Abstract
Macrophages that accumulate in the synovium of rheumatoid arthritis patients play an important role in the pathogenesis of this inflammatory disease. However, the mechanism by which macrophages are attracted into the inflamed synovium and accumulate there has not been completely delineated. The results of this study show that rheumatoid arthritis synovial stromal cells produce the chemokines monocyte chemotactic protein-1 and IL-8, and these have the capacity to attract peripheral monocytes. These results suggest that one of the mechanisms by which macrophages accumulate in the inflamed synovium is by responding to the chemokines produced locally.
Keywords: chemokine, monocyte, rheumatoid arthritis
Introduction
The synovial tissue in rheumatoid arthritis (RA) contains synovial fibroblasts and stromal cells as well as macrophages. Synovial stromal cells and fibroblasts are thought to proliferate in situ [1]. On the contrary, macrophages do not proliferate, but rather differentiate from monocytes that migrated from the peripheral blood, and are activated to differentiate in the synovial tissue. Macrophages in RA synovium secrete many inflammatory mediators (IL-1, IL-6, IL-8, tumor necrosis factor-α [TNF-α] and PGE2) and a variety of matrix metalloproteinases, and are thought to play a central role in the inflammation and joint destruction characteristic of RA [2]. The number of macrophages in RA synovium correlates significantly with clinical symptoms and the degree of joint damage [3]. Understanding the regulation of macrophage accumulation in the RA synovium should therefore provide insight into the inflammatory nature of rheumatoid synovitis.
Migration of peripheral blood monocytes is likely to be influenced by chemokines. A number of chemokines, including IL-8, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, epithelial-derived neutrophil attractant 78, and regulated upon activation, normal T cell expressed and secreted (RANTES), are known to be found in RA synovial fluid [4,5,6,7,8]. A number of specific chemokine receptors including CCR1, CCR2, CCR5, CCR8, and CXCR4 are also known to be expressed by peripheral blood monocytes [9,10]. Despite this information, the specific chemokine–chemokine receptor interactions involved in the recruitment of monocytes into the rheumatoid synovium have not been fully delineated.
To address this issue, we examined chemokine receptor expression by peripheral blood monocytes, and also analyzed the capacity of supernatants from RA synovial stromal cells to induce monocyte migration. The data indicate that MCP-1 secreted by synovial stromal cells plays a major role in attracting monocytes to the synovium, and that IL-8 may also contribute.
Materials and methods
Antibodies and reagents
Biotinylated mouse anti-human CCR1 monoclonal antibody (mAb), mouse anti-human CCR2 mAb conjugated with phycoerythrin (PE), mouse anti-human CCR6 mAb conjugated with PE, mouse anti-human CXCR1 mAb conjugated with PE, mouse anti-human CXCR2 mAb conjugated with PE, mouse anti-human CXCR5 mAb conjugated with PE, mouse anti-human CCR3 mAb conjugated with FITC, mouse anti-human MCP-1 mAb (24822.111), mouse anti-human IL-8 mAb (6217.111), mouse anti-human IP-10 mAb (33036.211), mouse anti-human CCR5 mAb (45531.111), and biotinylated goat IgG anti-human IP-10 were purchased from R&D Systems Inc (Miami, FL). Mouse anti-human CCR5 mAb conjugated with FITC and mouse anti-human CXCR4 mAb conjugated with PE were purchased from Pharmingen (San Diego, CA). Mouse anti-human CD14 mAb conjugated with FITC or PE, and Streptavidin conjugated with PE were obtained from Sigma (St Louis, MO). Mouse IgG1 mAb was prepared from hybridoma cell lines purchased from ATCC (Rockville, MD). Mouse IgG2A conjugated with PE (Pharmingen), mouse IgG2B conjugated with PE (R&D Systems), and mouse IgG1 conjugated with FITC (Becton Dickinson, San Jose, CA) were used for negative controls for flow cytometry.
Recombinant human TNF-α, RANTES, interferon-gamma inducible protein 10 (IP-10), and stromal cell derived factor-1α were purchased from R&D Systems. Trizol reagent, deoxyribonucleaseI, and SuperScriptII reverse transcriptase were purchased from Gibco BRL (Rockville, MD). Taq polymerase was purchased from Promega (Madison, WI), and Oligo dT and ficoll/isopaque were purchased from Pharmacia (Piscataway, NJ). DMEM with high-glucose, RPMI-1640, and FBS were purchased from Gibco BRL.
Enzyme-linked immunosorbent assays (ELISAs) for MCP-1, IL-8, MIP-1β, and RANTES were purchased from R&D Systems, and transwell membranes (5 μm pore size in 24 wells) were purchased from Costar (Cambridge, MA). The TMB microwell peroxidase substrate system was purchased from KPL (Gaithersburg, MD).
