Abstract
Cystic fibrosis females have a worse prognosis compared to male patients. Furthermore, cystic fibrosis patients infected with Pseudomonas aeruginosa have been shown to have dysregulated cytokine profiles, as higher levels of tumour necrosis factor alpha (TNF-α), interleukin (IL)-8, and lower levels of IL-10 are found in the bronchoalveolar lavage fluid compared to healthy controls. The present study was aimed at investigating the importance of gender and IL-10 in the susceptibility of C57BL/6 mice to pulmonary infection with Pseudomonas aeruginosa. We found that wildtype females were more susceptible than males to infection, as we observed greater weight loss, higher bacterial load, and inflammatory mediators in their lungs. IL-10 knockout mice, both females and males, had higher levels of TNF-α in the lungs compared to wildtype mice and maintained higher levels of polymorphonuclear cells and lower levels of macrophages for a longer period of time. Our results demonstrate that the number of bacteria recovered from the lungs of IL-10 knockout male mice was significantly higher than that observed in their wildtype male counterparts and we show that neutralization of IL-10 in infected female mice for a prolonged period of time leads to increased susceptibility to infection. Results reported in this study clearly demonstrate that females, both wildtype and IL-10 knockout mice are more susceptible to Pseudomonas aeruginosa infection than males, and that they mount a stronger inflammatory response in the lungs.
Introduction
Cystic fibrosis (CF), a disease characterized by a dysregulated inflammatory response in the lung environment, appears to be caused by an imbalance in cytokine production. The dysregulation of the immune response would impair the proper clearance of the bacterial infection in the lungs by Pseudomonas aeruginosa. Furthermore, a recent study showed a correlation between cytokine pattern and the clinical course of CF.1 Cytokine profile analyses in bronchoalveolar lavage fluids (BALF), respiratory epithelial cells obtained from CF patients, as well as sputum of CF children, indicated higher levels of tumour necrosis factor-α (TNF-α), interleukin (IL)-1 and IL-8 and less IL-10 compared to healthy subjects.2–6 These results correlated with the predominance of neutrophils found in the lungs of CF patients infected with P. aeruginosa.7,8 Other studies showed no significant differences in the IL-10 levels in BALF of young CF patients compared to healthy controls.9 From these divergent observations, it is not yet clear what the actual importance of IL-10 is in the regulation of the inflammation in CF lung.
Previously reported data suggest that the prognosis of the disease is worse in CF female patients compared to CF male patients.10 It has been shown extensively that sex hormones can affect the immune response,11,12 governing the gender-related differences observed in many diseases and disorders.13–18 Unfortunately, human CF studies using both male and female adults and/or children did not analyse or report any differences in IL-10 levels between genders.1–3,6,8,9,19 Nevertheless, the influence of IL-10 has already been shown in gender-related differences of the immune response.20–22
Human IL-10 (hIL-10) is an 18 000 MW protein that was originally identified as a T helper 2 (Th2) mediator of the immune response.23 IL-10 is produced relatively late following activation of T cells or monocytes/macrophages and has miscellaneous functions depending on the cell type, stimulus, and the target cell.24 Among others, IL-10 has previously been shown to inhibit the production of pro-inflammatory cytokines like TNF-α and chemokines like IL-8 (human homologue of murine KC).25 This pleiotropic cytokine has been shown to be involved in many aspects of the immune response; consequently, it is not surprising that it plays a pivotal role in the pathogenesis of several diseases and disorders. Because the proper balance, as well as the adequate temporal and spatial expression of IL-10, is essential for efficient immune response, the production of IL-10 can also lead to undesirable effects during the course of infection. In fact, neutralization of IL-10 or administration of anti-IL-10 is shown to enhance clearance of Mycobacterium avium26 as well as augment the survival in Klebsiella pneumonia infection in mice.27 In contrast, the lack or insufficient production of IL-10 can also be detrimental to the host.28–31 A study with IL-10-deficient transgenic mice, repeatedly exposed to mucoid P. aeruginosa, showed higher mortality and lung pathology compared to C57BL/6 (B6) control animals.32 It was also demonstrated that in P. aeruginosa pneumonia, systemic administration of IL-10 improved the host condition.33,34 Chmiel and colleagues observed that IL-10 attenuates excessive inflammation in P. aeruginosa infected male mice. More severe weight loss, increased area of lung inflammation was observed in IL-10 knockout (KO) compared to their male wildtype (WT) controls.35 Although the authors found a trend toward lower bacterial count at day 3 postinfection, no statistical significance was found. However, these authors observed substantial improvement in the extent of inflammation when the male CD1 mice were treated with the recombinant human IL-10. We were wondering whether similar or different improvement in handling P. aeruginosa lung infection and inflammation could also be observed in female mice. To test this hypothesis, we first defined the influence of gender on susceptibility using WT B6 mice infected with 105P. aeruginosa entrapped in agar beads. Next, we looked at the influence of gender on the susceptibility to P. aeruginosa infection and development of inflammation in the presence or absence of IL-10, and finally, we analysed the effects of IL-10 neutralization on bacteriology, secretory activity and inflammatory cell distribution in the lungs of female mice.
Materials and methods
Mice
Age- and gender-matched C57BL/6 mice, 8–10 weeks old, purchased from Charles River (St-Constant, Quebec, Canada) were used in the IL-10 neutralization experiments. C57BL/6 mice (n = 91; females 11·2 ± 2·2 weeks old and males 14·0 ± 6·0 weeks old) and C57BL/6-IL-10 KO mice (n = 83; females 13·2 ± 1·4 weeks old and males 14·6 ± 5·1 weeks old), were used for the IL-10 KO study according to the guidelines and regulations of the Canadian Council on Animal Care. IL-10 KO mice were confirmed by histology to be free of inflammatory bowel disease. Mice were kept in a specific pathogen free (SPF) facility; food and water were provided ad libitum.
