Abstract
Dendritic cells (DCs) are professional antigen-presenting cells with a highly immunostimulatory function and the capacity to activate naïve T cells. In recent years the rapid progress in mouse and human DC research can be mainly attributed to the generation of DCs from precursor cells in vitro, although a lack of reagents has hampered DC research in many large animal models. Here we describe the generation and characterization of ovine monocyte-derived DCs in vitro. In addition to the characteristic morphology and non-adherence of DCs, peripheral blood mononuclear cell monocytes cultured with ovine granulocyte–macrophage colony-stimulating factor (GM–CSF) and interleukin-4 (IL-4) expressed CD11c and major histocompatibility complex (MHC) class II, but did not express CD14. High levels of endocytosis and an ability to stimulate antigen-specific proliferation of CD4 T lymphocytes were also demonstrated.
Introduction
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that have the capacity to capture, process and present antigen to lymphocytes.1–3 Cell types such as macrophages may also serve as APCs under certain circumstances, but only DCs are capable of presenting antigen to naïve T cells, stimulating their proliferation and expansion. DCs can exist in two stages of maturation: immature and mature. In their immature state DCs are highly endocytic,4 enabling them to capture antigen when they are localized in the periphery, and at the same time have functionally poor immunostimulatory ability. In response to danger signals, DCs mature, leading to their migration from the periphery to secondary lymphoid tissues via the lymphatics. During migration the ability to capture antigen is down-regulated and there is a shift towards presentation of captured antigen coupled with an up-regulation of major histocompatibility complex (MHC) class II and costimulatory molecules, all features of DC maturation. Therefore, when DCs arrive at lymphoid tissues, such as the lymph node, they are highly immunostimulatory with the ability to prime naïve T cells for the generation of primary T-cell responses.5 The existence of several DC subsets (as identified by phenotype), in addition to experiments indicating that certain DC subsets are tolerogenic as opposed to immunogenic, further demonstrates the immunoregulatory role that DCs play as ‘gatekeepers’ of the immune response.
DCs are found at very low frequencies (despite their occurrence in virtually all tissues, with the exception of those that are immunologically privileged). Therefore, at present the majority of DC research focuses on the use of human and murine model systems, culturing proliferating progenitors and/or non-proliferating precursors from the bone marrow, blood and lymphoid organs (such as the spleen). With a combination of cytokines in vitro, useable numbers of DCs that would not normally be obtainable ex vivo can be generated. Such methods have been used to produce DC for other large animal models such as the pig,6 and horse,7 although a lack of available cytokine reagents has been one of the reasons why DC biology has not progressed to the same extent as the human and murine systems.
The sheep has been of particular interest as a large animal model for immunology and offers certain experimental opportunities that are not available in murine systems. A unique source of DCs in the sheep is via cannulation of pseudoafferent lymphatic vessels. DCs obtained from this route represent a highly physiologically relevant source of DCs, although (as one would expect) these DCs are not homogeneous with respect to subset or maturity. There are times when it would be advantageous to be able to study the interaction of a pathogen with a homogeneous population of DCs as well as to have access to a readily available in vitro-generated source of DCs. In an attempt to generate such an in vitro homogeneous population we show that it is possible to generate ovine DCs in vitro from adherent peripheral blood mononuclear cells (PBMC), adapting methods developed for human and murine models.
Materials and methods
Animals
The sheep used in this study were adult Finnish Landrace crossed sheep of either sex, 4–6 years of age, purchased from the Moredun Research Institute (Edinburgh, UK). Animals were maintained on site at the Centre for Veterinary Science, Department of Clinical Veterinary Medicine, Cambridge.
Culture medium
RPMI-1640 with L-glutamine (cat. no. 21875-034; Invitrogen, Paisley, UK) was supplemented with 10% heat-inactivated fetal calf serum (FCS), antibiotics (200 U penicillin/ml, 100 µg of streptomycin/ml) and 50 µmβ-mercaptoethanol.
