE+) in serum of patients with respiratory atopy was determined by stimulating stripped human basophils sensitized by serum with peripheral blood mononuclear cell (PBMC)-derived HRF, and measuring the release of histamine. In parallel, these sera were screened for the presence of IgE autoantibodies to nitrocellulose-blotted human cellular extracts. The capacity of IgE autoantigen-containing preparations to induce histamine release was tested in the stripped basophil assay. Eleven out of 52 sera contained IgE autoantibodies to blotted cellular extracts of human PBMCs or of the human epithelial cell line A431. No significant association was found between IgE autoreactivity and IgE-dependent responsiveness to HRF: 7/26 IgE+ sera contained IgE to human cellular extracts, and 4/26 of the sera without IgE+ did also. IgE autoantigen-containing extracts did not induce histamine release of appropriately sensitized basophils. By size-exclusion chromatography it was shown that a 32 000 MW autoantigen eluted in the >55 000 MW fraction, which indicates that this protein forms polymers or complexes with other macromolecules. This might explain the discrepancy between binding and histamine-releasing activity. A 20 000 MW IgE-defined autoantigen cross-reacted with a shrimp allergen. Our results indicate that IgE-reactivity to immunoblotted human protein and IgE-dependent HRF activity are distinct entities that may co-occur in atopic patients.
Introduction
Acute allergic symptoms are induced by the crosslinking of immunoglobulin E (IgE) antibodies on the surface of an effector cell (e.g. mast cell, basophil) by an allergen, which results in degranulation of mediators such as histamine.1 It is also well established that basophils can be activated to release mediators by IgE-independent histamine-releasing factors (HRF).2 Evidence for the presence of another type of HRF came from studies indicating that culture supernatants of human cells contained IgE-dependent HRF.3,4 IgE-dependent HRF, by definition, require the presence of IgE to induce histamine release and only certain types of IgE, designated IgE+, exert this reactivity.3 By definition, sera that fail to sensitize basophils for responsiveness to HRF were termed IgE−. Our group investigated the IgE-dependent histamine-releasing activity in culture supernatants of stimulated human peripheral blood mononuclear cells (PBMCs) using the stripped basophil bioassay.4,5 In this assay IgE antibodies are removed from human basophils with an acidic buffer, the cells are re-sensitized by serum, and the histamine release is investigated in response to stimuli. IgE-dependent responsiveness to HRF produced by mononuclear cells (HRFmn) was shown to be associated with atopic sensitization.5 HRFmn-responsive IgE was present in 40% of the sera from allergic rhinitis and asthma patients whereas it was absent in non-atopics.5 Sampson et al.6 showed that mononuclear cells from children with atopic dermatitis (AD) and food hypersensitivity produced IgE-dependent HRFmn, which declined with allergen avoidance and clinical remission of the skin disorder. Moreover, serum of these AD patients rendered basophils capable of secreting histamine in response to HRFmn, whereas serum from control subjects did not.
There are several possibilities to explain the responsiveness of IgE+. The feature discriminating IgE+ from other IgE antibodies might be present in the constant regions of IgE. IgE+ might be a differently glycosylated IgE3 or an alternative splice variant of the IgE molecule.7 It is known that a combination of two partial agonists can activate basophils. Accordingly, it is possible that both HRF and a structurally different form of IgE are necessary to achieve full basophil activation. HRF might then exert a priming effect on basophils. The histamine-releasing activity of the IgE-dependent HRF p23 cloned by MacDonald et al.8 might be due to such a priming process.
Alternatively, IgE+ reactivity might not be mediated by IgE alone, but in combination with IgG anti-IgE antibodies.9,10 Anti-IgE antibodies have been described to modulate basophil histamine release in a stimulating as well as in a blocking manner.10,11
A third possibility would be that IgE+ is a HRF-specific IgE antibody, and HRF an IgE autoantigen. HRF would then exert its reactivity, similar to conventional allergens, by cross-linking IgE on the surface of basophils. In this context it is noteworthy that several studies reported that patients suffering from severe forms of atopy contain IgE autoantibodies.12 IgE autoantibodies have been detected in sera from atopic dermatitis (AD) patients, recognizing different proteins in human cellular extracts on immunoblot.13 A strong IgE binding was associated with severe forms of atopic eczema.14 Several IgE-binding human proteins have been cloned by screening cDNA expression libraries from human tissues:15,16 Hom s 1 (55 kDa), Hom s 2 (10 000 MW), Hom s 3 (20 000 MW), Hom s 4 (36 000 MW), and Hom s 5 (43 000 MW).14 Other IgE autoantigens have been described by the group of Crameri et al.17–19 The allergens manganese-dependent superoxide dismutase (26 000 MW), acidic ribosomal phosphoprotein type 2 (11 000 MW) and cyclophilin B (22 000 MW) from Aspergillus fumigatus were IgE cross-reactive with their human homologues. It is not clear whether autoreactive IgE is produced during an allergic reaction to exogenous allergens, or, alternatively, that autoreactive IgE is induced by endogenous allergens released during the chronic allergic reaction. The fact that some autoreactive IgE antibodies are cross-reactive with an exogenous allergen indicates that autoreactive IgE production is in some instances induced by an exogenous allergen.
We have not been able to show binding of IgE to HRFmn with Western blotting and immunoprecipitation experiments. However, the concentration and the purity of HRFmn in our preparation might be too low for detection in these tests. In the basophil histamine release assay it is possible to detect low amounts of allergen and in the presence of a high concentration of irrelevant proteins.20 From model systems with common allergen we know that the sensitivity of the basophil assay is <0·05 ng/ml. The material used for stimulation with HRFmn had a total protein content of 537 µg/ml, which reflects the low purity of the HRFmn preparation.