Pseudomonas aeruginosa
P. aeruginosa strain 508 was kindly provided by Dr Jacqueline Lagacé (University of Montreal, Montreal, Quebec, Canada). This strain has a mucoid appearance when grown on blood agar and was originally isolated from the sputum of a CF patient at Ste-Justine Hospital, Montreal, Quebec, Canada.
Inoculum preparation and infection of mice
In order to establish a model of prolonged infection, bacteria-impregnated agar beads were prepared as previously described36 and were used for lung infection. Briefly, log phase bacteria were concentrated and added to warm 1·5% trypticase soy agar (TSA; Difco, Detroit, MI). This mixture was added to heavy mineral oil and stirred rapidly first at 52°, followed by cooling with continuous stirring. The oil–agar mixture was centrifuged to sediment the beads. The oil was removed and the beads washed. The size of the beads was verified microscopically and only those preparations containing beads predominantly 100–150 µm in diameter were used as inoculum. The number of bacteria was estimated after homogenizing the bacteria-impregnated bead suspension. Inoculum was prepared by diluting the bead suspension in sterile Dulbecco's phosphate-buffered saline (PBS; Invitrogen, Mississauga, Ontario, Canada) to 2–4 × 106 colony-forming units (CFU)/ml. Mice were anaesthetized with a combination of ketamine (15 mg/ml) and xylazine (2 mg/ml) administered intramuscularly at a dose of 0·2 ml. Following a transverse cervical incision, the trachea was exposed and intubated with a sterile, flexible 22-gauge cannula attached to a 1·0-ml syringe. An inoculum of 50 µl was implanted via the cannula into the lung. After inoculation, all incisions were closed by suture. No animal developed wound infection and healing occurred in 2–3 days. Previously published data showed only mild signs of inflammation in lungs of mice injected with sterile agar beads.35,37 Two doses were tested in this model of infection using B6 WT and B6 IL-10 KO mice. When using an infection dose of 2 × 105 CFU, high mortality rates were observed; about 40% of the WT mice and more than 80% of the IL-10 KO mice did not survive the infection. When the infection dose was lowered to 105 CFU, we were able to observe more than 90% survival for all the mice infected. It was therefore possible to study prolonged lung infection in both WT and IL-10 KO mice.
Bronchoalveolar lavage
Mice were killed by CO2 overdose, exsanguinated by cutting the vena cava, and the circulation was flushed by slow intracardiac infusion of divalent cation-free Hank's balanced salt solution (HBSS; Invitrogen). The trachea was cannulated with a 22-gauge intravenous catheter placement unit (Critikon) connected to two 5 ml syringes via a three-way stopcock with rotating collar (Namic USA, Glens Falls, NY). The alveoli of infected mice were washed three times with 1·4 ml of divalent cation-free HBSS. The volume of BALF recovered was approximately 1·2 ml. Alveolar cells were centrifuged and the supernatant was used for CFU counts determination; it was then stored at −20° until assayed for cytokine concentrations. Cells were resuspended in 0·5 ml of Dulbecco's modified Eagle's minimal essential medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), diluted in Turk's solution and counted using a haemocytometer. The proportions of macrophages, lymphocytes, and polymorphonuclear cells (PMN) were calculated after counting approximately 300 alveolar cells on cytospin preparations stained with Diff-Quick (American Scientific Products, McGaw Park, IL).
Lung homogenates
Lungs from infected mice were harvested and homogenized for 60 s at high speed (homogenizer PT10135 Brinkmann Instruments Co., Mississauga, Ontario, Canada) in 4 ml of sterile PBS (Invitrogen). Serial 10-fold dilutions of lung homogenates were plated on Petri dishes containing TSA. The number of CFU per lung was counted after overnight incubation at 37°. For cytokines measurements, lung homogenates were centrifuged at 1500 g at 4° for 10 min and the supernatants were removed and stored at −20° until assayed for cytokine concentrations.
Cytokine measurements
The concentrations of TNF-α protein in lung tissue homogenates from infected animals were determined by a double sandwich enzyme-linked immunosorbent assay (ELISA) as previously described.38 Briefly, 96-well polyvinyl chloride microtitre Immulon II plates (Dynatech, Chantilly, VA) were coated at 4° overnight with hamster anti-murine TNF-α monoclonal antibody (mAb) (R&D Systems, Minneapolis, MN). Plates were then washed, incubated with blocking buffer [PBS, 0·1% Tween-20, and 1% bovine serum albumin (BSA; Sigma, Oakville, Ontario, Canada)], and sequentially incubated at room temperature with various dilutions of lung homogenate samples, polyclonal rabbit antimurine TNF-α antibody, peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody (Bio-Rad, Hercules, CA), and peroxidase substrate (ABTS; Roche, Laval, Quebec, Canada). The intensity of the colorimetric reaction was determined by spectrophotometry at 405 nm. The levels of TNF-α were calculated with reference to a standard curve established with recombinant murine TNF-α (R & D Systems). KC protein concentrations were measured by ELISA kit (R & D Systems) according to the manufacturer's instructions.
Anti-murine IL-10 monoclonal antibody and neutralization protocol
Anti-IL-10 mAb was raised as ascites in BALB/c mice. Briefly, mice previously primed with pristane intraperitoneally (i.p) were given 5 × 106 JES-2A5 hybridoma cells i.p. (American Type Culture Collection, Rockville, MD). Ascites fluid was drained from peritoneal cavity after 10–14 days. The IgG fraction from the ascites fluid was purified by affinity chromatography, using protein G-Sepharose columns. Purified antibody was further dialysed and the concentration was determined by ELISA. For IL-10 neutralization experiments, randomly designated animals were treated with anti-murine IL-10 mAb JES-2A5 or rat IgG (Sigma) as control antibody.33,39 Mice were administered 200 µg of JES-2A5 or rat IgG i.p. on days −2, 0, 2, and 4 during the course of P. aeruginosa infection.