Cytokines and maturation factors
The Chinese hamster ovary cell line (CHO cells) transfected with cDNA encoding ovine granulocyte–macrophage colony-stimulating factor (GM–CSF)8 was a kind gift from Dr Gary Enterican, Moredun Research Institute. Recombinant ovine GM–CSF was produced as previously described and quantified using a sandwich enzyme-linked immunosorbent assay (ELISA),9 with reagents again kindly provided by Dr Gary Enterican. The ovine interleukin (IL)-4 cDNA was cloned from ovine mesenteric concanavalin blasts in our laboratory (GenBank acc. no.: AY096800) and expressed in the BAC-to-BAC baculovirus expression system (Cat. no. 10359-016; Life Technologies Ltd). The functional activity of supernatants from Sf9 insect cells infected with the recombinant ovine IL-4 baculovirus were quantified by assessing T-cell stimulatory activity. Briefly, sheep PBMCs were treated with phytohaemagglutinin (PHA) (0·25 µg/ml) for 3 days to produce PHA-activated lymphoblasts. Blasts were washed, resuspended at a concentration of 5 × 105/ml and dispensed in 100-µl aliquots into wells of 96-well round-bottomed plates (5 × 104 blasts/well). A 100-µl volume of serial dilutions of ovine IL-4-containing culture supernatant were added and the cells incubated at 37° for 4 days. Proliferation was measured by pulsing with 1 µCi of [3H]thymidine ([3H]TdR) per well for the final 16 hr of the incubation. [3H]TdR incorporation was measured by harvesting the cells onto glass-fibre mats using a Tomtek 96-well harvester (Tomtek, Orange, CT, USA) and counting by liquid scintillation in a Wallac Microbeta counter (PerkinElmer Life Sciences, Zaventem, Belgium).
Cell isolation and culture
Sheep PBMCs were obtained from whole blood collected into heparin at a final concentration of 10 U/ml. Buffy coats were underlaid with Lymphoprep (product no. 1053980, Nycomed Pharma AS, Oslo, Norway) and interface cells collected after density centrifugation at 1430 g for 15–20 min. PBMCs were then washed several times with phosphate-buffered saline (PBS) to remove platelets, resuspended in culture medium and plated into six-well plates (1·2 × 107 cells/3 ml per well). After incubation at 37° for 2 hr, non-adherent cells were removed by gentle washing with PBS. Adherent cells were maintained in culture medium at 37° with the addition of 40 ng/ml ovine GM–CSF and ovine IL-4-containing supernatant at the dilution required for maximum PHA lymphoblast stimulation (1 : 100 in these experiment). Cell cultures were fed every 2–3 days, for a total of 4–7 days, by aspirating 1 ml of media and replacing with 1·5 ml of fresh culture medium supplemented with cytokines. For lipopolysaccharide (LPS) stimulation, cells were cultured for the last 16 hr with 1 µg/ml Salmonella typhimurium LPS.
DC proliferation assays
Adherent PBMCs were harvested and divided into two fractions; one was irradiated with 3000 Gy before culture. Both fractions were set up at 1 × 105 cells/well with ovine GM–CSF and ovine IL-4, then were pulsed with 1 µCi of [3H]TdR for 16 hr on days 2, 4 and 6. [3H]TdR incorporation was measured by harvesting onto glass-fibre mats using a Tomtek 96-well harvester (Tomtek) and counting by liquid scintillation in a Wallac Microbeta counter (Wallac). Irradiated cell counts per minute (c.p.m.) were always < 600 c.p.m.
Flow cytometry and antibodies
Cell-surface phenotype was analysed by flow cytometry. Monoclonal antibodies (mAbs) were either against ovine cell-surface antigens or were mAbs known to cross-react with their ovine homologue. Briefly, cells were washed in PBS containing 0·1% bovine serum albumin (BSA) and 0·01% sodium azide [fluorescence-activated cell sorter (FACS) wash buffer] and incubated, for 40 min on ice, with 25 µl of either saturated supernatant or 1–2 µg/ml purified primary antibody. After two washes in FACS wash buffer, cells were incubated for 20 min on ice with isotype-specific, fluorescein isothiocyanate (FITC)-conjugated, anti-mouse antibodies. Dead cells were gated out using propidium iodide (PI) staining, and forward- and side-scatter profiles. Isotype-control antibodies were used to determine background staining and autofluorescence. mAbs used were: SBU-T4 for CD4,10 SBU-T8 for CD8αβ,10 DU2-104 as a pan B-cell marker,11 VPM65 for CD14,12 VPM36 for MHC class II DQ α-chain,13 VPM54 for MHC class II DR α-chain,13 OM1 for CD11c α-chain,14,15 SBU-T6 for CD1 (all isoforms),16 CC20 for CD1b,17 VPM19 for MHC class I,18 3.29 for mannose receptor4 and CC125 for CD11b.17
Endocytosis studies
Cells were resuspended in culture medium, at 37°, pulsed with either lucifer yellow (1 mg/ml) or FITC-dextran (1 mg/ml). Uptake was stopped by the addition of ice-cold PBS containing 0·1% BSA and 0·01% sodium azide, in which cells were washed four times. Uptake was compared with cells incubated with lucifer yellow or FITC-dextran for the same length of time on ice.