The primary question of the current study was whether two published observations, IgE autoreactivity and IgE-dependent histamine-releasing activity, were related. For this purpose we screened sera from atopic patients for the presence of IgE autoantibodies to blotted proteins and analysed some IgE-defined autoantigens in more detail. In parallel, we tested sera for their capacity to sensitize stripped basophils to release histamine in response to PBMC-derived HRF. Furthermore it was studied whether IgE autoantigen-containing preparations induce histamine release in passively sensitized basophils. Our finding that IgE autoreactivity and IgE-dependent HRF activity seem to be distinct entities is discussed.
Materials and Methods
Preparation of human cellular extracts
Cellular extracts of the human cervix carcinoma cell line HeLa S3, and the monocyte-like cell lines U937 and MonoMac 6 were prepared according to the method described in the protocol for cytoplasmic extracts by Verheijden et al.21 Preparing extracts from the human epithelial cell line A431 and peripheral blood mononuclear cells was performed as described previously.13,14
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotting
SDS–PAGE, blotting to nitrocellulose and IgE immunodetection of extracts from A431 and PBMCs were performed as described previously14 and of HeLa, MonoMac, and U937 extracts as described by Roberts et al.22 Extracts from A431 and PBMCs were run on a reduced 12·5% polyacrylamide gel (0·2 mg protein/cm), the other extracts on a reduced 15% gel (0·5 mg protein/cm). In short, sera were added to the nitrocellulose strips 1 : 10 diluted in buffer containing 0·5% bovine serum albumin (BSA). In order to mimic the in vivo situation as closely as possible, the amount of serum was not adjusted for the total IgE. After overnight incubation, immunodetection was performed following similar incubation with 125I-labelled anti-IgE. To visualize IgE binding, the blots were exposed to X-ray film (Kodak, New York, NY) at −70° for 1 week. In the case-control study serum from patients with AD as classified according to Hanifin and Rajka23 were used as positive control, because AD sera are reported to contain IgE autoantibodies.12 Serum from AD patients D4, D5, D8, D11, MD, S, and M contained a total IgE of 4668, 6951, 9070, 1251, 1800, 2600, and 11 000 IU/ml, respectively, and were radioallergosorbent test (RAST) positive to at least one inhalant allergen. Since it has been described that Aspergillus allergens might cross-react with human proteins17 plasma #141, #144, and #145 which are RAST positive to Aspergillus (>15 IU specific IgE/ml) were also tested. IgE binding of serum from patients with respiratory atopy was compared to that of a serum of a non-atopic subject (serum VR, total IgE: 19 IU/ml, RAST negative to common inhalant allergens). Bands present in the negative control were excluded from analysis. The scoring of the blots was performed independently by two persons. IgE binding was classified into three categories: positive, negative and doubtful. Serum with a clear IgE binding to one or more human proteins was scored as positive. Only serum judged as positive by both was termed IgE autoreactive serum; serum with a doubtful or no IgE binding negative serum.
Stripped basophil histamine release bioassay
The histamine release assay was performed as described previously.24,25 In short, after removal of IgE from surface of basophils by an acidic buffer, the cells were sensitized by 150 µl human serum for 90 min at 37°. Stimulations (37°, 60 min) were performed in 1 mm CaCl2 using 2·5×106 cells in 250 µl and 50 µl allergen or 100 µl cellular extract or HRF preparation. To up-regulate IgE-mediated histamine release, the stimuli were diluted in HEPES buffer/1 mm CaCl2 containing recombinant interleukin-3 (IL-3, final concentration 600 pm; Pepro Tech Inc., Rocky Hill, NJ). Histamine was determined by fluorometric analysis as described by Siraganian.26
Production of IgE-dependent HRFmn
IgE-dependent HRFs produced by human PBMC were prepared as described elsewhere.27 In short, PBMC obtained by leucapheresis were isolated by Percoll centrifugation and elution, and stimulated for 18 hr with streptokinase/streptodornase (SK/SD) (Lederle Laboratories, Pearl River, NY). IgE-independent HRFs were removed from the culture supernatant by heparin Sepharose CL-6B (Pharmacia Biotech, Uppsaala, Sweden). Eleven HRF-preparations from different donors selected out of 29 preparations on basis of high reactivity were pooled and further purified by size-exclusion chromatography (Sephadex G75). Five fractions containing IgE-dependent histamine-releasing activity were pooled, and 10 times concentrated. The concentration of protein was 9·4 mg/ml. A culture medium preparation was produced in the same way for control experiments.
Calculation of HRF-responsive IgE levels
Since the histamine release experiments were performed on different days, an adjustment had to be made for interassay variability. The histamine release of basophils sensitized by 150 µl of serum was determined upon challenge with different concentrations of HRFmn (threefold dilutions, final protein concentration: 20–537 µg/ml). The HRF-response of the patients was related to the HRF dose–response curve of reference plasma #151. Plasma #151 was allotted 100 sensitizing units/ml, which corresponded to a histamine release of 66±9% (n = 8, mean±SD) to 537 µg/ml of HRFmn preparation. A serum containing more than three sensitizing units/ml was defined as HRFmn-reactive serum (>15% histamine release to 537 µg/ml of HRFmn preparation).
Characterization of atopic patients
This study was performed after written informed consent of the patients and was approved by the local ethical committee.
Twenty-six IgE+ and 26 IgE− sera from atopic non-asthmatics and asthmatics were described before28 (Table 1). These sera were tested in the case-control analysis to determine the correlation between IgE auto-reactivity to cellular extracts of human cell lines and IgE-dependent responsiveness to HRF. Moreover, an additional group of atopic non-asthmatic or mild asthmatic patients (15 IgE+ and 19 IgE−) and non-atopics (n = 15) were tested for IgE binding to extracts of the HeLa cell line. These latter sera were not included in the case–control analysis.
Table 1.
