Abstract
T helper 2 (Th2) cytokines [interleukin (IL)-4 and IL-5] play a central role in the development of allergic immune responses. After allergen provocation, the expression of Th2 cytokines is rapidly up-regulated in atopy and asthma. IL-6 is a multifunctional cytokine that is able to direct Th2 immune responses and is secreted by multiple tissue cell types. This study shows that IL-6 induces up-regulation of IL-4 and IL-5 after short (5 min) preincubation periods in freshly isolated, α-CD3/α-CD28-stimulated T cells. After longer preincubation periods with IL-6 (12 and 24 hr), the priming effect on IL-4 production gradually disappears, whereas the effect on IL-5 becomes more pronounced. In contrast, a small but significant inhibitory effect is found on the production of the Th1 cytokine interferon-γ. Additional experiments indicate that the long-term priming effect of IL-6 on IL-5 production is dependent on IL-2 signalling. This is not the case for the short-term IL-6 effect on IL-5 secretion, where the p38 mitogen-activated protein kinase-dependent induction of activator protein-1 DNA-binding activity is involved, independent of signal transducer and activator of transcription 3 phosphorylation. In summary, these data demonstrate that the short-term and long-term priming effects of IL-6 on Th2 cytokine production are regulated by different mechanisms.
Introduction
The development of allergic immune responses, like atopic dermatitis, allergic rhinitis and atopic asthma is mediated by T helper 2 (Th2)-like cytokines, particularly by interleukin-4 (IL-4) and IL-5. IL-4 plays a central role in immunoglobulin E (IgE)-mediated responses, whereas IL-5 is crucial for eosinophil recruitment and activation, which is observed in the inflammatory reaction of cutaneous late phase reactions and asthma. Increased numbers of CD4+ T cells expressing IL-4 and IL-5 mRNA have been found in bronchoalveolar lavage (BAL) of asthmatic subjects1–3 and elevated levels of IL-4 and IL-5 expression have been observed in nasal and bronchial mucosa from allergic subjects.4–7 Furthermore, it has been demonstrated that peripheral blood lymphocytes from asthmatic subjects are primed for enhanced productions of IL-4 and IL-5 mRNA.8 Thus, a primed T-cell subset may migrate to sites of allergen-induced reactions. At present, it is not clear which factors are responsible for priming of peripheral T cells to produce higher amounts of Th2 cytokines. Possibly, soluble mediators produced during the inflammatory reaction are released into the circulation and prime the T lymphocytes for enhanced Th2 cytokine production.
In this respect, IL-6 might be of interest. IL-6 is a multifunctional cytokine produced at sites of tissue inflammation. It has been demonstrated that IL-6 derived from antigen-presenting cells (APCs) is able to induce initial IL-4 production in naive CD4+ T cells, thereby polarizing these cells into Th2 cells.9 Furthermore, IL-6 protein levels secreted by alveolar macrophages positively correlate with the enhancement of IL-5 production in CD4+ T cells.10 The capacity of alveolar macrophages from atopic asthmatics to increase IL-5 production in CD4+ T cells was abolished in the presence of neutralizing antibodies to IL-6,10 clearly demonstrating that IL-6 has Th2-like promoting capacity. Moreover, monocytes from newborns with a high risk to develop allergy produced significantly larger amounts of IL-6 than monocytes from newborns with a low risk to develop allergy.11 These findings suggest that IL-6 might have an important role in the development of Th2 mediated diseases, like allergy. In addition, it has been demonstrated that IL-6 plays a role in regulation of Th1 responses by inhibition of interferon-γ (IFN-γ) production.12,13 In this study we investigate the ability of IL-6 to promote the production of Th2 type cytokines IL-4 and IL-5 in human T cells and the mechanisms involved in this process.