Antigen-presentation assays
PBMCs from sheep primed to purified protein derivative (PPD) were stained for CD4 using the mAb SBU-T4 conjugated to biotin and incubated with streptavidin-conjugated paramagnetic beads (Miltenyi Biotec Ltd, Bisley, UK). CD4 lymphocytes were positively selected on a magnetized column. CD4 lymphocytes were set up at 1 × 105 cells/well in triplicate with PPD antigen (12·5 µg/ml) and incubated with different ratios of autologous irradiated (3000 Gy) APCs (PBMCs or DCs derived from adherent PBMCs using ovine GM–CSF and IL-4). Cells were cultured for 5 days at 37°, with 1 µCi [3H]TdR added for the final 16 hr. [3H]TdR incorporation was measured by harvesting onto glass-fibre mats using a Tomtek 96-well harvester (Tomtek) and counting by liquid scintillation in a Wallac Microbeta counter (Wallac).
Results
Generation of recombinant ovine IL-4 and quantification of activity
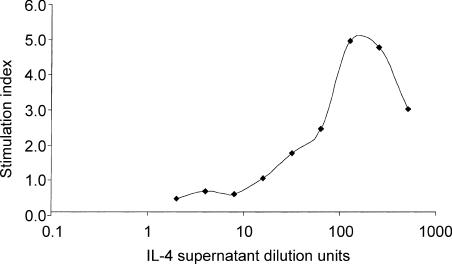
Some recombinant human cytokines, such as IL-2, are functionally cross-reactive on ovine cells. However, murine and human IL-4 are not biologically active in the ovine system, as IL-4 appears to be highly species specific. Therefore an ovine IL-4 (OvIL-4) cDNA cloned in our laboratory was expressed from a recombinant baculovirus. Cell lysates of Sf9 insect cells infected with the recombinant baculovirus showed the presence of a 17 000-molecular weight protein that corresponded to OvIL-4. This protein was not present in cells infected with wild-type baculovirus (data not shown). To ensure that supernatants from recombinant baculovirus-infected cells encoded a biologically active protein, ovine lymphoblast proliferation caused by recombinant baculovirus supernatants was assessed. Ovine lymphoblasts were found to proliferate in a dose-dependent manner with a dose–response curve comparable to previously published human IL-4 dose–response curve data. Proliferation was not observed with supernatants from wild-type baculovirus. OvIL-4 activity was quantified by the least amount of OvIL-4-containing supernatant that provided maximal proliferation activity (Fig. 1) and was given by a 1 : 100 dilution.
Figure 1.
Generation of ovine in vitro-derived DCs from adherent PBMCs
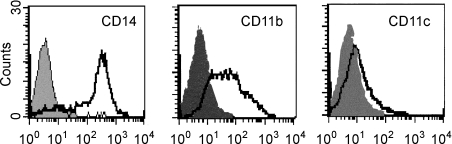
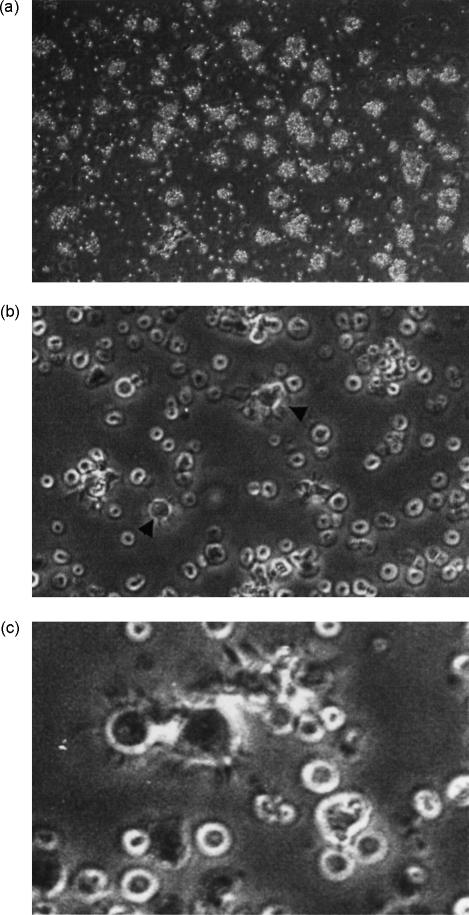
Most (± 78%) of the adherent PBMCs displayed the forward- and side-scatter light properties of monocytes (data not shown), with cells being CD11b+, very weakly CD11c+ and CD14hi (Fig. 2). This phenotypic profile is identical to that of ovine peripheral blood monocytes.12,14,19 After 3 days of culture with ovine GM–CSF and IL-4, the vast majority of adherent cells had enlarged and were either loosely or non-adherent, with a small proportion of cells adhering to the plate. This was associated with the development of non-adherent cellular aggregates that appeared to expand with time (Fig. 3a). On day 5, and in some cases as early as day 3, the peripheral cells of some of these aggregates could be seen to have a veiled or dendritic appearance visible under inverted phase-contrast microscopy (Fig. 3b, 3c). These processes became more prominent with the addition of LPS to the culture medium (data not shown). The yield of cells with the aforementioned morphology derived from PBMC ranged from 1 × 106 to 5 × 106/100 ml of peripheral blood (1–5%). The relatively low yields of cells with a DC-like morphology suggests that adherent monocyte precursors do not proliferate during DC generation. This was shown by the lack of [3H]TdR uptake (Table 1). Similar results have also been obtained with adherent PBMCs in the murine model.20 Interestingly, irradiated adherent PBMCs were also able to develop into enlarged cells with processes (data not shown). Overall, the cultured PBMCs displayed the characteristic morphology of DCs.