Statistical analyses
Data were analysed using SPSS V10.0.7 software (SPSS Inc, Chicago, IL) for Kaplan–Meier survival analysis followed by a multiple comparison log rank test, and Sigma Stat V2.03 software for all other analyses (SPSS Inc). Statistically significant differences between means and medians of studied groups were evaluated using Student's t-test and non-parametric Mann–Whitney U-test, respectively. Kruskal–Wallis anova on ranks combined with pair wise multiple comparison procedures (Dunn's method) were used to evaluate differences between multiple groups. Significance was set at a two-tailed P-value of ≤ 0·05.
Results
Gender differences in susceptibility to P. aeruginosa lung infection in B6 mice
In order to evaluate the role of gender during lung infection with P. aeruginosa, B6 male and female mice were infected intratracheally with 105P. aeruginosa entrapped in agar beads. We wanted to verify how early postinfection a difference in susceptibility to infection could be seen between males and females. In fact, striking differences between the males and the females were already observed at day 3–4 postinfection.
Survival of mice infected with P. aeruginosa
No significant difference was observed in survival when we compared the males with the females (P = 0·055, data not shown). No mouse died from surgery or anaesthesia, and no bacteria were found in the spleens of infected animals. Survival was monitored three times per day.
Variations in body weight during lung infection
The progression of the infection was monitored by the weight losses and gains of mice every day over the period of infection. The maximal loss was seen during the first 2–3 days postinfection, when the percentage of weight loss ranged from 10 to 22% (data not shown). After 3 days postinfection, the animals started to regain weight. We observed that males were regaining their weight faster than females (P ≤ 0·001) (Table 1). However, 14 days postinfection, the majority of mice recovered their original weight.
Table 1. Sex differences in B6 mice during P. aeruginosa lung infection.
| Female | Male | ||||
|---|---|---|---|---|---|
| CFU counts | BALF (× 104) | 210·0† | (79·0–745·0) | 2·0 | (0·0–10·8) |
| Lungs ( × 106) | 160·0† | (131·0–228·0) | 1·4 | (0·6–3·3) | |
| Alveolar cells (%) | PMN | 88·1 | (79·7–89·3) | 87·9 | (84·0–88·2) |
| Macrophages | 11·9 | (10·7–20·3) | 11·9 | (11·8–16·0) | |
| Lymphocytes | 0·0 | (0·0–0·0) | 0·0 | (0·0–0·0) | |
| Cytokines (ng/ml) | TNF | 1·3† | (0·6–2·0) | 0·5 | (0·4–0·5) |
| KC | 0·5† | (0·3–1·9) | 0·2 | (0·1–0·2) | |
| Weight* change (%) | 22·2 ± 0·7† | 18·0 ± 0·7 | |||
Values are medians of seven animals with quartiles in brackets and represent three independent experiments performed in the same conditions.
Values are means ± SEM.
Medians of the females compared to the males are significantly different at day 3 postinfection (P≤0·05).
Recruitment of alveolar cells to the site of infection
No significant difference was seen between male and female mice in the numbers of alveolar cell recruited to the infected lungs or in the numbers of PMN, macrophages and lymphocytes in the BALF.
Cytokine levels in the lungs of infected B6 mice
We assessed the concentration of TNF-α and KC protein secretion in the lung during P. aeruginosa infection. Females had significantly higher TNF-α (P = 0·029) and KC (P = 0·004) levels (Table 1), which coincided with a significantly higher weight loss, and bacterial burden in the lung.
Bacterial burden in the BALF and lungs
Susceptibility to P. aeruginosa lung infection in female mice in comparison to their male counterparts was evaluated by the bacterial load found in the BALF samples, as well as in the lung tissue homogenates of infected mice. We observed a 100–1000-fold difference in CFU counts between the BALF and the lung tissue homogenates obtained from infected mice throughout the infection (Table 1). We also observed significant lower CFU counts for male compared to female mice at day 3 postinfection in the BALF (P = 0·003) and in the lungs (P = 0·010). Similar significant differences could also be observed at day 4 postinfection (data not shown).
Overall, our results clearly demonstrate major differences in the inflammatory response to P. aeruginosa lung infection between the males and females.
Influence of IL-10 in P. aeruginosa lung infection in mice
Next, we wanted to assess the role of IL-10 during lung infection in the context of mouse gender. To evaluate the role of IL-10, we used IL-10 KO male and female mice on the B6 genetic background. Mice were intratracheally infected with 105P. aeruginosa entrapped in agar beads. We then assessed their susceptibility and inflammatory parameters during the course of infection. We evaluated the weight loss throughout the infection, the bacterial load found in the BALF samples, as well as in the lung homogenates of infected mice, and the numbers of alveolar cells recruited to the lung (PMN, macrophages, and lymphocytes). We also looked at different cytokines present at specific time points.
Survival of mice infected with P. aeruginosa
No mouse died from surgery or anaesthesia, and no bacteria were found in the spleens of infected animals. Survival was monitored three times per day. The survival curves of the IL-10 KO and WT mice did not significantly differ from each other for either male (P = 0·970) or female (P = 0·232) animals (data not shown).
Variations in body weight during lung infection
Several reports have demonstrated that substantial weight loss occurs in CF patients during acute, exacerbated pulmonary infection.40,41 Therefore, the weight of mice was monitored every day over the course of infection. The maximal weight loss was seen during the first 3–4 days postinfection, when the percentage of weight loss ranged from 19 to 23% (data not shown). Three to 4 days postinfection, the animals started to regain weight, and after 14 days postinfection, the majority of mice recovered their original weight. We observed significant differences in the kinetics of weight loss variation during infection between the WT (18·0 ± 0·7) and IL-10 KO (23·3 ± 0·6) males at day 3 postinfection (P ≤ 0·001). Similar significant differences in the weight loss were observed at day 4 (P ≤ 0·001) postinfection between the WT (13·3 ± 4·9) and IL-10 KO (23·2 ± 5·3) males. As for the female mice, IL-10 KO (22·4 ± 2·0) mice lost significantly more weight at day 4 postinfection (P = 0·038) compared to the WT mice (15·9 ± 2·0) (data not shown).