Stromal cell lines and fibroblast lines
One RA stromal cell line (SCL) was established from synovium as previously described [11]. In brief, synovial tissue of a patient with RA who met American College of Rheumatology criteria [12] was obtained following informed consent, and dissociated with collagenase and trypsin. Dissociated cells were cultured in DMEM supplemented with 10% FBS and 10% conditioned medium, which was prepared by incubation of peripheral blood mononuclear cells from 10 healthy donors in RPMI-1640 medium with 10% FBS for 48 h. The cultures were then maintained for more than 2 months, and the SCLs were cloned by limiting dilution. Clones were thereafter maintained and replenished with fresh DMEM medium with 10 or 20% FBS every 3–4 days. One clone (Sy77) was used in the present experiments. RA tissues from two other patients were also dissociated with collagenase and trypsin. Dissociated cells were cultured in DMEM supplemented with 10% FBS and 10% conditioned medium and used as RA SCLs (RA6/1 and RA8/3) after 4–10 passages. An SCL was also established from osteoarthritis synovium (OA5/26) using the same procedure. A skin fibroblast line (FBHG) was also established from a healthy human skin sample.
SCL and fibroblast culture
RA SCL, osteoarthritis (OA) SCL, and skin fibroblasts (1 × 105) were seeded in six-well culture plates (Costar) with DMEM containing 10% FBS, 200 U/ml penicillinG, 10 μg/ml gentamicin and 0.3 mg/ml L-glutamine, and cultured for 3 days. After culturing, the cells were washed once and the medium was changed to DMEM containing 5% FBS, 200 U/ml penicillinG, 10 μg/ml gentamicin, and 0.3 mg/ml L-glutamine with or without 2 ng/ml TNF-α. To assess chemokine mRNA expression, cell lines were harvested after 4 h of culture, then suspended in Trizol and stored at -80°C. Supernatants were collected after 24 h and stored at -80°C until use for migration assay and quantification of chemokines.
Preparation of mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood of healthy adult volunteers by density sedimentation using ficoll/isopaque. PBMC were cultured with sheep red blood cells (SRBC) treated for 70 min at 4°C, and rosette negative cells were collected by ficoll/isopaque sedimentation.
Chemokine receptor expression by monocytes
Chemokine receptor expression by monocytes was assessed by dual immunofluorescence flow cytometry following staining with anti-CD14 mAb and anti-chemokine receptor mAbs (FACScan; Becton Dickinson). For staining chemokine receptors, 10 μl of each mAb was used for 1 × 105 cells suspended in 100 μl staining buffer (PBS + 2% FBS) in accordance with the manufacturer's instructions.
Migration assay
Cell migration was assessed in 24-well chemotaxis chambers fitted with 5.0 μm transwell membranes. Supernatants (550 μl) of cell lines cultured in various conditions were added to the lower wells, and 5 × 105 SRBC rosette negative PBMC in 100 μl DMEM containing 5% FBS were added to the upper wells. In blocking experiments, the supernatants were cultured with blocking mAbs specific for chemokines overnight at 4°C before assay. SRBC rosette negative PBMC were incubated with blocking mAb to chemokine receptors for 1 h at 4°C before assay in some experiments. The transwell membranes were removed after 90 min of incubation at 37°C, and migrated cells in the lower chamber were harvested by pipetting. The cells were stained with anti-CD14 mAb conjugated with FITC, and they were suspended in 110 μl PBS containing 3% FBS. All of the cells were analyzed by flow cytometry and CD14+ cells were counted.
RNA isolation and reverse transcriptase-polymerase chain reaction
RNA was extracted from SCLs and fibroblasts using Trizol reagent in accordance with the manufacturer's instructions. Two micrograms of the extracted RNA was treated with 2 U DNaseI to eliminate DNA, and reverse transcribed with 200 U SuperScriptII reverse transcriptase at 42°C for 70 min using oligo dT primers in accordance with the manufacturer's instructions. Polymerase chain reaction (PCR) was carried out with Taq polymerase using 0.1–0.3 μl cDNA (1.5 mM MgCl2). Denaturation and extension conditions were 94°C for 1 min and 72°C for 1 min, respectively. The annealing period was 1 min for each PCR, and the temperature was 62°C for RANTES and 56°C for the other chemokines and β-actin. PCR products were resolved by electrophoresis on 1.5% agarose gels and identified with ethidium bromide staining. Firstly, β-actin expression was examined using 26-30 cycles of reverse transcriptase-PCR (RT-PCR) to amplify 0.1, 0.2, and 0.3 μl cDNA to adjust the amount of cDNA of each sample precisely. After resolving the PCR products on agarose gels and identifying the relevant bands with ethidium bromide, the optimal amounts of cDNA for analysis were then determined. Chemokine expression in this amount of cDNA was examined using 30, 32, 35, 38, and 40 cycles of PCR amplification, and the results in the linear part of the amplification curve are reported in the figures.