Characteristics of patients with respiratory atopy
| IgE autoreactivity | ||||||
|---|---|---|---|---|---|---|
| Patient no. | Total IgE (IU/ml) | Symptoms | IgE-dependent HRA (units/ml) | A431 | PBMC | HeLa |
| IgE+ sera | ||||||
| B2 | 98 | ANA | 67 | − | − | − |
| R3 | 230 | ANA | 58 | − | − | − |
| B3 | 206 | ANA | 32 | + | − | − |
| R4 | 1151 | ANA | 28 | − | − | − |
| R5 | 250 | ANA | 27 | − | − | − |
| R6 | 323 | ANA | 27 | − | − | − |
| R7 | 128 | ANA | 24 | − | − | − |
| R8 | 69 | ANA | 18 | − | − | − |
| R9 | 58 | ANA | 14 | − | − | − |
| R10 | 285 | ANA | 13 | − | − | − |
| R11 | 79 | ANA | 4 | − | − | − |
| A10 | 260 | AMA | 40 | − | + | − |
| A11 | 507 | AMA | 39 | − | − | − |
| A12 | 3568 | AMA | 28 | + | + | − |
| A13 | 716 | AMA | 26 | + | + | − |
| A15 | 189 | AMA | 18 | − | − | − |
| A16 | 365 | AMA | 13 | − | − | − |
| A17 | 63 | AMA | 12 | − | − | − |
| A19 | 52 | AMA | 10 | − | − | − |
| A20 | 178 | AMA | 9 | − | − | − |
| A21 | 311 | AMA | 9 | − | − | − |
| A23 | 349 | AMA | 8 | − | + | − |
| A24 | 247 | AMA | 6 | − | − | − |
| SA4 | 916 | ASA | 115 | + | ND | ND |
| SA7 | 293 | ASA | 14 | − | ND | ND |
| SA8 | 1800 | ASA | 7 | + | ND | ND |
| IgE− sera | ||||||
| B4 | 450 | ANA | 3 | + | + | − |
| B5 | 42 | ANA | 3 | − | − | − |
| B6 | 183 | ANA | 3 | − | − | − |
| B7 | 44 | ANA | 3 | − | − | − |
| R9 | 58 | ANA | 3 | − | − | − |
| R12 | 86 | ANA | 3 | − | − | − |
| R13 | 291 | ANA | 3 | − | − | − |
| R14 | 68 | ANA | 3 | − | − | − |
| R15 | 27 | ANA | 3 | − | − | − |
| R16 | 108 | ANA | 3 | − | − | − |
| R17 | 31 | ANA | 3 | − | − | − |
| R19 | 79 | ANA | 3 | − | − | − |
| R20 | 171 | ANA | 3 | + | − | + |
| R23 | 39 | ANA | 3 | − | − | − |
| R24 | 23 | ANA | 3 | − | − | − |
| R25 | 483 | ANA | 3 | − | − | − |
| A28 | 331 | AMA | 3 | − | + | − |
| A30 | 264 | AMA | 3 | − | − | − |
| A31 | 370 | AMA | 3 | − | − | − |
| A32 | 571 | AMA | 3 | − | − | − |
| A36 | 37 | AMA | 3 | − | − | − |
| A37 | 96 | AMA | 3 | − | − | − |
| A41 | 1197 | AMA | 3 | − | + | − |
| A44 | 163 | AMA | 3 | − | − | − |
| A47 | 418 | AMA | 3 | − | − | − |
| SA11 | 58 | ASA | 3 | − | ND | ND |
ND, not done; ANA, atopic non-asthma; AMA, atopic mild/moderate asthma; ASA, atopic severe asthma; HRA, histamine-releasing activity.
+ serum with IgE binding to blotted proteins.
− serum with doubtful or no IgE binding to blotted proteins.
Besides sera described above, five plasma samples from the diagnostic panel of the CLB were tested (Table 2). Plasma was defibrinated by recalcification and dialysed. The plasma samples were used to study the specificity and cross-reactivity of the IgE binding in more detail.
Table 2.
Reference plasma samples
| IgE autoreactivity | RAST (IU/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | IgE-dependent HRA | HeLa | MonoMAc | U937 | Total IgE (IU/ml) | M | CH | C | S | T |
| #13 | + | + | + | + | 850 | 0·2 | <0·1 | 2 | <0·1 | <0·1 |
| #164 | − | + | − | + | 360 | 66 | 24 | 23 | >100 | 33 |
| #36 | + | + | + | + | 9100 | 40 | ND | ND | ND | ND |
| #193 | + | + | − | + | 360 | 4 | ND | ND | ND | ND |
| #151 | + | + | − | + | 1600 | 24 | ND | ND | ND | ND |
ND, not done; HRA, histamine-releasing activity; M, house dust mite; CH, chironomids; C, cod; S, shrimp; T, shrimp tropomyosin.
Analysis of IgE-reactive autoantigens
Ammonium sulphate fractionation
HeLa cellular proteins were precipitated with ammonium sulphate (30%, 50%, 100% saturation) at 4°. In the 30%, 50% and 100% saturated ammonium sulphate (SAS) precipitates, respectively, 22%, 21%, and 14% of the total protein was present.
Size exclusion chromatography
One ml of 100% SAS precipitate (10·7 mg protein) was fractionated by size-exclusion chromatography using Sephadex G75 (35×1·2 cm). After elution of 14 ml (35% of column volume), fractions of 3 ml were collected. Fraction A contained the bulk of the protein (molecular weight >55 000 MW); fractions B (25 000–55 000 MW) and C (10 000–25 000 MW) were the next fractions. The protein concentration of the fractions was 0·94 mg/ml, 0·36 mg/ml, and 0·19 mg/ml, respectively.