Recruitment of alveolar cells to the site of infection
In order to determine if IL-10 is involved in the regulation of the inflammatory cell recruitment to the lungs during the course of P. aeruginosa infection, we measured the number of alveolar cells recruited to the lung and the percentages of PMN, macrophages, and lymphocytes found in the lungs throughout the infection. As shown in Fig. 1(a), there was a significantly higher number of alveolar cells in the lung of IL-10 KO males than the WT controls at day 4 postinfection (P ≤ 0·001). No difference in the amount of alveolar cells could be seen earlier in the infection. We did not observe any significant difference in the recruitment of alveolar cells between IL-10 KO and WT female mice neither at day 3 or 4 postinfection (Fig. 1b).
Figure 1.
Alveolar inflammatory cells in the lungs of P. aeruginosa infected mice. The number of alveolar cells from the BALF was evaluated in IL-10 KO (open) and WT (solid) male (a) and female (b) mice 4 days postinfection. Data are presented as individual values of seven to 10 mice and the horizontal line represents the median. Significant (*) difference was found between IL-10 KO and WT males (P ≤ 0·001). These results illustrate three independent experiments performed under the same conditions.
As shown in Table 2, there were differences in the subtypes of inflammatory cells found in the lung milieu of IL-10 KO and WT animals. At the early stage of infection (day 1 postinfection), there was no difference between the IL-10 KO and the WT mice either in the absolute number of alveolar cells found in the lungs, nor in the relative abundance of the subtype of alveolar cells (data not shown). However, we observed more PMN and fewer macrophages in the lungs of IL-10 KO mice compared to their WT counterparts at day 4 postinfection, for both males and females (Table 2). At day 6 and day 14 postinfection, the percentage of PMN in the lungs decreased as the percentage of macrophages augmented in WT males compared to earlier days postinfection (data not shown). This switch in cell population also occurred, although at a much slower pace in IL-10 KO mice. Altogether, the absolute and relative numbers of PMN and macrophages are clearly demonstrating the dysregulation in cellular recruitment because of the lack of IL-10 protein expression in the lung. Overall, the lowest number of inflammatory cells observed in the WT males compared to all other groups (IL-10 KO males, IL-10 KO females and WT females), correlate with the lowest amount of CFU counts found in the lung of these mice compared to the other three groups at day 4 postinfection.
Table 2. Recruitment of inflammatory cells into the lungs of infected mice.
| Alveolar cells (%) | |||||
|---|---|---|---|---|---|
| Sex | Genotype | n† | PMN | Macrophages | Lymphocytes |
| Females | WT | 10 | 68·1* (57·5–73·8) | 31·7* (25·9–39·7) | 0·2 (0·0–1·7) |
| IL-10 KO | 8 | 86·2 (75·3–88·4) | 13·4 (11·6–24·6) | 0·0 (0·0–0·5) | |
| Males | WT | 7 | 68·2* (45·1–70·8) | 30·3* (26·4–48·9) | 1·5 (0·2–4·6) |
| IL-10 KO | 8 | 78·1 (68·9–83·5) | 21·4 (16·0–30·0) | 0·4 (0·0–1·2) | |
Values are medians with quartiles in brackets.
Medians of WT mice compared to those of IL-10 KO mice are significantly different at 4 days postinfection (P ≤0·05) for the same sex.
Number of animals; represent three different experiments performed under the same conditions.
Levels of TNF-α in the lungs of infected IL-10 KO and WT mice
To establish whether the higher susceptibility to lung infection observed in IL-10 KO mice was associated with TNF-α protein secretion, the levels of TNF-α were measured in the lung tissue homogenates following P. aeruginosa infection. In IL-10 KO male mice, we found significantly higher levels of TNF-α (P = 0·029; data not shown) 3 days following P. aeruginosa infection, which coincided with a significantly higher weight loss, bacterial burden and cellularity in the lung compared to WT males. In females, IL-10 KO mice had significantly higher TNF-α levels in the lungs (0·56 ng/ml (0·37–0·73)) compared to WT mice (0·25 ng/ml (0·20–0·26)) at day 4 postinfection (P ≤ 0·001). Because the differences were seen earlier in males than females, the overabundance of TNF-α in the lung of infected animals seems to correlate with more severe outcome in a time dependent fashion.
Levels of KC in the lungs of infected IL-10 KO and WT mice
Since we observed high numbers of PMN in infected IL-10 KO mice compared to WT animals, we assessed the implication of KC chemoattractant in the lung of IL-10 KO infected mice. We found significantly higher KC levels in females IL-10 KO [94·9 pg/ml (30·6–157·6)] compared to females WT [26·7 pg/ml (23·8–33·0)] (P = 0·026). We also observed a tendency towards higher KC levels in IL-10 KO male mice compared to WT animals both at day 3 and 4 postinfection, although these differences were not statistically significant.
Bacterial burden in the BALF and lungs
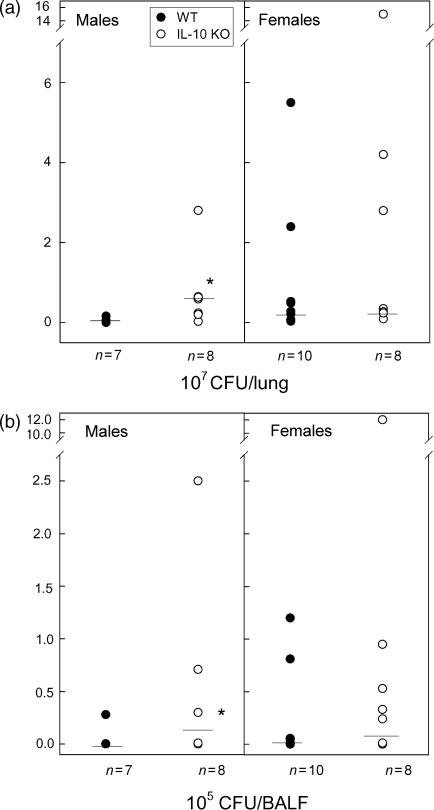
We also observed a significantly higher number of CFU counts in the IL-10 KO compared to WT males in both lungs (P = 0·022) and BALF (P = 0·008) at day 3 postinfection (data not shown). Similar significant differences between WT and IL-10 KO males were observed at 4 days postinfection again in both lungs (P = 0·021; Fig. 2a) and BALF (P = 0·021; Fig. 2b) suggesting that IL-10 KO males are more susceptible to P. aeruginosa lung infection compared to the WT controls. Both WT and IL-10 KO female mice were very sensitive to lung infection, and we did not observe any significant differences in CFUs in the BALF and lungs of these mice (Fig. 2). Significant differences were observed at day 4 postinfection between males and females WT mice in the CFU counts in BALF (P = 0·042) and lung tissue homogenates (P ≤ 0·001) (data not shown). No difference in the bacterial burden was observed between female and male IL-10 KO mice (data not shown).