PCR primers
The primer pair for β-actin was GTC CTC TCC CAA GTC CAC ACA (forward) and CTG GTC TCA AGT CAG TGT ACA GGT AA (reverse), that of IL-8 was CTG CGC CAA CAC AGA AAT TA (forward) and ATT GCA TCT GGC AAC CCT AC (reverse), that of MCP-1 was GCC TCC AGC ATG AAA GTC TC (forward) and TAA AAC AGG GTG TCT GGG GA (reverse), that of IP-10 was CCA CGT GTT GAG ATC ATT GC (forward) and TGG AAG ATG GGA AAG GTG AG (reverse), that of RANTES was CGC TGT CAT CCT CAT TGC TA (forward) and GCT GTC TCG AAC TCC TGA CC (reverse), that of MIP-1α was TGC AAC CAG TTC TCT GCA TC (forward) and ACA GGG GAA CTC TCA GAG CA (reverse), and that of MIP-1β was CTG GGT CCA GGA GTA CGT GT (forward) and ACA GTG GAC CAT CCC CAT AG (reverse).
ELISA
Concentrations of chemokines were measured by sandwich ELISA according to the manufacturer's instructions (R&D Systems).
Statistical analysis
The Student paired t test was used to compare the effect of CD14 cell migration, and the blocking effect of mAbs. The Student t test was used to evaluate chemokine production by SCLs and the fibroblast line.
Results
Chemokine receptor expression by peripheral blood monocytes
Chemokine receptor expression by CD14+ monocytes was examined using flow cytometry. Representative examples (Fig. 1) and a summary of staining results (Table 1) are presented. CXCR3 and CXCR4 were expressed by a high frequency of monocytes in all healthy donors. More than one-half of monocytes expressed CCR2, CCR5, CXCR1, and CXCR2 in seven of nine cases, although a minimal number of monocytes from two donors expressed these receptors. Expression of CCR6 and CXCR5 by monocytes was minimal in all subjects. CCR1 was expressed by few monocytes in three donors, whereas 20-40% of monocytes of the other donors expressed CCR1. These results suggest that CCR1, CCR2, CCR5, and CXCR1, CXCR2, CXCR3, and CXCR4 are candidates to be involved in chemokine-mediated trafficking of monocytes.
Figure 1.
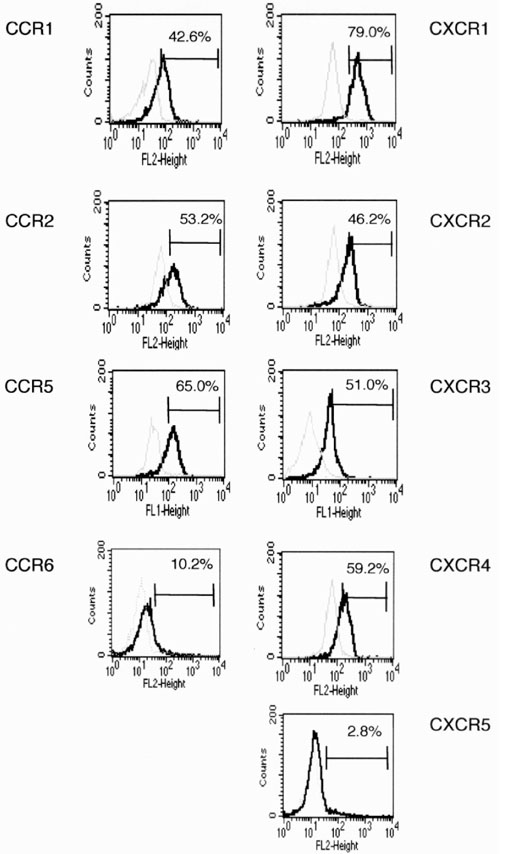
Chemokine receptor expression by peripheral blood monocytes. Peripheral blood SRBC rosette negative cells (1 × 105) were stained with anti-CD14 mAb and various chemokine receptor mAbs, and were analyzed by flow cytometry. CD14+ cells were gated, and chemokine receptor expression by the CD14+ monocytes is shown (solid line). Dotted lines show staining by isotype-matched control mAb. Percentage of positive cells is also shown in the histograms. Staining was from one of nine experiments. FL-1 height, FITC fluorescence; FL-2 height, PE fluorescence.
Table 1.
Chemokine receptor expression by CD14+ monocytes
| Positive expression | |||||||||
| CCR1 | CCR2 | CCR5 | CCR6 | CXCR1 | CXCR2 | CXCR3 | CXCR4 | CXCR5 | |
| Mean (%) | 26.8 | 51.1 | 41.6 | 9.5 | 58.2 | 51.3 | 73.6 | 68.2 | 2.8 |
| Range | 3–43 | 12–81 | 0.8–76 | 5–14 | 7–79 | 2–81 | 51–88 | 34–88 | 2–4 |
Sheep red blood cell rosette negative cells from nine healthy donors were stained with anti-CD14 monoclonal antibody and anti-chemokine receptor monoclonal antibodies, and were analyzed by flow cytometry. CD14-positive cells were gated, and chemokine receptor expression by CD14+ cells was analyzed. The mean percent and range of positive cells are shown.
Supernatants of cell lines attract monocytes
To examine whether SCLs derived from RA synovium can attract monocytes efficiently, migration of monocytes in response to culture supernatants of RA and OA SCLs and fibroblasts was examined (Fig. 2). Supernatants of all cell lines attracted significantly more monocytes than medium alone (P < 0.01). Those supernatants from RA SCLs induced the migration of significantly more monocytes than the supernatants of the OA SCL and skin fibroblasts.