Materials and methods
Isolation of T cells
Peripheral blood cells were obtained from healthy volunteer platelet donors. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque (Lymphoprep; Nycomed, Oslo, Norway) density-gradient centrifugation. T cells were isolated by rosetting with 2-aminoethylisothioronium bromide (AET) treated sheep red blood cells (SRBC). The SRBC were lysed with 155 mmol/l NH4Cl, 10 mmol/l KHCO3 and 0·1 mmol/l ethylenediaminetetraacetic acid (EDTA). The remaining cell preparations contained more than 98% T cells, as assessed by flow cytometric analysis after staining with α-CD2 (Becton Dickinson, Erebodegem-Aalst, Belgium). To obtain naive T cells, CD4+/CD45RO− cells were sorted using a MoFlo™ flow cytometer (Cytomation, Fort Collins, CO), as previously described.14 After isolation, T cells were incubated overnight at 37° in RPMI-1640 (BioWhittaker, Verviers, Belgium) containing 5% fetal calf serum (FCS; Hyclone, Logan, UT), supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin. CD4+/CD45RO− cells were cultured for 7 days in RPMI-1640 medium containing 10% FCS, in presence of phytohaemagglutinin (PHA), IL-2, irradiated allogenic PBMC (feeder cells) and either IL-12 (2 ng/ml, R & D systems, ITK diagnostics, Uithoorn, the Netherlands) plus α-IL-4 (200 ng/ml, Becton Dickinson) to generate polarized Th1 cells, or IL-4 (200 U/ml, Becton Dickinson) and α-IL-12 (2 µg/ml, R & D systems) to generate polarized Th2 cells, as described before.14
Stimulation of T cells
Freshly isolated T cells, naive CD45RO− cells, or polarized Th2 cells were incubated in RPMI-1640 medium containing 5% FCS and stimulated with 50 µl/ml of α-CD3 and α-CD28 antibodies as previously described by Borger et al.,8 which evokes a T-cell specific reponse. T cells were stimulated in the presence or absence of inhibitors of the p38 mitogen-activated protein (MAP) kinase pathway, the phosphatidylinositol 3-kinase (PI3-kinase) pathway, the calcineurin-dependent pathway and the MAPK/extracellular signal-related kinase (ERK)-1 (MEK-1) pathway; SB203580 (SmithKline Beecham Pharmaceuticals, King of Prussia, PA) LY294002 (Alexis Corporation, Läufelfingen, Switzerland), cyclosporin A (CsA) and PD98059 (New England Biolabs, Beverly, MA), respectively, in a final concentration of 10 µm. Antibodies against IL-2 (Diaclone Research, Besançon, France) or isotype-matched antibodies (IgG1) were added in a final concentration of 2 µg/ml. To investigate priming mechanisms of IL-6 and IL-2, cells were preincubated for 5 min, 6, 12 or 24 hr before stimulation in presence or absence of 10 ng/ml recombinant human (rh)IL-6 or 2 ng/ml rhIL-2.
Measurement of cytokine protein
T cells (3 × 106 ml−1) were stimulated with α-CD3/α-CD28 during 8 hr. Secreted IL-4, IL-5 and IFN-γ protein were measured in cell-free supernatants, using enzyme-linked immunosorbent assay (ELISA) kits for IL-4 and IFN-γ (CLB, Amsterdam, the Netherlands). The IL-5 ELISA was performed as previously described by Hoekstra et al.15
FACS analysis of intracellular IFN-γ and IL-4
Intracellular IFN-γ and IL-4 accumulation was studied as previously described14 using the Cytodetect™ kit [a gift from Immune Quality Products (IQP), Groningen, the Netherlands]. In short, 1 × 106 ml−1 cells were stimulated for 4 hr with PMA (10 ng/ml) and ionomycin (1 µg/ml) in the presence of monensin (2 µm, IQP). Cells were harvested, fixed with 4% paraformaldehyde and permeabilized in 0·1% saponin/0·1% azide. Cells were stained with anti-CyQ-CD4(B-F5, IQP), anti-CD45RO-FITC (UCHL1), anti-IFN-γ–fluoroscein isothiocyanate (FITC; 45-15, IQP) and anti-IL-4–phycoerythrin (PE; B-T4, IQP). Irrelevant isotype-matched antibodies were used for gate setting. Analysis was performed usong an Elite™ flow cytometer (Beckman Coulter, Hialeah, FL) calibrated using Flow-Check™ Fluorospheres (Beckman Coulter) in combination with Winlist™ software (Verity, Topsham, ME). Lymphocyte events were gated on the basis of forward and side scatter characteristics. To confirm that Th1 and Th2 phenotypes were obtained after 7 days of culture under polarizing conditions, the polarized T helper cells were replated in RPMI medium containing 5% FCS and cultured overnight before stimulation. The intracellular cytokine stainings indicated that highly divergent cytokine production patterns were obtained after one week of culture. Virtually all cells became CD45RO+ after culturing under polarizing conditions. In the cell population polarized under Th1 conditions, approximately 40% of the CD4+ cells was IFN-γ+/IL-4−, whereas in the cell population cultured under Th2 conditions, approximately 10% was IL-4+/IFN-γ−. In both cell populations, only a small percentage of the cells was positive for both cytokines.