Figure 2.
The phenotype of adherent monocytes from ovine peripheral blood mononuclear cells (PBMCs). Cells were stained with control isotype-matched antibodies (filled histograms) and a panel of cell-surface antigen markers (open histograms). Each histogram is representative of at least four different experiments.
Figure 3.
The morphology of adherent peripheral blood mononuclear cells (PBMCs) cultured with ovine granulocyte–macrophage colony-stimulating factor (GM–CSF) and interleukin-4 (IL-4) was evaluated by phase-contrast microscopy after 6 days. (a) Large, non-adherent cellular aggregates formed from adherent PBMCs that were cultured as described in the Materials and methods (magnification ×10). (b) These aggregates contained DCs with some DCs also observed in single suspension (magnification ×20). (c) The veiled morphology of in vitro-derived DCs (magnification ×40).
Table 1. [3H]Thymidine uptake of adherent peripheral blood mononuclear cell (PBMC) cultures during dendritic cell (DC) generation demonstrated that proliferation was not present in our DC cultures.
| Stimulation index | ||||
|---|---|---|---|---|
| Exp No. | Day 0 | Day 2 | Day 4 | Day 6 |
| 1 | ND | 1·5 | 1·8 | 1·8 |
| 2 | ND | 2·0 | 1·6 | 1·5 |
Results are given as stimulation indices (SI) of the c.p.m. of non-irradiated cells ÷ the c.p.m. of irradiated cells.
An SI of >2 indicates proliferation.
c.p.m., counts/min; ND, not done.
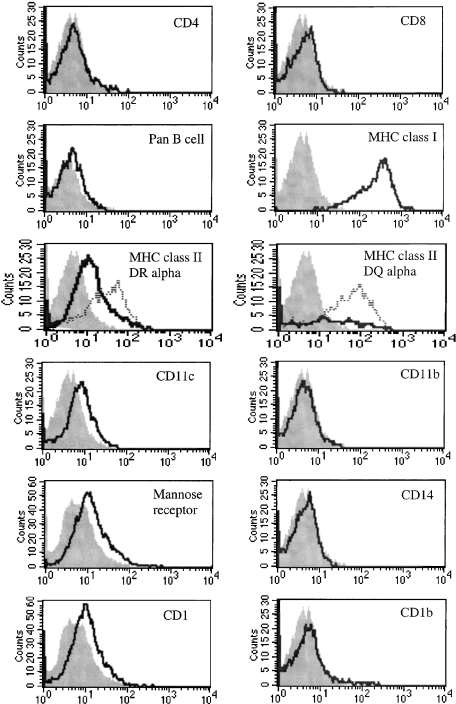
After 5–6 days of culture, phenotypic analysis of these non-adherent cells with DC-like morphology was carried out by flow cytometry. Large live cells were gated as defined by their forward- and side-scatter profiles and PI exclusion. Cells were analysed for the expression of markers associated with monocytes/macrophages and DCs (Fig. 4). Expression of T-cell (CD4 or CD8) and B-cell markers was not detected on large cells. Cells expressed CD11c weakly, and intermediate levels of MHC class II DR and DQ were observed as were high levels of MHC class I and MHC class II upon LPS stimulation. All cells expressed CD1 weakly, although this does not appear to be the result of expression of CD1b as this was variable between cultures, with no, or only weak, CD1b expression detected (0–10%). Furthermore, the CD11b and CD14 expression found on freshly adherent monocytes was lost after culture with GM–CSF and IL-4. The anti-mannose receptor antibody, 3.29, has previously been reported to stain DCs in ovine tissue sections4 and DCs found in ovine afferent lymph.21 A proportion of large cells weakly expressed the mannose receptor. Based on this phenotype and the above morphology, these cells were defined as ovine monocyte-derived immature DCs.