Removal of specific IgE from sera
To 300 mg CNBr-activated Sepharose 4B (Pharmacia) 12 mg shrimp protein, or 6 mg chironomid protein was coupled. Allergen-specific IgE antibodies were removed from 500 µl serum by absorption with an equal volume of allergen Sepharose (50 mg Sepharose/ml) for 4 h while rotating. Removal of allergen-specific IgE was confirmed by RAST performed as described elsewhere.29,30
Results
Lack of association between IgE autoreactivity and HRF-reactivity
As positive control for the study to IgE autoreactivity, IgE binding of serum from AD patients was analyzed. In all four AD patients (one IgE− and three IgE+ sera), IgE binding to cellular extracts of the cell line A431 as well as of PBMCs was found. This result is in agreement with data published elsewhere.12 IgE autoantibodies in sera of three atopic patients with aspergillosis were also studied. Plasma #145 bound to a protein in the A431 extract, and plasma #144 bound to a protein in the PBMC extract. No binding to an antigen with a molecular weight similar to Aspergillus allergens reported to be cross-reacting with human proteins was found.17
To investigate a possible association of IgE-dependent responsiveness to HRF and IgE autoreactivity, 26 IgE+ and 26 IgE− sera were tested for the presence of IgE autoantibodies (Table 1). Seven of the 26 IgE+ sera and 4 of the 26 IgE− sera contained immunoblot-detectable IgE autoantibodies to human cellular extracts. IgE-reactivity of sera to nitrocellulose-blotted A431 and PBMC cell extracts are shown in Fig. 1a and b, respectively. IgE antibodies in two IgE− and five IgE+ sera (weakly) bound to human protein in cellular extracts from the epithelial cell line A431. IgE binding to PBMC extracts was stronger than to A431 extracts. It was found in three IgE− and four IgE+ sera. Three sera (one IgE− and two IgE+ sera) recognized proteins in both extracts. The lack of a significant association between IgE autoreactivity and HRF-reactivity is summarized in Fig. 2. Of the 12 IgE+ sera with >20 units HRF-IgE/ml, five contained blot-detectable IgE autoantibodies while seven sera did not. Four IgE− sera contained IgE autoantibodies while 22 IgE− sera did not show IgE autoreactivity (Fig. 2).
Figure 1.
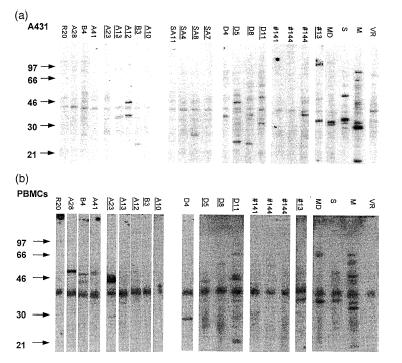
Detection of IgE autoantibodies in sera of atopic patients. Extracts from the (a) epithelial cell line A431 and (b) human PBMCs. Sera tested: atopic non-asthmatics and mild asthmatics (n = 48), only the nine sera with autoreactive IgE are presented; severe asthmatic patients (SA); atopic AD patients (d); patients with aspergillosis (#141, #144, #145); plasma sample #13; positive controls MD, S, M (AD); negative control VR. A 40 000 MW component non-specifically binds radioactive anti-IgE. Underlined lane numbers: IgE+ serum. MD, S, M, and VR were not tested for IgE+ reactivity.
Figure 2.
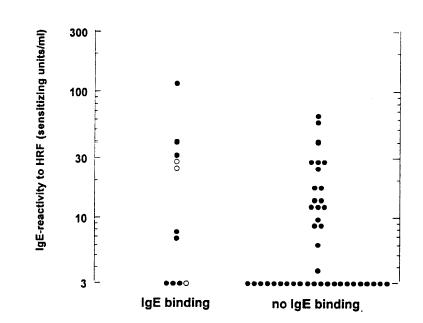
Lack of association between HRFmn-reactivity and IgE autoreactivity. IgE binding on immunoblot was determined to extracts of the human epithelial cell line A431 and PBMCs. Open circle: serum with IgE binding to both extracts.
Twenty-five IgE− and 23 IgE+ sera were also tested for reactivity to cellular extracts of the cervix carcinoma cell line HeLa (Table 1). Only serum R20 had autoantibodies to this cellular extract; a 38 000 MW protein was recognized. In an additional group of atopic non-asthmatic or mild asthmatic patients (15 IgE+ and 19 IgE− sera), one IgE− serum was found binding a protein of 33 000 MW. No binding was found with sera from 15 non-atopic subjects.
Analysis of HeLa-derived IgE autoantigens
IgE binding to HeLa extracts was studied in more detail using plasma samples from atopic subjects (Table 2). In Fig. 3 IgE binding of five plasma samples is shown. Both a IgE+ plasma (#13) and a IgE− plasma (#164) bound strongly to a 32 000 MW protein. In addition, IgE antibodies in plasma #164 also bound a smaller protein (20 000 MW). IgE autoreactivity was also detected to extracts of the monocyte cell lines U937 and MonoMac (Fig. 3). Plasma #13 recognized a 32 000 MW protein also in extracts of these cell lines.
Figure 3.
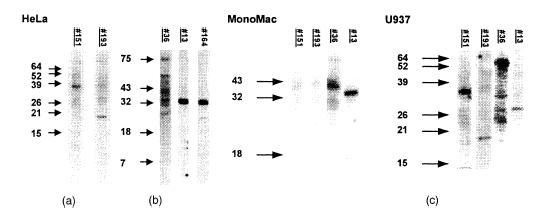
IgE immunoblot of extracts from (a) the cervix carcinoma cell line HeLa, and the monocyte-like human cell lines (b) MonoMac and (c) U937. Plasma samples from atopic patients were analysed for IgE binding. Underlined lane numbers: IgE+ serum.