Immunodetection by Western blotting
Phosphorylation of p38 and signal transducer and activator of transcription-3 (STAT3) was analysed by Western blotting. T cells (3 × 106 ml−1) were cultured overnight in RPMI-1640 medium containing 0·5% FCS. T cells were stimulated with IL-6 alone, with α-CD3/α-CD28 alone or with the combination for 60 min in presence or absence of SB203580, PD98059 or CsA. T cells were harvested and spun down at maximum speed during 30 s. Next, total cell lysates were obtained by resuspension of the pellets in 1× sample buffer (containing 2% sodium dodecyl sulphate (SDS), 10% glycerol, 2% β-mercapoethanol, 60 mm Tris–Cl pH 6·8 and bromophenol blue) and boiling for 5 min Samples were loaded on a SDS 10% polyacrylamide gel electrophoresis (PAGE) gel (acrylamide : bisacrylamide, 173 : 1) and transferred to a cellulose nitrate membrane (Schleicher & Schuell, Dassel, Germany). Immunodetection of phospho-p38, phospho-Stat3 (New England Biolabs, Hitchin, UK), pan-p38 and pan-Stat3 (Santa Cruz Biotechnology, Santa Cruz, CA) was performed by standard procedures and the detection was performed according the manufacturer's guidelines (ECL, Amersham, UK). Relative protein levels were quantified using the gelscan program Diversity One (Pharmacia).
Electrophoretic mobilily shift assay
T cells (5 × 106 ml−1) cells were stimulated during 4 hr in the presence or absence of IL-6 and in the presence or absence of SB203580, PD98059 and CsA. Cells were harvested and nuclear extracts were produced according to the mini-scale procedure described.16 In short, cells were harvested by centrifugation at 500 g for 5 min, washed once with phosphate-buffered saline (PBS) and resuspended in Buffer A (10 mm HEPES pH 7·9, 10 mm KCl, 0·1 m EDTA, 0·1 mm egtazic acid (EGTA), 1 mm dithiothreitol (DTT)) supplemented with protease inhibitors (Complete™, Boehringer, Mannheim, Mannheim, Germany). After 15 min of incubation on ice, cells were lysed by adding Nonidet P-40 (25 µl). T-cell lysates were centrifuged at maximal speed for 1 min at 4° and nuclear pellets were resuspended in buffer C (20 mm HEPES, 400 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm DTT) supplemented with Complete™. The suspension was incubated on ice for 20 min with regular vortexing to extract the nuclear proteins. Finally pellets were spun down at maximum speed for 30 s and the supernatants containing nuclear extracts were stored at −80°. The concentration of the nuclear proteins was determined using the Bradford assay.17 A double-stranded oligonucleotide probe (5′-AGCTCGCGTGACTCAGCTG-3′) containing the activator protein-1 (AP-1) binding sequence was used in the gel retardation assay. Annealing of the two strands was performed by heating for 2 min at 94° and slow cooling down to room temperature. Hundred ng of the double-stranded oligo was labelled with 32P α-ATP (3000 Ci/mmol, Amersham) and separated from non-incorporated radiolabel by Sephadex G50 chromatography. Next, 5 µg of nuclear extract and 0·1 ng double-stranded labelled oligo were incubated in 20 mm HEPES (pH 7·9), 60 mm KCl, 0·06 mm EDTA, 0·6 mm DTT, 2 mm spermidine and 10% glycerol, supplemented with 2 µg poly dI-dC) at 26° for 30 min The supershift experiment was performed by incubating the nuclear extracts with 1 µg of polyclonal antibody against JunB (Santa Cruz Biotechnology). The samples were loaded on prerun (30 min, 100 V) 4% polyacrylamide gels and run for 1·5 hr at 150 V in 1·0 × TRIS boric acid EDTA (TBE) at room temperature. Gels were dried and quantification of the binding signals was performed using a Phosphor Imaging (Molecular Dynamics, Sunnyvale, CA).