Figure 2.
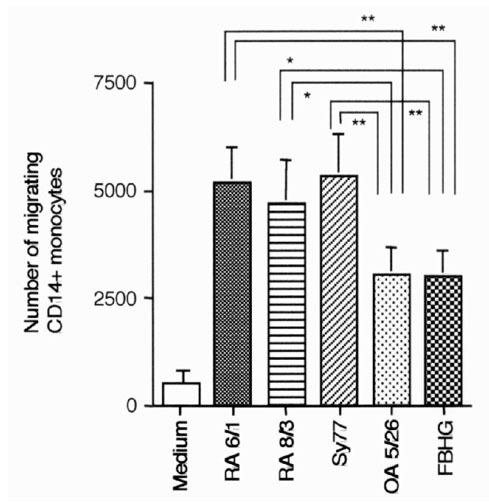
Monocyte migration induced by supernatants of various cell lines. An assay of monocyte migration was performed using supernatants of various cells. Each cell line (1 × 105/well) was cultured in six-well culture plates with 2 ml DMEM supplemented with 5% FBS. Supernatants were collected after 24 h of incubation, and were used for assay. Migrated cells were stained with anti-CD14 mAb, then the number of migrated CD14+ cells was assessed by flow cytometry. RA6/1, RA8/3, and Sy77 are RA SCLs, OA5/26 is a SCL from OA, and FBHG is a skin fibroblast line. The mean and SEM were calculated with data from 11 independent experiments. Statistical analysis was performed with the paired Student t test. *P < 0.05 and **P < 0.01.
Chemokine mRNA production by cell lines
Production of proinflammatory chemokine mRNA by the various cell lines was assessed using RT-PCR (Fig. 3). RA SCLs expressed a number of proinflammatory chemokine mRNAs, including MCP-1, IL-8, MIP-1α, MIP-1β, RANTES, and IP-10. OA SCL also expressed these same chemokine mRNAs with the exception of MIP-1β. The fibroblast line expressed mRNAs for IL-8, MCP-1, and IP-10, but not RANTES, MIP-1α, and MIP-1β.
Figure 3.
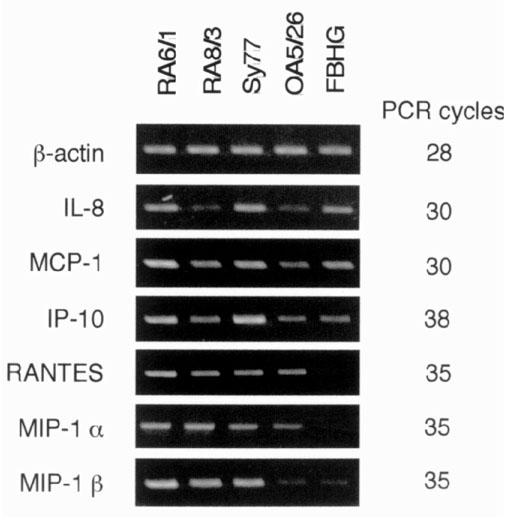
Chemokine mRNA expression by various cell lines. Chemokine mRNA expression was examined with RT-PCR. PCR products were separated in 1.5% agarose gels and analyzed after ethidium bromide staining. Each PCR was performed using 0.1 μl cDNA sample and the number of PCR cycles indicated. RA6/1, RA8/3, and Sy77 are RA SCLs, OA5/26 is a SCL from OA, and FBHG is a skin fibroblast line.
Chemokine production by cell lines
Chemokines secreted into the culture supernatants of cell lines were measured by ELISA (Fig. 4). Production of MCP-1 and IL-8 by RA SCLs was greater than that by OA SCL and the fibroblast line. The cell lines produced low levels of RANTES, whereas IP-10 and MIP1-β were not detected (data not shown).
Figure 4.
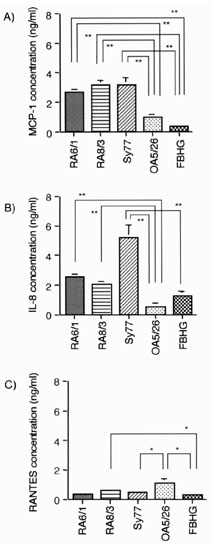
Chemokine production by various cell lines. Production of MCP-1, IL-8, RANTES, IP-10, and MIP-1β was measured by ELISA. Various cell lines (1 × 105/ml) were cultured for 24 h with 2 ml DMEM containing 5% FBS, and supernatants were collected. The contents of (A) MCP-1, (B) IL-8, and (C) RANTES are shown; MIP-1β and IP-10 were not detected (less than 10 pg/ml). RA6/1, RA8/3, and Sy77 are RA SCLs, OA5/26 is a SCL from OA, and FBHG is a skin fibroblast line. The mean and SEM were calculated from triplicates of one representative experiment out of three with similar results. Statistical analysis was performed with the Student t test. *P < 0.05 and **P < 0.01.