In Fig. 4a it is shown that plasma #13 also recognized the protein under non-reducing conditions (apparent MW of 41 000). Size-exclusion chromatography of the 100% SAS fraction of HeLa extract might indicate that the 32 000 MW protein is in complex with other protein(s). IgE binding of plasma #13 was strongest to the high molecular weight fraction (>55 000 MW) whereas this protein migrated at 32 000 MW on SDS–polyacrylamide gel (Fig. 4b).
Figure 4.
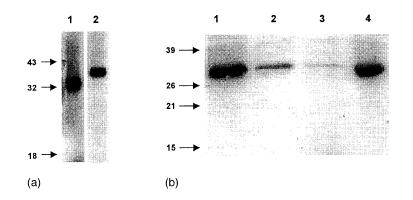
Analysis of a HeLa cell-derived IgE autoantigen bound by plasma #13. (a) IgE binding on immunoblot of plasma #13 to extract reduced by dithiothreitol (lane 1) and under non-reducing conditions (lane 2). (b) IgE immunoblot of fractions obtained by size exclusion chromatography of the 50–100% SAS precipitate of extracts of HeLa. Proteins present in the extract precipitating at a saturation of 50–100% ammonium sulphate were fractionated. Pool A (>55 000 MW) (lane 1); pool B (25–55 000 MW) (lane 2); pool C (10–25 000 MW) (lane 3), HeLa 100% SAS precipitate (lane 4).
Because the IgE antibodies present in plasma samples #13 and #164 bound to an autoantigen of the same molecular weight, these samples were studied in more detail. Because both plasma samples happened to be RAST positive to seafood extracts (shrimp and/or cod) (Table 2), it was studied whether the 32 000 MW protein on immunoblot was human tropomyosin31,32 an important cross-sensitizing allergen. IgE against shrimp or chironomid were removed from the plasma samples after which IgE binding to HeLa proteins was studied. IgE from the absorbed plasma samples still bound to the 32 000 MW protein; however, the binding to the 20 000 MW protein of plasma #164 was abolished by absorption with shrimp extract (Fig. 5). Absorption with chironomid extract did not change IgE binding. HeLa cells thus contain a 20 000 MW IgE autoantigen that cross-reacts with a shrimp allergen.
Figure 5.
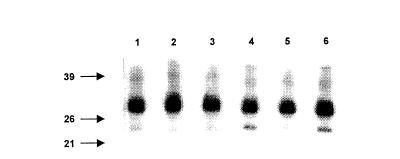
Cross-reactivity between a human protein and a shrimp allergen. IgE binding on immunoblot to HeLa extract by plasma samples before and after absorption was compared. Plasma #13 and #164 not absorbed (lane 1,4), absorbed Sepharose-coupled shrimp extract (lane 2,5), or by Sepharose-coupled chironomid extract (lane 3,6).
Preparations containing native IgE autoantigens do not induce histamine release
To determine whether the IgE binding proteins in extracts of human cells had histamine-releasing activity, the stripped basophil assay was used. Plasma samples with IgE autoantibodies (Fig. 3) were tested. Despite strong IgE-reactivity to HeLa cell-derived autoantigens on immunoblot, the histamine released by basophils sensitized by plasma samples #13, or #164 in response to cell extracts of HeLa (3 mg/ml) was below 5%. Even in the presence of the priming cytokine IL-3, no histamine release was induced. In another stripped basophil experiment, no histamine release was found with basophils sensitized either by plasma samples #13, or #151 in response to HeLa (3·5 mg/ml), U937 (1·5 mg/ml), or MonoMac (1 mg/ml) extracts. Plasma samples #193 and #36 were both not responsive to HeLa (3·1 mg/ml) or U937 (1·9 mg/ml) extracts. The same cellular extracts were used in the immunoblot experiments as in the histamine release experiments.
The results were remarkable because the IgE binding to human cellular extracts of especially plasma samples #13 and #164, was very strong on immunoblot. The presence of a factor inhibiting the histamine release in cellular extracts was excluded by stimulating basophils sensitized by #13 with grass pollen (1·3 µg/ml) in the presence of HeLa extract (3 mg/ml). The grass-pollen-induced-histamine release of basophils sensitized by #13 was not changed by cellular proteins: 47% and 48%, respectively, in the presence and absence of human cell extract.
The association between IgE binding and histamine-releasing activity was also studied in serum from atopic subjects without asthma or with mild asthma. As described above, 2 of the 82 sera contained IgE autoantibodies to HeLa cellular extracts. Histamine release of basophils sensitized by these sera in response to extract of HeLa (3 mg/ml) was below 5%.
Discussion
The main aim investigated in the present study was whether IgE autoreactivity and reactivity to IgE-dependent HRF were related phenomena. IgE-dependent responsiveness to HRF has been defined as the ability of certain atopic sera to sensitize stripped basophils to release histamine when exposed to culture supernatants of human cells. This activity depends on special features of the IgE antibodies in such sera and accordingly these sera were termed IgE+.3 In view of studies reporting that sera from atopic patients contain IgE autoantibodies13,14,33 we considered the possibility that IgE+ sera may contain autoreactive IgE antibodies that are directed to cell-derived autoantigens. In this scenario IgE+ would be autoreactive IgE and HRF an IgE autoantigen, which might be released into the culture medium by secretion or cell death. By receptor cross-linking of IgE+ by HRF on the surface of basophils, histamine release might be induced, such as is the case for exogenous allergens. To address this hypothesis, HRF-reactivity of serum was determined in the stripped basophil assay using culture supernatant of human peripheral mononuclear cells as stimulus. On the other hand we determined the presence of IgE autoantibodies in atopic sera by IgE immunoblotting, using a variety of human cell extracts (HeLa cells, epithelial cells, mononuclear cells) in order to cover a broad spectrum of IgE autoantigens. However, we found no significant association between IgE+ and IgE autoantibodies. Seven out of the 26 IgE+ sera contained IgE antibodies, and 4 out of 26 IgE− sera exhibited IgE autoreactivity. Even if sera with doubtful IgE binding were scored ‘positive’, no association was found (seven IgE+ and two IgE− with ‘doubtful IgE binding’ to PBMC or A431 extract). These results suggested that IgE autoreactivity and IgE-dependent HRF reactivity are unrelated phenomena.