Figure 4.
Phenotypic analysis of ovine dendritic cell (DC) cultures by flow cytometry. Cells were stained with control isotype antibodies (filled histograms) and a panel of cell-surface antigen markers (open histograms). Major histocompatibility complex (MHC) class II up-regulation in response to lipopolysaccharide (LPS) is denoted by dotted histograms. Each histogram is representative of at least three different experiments.
Endocytic properties of ovine in vitro-derived DCs
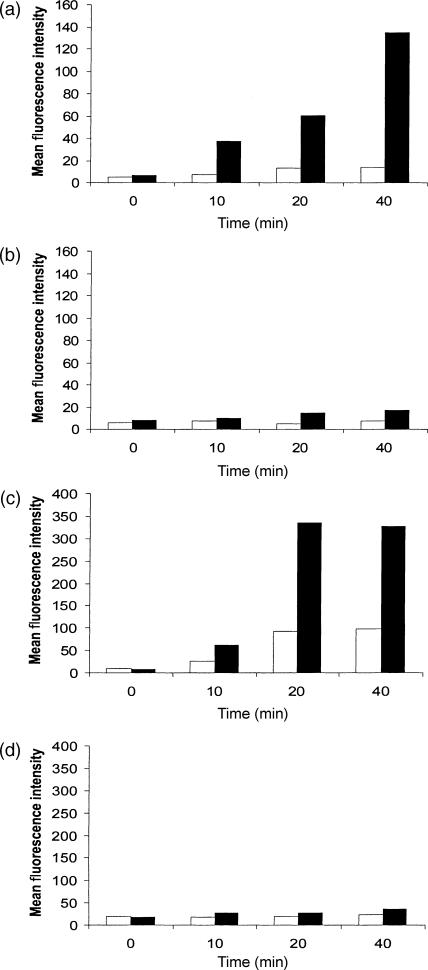
A prominent feature of immature DCs is their ability to capture antigen, which is subsequently processed and presented to T lymphocytes. We investigated the ability of ovine in vitro-derived DCs to take up antigen by two distinct mechanisms: macropinocytosis; and receptor-mediated endocytosis. High levels of fluid endocytosis by macropinocytosis and receptor-mediated mechanisms are characteristic of immature DCs and can be measured by the uptake of the lucifer yellow and FITC-dextran, respectively. As shown in Fig. 5, ovine monocyte-derived DCs rapidly and efficiently accumulated the soluble antigens lucifer yellow and FITC-dextran when compared to PBMC. These properties were greatly reduced when control cells were incubated at 0° or when LPS was added to the culture 24 hr before endocytosis was measured (data not shown).
Figure 5.
Ovine dendritic cells (DCs) rapidly take up soluble antigen by macropinocytosis and receptor-mediated endocytosis. Fluorescence was quantified by flow cytometry. Cells were incubated either at 0° (□) or 37° (▪) prior to and during the experiments. (a) DCs and lucifer yellow; (b) peripheral blood mononuclear cells (PBMCs) and lucifer yellow; (c) DCs and fluorescein isothiocyanate (FITC)-dextran; (d) PBMCs and FITC-dextran. Bar graphs represent the mean fluorescence of the markers taken up by ovine in vitro-derived DCs or PBMCs.
Analysis of the uptake of these two soluble antigens over a short time-period revealed that the fluorescent signal obtained with lucifer yellow continued to increase with time (up to the last 40-min time-point), while FITC-dextran loading appeared to be maximal after 20 min. Uptake of FITC-dextran has been shown to be mannose-receptor dependent, with the maximal fluorescence obtained after 20 min probably representing saturation of mannose receptors. In contrast, the continual uptake of lucifer yellow, with time, is known to be the result of macropinocytosis and is receptor independent.
Ovine monocyte-derived DCs efficiently present antigen to CD4 T lymphocytes
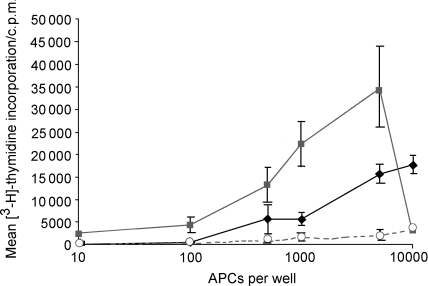
DCs are known to be able to stimulate antigen-specific T lymphocytes in a secondary response at an efficiency that exceeds that of PBMC on a cell-per-cell basis. Therefore, to compare the ability of ovine in vitro-derived DCs and PBMC to process and present antigen, sheep were primed to PPD. Ovine in vitro-derived DCs and PBMC from these sheep were then co-cultured with PPD antigen and autologous CD4 purified lymphocytes. On a per-cell basis, DC were four- to fivefold more potent at stimulating PPD-specific CD4 lymphocytes to proliferate than PBMC and therefore were able to cause proliferation of CD4 lymphocytes when present at low numbers (Fig. 6). CD4 lymphocytes did not proliferate in response to APC co-cultured with non-specific antigen, confirming the antigen specificity of lymphocyte proliferation.