MCP-1 and IL-8 play a role in migration of monocytes
Blocking experiments were carried out using mAbs to determine the chemokines that contribute to monocyte migration induced by RA SCLs (Fig. 5). Anti-MCP-1 and anti-IL-8 mAbs decreased monocyte migration significantly, with the effect of anti-MCP-1 being consistently greater than that of anti-IL-8. Anti-CCR5 blocking mAb did not effect monocyte migration. The anti-CCR5 mAb did have blocking activity, however, since migration of T cells induced by RANTES (500 ng/ml) was blocked by 95% (data not shown).
Figure 5.
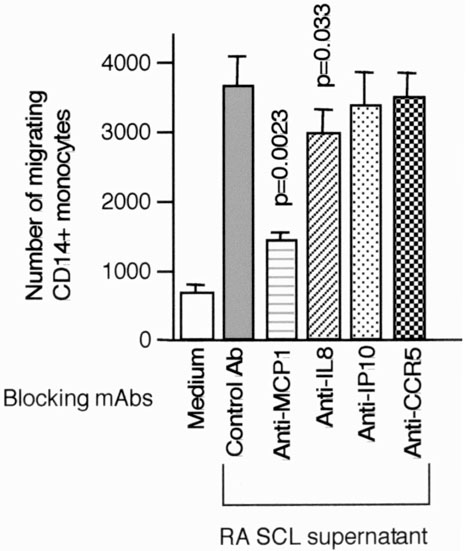
Blocking of monocyte migration induced by RA SCL supernatants with blocking anti-chemokine or chemokine receptor mAbs. Monocyte migration induced by supernatants of RA SCLs (RA6/1 and RA8/3) was assessed using mAbs, which can neutralize specific chemokines or chemokine receptors. Concentrations of mAbs were 10 μg/ml for MCP-1, IL-8, and IP-10, and 50 μg for anti-CCR5 mAb with 5 × 105 SRBC rosette negative cells. Migrating cells were stained with anti-CD-14 mAb, and migrating CD14+cells were counted by flow cytometry. The mean and SEM were calculated from the results of nine independent experiments. The paired Student t test was used to assess statistical differences versus the result with control mAb.
Stimulation with TNF-α induces RA SCL to produce more chemokines and attract more monocytes
RA SCLs were stimulated with TNF-α, and chemokine production and the capacity of supernatants to influence monocyte migration were examined to assess the influence of inflammatory cytokines on chemokine production and monocyte trafficking. Supernatants from RA SCLs stimulated with 2 ng/ml TNF-α produced more MCP-1, IL-8, RANTES and IP-10 (Fig. 6), and attracted more monocytes (Fig. 7) than those that were unstimulated.
Figure 6.
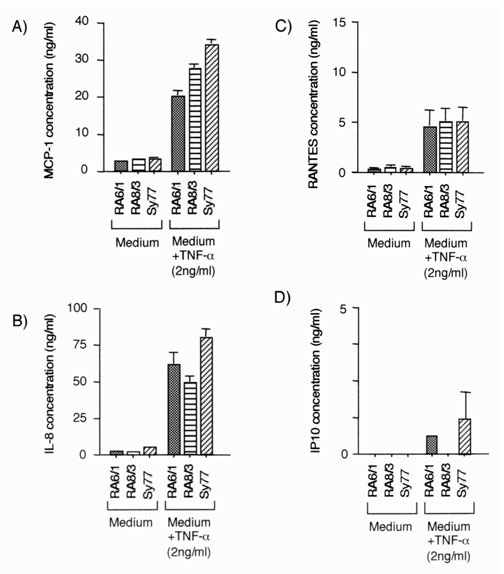
Chemokine production by RA SCL stimulated with TNF-α. Production of MCP-1, IL-8, RANTES, IP-10, and MIP-1β was measured by ELISA. Various cell lines (1 × 105/ml) were cultured for 24 h in 2 ml DMEM containing 5% FBS with or without 2 ng/ml TNF-α, and supernatants were collected. The content of (A) MCP-1, (B) IL-8, (C) RANTES and (D) IP-10 are shown; MIP-1β was not detected (less than 10 pg/ml). RA6/1, RA8/3, and Sy77 are RA SCLs. The mean and SEM were calculated from triplicates of one representative experiment out of three with similar results. Statistical analysis was performed with the Student t test. *P < 0.05 and **P < 0.01.
Figure 7.