In a group of atopic patients, five plasma samples with IgE binding to cellular extracts of the human cervix carcinoma cell line HeLa and/or monocyte-like cell lines were studied in more detail. The assumption that IgE autoantibodies to blotted proteins are not IgE+ antibodies was further supported by the observation that both an IgE+ plasma (#13) and a IgE− (plasma #164) bound very strongly to a HeLa derived autoantigen of the same molecular weight (32 000 MW). This protein was shown to be expressed in all cell types investigated in this study. The similarity between these two plasma samples was that they both contained IgE antibodies to fish and/or shrimp. We therefore were interested if the 32 000 MW IgE-defined autoantigen could be related to the major cross-reactive shrimp allergen tropomyosin.34 While we found no cross-reactivity of the 32 000 MW allergen with a shrimp allergen, a 20 000 MW autoantigen cross-reacted with shrimp extract. These results support observations of Valenta et al.12 and Mayer et al.17 that sensitization by an exogenous allergen may lead to IgE autoreactivity.
Another argument that IgE autoreactivity and IgE-responsiveness to HRF are distinct phenomena is that human cellular proteins failed to induce histamine release from basophils sensitized by five plasma samples or two sera from patients with respiratory atopy containing Western blot-detectable IgE. Despite IgE binding on immunoblot, no histamine-releasing activity was found in cellular extracts from HeLa, MonoMac, or U937. Even in the presence of IL-3, a cytokine which enhances IgE-dependent histamine release,35–37 basophils sensitized by plasma #13 failed to release histamine in response to cellular proteins. There are several possibilities to explain the discrepancy between IgE autoreactivity on immunoblot and the absence of histamine-releasing activity. First, the absence of histamine-releasing activity might be caused by a histamine-release inhibitory factor. However, HeLa cellular proteins did not influence histamine release to grass-pollen-extract Dactylis glomerata. The second possibility is that the IgE-binding sites for autoreactive IgE are exposed only after denaturation and/or reduction on immunoblot. However, the 32 000 MW protein was also recognized by plasma #13 under non-reducing condition on immunoblot. By size exclusion chromatography it was shown that this protein eluted in the >55 000 MW fraction, which might indicate that this protein forms polymers or complexes with other macromolecules, possibly explaining the discrepancy. Whether these putative complexes are physiological, or formed during the preparation of the extract, is not known. The IgE autoantibodies might also be directed to cross-reactive carbohydrate determinants, which have been described to have a poor biological activity.38 Finally, it is possible that some autoantigens identified on immunoblot are monovalent, and thus fail to crosslink IgE on the surface of basophils.
The lack of association between IgE autoantibodies and IgE+ might be caused by the fact that HRFmn is not an IgE autoantigen, but rather acts like a priming factor on basophils. This is the case for recombinant IgE-dependent HRF p23,8 which does not to bind IgE.39,40 However, we recently found that the reactivity to HRFmn is not identical to recombinant HRF p23.41 Assuming that HRFmn is an IgE autoantigen, the lack of association between IgE binding on immunoblot and IgE-responsiveness to HRF might be caused by the use of different allergen sources: cellular extracts in case of blotted proteins, culture supernatant of stimulated cells in case of HRF. Moreover, by conformational changes of the IgE autoantigens caused by dissociation, denaturation and reduction, the epitope recognition by IgE might be suppressed or enhanced on immunoblot. Previous results indicated that reducing conditions increase exposure of epitopes for IgE.42 Alternatively, it is possible that the cells express different profiles and/or amounts of IgE autoantigens depending on the culture conditions. Most likely, the concentration of HRF in the cellular extracts is low compared to total protein, particularly as the cells used for the preparation of the extracts were not stimulated.
In conclusion, no association was found between IgE-responsiveness to HRFmn and IgE autoantibodies to blotted human proteins. Moreover, the lack of certain cellular IgE autoantigens to activate basophils to release histamine is in striking contrast to the definition of IgE-dependent HRF. Our study indicated that IgE autoreactivity on Western blot and IgE-dependent responsiveness to HRFmn are distinct entities that may coexist in atopic sera.
Acknowledgments
The authors wish to thank donors of the Department of Plasmapheresis for donating blood, and Anneke Vogelaar-Vermeulen and Endah Tjokrosoeseno for the preparation of the buffy coats. Marjolein van Maanen and Tjalling Siersma are thanked for their technical assistance. This project was funded by the Netherlands Asthma Foundation (project no. 32.95.13). Part of this work was supported by grant F0506 of the Austrian Science Fund.