Reverse transcription (RT)–polymerase chain reaction (PCR)
T cells (5–10 × 106 1·5 ml−1) were incubated with and without IL-2 or IL-6 for 24 hr or stimulated for 6 hr with IL-6 alone, with α-CD3/α-CD28 or with IL-6 in combination with α-CD3/α-CD28, in presence and absence of SB203580 and CsA. After stimulation, cells were harvested and RNA was isolated using the TRIzol method (GibcoBRL, Burlington, Ontario, Canada). Total cellular RNA was resuspended in diethyl-pyrocarbonate (DEPC; Sigma, St. Louis, MO)-treated H2O. RNA (1–3 µg) was used for cDNA synthesis. First, the samples were incubated during 10 min at 65° with random hexamer (pdN6). After cooling on ice, the RT mix containing 5× RT buffer (GibcoBRL), 0·1 m DTT, 5 mm of each dNTP and 3 units of RT (GibcoBRL) was added and the samples were incubated at 37° for 1 hr. For the PCR reaction, 10× PCR buffer (GibcoBRL), 50 µm of forward and reverse primer, 0·25 µl Taq polymerase, 2 mm dinitrotriphenols (dNTPs), and 75 µl MgCl2 in 25 µl total volume were added. The following specific primer pairs for β-2-microglobulin (β2µG, housekeeping gene), GATA-3 and JunB were obtained from Biolegio BV (Malden, the Netherlands):
β2µG: 5′-CCAGCAGAGAATGGAAAGTC-3′ sense and 5′-GATGCTGCTTACATGTCTCG-3′ antisense. SOCS1: 5′-CACGCACTTCCGCACATTCC-3′ sense and 5′-TCCAGCAGCTCGA AGAGGCA-3′ antisense. JunB: 5′-CCAGTCCTTCCACCTACCTCGACGTTTACAA-3′ sense and 5′-GACTAAGTGCGTGTTTCTTTTCCACAGTAC-3′ antisense.
GATA3: 5′-GAACATCATCTCGGG-3′ sense and 3′-CACGTACTGAGTGACCTTTC-5′ antisense.
PCR conditions were a denaturation step at 94° for 5 min followed by 20 cycles of 94°, 30 s; 55°, 30 s; 72°, 30 s for detection of β2µG, 25 cycles of 94°, 30 s; 60°, 30 s; 72°, 30 s for detection of suppressor of cytokine signalling (SOCS1), 30 cycles of 94°, 30 s; 55°, 30 s; 72°, 30 s for detection of GATA-3 and 35 cycles of 94°, 30 s; 57°, 45 s; 72°, 45 s for detection of JunB. With these primers the amplified products were 268 bp, 300 bp, 150 bp and 257 bp long for β2µG, SOCS1, GATA-3 and JunB, respectively. After PCR, 10 µl of the reaction mixture was run on a 1·5% agarose gel, containing 0·2 µg ethidium bromide in 1 × TAE buffer. A 100-bp ladder (Pharmacia, Uppsala, Sweden) was used as DNA marker. Relative mRNA levels of the PCR products were quantified using the gelscan program Diversity One (Pharmacia).