Figure 2.
Bacterial burden in the lungs of P. aeruginosa infected mice. The CFU counts were assessed in the lung tissue homogenates (a) and BALF (b) of IL-10 KO (open) and WT (solid) mice 4 days postinfection. Data are presented as individual values of seven to 10 mice and the horizontal line represents the median. Significant (*) differences were found between IL-10 KO and WT males in the CFU counts in lung tissue homogenates (P = 0·021), and BALF (P = 0·021). These results illustrate three independent experiments performed under the same conditions.
Effects of anti-IL-10 neutralization on the course of P. aeruginosa infection
Because we found that female mice, both WT and IL-10 KO, were extremely susceptible to infection with P. aeruginosa, it was possible that the absence of IL-10 during their development affected the overall maturation of the immune system. Alternatively, it is possible that the kinetics of the infection differ between males and females and to observe any difference in CFU, later time points postinfection would have been more appropriate for the analysis of female mice. To test those two possibilities, we decided to assess the susceptibility of female mice at day 7 and day 14 postinfection, and instead of using IL-10 KO mice, we performed systemic IL-10 neutralization using anti-IL-10 neutralizing antibodies. Groups of B6 female mice were treated intraperitoneally with four doses of neutralizing anti-IL-10 (200 µg/dose) or isotype-matched rat IgG (200 µg/dose). As shown in Table 3, treatment of animals with anti-IL-10 resulted in a significantly higher bacterial load in the lung (P = 0·050) at day 7 postinfection compared to IgG-treated mice. Interestingly, the increased bacterial proliferation following P. aeruginosa infection was associated with significantly higher concentrations of TNF-α in the lungs of mice (P = 0·012). In order to determine if IL-10 influenced the composition of the cells at the site of infection, the cell composition of BALF was also analysed. Neutralization of IL-10 also resulted in a significantly higher influx of PMN (P = 0·001) and a significant decrease in the numbers of macrophages recovered from the airways (P = 0·001) compared to WT control infected animals (Table 3). No significant differences in the number of lymphocytes were observed following systemic depletion of IL-10. At day 14 postinfection, both experimental groups almost resolved the infection and inflammation (data not shown). Overall, the females used in the above study were very much more susceptible to infection and developed more inflammation when IL-10 protein was neutralized.
Table 3. Effects of IL-10 neutralization on P. aeruginosa lung infection in B6 mice.
| Quartiles | ||||||
|---|---|---|---|---|---|---|
| Antibody treatment | n* | 25% | 50% (Median) | 75% | P-value | |
| CFU per lung† | IgG | 15 | 1·6 × 104 | 4·8 × 104 | 9·2 × 104 | P = 0·050 |
| Anti-IL-10 | 16 | 3·6 × 104 | 24·0 × 104 | 190 × 104 | ||
| TNFα (ng/ml)† | IgG | 13 | 12·1 | 20·7 | 38·4 | P = 0·012 |
| Anti-IL-10 | 16 | 29·0 | 50·8 | 88·7 | ||
| PMN (%)†‡ | IgG | 11 | 58·2 | 66·0 | 69·8 | P = 0·001 |
| Anti-IL-10 | 17 | 72·8 | 76·0 | 80·5 | ||
| Macrophages (%)†‡ | IgG | 11 | 23·2 | 26·0 | 34·8 | P ≤ 0·001 |
| Anti-IL-10 | 17 | 12·0 | 16·0 | 20·2 | ||
| Lymphocytes (%)‡ | IgG | 11 | 5·0 | 7·0 | 10·0 | P = 0·495 |
| Anti-IL-10 | 17 | 4·0 | 6·0 | 8·2 | ||
Number of animals; represent three different experiments performed under the same conditions.
Medians of the two treatment groups are significantly different at 7 days postinfection (P≤0·05).
Values are calculated based on the cytospin differential staining analysis of BALF harvested from P. aeruginosa infected B6 mice.
The studies illustrated in this manuscript represent, to our knowledge, the first comprehensive analysis of the effects of gender and IL-10 on the inflammatory response and susceptibility to lung infection with P. aeruginosa.