Abbreviations
- AD
atopic dermatitis
- PBMC
peripheral blood mononuclear cells
- HRF
histamine-releasing factor
- HRFmn
HRF produced by mononuclear cells
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- SAS
saturated ammonium sulphate
References
- 1.Liu MC, Hubbard WC, Proud D, Stealey BA, Galli SJ, Kagey-Sobotka A, Bleecker ER, Lichtenstein LM. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am Rev Respir Dis. 1991;144:51–8. doi: 10.1164/ajrccm/144.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Kuna P, Reddigari SR, Schall TJ, Rucinski D, Sadick M, Kaplan AP. Characterization of the human basophil response to cytokines, growth factors, and histamine releasing factors of the intercrine/chemokine family. J Immunol. 1993;150:1932–43. [PubMed] [Google Scholar]
- 3.MacDonald SM, Lichtenstein LM, Proud D, Plaut M, Naclerio RM, MacGlashan DW, Kagey-Sobotka A. Studies of IgE-dependent histamine releasing factors: heterogeneity of IgE. J Immunol. 1987;139:506–12. [PubMed] [Google Scholar]
- 4.Pasmans SGMA, Witteman AM, Aalbers M, et al. Variability of IgE-dependent activity in supernatants of human mononuclear cells. Int Arch Allergy Immunol. 1994;103:44–52. doi: 10.1159/000236604. [DOI] [PubMed] [Google Scholar]
- 5.Pasmans SGMA, Aalbers M, van der Veen MJ, Knol EF, van der Zee JS, Jansen HM, Aalberse RC. Reactivity to IgE-dependent histamine-releasing activity in asthma or rhinitis. Am J Respir Crit Care Med. 1996;154:318–23. doi: 10.1164/ajrccm.154.2.8756800. [DOI] [PubMed] [Google Scholar]
- 6.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989;321:228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 7.Saxon A, Max EE, Diaz-Sanchez D, Zhang K. Alternative RNA of epsilon transcripts produces mRNAs encoding two membrane and four secreted IgE isoforms. Int Arch Allergy Immunol. 1995;107:45–7. doi: 10.1159/000236926. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995;269:688–90. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- 9.Marone G, Casolaro V, Paganelli R, Quinti I. IgG anti-IgE from atopic dermatitis induces mediator release from basophils and mast cells. J Invest Dermatol. 1989;93:246–52. doi: 10.1111/1523-1747.ep12277582. [DOI] [PubMed] [Google Scholar]
- 10.Marone G, Spadaro G, Palumbo C, Condorelli G. The anti-IgE/anti-FcepsilonRIalpha autoantibody network in allergic and autoimmune diseases. Clin Exp Allergy. 1999;29:17–27. doi: 10.1046/j.1365-2222.1999.00441.x. [DOI] [PubMed] [Google Scholar]
- 11.Shakib F, Smith SJ. In vitro basophil histamine-releasing activity of circulating IgG1 and IgG4 autoanti-IgE antibodies from asthma patients and the demonstration that anti-IgE modulates allergen-induced basophil activation. Clin Exp Allergy. 1994;24:270–5. doi: 10.1111/j.1365-2222.1994.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 12.Valenta R, Seiberler S, Natter S, Mahler V, Mossabeb R, Ring J, Stingl G. Autoallergy: a pathogenetic factor in atopic dermatitis? J Allergy Clin Immunol. 2000;105:432–7. doi: 10.1067/mai.2000.104783. [DOI] [PubMed] [Google Scholar]
- 13.Valenta R, Maurer D, Steiner R, et al. Immunoglobulin E response to human proteins in atopic patients. J Invest Dermatol. 1996;107:203–8. doi: 10.1111/1523-1747.ep12329617. [DOI] [PubMed] [Google Scholar]
- 14.Natter S, Seiberler S, Hufnagl P, et al. Isolation of cDNA clones coding for IgE autoantigens with serum IgE from atopic dermatitis patients. FASEB J. 1998;12:1559–69. doi: 10.1096/fasebj.12.14.1559. [DOI] [PubMed] [Google Scholar]
- 15.Valenta R, Natter S, Seiberler S, Grote M. Isolation of cDNAs coding for IgE autoantigens. A link between atopy and autoimmunity. Int Arch Allergy Immunol. 1997;113:209–12. doi: 10.1159/000237549. [DOI] [PubMed] [Google Scholar]
- 16.Valenta R, Natter S, Seiberler S, et al. Molecular characterization of an autoallergen, Hom s 1, identified by serum IgE from atopic dermatitis patients. J Invest Dermatol. 1998;111:1178–83. doi: 10.1046/j.1523-1747.1998.00413.x. [DOI] [PubMed] [Google Scholar]
- 17.Mayer C, Hemmann S, Faith A, Blaser K, Crameri R. Cloning, production, characterization and IgE cross-reactivity of different manganese superoxide dismutases in individuals sensitized to Aspergillus fumigatus. Int Arch Allergy Immunol. 1997;113:213–5. doi: 10.1159/000237550. [DOI] [PubMed] [Google Scholar]
- 18.Appenzeller U, Meyer C, Menz G, Blaser K, Crameri R. IgE-mediated reactions to autoantigens in allergic diseases. Int Arch Allergy Immunol. 1999;118:193–6. doi: 10.1159/000024064. [DOI] [PubMed] [Google Scholar]
- 19.Mayer C, Appenzeller U, Seelbach H, Achatz G, Oberkofler H, Breitenbach M, Blaser K, Crameri R. Humoral and cell-mediated autoimmune reactions to human acidic ribosomal P2 protein in individuals sensitized to Aspergillus fumigatus P2 protein. J Exp Med. 1999;189:1507–12. doi: 10.1084/jem.189.9.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleine Budde I, de Heer PG, van der Zee JS, Aalberse RC. The stripped basophil histamine release bioassay as a tool for the detection of allergen-specific IgE in serum. Int Arch Allergy Immunol. 2001;126:277–85. doi: 10.1159/000049524. [DOI] [PubMed] [Google Scholar]