Statistical analysis
For the protein measurements, statistical analysis was performed using Wilcoxon's signed rank test for paired observations. Statistical significance of the secretion data was set at P < 0·05.
Results
Preincubation with IL-6 up-regulates IL-4 and IL-5 protein secretion
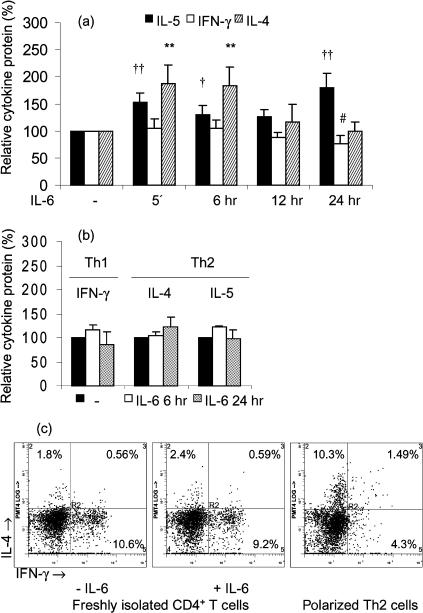
Freshly isolated T cells were preincubated with or without IL-6 for 5 min, 6, 12 or 24 hr and subsequently stimulated. Incubation of T cells with IL-6 alone did not induce a detectable amount of cytokines (data not shown). Because there was a wide range in the levels of cytokines secreted by the different blood donors, yet IL-6 exerted similar effects in low and high cytokine producers, the cytokine protein levels are expressed as a percentage of the cytokine secretion upon stimulation in absence of IL-6. Figure 1(a) shows that IL-6 preincubation for 5 min followed by α-CD3/α-CD28 stimulation significantly enhanced IL-4 secretion 1·9 ± 0·3-fold (from 16 ± 12 to 29 ± 22 pg/ml, P < 0·01) and IL-5 secretion 1·5 ± 0·2-fold (from 56 ± 53 to 86 ± 71 pg/ml, P < 0·01) compared to α-CD3/α-CD28 stimulation alone. After 6 hr of preincubation, IL-6 had a similar effect on IL-4 protein secretion (fold increase 1·8 ± 0·3, P < 0·01), which was reduced after 12 h and completely abolished after 24 hr of preincubation. In contrast, the priming effect of IL-6 on IL-5 secretion was most pronounced after 24 hr of preincubation (fold increase 1·8 ± 0·1, P < 0·01). No effect of IL-6 was observed on IFN-γ secretion within 12 hr, whereas a slight but significant inhibitory effect on IFN-γ protein secretion was observed after 24 hr of preincubation (from 4·1 ± 5·8 to 2·8 ± 3·6 ng/ml, P < 0·05, Fig. 1a). We also examined the effect of IL-6 in naive (CD45RO−/CD4+) T cells; however, in these cells no cytokine secretion could be detected after α-CD3/α-CD28 stimulation in absence or presence of IL-6. This indicates that in our setting CD45RO+ T cells are responsible for the effects of IL-6 on cytokine secretion, although we cannot exclude that interactions between naive and memory T cells could be involved in the effect of IL-6. Furthermore, Fig. 1(b) demonstrates that IL-6 did not significantly alter the cytokine secretion in polarized Th1 and Th2 cells (absolute amounts: 96 ± 31 pg/ml for IL-4 and 471 ± 252 pg/ml for IL-5 in Th2 cells and 15·3 ± 9·8 ng/ml for IFN-γ in Th1 cells, n = 4). This indicates that the T cells affected by IL-6 are rather of an intermediate phenotype (Th0) than highly restricted to a Th1 or Th2 phenotype. In polarized Th2 cells 10·3% of the CD4+ cells expressed IL-4, while in freshly isolated T cells only approximately 2% of the CD4+ cells expressed IL-4, which was not clearly different in presence of IL-6 (Fig. 1c). In addition, presence of IL-6 did not clearly alter the percentage of IFN-γ+ cells in CD4+ cells, nor the percentages of IL-4+ and IFN-γ+ cells in CD4− cell populations (2·0% versus 2·0% for IL-4 and 7·3% versus 6·1% for IFN-γ, in the absence and presence of IL-6, respectively). Accordingly, it has been demonstrated that expression of effector cytokines in T helper cells is cell-cycle dependent and the induction of IL-4 expression requires at least three cell divisions.18 Thus, instead of inducing the expression of Th2 cytokines in naive T cells, IL-6 appears to enhance the capacity to produce Th2 cytokines in T cells unrestricted to a T helper phenotype, possibly by modulating intracellular pathways that lead to Th2 cytokine gene transcription.