Figure 6.
Ovine dendritic cells (DCs) present antigen to antigen-specific CD4 T cells more efficiently than peripheral blood mononuclear cells (PBMCs). PBMCs from sheep primed to purified protein derivative (PPD) were used to generate DCs with granulocyte–macrophage colony-stimulating factor (GM–CSF) and interleukin-4 (IL-4). These were irradiated and co-cultured with autologous CD4 lymphocytes and PPD antigen for 4 days before proliferation was determined by [3H]thymidine incorporation over 16 hr. Proliferation of antigen-specific CD4 lymphocytes by autologous irradiated in vitro-derived DCs (▪) was compared to proliferation induced by autologous irradiated PBMCs (♦). Levels of proliferation to a mock antigen pRSET C with DCs are also shown (○). Results are representative of two experiments repeated in triplicate. c.p.m., counts/min.
Discussion
Ovine IL-4 cDNA has been cloned and sequenced previously,22–24 and has also previously been expressed in a baculovirus expression system.23 The cDNA clone generated for use here differed from the Seow sequence at amino acid positions 26, 38, 44, 53, 72 and 129 and from the Chaplin sequence at amino acid positions 26 and 129 of the mature protein. The recombinant protein expressed by Chaplin et al.23 stimulates proliferation of both T and B cells, but was found to be ‘limitingly effective’ when used together with GM–CSF for the generation of monocyte-derived DCs (personal communication: Toby Coates, The Queen Elizabeth Hospital, Woodville, Australia).
Based on morphology, our adherent monocyte-containing PBMCs cultured with GM–CSF and IL-4 developed into DCs, which were phenotypically immature DCs expressing CD11c, mannose receptor and MHC class II DR and DQ. Compared with untreated cells, MHC class II DR and DQ were up-regulated in response to LPS, indicative of the ability of DCs to enter a mature state. The loss of CD14 and a lack of CD11b, CD4, CD8 and a pan B-cell marker expression discounted the possibility that our cultures were macrophages, monocytes or lymphocytes. In other species, the up-regulation of costimulatory molecules such as CD40, CD80 and CD86 has been demonstrated. Unfortunately, in the absence of cross-reactive or ovine-specific antibodies the expression of these molecules could not be investigated.
Double staining of afferent lymph DCs has previously revealed that 60–75% of afferent lymph DCs are highly positive for CD1b and that these were either CD14− or CD14lo.21 The absence of CD1b expression in monocytes, macrophages and lymphocytes makes it an ideal marker for identifying afferent lymph DCs. To date, CD1b has been found in ovine afferent lymph DCs, Langerhans’ cells and cortical thymocytes. CD1b belongs to the CD1 family composed of five genes25 – CD1a, CD1b, CD1c, CD1d and CD1e – the first four of which encode protein products involved in lipid antigen presentation. As mice and rats only possess genes that are homologues for CD1d, their DCs do not express CD1b. CD1b genes have also been identified in humans and rabbits. Our own in vitro DC cultures displayed up to 10% CD1b+ cells, unlike expression on afferent lymph DC, and this perhaps underlines the differences between DCs obtained from in vitro and in vivo sources. The in vitro sources probably represent an early ‘stage’ of DC differentiation. Another possibility, not excluded, is the presence of CD1b in intracellular compartments in our immature DCs. However, the weak expression of CD1 on all ovine in vitro-derived DCs suggests that at least one or more of the other isoforms of CD1 is present on the cell surface.