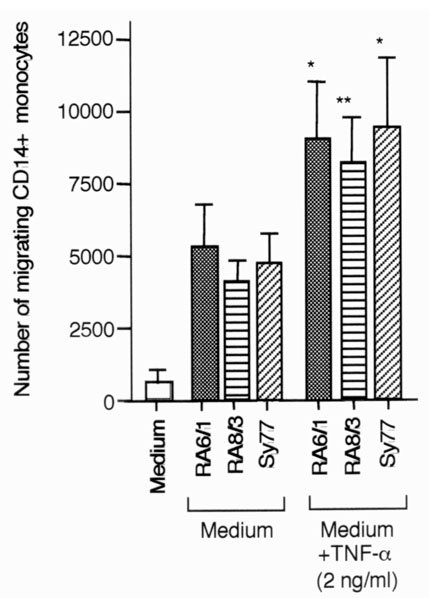
Monocyte migration induced by supernatants of RA SCLs stimulated with TNF-α. Assay of monocyte migration was performed using supernatants of RA SCLs stimulated with TNF-α. Each RA SCL (1 × 105/well) was cultured in six-well culture plates with 2 ml DMEM supplemented with 5% FBS with or without 2 ng/ml TNF-α. Supernatants were collected after 24 h of incubation, and were used for assay. Migrated cells were stained with anti-CD14 mAb, then the number of migrated CD14+ cells was assessed by flow cytometry. RA6/1, RA8/3, and Sy77 are RA SCLs. The mean and SEM were calculated from three independent experiments. Statistical analysis was performed with the paired Student t test. *P < 0.05 and **P < 0.01.
MCP-1 and IL-8 play major roles in monocyte migration by RA SCLs after TNF-α stimulation
Anti-MCP-1 mAb and anti-IL-8 mAb significantly inhibited monocyte migration by supernatants of RA SCLs stimulated with TNF-α. Anti-IP-10 mAb and anti-CCR5 mAb did not inhibit monocyte migration significantly (Fig. 8).
Figure 8.
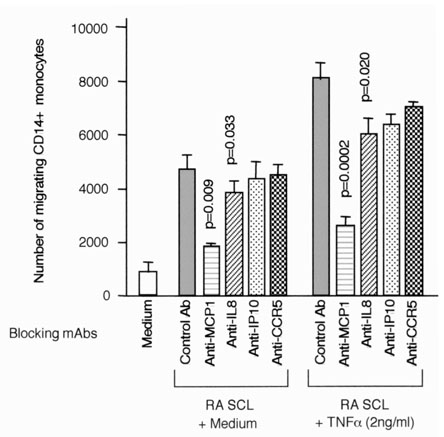
Blocking of monocyte migration induced by supernatant of RA SCL stimulated with TNF-α. Monocyte migration induced by supernatants of RA SCLs (RA6/1 and RA8/3) stimulated with 2 ng/ml TNF-α was assessed using mAbs, which can neutralize specific chemokines or chemokine receptors. Concentrations of mAbs were 10 μg/ml for MCP-1, IL-8, and IP-10, and 50 μg for anti-CCR5 mAb with 5 × 105 SRBC rosette negative cells. Migrating cells were stained with anti-CD-14 mAb, and migrating CD14+cells were counted by flow cytometry. The mean and SEM were calculated from the results of three independent experiments. The paired Student t test was used to assess statistical differences versus the result with control mAb.
Discussion
The results of this study indicate that supernatants of SCL derived from RA synovial tissue can attract more monocytes from peripheral blood than OA SCL and skin fibroblasts. MCP-1 and, to a lesser degree, IL-8 played the major roles in SCL-induced trafficking of monocytes. These results begin to provide an explanation for the extensive accumulation of myeloid cells in rheumatoid synovium. Importantly, RA SCL induced more monocyte migration after stimulation with TNF-α, one of the major inflammatory cytokines produced in the rheumatoid synovium.
Peripheral blood monocytes express many chemokine receptors [9,10], including CCR1, CCR2, CCR5, CCR8, and CXCR4. The current analysis indicates that, along with these receptors, CXCR1, CXCR2, and CXCR3 are also expressed by monocytes. CCR1, CCR2, CCR5, CXCR1, CXCR2, CXCR3, and CXCR4 notably appear to be expressed by monocytes obtained from most donors.
Ligands of these chemokine receptors are candidates to be involved in monocyte trafficking. These ligands would include RANTES, MIP-1α and MCP-3 (CCR1), MCP-1, MCP-2, MCP-3, MCP-4, MCP-5 (CCR2), RANTES, MIP-1α, and MIP-1β (CCR5), IL-8 and CGP-2 (CXCR1), IL-8, GROα, and epithelial-derived neutrophil attractant 78 (CXCR2), IP-10 and MIG (CXCR3), and stromal cell derived factor-1 (CXCR4) [13]. Migration assays using supernatants of RA SCLs were employed to determine which of these chemokine and receptor interactions might be involved in monocyte migration in the RA synovium.
Stromal cells are one of the important cell populations in RA synovium. SCL can produce many cytokines, chemokines, and can promote viability and functional activation of T cells and B cells [14,15,16]. The current findings also indicate that supernatants of RA SCLs attract more monocytes than the supernatants of OA SCL and skin fibroblasts. This result suggests that the supernatants from RA SCLs may contain more chemokines than or different chemokines to those of OA SCL or fibroblast lines and, therefore, are able to attract additional monocytes. This is likely to contribute to the more marked accumulation of monocytes in RA compared with OA synovium.