Figure 1.
(a) IL-6 up-regulates the production of Th2-like cytokines. Effects of different IL-6 (10 ng/ml) preincubation periods (5 min, 6 hr, 12 hr or 24 hr) on IL-5, IFN-γ and IL-4 secretion were studied in T lymphocytes from healthy donors. After preincubation with or without IL-6, T cells were stimulated with α-CD3/α-CD28 during 8 hr. Cytokine protein levels are expressed as percentage (x ± SEM, n = 9) of the secretion after stimulation with α-CD3/α-CD28 in the absence of IL-6. †, P < 0·05 and ††, P < 0·01 for the IL-5 secretion level after preincubation with IL-6 compared to the level after preincubation without IL-6. #, P < 0·05 for the IFN-γ secretion level after preincubation with IL-6 compared to the level after preincubation without IL-6. **, P < 0·01 for the IL-4 secretion level after preincubation with IL-6 compared to the level after preincubation without IL-6. (b) IL-6 does not affect cytokine production in polarized T helper cells. The effect of IL-6 (10 ng/ml) on IFN-γ, IL-4 and IL-5 secretion was studied in naive CD4+ T cells from healthy donors that were cultured under Th1 and Th2 polarizing conditions for 7 days, rested overnight in RPMI-1640 containing 5% FCS, preincubated for 6 or 24 hr with or without IL-6 and stimulated with α-CD3/α-CD28 during 6 hr. Cytokine protein levels are expressed as percentage (x ± SEM, n = 4) of the secretion after stimulation with α-CD3/α-CD28 in absence of IL-6. (c) Characterization of cytokine profiles in freshly isolated T cells and polarized Th2 cells. Intracellular cytokine stainings were performed after stimulation of the T cells with PMA/ionomycin for 4 hr, in the presence or absence of IL-6 (10 ng/ml). Results shown are representative of two independent experiments.