- 21.Verheijden R, Salden M, Venrooij van WJ. Protein blotting. Man Biol Markers Dis. 1993;A4:1–25. [Google Scholar]
- 22.Roberts AM, van Ree R, Cardy SM, Bevan LJ, Walker MR. Recombinant pollen allergens from Dactylis glomerata: preliminary evidence that human IgE cross-reactivity between Dac g II and Lol p I/II is increased following grass pollen immunotherapy. Immunology. 1992;76:389–96. [PMC free article] [PubMed] [Google Scholar]
- 23.Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Suppl) (Stockh) 1980;92:44–7. [Google Scholar]
- 24.Kleine Budde I, Knol EF, Aalbers M, van der Zee JS, Aalberse RC. Reactivity to IgE-dependent histamine-releasing factor is due to monomeric IgE. Allergy. 2000;55:653–7. doi: 10.1034/j.1398-9995.2000.00573.x. [DOI] [PubMed] [Google Scholar]
- 25.Knol EF, Kuijpers TW, Mul FP, Roos D. Stimulation of human basophils results in homotypic aggregation. A response independent of degranulation. J Immunol. 1993;151:4926–33. [PubMed] [Google Scholar]
- 26.Siraganian RP. Refinements in the automated fluorometric histamine analysis system. J Immunol Meth. 1975;7:283–90. doi: 10.1016/0022-1759(75)90025-3. [DOI] [PubMed] [Google Scholar]
- 27.Pasmans SGMA, Aalbers M, Daha MR, Knol EF, Jansen HM, Aalberse RC. Histamine-releasing activity in supernatants of mononuclear cells. Contribution of monocyte chemotactic protein-1 activity compared with IgE-dependent activity. J Allergy Clin Immunol. 1996;98:962–8. doi: 10.1016/s0091-6749(96)80013-3. [DOI] [PubMed] [Google Scholar]
- 28.van der Veen MJ, Lopuhaa CE, Aalberse RC, Jansen HM, van der Zee JS. Bronchial allergen challenge with isolated major allergens of Dermatophagoides pteronyssinus: the role of patient characteristics in the early asthmatic response. J Allergy Clin Immunol. 1998;102:24–31. doi: 10.1016/s0091-6749(98)70051-x. [DOI] [PubMed] [Google Scholar]
- 29.Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68:356–64. doi: 10.1016/0091-6749(81)90133-0. [DOI] [PubMed] [Google Scholar]
- 30.van der Zee JS, de Groot H, van Swieten P, Jansen HM, Aalberse RC. Discrepancies between the skin test and IgE antibody assays: study of histamine release, complement activation in vitro, and occurrence of allergen-specific IgG. J Allergy Clin Immunol. 1988;82:270–81. doi: 10.1016/0091-6749(88)91011-1. [DOI] [PubMed] [Google Scholar]
- 31.Lehrer SB. The complex nature of food antigens: studies of cross-reacting crustacea allergens. Ann Allergy. 1986;57:267–72. [PubMed] [Google Scholar]
- 32.Halmepuro L, Salvaggio JE, Lehrer SB. Crawfish and lobster allergens. Identification and structural similarities with other crustacea. Int Arch Allergy Appl Immunol. 1987;84:165–72. doi: 10.1159/000234418. [DOI] [PubMed] [Google Scholar]
- 33.Crameri R, Faith A, Hemmann S, Jaussi R, Ismail C, Menz G, Blaser K. Humoral and cell-mediated autoimmunity in allergy to Aspergillus fumigatus. J Exp Med. 1996;184:265–70. doi: 10.1084/jem.184.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanti KN, Martin BM, Nagpal S, Metcalfe DD, Rao PV. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J Immunol. 1993;151:5354–63. [PubMed] [Google Scholar]
- 35.Kurimoto Y, de Weck AL, Dahinden CA. The effect of interleukin 3 upon IgE-dependent and IgE-independent basophil degranulation and leukotriene generation. Eur J Immunol. 1991;21:361–8. doi: 10.1002/eji.1830210217. [DOI] [PubMed] [Google Scholar]
- 36.Hirai K, Morita Y, Misaki Y, Ohta K, Takaishi T, Suzuki S, Motoyoshi K, Miyamoto T. Modulation of human basophil histamine release by hemopoietic growth factors. J Immunol. 1988;141:3958–64. [PubMed] [Google Scholar]
- 37.Miadonna A, Roncarolo MG, Lorini M, Tedeschi A. Inducing and enhancing effects of IL-3, -5, and -6 and GM-CSF on histamine release from human basophils. Clin Immunol Immunopath. 1993;67:210–5. doi: 10.1006/clin.1993.1067. [DOI] [PubMed] [Google Scholar]
- 38.van der Veen MJ, van Ree R, Aalberse RC, Akkerdaas J, Koppelman SJ, Jansen HM, van der Zee JS. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100:327–34. doi: 10.1016/s0091-6749(97)70245-8. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald SM. Histamine-releasing factors. Curr Opin Immunol. 1996;8:778–83. doi: 10.1016/s0952-7915(96)80004-5. [DOI] [PubMed] [Google Scholar]
- 40.Wantke F, MacGlashan DW, Langdon JM, MacDonald SM. The human recombinant histamine releasing factor: functional evidence that it does not bind to the IgE molecule. J Allergy Clin Immunol. 1999;103:642–8. doi: 10.1016/s0091-6749(99)70237-x. [DOI] [PubMed] [Google Scholar]
- 41.Kleine Budde I, Lopuhaa CE, de Haar PG, Langdon JM, MacDonald SM, van der Zee JS, Aalberse RC. Lack of correlation between bronchial late allergic reaction to D. pteronyssinus and in vitro IgE-reactivity to histamine-releasing factor derived from mononuclear cells. Ann Allergy Asthma Immunol. 2002 doi: 10.1016/S1081-1206(10)62109-6. in press. [DOI] [PubMed] [Google Scholar]
- 42.Seiberler S, Natter S, Hufnagl P, Binder BR, Valenta R. Characterization of IgE-Reactive autoantigens in atopic dermatitis. 2. A pilot study on autoantigens IgE versus IgG subclass response and seasonal variation of IgE autoreactivity. Int Arch Allergy Immunol. 1999;120:117–25. doi: 10.1159/000024229. [DOI] [PubMed] [Google Scholar]