An analysis of chemokines produced by RA SCL indicated that these cells expressed MCP-1 and IL-8 mRNAs, and also secreted MCP-1 and IL-8. It is noteworthy that there were some discrepancies between mRNA expression and protein production. From the mRNA expression, we expected RA SCLs to produce reasonable amounts of IP-10, RANTES, MIP-1α, and MIP-1β. RA SCLs, however, secreted only small amounts of RANTES, and IP-10 and MIP-1β secretion was not detected. According to previous reports, mRNAs of MCP-1, IL-8, and RANTES were found in RA SCL or fibroblasts, but only MCP-1 was reported to be secreted by RA SCL or fibroblasts without cytokine stimulation [4,5,17,18]. The current study indicates a wider profile of chemokine expression and secretion by RA SCL, and also a greater production of IL-8 and MCP-1 by RA SCL compared with OA SCL, implying a greater proinflammatory potential.
Experiments with blocking mAbs were carried out to examine the chemokines in the supernatants of SCL that accounted for monocyte migration. The data were consistent with the conclusion that MCP-1 and, to a lesser extent, IL-8 accounted for the capacity of RA SCL supernatants to stimulate monocyte migration. Whether additional cytokines produced by RA SCL also contributed to monocyte migration is currently not known, but the combination of the chemokine data and the mAb blocking results suggests that MCP-1 and IL-8 play a dominant role in monocyte migration measured by SCL.
TNF-α is one of the major cytokines produced in inflammatory sites such as RA synovium, and it is thought to play a central proinflammatory role [2]. We examined the influence of TNF-α on monocyte migration induced by RA SCL. RA SCLs stimulated by TNF-α secreted more MCP-1, IL-8, and RANTES than those that were unstimulated, and began to produce IP-10. Moreover, supernatants of TNF-α-stimulated RA SCLs attracted more monocytes in migration assay. MCP-1 and IL-8 played the main roles in monocyte migration induced by TNF-α-stimulated RA-SCL, as documented by blocking experiments.
It has recently been reported that T cells expressing CCR5 gather at inflammatory sites such as RA or multiple sclerosis, and that interactions between CCR5 and its ligands (RANTES, MIP-1α, MIP-1β) are thought to be important in the accumulation of inflammatory cells at these sites [19,20,21]. CCR5 expressed by monocytes, however, was not apparently active in transmitting transmigratory signals. RANTES is also the ligand of CCR1, but the major receptor for RANTES is thought to be CCR5. In this regard, expression of CCR1 by monocytes was lower than that of CCR5. Moreover, antibody to MCP-1 and IL-8 blocked 75% and 20% of migration of monocytes, respectively. According to this information, RANTES is unlikely to play a major role in monocyte migration by RA SCL supernatants.
MCP-1 has been shown to play an important role in the development of arthritis in MRL-lpr mice [22], whereas the migration of monocytes into inflammatory sites was reduced in the CCR2-deficient mouse [23]. These results are consistent with the current findings, demonstrating the important role of MCP-1 in monocyte migration. Blocking the effect of RANTES has, however, also been shown to be effective in ameliorating collagen-induced arthritis in DAB/1 mice and adjuvant-induced arthritis in rats [24,25]. RANTES might, therefore, play a different role in the initiation of inflammatory arthritis in experimental animals compared with propagating chronic inflammation in RA.
The overlapping roles of MCP-1 and IL-8 in the migration of inflammatory cells have recently been highlighted in studies of neutrophil trafficking. It was reported that MCP-1 plays a role in neutrophil trafficking in inflammation, although neutrophil trafficking was previously believed to be regulated only by IL-8, and not by MCP-1 [26]. These previous results, together with the current data, indicate that both MCP-1 and IL-8 play major roles in regulating the trafficking of myeloid cells into inflammatory sites.
The role of specific chemokines in arthritis is still controversial, and further investigation is necessary to delineate to specific roles of these effector molecules. Moreover, the role of tissue cells in regulating the migration of inflammatory cells into the synovium is also not fully established. The current data, however, strongly imply that monocyte accumulation in rheumatoid synovium is regulated by SCL via production of the chemokines MCP-1 and IL-8.
Abbreviations
ELISA = enzyme-linked immunosorbent assay; IP-10 = interferon-gamma inducible protein 10; mAb = monoclonal antibody; MCP-1 = monocyte chemotactic protein-1; MIP = macrophage inflammatory protein; OA = osteoarthritis; PBMC = peripheral blood mononuclear cells; PE = phycoerythrin; RA = rheumatoid arthritis; RANTES = regulated upon activation, normal T cell expressed and secreted; RT-PCR = reverse transcriptase-polymerase chain reaction; SCL = stromal cell line; SRBC = sheep red blood cells; TNF-α = tumor necrosis factor-α.
Acknowledgments
Acknowledgements
This research was supported by National Institutes of Health grant AR-39169 and by the Organization for Pharmaceutical Safety and Research.
References
- Qu Z, Garcia CH, O'Rourke LM, Planck SR, Kohli M, Rosenbaum JT. Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia. Arthritis Rheum. 1994;37:212–220. doi: 10.1002/art.1780370210. [DOI] [PubMed] [Google Scholar]
- Roitt IM, Brostoff J, Male DK. Immunology, edn 5 London: Mosby, 1998.