Long-term effect of IL-6 on IL-5 production is dependent on IL-2
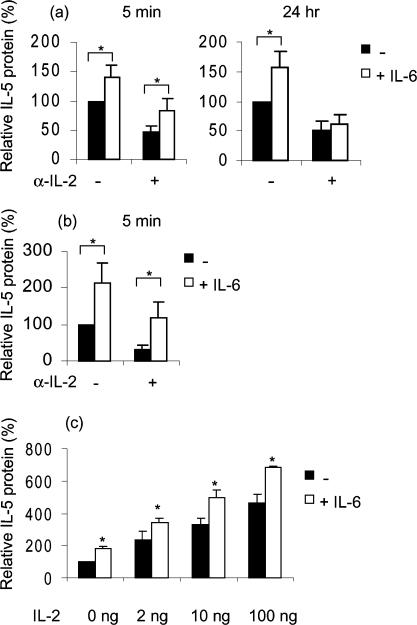
Th2 cytokine production has been reported to be dependent on IL-2 activation.19 Neutralizing antibodies to IL-2 (α-IL-2) significantly inhibited the secretion of α-CD3/α-CD28 induced IL-4 and IL-5 (see Fig. 2; P < 0·001). As a control, isotype-matched antibodies (IgG1) were added prior to stimulation, which did not result in a reduction in IL-5 secretion (data not shown). Figure 2(a) shows that the immediate early up-regulatory effect of IL-6 on IL-5 secretion was not blocked by the addition of α-IL-2. Similarly, the early up-regulatory effect of IL-6 on IL-4 protein secretion was not dependent on the endogenous levels of IL-2, because 5 min of preincubation with IL-6 still significantly up-regulated IL-4 in the presence of neutralizing α-IL-2 antibodies (P < 0·05, Fig. 2b). In contrast, the IL-6-induced up-regulation of IL-5 secretion after 24 hr of preincubation (1·6 ± 0·3-fold, P < 0·01) was abrogated by addition α-IL-2; in presence of α-IL-2, IL-6 was no longer able to induce a significant increase in IL-5 secretion, see Fig. 2(a). These results indicate that the long-term preincubation effect of IL-6 on IL-5 production is dependent on the presence of endogenously produced IL-2.
Figure 2.
The long-term preincubation effect of IL-6 on IL-5 secretion is dependent on IL-2 availability. (a) Effects of preincubation with IL-6 on IL-5 production in freshly isolated T lymphocytes during 5 min or 24 hr in the absence and presence of neutralizing antibodies to IL-2. After preincubation with or without IL-6, T cells were stimulated with α-CD3/α-CD28 during 8 hr. IL-5 protein levels are expressed as percentage (x ± SEM, n = 8) of the production after stimulation in the absence of IL-6 and α-IL-2. (b) Effects of preincubation with IL-6 on IL-4 production in freshly isolated T lymphocytes during 5 min in the absence and presence of neutralizing antibodies to IL-2. After preincubation with or without IL-6, T cells were stimulated with α-CD3/α-CD28 during 8 hr. IL-4 protein levels are expressed as percentage (x ± SEM, n = 5) of the production after stimulation in absence of IL-6 and α-IL-2. (c) Effect of IL-6 preincubation during 24 h on IL-5 production in T lymphocytes, in the presence of increasing IL-2 concentrations (0, 2, 10, 100 ng). After preincubation with IL-6, T cells were stimulated with α-CD3/α-CD28 during 8 hr. IL-5 protein levels are expressed as a percentage (x ± SEM, n = 4) of the production after stimulation with α-CD3/α-CD28 alone. *, P < 0·05 between values of relative IL-5 protein in presence and in absence of IL-6.
To further underscore the role of IL-2 in IL-5 protein secretion, T lymphocytes were preincubated with IL-2 for 5 min or 24 hr. Five min of preincubation with IL-2 followed by α-CD3/α-CD28 stimulation did not significantly alter IL-5 production compared to α-CD3/α-CD28 stimulation alone (data not shown). Preincubation with 2, 10 and 100 ng IL-2 during 24 hr followed by α-CD3/α-CD28 stimulation resulted in significantly higher levels of IL-5 protein secretion compared to preincubation without IL-2. Furthermore, an additional enhancement of IL-5 secretion was observed with all IL-2 concentrations when IL-6 was added as well (P < 0·05, Fig. 2c). These data suggest that the effect of IL-6 exposure to T lymphocytes is not due to additional production of IL-2, but the intracellular IL-2 signalling is more likely to be involved. In vitro, IL-2 is required for Th2 differentiation and IL-2 may therefore be of importance for the expression of Th2 specific transcription factors. Previously, it has been demonstrated that GATA-3, a Th2 specific transcription factor, is crucial for activation of the IL-5 promoter and that ectopic expression of GATA-3 is sufficient to drive IL-5 gene expression.20 As shown in Fig. 3, GATA-3 mRNA expression is up-regulated by IL-2 already after 6 hr but more strongly after 24 hr of incubation (induction ± 2-fold), compared to 6 and 24 hr of culture in the absence of IL-2. Thus, one of the events induced by IL-2 leading to enhanced production of IL-5 may be the up-regulation of GATA-3 expression. In contrast to IL-2, IL-6 did not elevate the expression of GATA-3 mRNA. Furthermore, IL-6 did not increase the IL-2-induced GATA-3 mRNA expression (data not shown). Whether the expression levels of GATA-3 are of relevance for the long-term effect of IL-6 on IL-5 secretion still remains to be elucidated.