Discussion
In the current study, we assessed the effect of gender on infection and inflammation using a mouse model of lung infection with P. aeruginosa. Using B6 male and female mice infected with P. aeruginosa, we found differences in the inflammatory response to the bacteria between both sexes. Females had a higher inflammatory response in terms of cytokines secreted, which correlated with a higher bacterial load, and a more severe weight loss compared to males (Table 1). These differences in inflammation influenced by gender prompted us to look at the effect of IL-10 in P. aeruginosa lung infection focusing on the gender issue. Therefore, we infected IL-10 KO male and female mice and their respective controls, and extensive analysis of various parameters of susceptibility was performed. We observed that IL-10 KO males were much more susceptible to P. aeruginosa lung infection compared to WT males. This was observed in the weight loss, the bacterial load in the lung, and in the cellular inflammatory response (Figs 1 and 2, Table 2). As for the females, WT and IL-10 KO mice were extremely sensitive to the infection, comparable with the CFU levels of males IL-10 KO. Overall, we were able to observe higher weight loss, inflammatory cytokine responses, and dysequilibrated PMN-macrophages ratio in the IL-10 KO females compared to WT females. We have chosen to analyse the parameters of inflammation between day 3 and 4 following P. aeruginosa lung infection since we could already see major differences in the inflammatory response between the IL-10 KO and the WT animals and these were the days most frequently used in other similar studies.35,37,42
Chmiel et al. also analysed early time points during the course of P. aeruginosa infection and reported a trend towards a lower bacterial burden for WT male mice after bacterial inoculation compared to their IL-10 KO counterparts at 3 days postinfection, although this difference was not significant. In the present study, we showed that IL-10 KO males have significantly more CFU counts in the lungs and BALF both at day 3 and 4 postinfection compared to WT mice. The number of infected animals used in our study most likely contributed to the statistical significance that we were able to achieve. At day 3–4 postinfection, both WT and IL-10 KO females were similarly susceptible to infection. However, we could already see a very clear difference in the level of inflammatory cytokines. These differences did not result from the altered maturation of the immunological system in IL-10 KO mice that were devoid of IL-10 during development since we saw similar differences in the inflammatory cytokine levels when we neutralized IL-10 in WT mice during the course of infection.
Several reports demonstrated that substantial weight loss occurred in CF patients during acute exacerbated pulmonary infection. This weight loss might be due to changes in energy expenditure, although the exact cause of these changes is still not well understood.41,43–46 Previous studies showed a correlation between weight loss or energy expenditure and inflammatory cytokines.40,42 In our mouse model, both IL-10 KO and control animals lost weight during the course of infection, but IL-10 KO showed a more pronounced weight loss. Interestingly, we found that WT mice were recovering from the weight loss much better than IL-10 KO mice. Overall, similar association between the weight loss and CFU was suggested by van Heeckeren et al. with a CF mouse model.42 The time points of maximal weight loss presented in this study corresponded with differences observed between the IL-10 KO and the WT mice in the CFU counts, number of inflammatory cells recruited, and levels of TNF-α observed in the lungs.
Upon P. aeruginosa colonization of the lungs, inflammatory cells are recruited into the lung to initiate an immune response cascade, which ultimately leads to clearance of the bacteria. Since IL-10 is principally an anti-inflammatory cytokine which downregulates TNF-α and membrane inflammatory protein-2, we hypothesized that IL-10 KO mice should have greater cell recruitment to the lung than WT, thereby allowing a better environment for the eradication of the infection. We demonstrated a significantly higher number of inflammatory cells present in the infected lungs in the IL-10 KO males (P = 0·001; Fig. 1a), that was associated with a higher bacterial burden. Our results showed that although the recruitment of an appropriate number of inflammatory cells is important, the type of cells recruited to the lung is also crucial. It was previously shown, in mouse models of P. aeruginosa lung infection, that an exaggerated inflammatory response dominated by PMN correlated with susceptibility to infection, whereas a modest inflammatory response dominated by macrophages correlated with resistance.36 In CF patients infected with P. aeruginosa, a persistent inflammation dominated by PMN neutrophils is observed.7 Moreover, BALF studies have suggested that CF infants, showing no clinically apparent lung disease, developed very early a predominant neutrophilic lung inflammation.47 We also observed in our study that the switch from PMN to macrophages predominance in the BALF was initiated at a later time point for IL-10 KO animals compared to WT controls, emphasizing the importance of IL-10 to initiate this change. Overall, our results suggest that PMN overabundance interferes with clearance of the bacteria from the lungs. Considering that PMN exert their antibacterial activity by releasing reactive oxygen species, proteases and diverse cytokines, an excess of PMN at the later phase of infection could provoke more harm than good by damaging lung tissues and thereby affecting the efficient eradication of the bacteria. Therefore, the amount of PMN, as well as the time point at which the percentage of alveolar cell switches from a predominantly PMN to a predominantly macrophage cell population, seems to be of crucial importance.
Because IL-10 inhibits the production of TNF-α and IL-8,25 we also investigated the effect of the lack of IL-10 on TNF-α and KC protein secretion kinetics in the lung. Our previous results show the beneficial effects of a transiently increased TNF-α level (during the first 24 hr) in P. aeruginosa-resistant mice strain compared to susceptible strains in order to efficiently eradicate the bacteria from the lungs.38 Our present data suggest that higher and prolonged expression of TNF-α in the lung is not always beneficial for the mice in terms of efficiency of the immune response and clearance of the bacteria throughout the time of infection. Furthermore, as demonstrated in adult respiratory distress syndrome, the ratio of IL-10 and TNF-α production may play a pivotal role in the balance between protection and pathogenesis in P. aeruginosa lung infection.48 Altogether, these results suggest that the proper timing and quantity of TNF-α protein secretion, as well as other inflammatory mediators in the lung environment, are mandatory in order to adequately control other immunological mediators which would lead to an efficient eradication of the bacterial infection.
In BALF as well as respiratory epithelial cells recovered from CF patients, high levels of the chemoattractant IL-8 (human homologue of murine KC and MIP-2) are observed corresponding to high amount of PMN.2,4,5,8 KC is known to be down regulated by IL-10 at the mRNA level.49,50 We found significantly higher KC levels in IL-10 KO P. aeruginosa- infected females compared to their WT controls. We also observed a trend towards higher KC levels in the IL-10 KO males, although these differences did not reach statistical significance neither at day 3 or 4 postinfection. Interestingly, there was a significant difference between WT male and female mice in their ability to produce KC, and higher susceptibility to P. aeruginosa infection observed in WT females correlated with higher levels of KC present in the infected lungs. We also observed a difference between WT male and female animals at day 3 postinfection (Table 1) where females had higher levels of KC in the lung. These results suggest that KC might be an important player during the course infection, but other mediators are also of importance.