- Tak PP, Smeets TJM, Daha MR, Kluin PM, Meijers KAE, Brand R, Meinders AE, Breedveld FC. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- Koch AE, Kunkel SL, Burrows JC, Evanoff HL, Haines GK, Pope RM, Strieter RM. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991;147:2187–2195. [PubMed] [Google Scholar]
- Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, Buridick MD, Pope RM, Strieter RM. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90:772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AE, Kunkel SL, Harlow LA, Mazaraakis DD, Haines GK, Burdick MD, Pope RM, Strieter RM. Epithelial neutrophil activating peptide-78: a novel chemotactic cytokine for neutrophils in arthritis. J Clin Invest. 1994;94:1012–1018. doi: 10.1172/JCI117414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AE, Kunkel SL, Shah MR, Fu R, Mazaraakis DD, Haines GK, Burdick MD, Pope RM, Strieter RM. Macrophage inflammatory protein-1b: a C-C chemokine in osteoarthritis. Clin Immunol Immunopathol. 1995;77:307–314. doi: 10.1006/clin.1995.1157. [DOI] [PubMed] [Google Scholar]
- Robinson E, Keystone EC, Scahll TJ, Gillett N, Fish EN. Chemokine expression in rheumatoid arthritis: evidence of RANTES and macrophage inflammatory protein-1b production by synovial T cells. Clin Exp Immunol. 1995;101:398–407. doi: 10.1111/j.1365-2249.1995.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells TNC, Power CA, Proudfoot EI. Definition, function, and pathophysiological significance of chemokine receptors. Trends Pharmacol Sci. 1998;19:376–380. doi: 10.1016/s0165-6147(98)01247-4. [DOI] [PubMed] [Google Scholar]
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka Y, Attrep JF, Hirano T, Ishihara K, Suzuki R, Toyosaki T, Ochi T, Lipsky PE. Nurse-like cells from bone marrow and synovium of patients with rheumatoid arthritis promote survival and enhance function of human B cells. J Clin Invest. 1998;102:606–618. doi: 10.1172/JCI3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Neustat DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi E, Tomita T, Toyosaki-Maeda T, Kaneko M, Takano H, Hashimoto H, Sugamoto K, Suzuki R, Ochi T. Establishment and characterization of nurse cell-like stromal cell lines from synovial tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:221–228. doi: 10.1002/1529-0131(199902)42:2<221::AID-ANR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Salmon M, Scheel-Toellner D, Huissoon AP, Pilling D, Shamsadeen N, Hyde H, D'Angeac AD, Bacon PA, Emery P, Akbar N. Inhibition of T cell apoptosis in rheumatoid synovium. J Clin Invest. 1997;99:439–446. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Shimaoka Y, Ochi T, Lipsky PE. Rheumatoid arthritis synovial stroma cells inhibit apoptosis and up-regulate Bcl-XL expression by B cells in a CD49d/CD29-CD106 dependent mechanism. J Immunol. 2000;164:1110–1116. doi: 10.4049/jimmunol.164.2.1110. [DOI] [PubMed] [Google Scholar]
- Hosaka S, Akahoshi T, Wada C, Kondo H. Expression of the chemokine superfamily in rheumatoid arthritis. Clin Exp Immunol. 1994;97:451–457. doi: 10.1111/j.1365-2249.1994.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathanaswami P, Hachicha M, Sadick M, Schall TJ, McColl SR. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. J Biol Chem. 1993;268:5834–5839. [PubMed] [Google Scholar]
- Qin S, Rottman JB, Myers P, Kassaam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1a and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M, Bruhl H, Gruber R, Jaeger C, Cihak J, Viktoria E, Plachy J, Stangassinger M, Uhlig K, Schattenkirchner M, Schlondorff D. Predominance of mononuclear cells expressing the chemokine receptor CCR5 in synovial effusions of patients with different forms of arthritis. Arthritis Rheum. 1999;42:981–988. doi: 10.1002/1529-0131(199905)42:5<981::AID-ANR17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gong JH, Ratkay LG, Waterfield JD, Clark-Lewis I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med. 1997;186:131–137. doi: 10.1084/jem.186.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plater-Zyberk C, Hoogewerf AJ, Proudfoot AEI, Power CA, Wells TNC. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol Lett. 1997;57:117–120. doi: 10.1016/s0165-2478(97)00075-8. [DOI] [PubMed] [Google Scholar]
- Barnes DA, Tse J, Kaufhold M, Owen M, Hesselgesser J, Strieter R, Horuk R, Perez HD. Polyclonal antibody directed against human RANTES ameliorates disease in the Lewis rat adjuvant-induced arthritis model. J Clin Invest. 1998;101:2910–2919. doi: 10.1172/JCI2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest. 1999;103:1269–1276. doi: 10.1172/JCI5208. [DOI] [PMC free article] [PubMed] [Google Scholar]