In mice, several studies refer to the existence of CD11b+ CD11c+ DCs.26–28 Our studies, and those of others, have shown that CD11b is expressed on the adherent fraction of monocyte-containing ovine PBMCs and ovine macrophages.29 A number of large granular cells in afferent lymph also express CD11b.30 However, double staining these afferent lymph cells for CD11b and CD1b revealed that CD11b expression was associated with a very small percentage of CD1b-expressing cells. Furthermore, in some sheep, CD11b was not co-expressed with CD1b+ large cells in afferent lymph. Given the wider association of CD11b with macrophages in the ovine model, others have speculated that these cells were more likely to be macrophages found in the afferent lymph.30 Therefore, the lack of CD11b in our monocyte-derived DCs was not too surprising, although we cannot disregard the possibility that CD11b is expressed on an as-yet unidentified subset of ovine DCs not generated by our in vitro methods. Contradictory results have been published with CD11b expression on in vitro human monocyte-derived DCs, with some groups showing expression31 and others not.32 The uptake of FITC-dextran by afferent lymph DCs is variable, with Sallusto and colleagues reporting receptor-mediated endocytosis on afferent lymph DCs, as measured by FITC-dextran uptake, while Ryan found a distinct lack of this ability.4,30 It is possible that such conflicting reports were a result of the afferent lymph DC maturation state, as DCs found in afferent lymph are a heterogeneous population of cells that mature as they migrate from the periphery towards secondary lymphoid organs. Certainly, down-modulation of endocytosis in response to inflammatory stimuli is a well-documented feature of DCs. However, functional characterization of our DC cultures demonstrated that they were highly endocytic, with rapid uptake of FITC-dextran via receptor-mediated endocytosis and lucifer yellow by macropinocytosis. This further confirmed the immature status of our in vitro-derived DCs.
It has been reported that DCs are able to process and present antigen to stimulate lymphocyte proliferation at levels that exceed those of other APCs. The in vitro-generated DCs possessed strong CD4 T-cell stimulatory capacity in antigen-specific presentation assays, with lower numbers of DCs required to obtain the same proliferative effect as PBMCs on CD4 lymphocytes. In other species this has been shown to be a result of the levels of MHC class II and costimulatory molecules that DCs express.
Sheep represent a large animal model for studying certain aspects of immunology and have a higher degree of similarity to humans than most small animal models. The results presented in this report demonstrate that ovine in vitro-derived DCs can be generated from adherent PBMCs using methods available for human DC generation. These cells may be used as an additional source of DCs to those found in afferent lymph, and should serve as a valuable tool in making interspecies comparisons of DC. Finally, these in vitro-derived DCs will aid further characterization of ovine DC biology and studies between ovine DCs and infectious agents.
Acknowledgments
We wish to thank Dr Gary Entrican and Sean Wattegedera of the Moredun Research Institute, Edinburgh, for the ovine GM–CSF expressing CHO cell line. This work was supported by a Wellcome Trust project grant to B.A.B and I.M. (060000/Z/99/z). S.S.M.C. is supported by a BBSRC studentship.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1999;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment. Downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inaba K, Turley S, Iyoda T, et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191:927–36. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paillot R, Laval F, Audonnet J-C, Andreoni C, Juillard V. Functional and phenotypic characterization of distinct porcine dendritic cells derived from peripheral blood monocytes. Immunology. 2001;102:396–404. doi: 10.1046/j.1365-2567.2001.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond SA, Horohov D, Montelaro RC. Functional characterization of equine dendritic cells propagated ex vivo using recombinant human GM2CSF and recombinant equine IL-4. Vet Immunol Immunopathol. 1999;71:197–214. doi: 10.1016/s0165-2427(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 8.McInnes CJ, Haig DM. Cloning and expression of a cDNA encoding ovine granulocyte–macrophage colony-stimulating factor. Gene. 1991;105:275–9. doi: 10.1016/0378-1119(91)90163-6. [DOI] [PubMed] [Google Scholar]
- 9.Entrican G, Deane D, MacLean M, Inglis L, Thomson J, McInnes C, Haig DM. Development of a sandwich ELISA for ovine granulocyte/macrophage colony- stimulating factor. Vet Immunol Immunopathol. 1996;50:105–15. doi: 10.1016/0165-2427(95)05468-5. [DOI] [PubMed] [Google Scholar]