Overall, differences observed between female and male WT mice, with respect to the weight loss, bacterial load, and cytokines production, are consistent with previously reported data suggesting that the prognosis of the disease is worse in CF female patients compared to CF male patients.10 Unfortunately, most of the CF human studies using both male and female adults and/or children did not analyse or report any differences in IL-10 levels between genders.1–3,6,8,9,19 Nevertheless, the importance of IL-10 is clearly demonstrated in the gender-related differences of the immune response,22 associating sex steroids with the secretion of IL-10,20 and gender-related difference in IL-10 administration therapy.21
Taken together, the results presented here demonstrate a major difference between the males and the females in the susceptibility to lung infection reinforcing the importance of studying pulmonary diseases taking gender into account. Our data also show the importance of a proper balance in the cytokine production and type of alveolar inflammatory cells in a timely manner, and reinforce the importance of IL-10 protein secretion during lung infection with P. aeruginosa.
Acknowledgments
This work was supported by Canadian Cystic Fibrosis Foundation grants (DR and MMS). C.G. is supported by a studentship from the Fonds de la Recherche en Santé du Québec et Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (FRSQ-FCAR), and D.R. is supported by the Chercheurs Nationaux scholarship from FRSQ-FCAR. We would like to thank Mary Fujiwara and Fabrice Rouah for the help with statistical analysis and Sergio Di Marco and Ellen Buschman for critical review of the manuscript.
Abbreviations
- CF
cystic fibrosis
- WT
wildtype
- KO
knockout
- B6
C57BL/6
- IL
interleukin
- PMN
polymorphonuclear cells
- BALF
bronchoalveolar lavage fluid
- CFU
colony-forming unit
- TNF-α
tumour necrosis factor-α
References
- 1.Wojnarowski C, Frischer T, Hofbauer E, Grabner C, Mosgoeller W, Eichler I, Ziesche R. Cytokine expression in bronchial biopsies of cystic fibrosis patients with and without acute exacerbation. Eur Respir J. 1999;14:1136–44. doi: 10.1183/09031936.99.14511369. [DOI] [PubMed] [Google Scholar]
- 2.Bonfield TL, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–61. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 3.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs [published erratum appears in Am J Respir Crit Care Med 1996; 154: 1217] Am J Respir Crit Care Med. 1995;152:2111–8. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 4.Dosanjh AK, Elashoff D, Robbins RC. The bronchoalveolar lavage fluid of cystic fibrosis lung transplant recipients demonstrates increased interleukin-8 and elastase and decreased IL-10. J Interferon Cytokine Res. 1998;18:851–54. doi: 10.1089/jir.1998.18.851. [DOI] [PubMed] [Google Scholar]
- 5.Massengale AR, Quinn FJ, Yankaskas J, Weissman D, McClellan WT, Cuff C, Aronoff SC. Reduced interleukin-8 production by cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 1999;20:1073–80. doi: 10.1165/ajrcmb.20.5.3243. [DOI] [PubMed] [Google Scholar]
- 6.Osika E, Cavaillon JM, Chadelat K, Boule M, Fitting C, Tournier G, Clement A. Distinct sputum cytokine profiles in cystic fibrosis and other chronic inflammatory airway disease. Eur Respir J. 1999;14:339–46. doi: 10.1034/j.1399-3003.1999.14b17.x. [DOI] [PubMed] [Google Scholar]
- 7.Berger M. Inflammation in the lung in cystic fibrosis. A vicious cycle that does more harm than good? Clin Rev Allergy. 1991;9:119–42. doi: 10.1007/978-1-4612-0475-6_8. [DOI] [PubMed] [Google Scholar]
- 8.Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol. 1999;104:72–8. doi: 10.1016/s0091-6749(99)70116-8. [DOI] [PubMed] [Google Scholar]
- 9.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis. 1997;175:638–47. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 10.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 11.D'Agostino P, Milano S, Barbera C, et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann N Y Acad Sci. 1999;876:426–9. doi: 10.1111/j.1749-6632.1999.tb07667.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller L, Alley EW, Murphy WJ, Russell SW, Hunt JS. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukoc Biol. 1996;59:442–50. doi: 10.1002/jlb.59.3.442. [DOI] [PubMed] [Google Scholar]
- 13.Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40. [PubMed] [Google Scholar]
- 14.Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol. 1995;13:217–26. [PubMed] [Google Scholar]
- 15.Cutolo M, Accardo S, Villaggio B, et al. Androgen and estrogen receptors are present in primary cultures of human synovial macrophages. J Clin Endocrinol Metab. 1996;81:820–7. doi: 10.1210/jcem.81.2.8636310. [DOI] [PubMed] [Google Scholar]
- 16.Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha- deficient mice. J Clin Invest. 1998;102:1083–91. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20:112–8. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Zuckerman SH, Bryan-Poole N, Evans GF, Short L, Glasebrook AL. In vivo modulation of murine serum tumour necrosis factor and interleukin-6 levels during endotoxemia by oestrogen agonists and antagonists. Immunology. 1995;86:18–24. [PMC free article] [PubMed] [Google Scholar]
- 19.Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol. 2000;25:518–25. doi: 10.1046/j.1365-2249.2000.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angele MK, Knoferl MW, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol. 1999;277:C35–C42. doi: 10.1152/ajpcell.1999.277.1.C35. [DOI] [PubMed] [Google Scholar]
- 21.Kahlke V, Dohm C, Brotzmann K, Schreiber S, Schroder J. Gender-related therapy: early IL-10 administration after hemorrhage restores immune function in males but not in females. Shock. 2000;14:354–9. doi: 10.1097/00024382-200014030-00020. [DOI] [PubMed] [Google Scholar]