- 10.Maddox JF, Mackay CR, Brandon MR. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985;55:739–48. [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay CR, Marston WL, Dudler L, Spertini O, Tedder TF, Hein WR. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992;22:887–95. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 12.Gupta VK, McConnell I, Dalziel RG, Hopkins J. Identification of the sheep homologue of the monocyte cell surface molecule – CD14. Vet Immunol Immunopathol. 1996;51:89–99. doi: 10.1016/0165-2427(95)05512-6. [DOI] [PubMed] [Google Scholar]
- 13.Dutia BM, MacCarthy-Morrogh L, Glass EJ, Knowles G, Spooner RL, Hopkins J. Discrimination between major histocompatibility complex class II DQ and DR locus products in cattle. Anim Genet. 1995;26:111–4. doi: 10.1111/j.1365-2052.1995.tb02643.x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta VK, McConnell I, Hopkins J. Reactivity of the CD11/CD18 workshop monoclonal antibodies in the sheep. Vet Immunol Immunopathol. 1993;39:93–102. doi: 10.1016/0165-2427(93)90168-4. [DOI] [PubMed] [Google Scholar]
- 15.Pepin M, Cannella D, Fontaine JJ, Pittet JC, Le Pape A. Ovine mononuclear phagocytes in situ: identification by monoclonal antibodies and involvement in experimental pyogranulomas. J Leukoc Biol. 1992;51:188–98. doi: 10.1002/jlb.51.2.188. [DOI] [PubMed] [Google Scholar]
- 16.Mackay CR, Maddox JF, Gogolin-Ewens KJ, Brandon MR. Characterization of two sheep lymphocyte differentiation antigens, SBU-T1 and SBU-T6. Immunology. 1985;55:729–37. [PMC free article] [PubMed] [Google Scholar]
- 17.Howard CJ, Naessens J. Summary of workshop findings for cattle (tables 1 and 2) Vet Immunol Immunopathol. 1993;39:25–47. doi: 10.1016/0165-2427(93)90161-v. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins J, Dutia BM. Monoclonal antibodies to the sheep analogues of human CD45 (leucocyte common antigen), MHC class I and CD5. Differential expression after lymphocyte activation in vivo. Vet Immunol Immunopathol. 1990;24:331–46. doi: 10.1016/0165-2427(90)90004-c. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins J, Gupta VK. Identification of three myeloid-specific differentiation antigens in sheep. Vet Immunol Immunopathol. 1996;52:329–39. doi: 10.1016/0165-2427(96)05584-5. [DOI] [PubMed] [Google Scholar]
- 20.Schreurs MW, Eggert AA, de Boer AJ, Figdor CG, Adema GJ. Generation and functional characterization of mouse monocyte-derived dendritic cells. Eur J Immunol. 1998;29:2835–41. doi: 10.1002/(SICI)1521-4141(199909)29:09<2835::AID-IMMU2835>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Ryan S, Tiley L, McConnell I, Blacklaws B. Infection of dendritic cells by the Maedi-Visna lentivirus. J Virol. 2000;74:10096–103. doi: 10.1128/jvi.74.21.10096-10103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engwerda CR, Sandeman RM. The isolation and sequence of sheep interleukin 4. DNA Seq. 1992;3:111–3. doi: 10.3109/10425179209034004. [DOI] [PubMed] [Google Scholar]
- 23.Chaplin PJ, Casey G, Rose RD, Buchan GS, Wood PR, Scheerlinck J-PY. The expression and biologic effects of ovine interleukin-4 on T and B cell proliferation. J Interferon Cytokine Res. 2000;20:419–25. doi: 10.1089/107999000312360. [DOI] [PubMed] [Google Scholar]
- 24.Seow H-F, Rothel JS, Wood PR. Cloning and sequencing an ovine interleukin-4 encoding cDNA. Gene. 1993;124:291–3. doi: 10.1016/0378-1119(93)90408-u. [DOI] [PubMed] [Google Scholar]
- 25.Martin LH, Calabi F, Milstein C. Isolation of CD1 genes: a family of major histocompatibility complex- related differentiation antigens. Proc Natl Acad Sci USA. 1986;83:9154–8. doi: 10.1073/pnas.83.23.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–62. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, Maliszewski CR, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand- treated mice. J Immunol. 1997;159:2222–31. [PubMed] [Google Scholar]
- 28.Shurin MR, Pandharipande PP, Zorina TD, et al. FLT3 ligand induces the generation of functionally active dendritic cells in mice. Cell Immunol. 1997;179:174–84. doi: 10.1006/cimm.1997.1152. [DOI] [PubMed] [Google Scholar]
- 29.Gupta VK, McConnell I, Pepin M, Davis WC, Dalziel RG, Hopkins J. Biochemical and phenotypic characterization of the ovine beta 2 (leucocyte) integrins. J Comp Pathol. 1995;112:339–49. doi: 10.1016/s0021-9975(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 30.Ryan S. PhD thesis. Cambridge, UK: Department of Clinical Veterinary Medicine, University of Cambridge; 1999. Infection of dendritic cells by Maedi Visna Virus; p. 172. [Google Scholar]
- 31.Pickl WF, Majdic O, Kohl P, Stockl J, Riedl E, Scheinecker C, Bello-Fernandez C, Knapp W. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol. 1996;157:3850–9. [PubMed] [Google Scholar]
- 32.Cavanagh LL, Saal RJ, Grimmett KL, Thomas R. Proliferation in monocyte-derived dendritic cell cultures is caused by progenitor cells capable of myeloid differentiation. Blood. 1998;92:1598–607. [PubMed] [Google Scholar]