- 22.Wilder RL. Hormones, pregnancy, and autoimmune diseases. Ann N Y Acad Sci. 1998;840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x. 45–50. [DOI] [PubMed] [Google Scholar]
- 23.Lalani I, Bhol K, Ahmed AR. Interleukin-10: biology, role in inflammation and autoimmunity [published erratum appears in Ann Allergy Asthma Immunol 1998; 80: A–6] Ann Allergy Asthma Immunol. 1997;79:469–83. doi: 10.1016/S1081-1206(10)63052-9. [DOI] [PubMed] [Google Scholar]
- 24.Moore KW, O'Garra A, de Waal M, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 25.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 26.Bermudez LE, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–7. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumoniae. J Immunol. 1995;155:722–9. [PubMed] [Google Scholar]
- 28.Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med. 1996;184:241–51. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J Immunol. 1995;155:2222–9. [PubMed] [Google Scholar]
- 30.Gudmundsson G, Bosch A, Davidson BL, Berg DJ, Hunninghake GW. Interleukin-10 modulates the severity of hypersensitivity pneumonitis in mice. Am J Respir Cell Mol Biol. 1998;19:812–8. doi: 10.1165/ajrcmb.19.5.3153. [DOI] [PubMed] [Google Scholar]
- 31.Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Hanes M, Chrisp CE, Boucher JC, Deretic V. Microbial pathogenesis in cystic fibrosis. pulmonary clearance of mucoid Pseudomonas aeruginosa and inflammation in a mouse model of repeated respiratory challenge. Infect Immun. 1998;66:280–8. doi: 10.1128/iai.66.1.280-288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawa T, Corry DB, Gropper MA, Ohara M, Kurahashi K, Wiener-Kronish JP. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol. 1997;159:2858–66. [PubMed] [Google Scholar]
- 34.Steinhauser ML, Hogaboam CM, Kunkel SL, Lukacs NW, Strieter RM, Standiford TJ. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J Immunol. 1999;162:392–9. [PubMed] [Google Scholar]
- 35.Chmiel JF, Konstan MW, Knesebeck JE, Hilliard JB, Bonfield TL, Dawson DV, Berger M. IL-10 attenuates excessive inflammation in chronic pseudomonas infection in mice [in process citation] Am J Respir Crit Care Med. 1999;160:2040–7. doi: 10.1164/ajrccm.160.6.9901043. [DOI] [PubMed] [Google Scholar]
- 36.Sapru K, Stotland PK, Stevenson MM. Quantitative and qualitative differences in bronchoalveolar inflammatory cells in Pseudomonas aeruginosa-resistant and -susceptible mice. Clin Exp Immunol. 1999;115:103–9. doi: 10.1046/j.1365-2249.1999.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosselin D, Stevenson MM, Cowley EA, et al. Impaired ability of Cftr knockout mice to control lung infection with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 1998;157:1253–62. doi: 10.1164/ajrccm.157.4.9702081. [DOI] [PubMed] [Google Scholar]
- 38.Gosselin D, DeSanctis J, Boule M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–8. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida H, Hastings R, Thompson-Snipes L, Howard M. Modified immunological status of anti-IL-10 treated mice. Cell Immunol. 1993;148:371–84. doi: 10.1006/cimm.1993.1119. [DOI] [PubMed] [Google Scholar]
- 40.Elborn JS, Cordon SM, Western PJ, Macdonald IA, Shale DJ. Tumour necrosis factor-alpha, resting energy expenditure and cachexia in cystic fibrosis. Clin Sci (Colch) 1993;85:563–8. doi: 10.1042/cs0850563. [DOI] [PubMed] [Google Scholar]
- 41.Naon H, Hack S, Shelton MT, Gotthoffer RC, Gozal D. Resting energy expenditure. Evolution during antibiotic treatment for pulmonary exacerbation in cystic fibrosis. Chest. 1993;103:1819–25. doi: 10.1378/chest.103.6.1819. [DOI] [PubMed] [Google Scholar]
- 42.van Heeckeren AM, Tscheikuna J, Walenga RW, Konstan MW, Davis PB, Erokwu B, Haxhiu MA, Ferkol TW. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med. 2000;9:271–9. doi: 10.1164/ajrccm.161.1.9903019. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd RW, Holt TL, Vasques-Velasquez L, Coward WA, Prentice A, Lucas A. Increased energy expenditure in young children with cystic fibrosis. Lancet. 1988;1:1300–3. doi: 10.1016/s0140-6736(88)92119-8. [DOI] [PubMed] [Google Scholar]
- 44.Shepherd RW, Holt TL, Cleghorn G, Ward LC, Isles A, Francis P. Short-term nutritional supplementation during management of pulmonary exacerbations in cystic fibrosis: a controlled study, including effects of protein turnover. Am J Clin Nutr. 1988;48:235–9. doi: 10.1093/ajcn/48.2.235. [DOI] [PubMed] [Google Scholar]
- 45.Steinkamp G, Drommer A, von der Hardt H. Resting energy expenditure before and after treatment for Pseudomonas aeruginosa infection in patients with cystic fibrosis. Am J Clin Nutr. 1993;57:685–9. doi: 10.1093/ajcn/57.5.685. [DOI] [PubMed] [Google Scholar]
- 46.Ward SA, Tomezsko JL, Holsclaw DS, Paolone AM. Energy expenditure and substrate utilization in adults with cystic fibrosis and diabetes mellitus. Am J Clin Nutr. 1999;69:913–9. doi: 10.1093/ajcn/69.5.913. [DOI] [PubMed] [Google Scholar]
- 47.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24:137–42. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong L, Millar AB. Relative production of tumour necrosis factor alpha and interleukin 10 in adult respiratory distress syndrome. Thorax. 1997;52:442–6. doi: 10.1136/thx.52.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim HS, Armstrong D, Hamilton TA, Tebo JM. IL-10 suppresses LPS-induced KC mRNA expression via a translation-dependent decrease in mRNA stability. J Leukoc Biol. 1998;64:33–9. doi: 10.1002/jlb.64.1.33. [DOI] [PubMed] [Google Scholar]
- 50.Kishore R, Tebo JM, Kolosov M, Hamilton TA. Cutting edge. clustered AU-rich elements are the target of IL-10- mediated mRNA destabilization in mouse macrophages. J Immunol. 1999;162:2457–61. [PubMed] [Google Scholar]