Figure 3.
GATA-3 mRNA expression is up-regulated by IL-2. Freshly isolated T cells were incubated with IL-2 (2 ng/ml) or without exogenously added IL-2 for 6 h and 24 hr. In the upper panel the GATA-3 signal is shown. In the lower panel the β2µG signal shows that comparable amounts of product were amplified for each condition. Results shown are representative of three independent experiments.
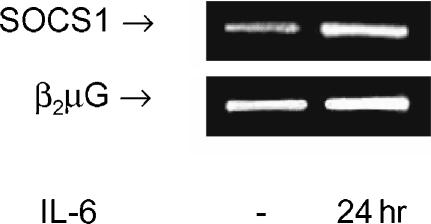
IFN-γ secretion was also inhibited in the presence of α-IL-2 by approximately 40%. However, the production of IFN-γ was not altered by exogenously added IL-2 in the absence or presence of IL-6, demonstrating that the effect of IL-2 is not a general effect on cytokine production (data not shown). In addition, the results indicate that the inhibitory effect of IL-6 on IFN-γ production is regulated independently from IL-2 signalling. A plausible explanation for the inhibitory effect of IL-6 on IFN-γ might be the up-regulation of SOCS1 expression. SOCS1 inhibits STAT1 mediated activation of the IFN-γ promoter, which may be crucial for IFN-γ expression. IFN-γ production was strongly enhanced in SOCS-deficient mice. Moreover, IL-6 was not able to inhibit IFN-γ production in these mice.12 As demonstrated in Fig. 4, incubation with IL-6 during 24 hr results in up-regulation (± 2·0-fold) of SOCS1 mRNA expression compared to culturing for 24 h without IL-6 in freshly isolated T cells.
Figure 4.
SOCS1 mRNA expression is up-regulated by IL-6. Freshly isolated T cells were incubated with IL-6 (10 ng/ml) or without IL-6 for 24 hr. In the upper panel the SOCS1 signal is shown. In the lower panel the β2µG signal shows that comparable amounts of product were amplified for each condition. Results shown are representative of three independent experiments.
Effect of IL-6 on signal transduction
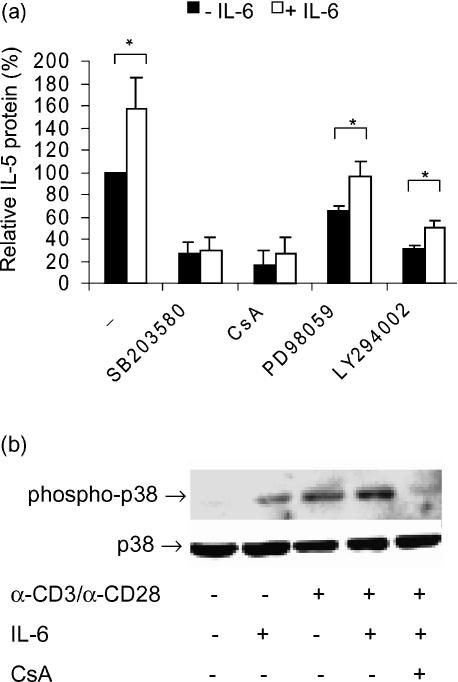
The short-term (5 min) preincubation effect of IL-6 on Th2-like cytokine secretion is most likely a direct effect, which could not be inhibited by α-IL-2. This suggests the involvement of alternative IL-6-specific signalling pathways. To study this in more detail, the up-regulatory effect of IL-6 was investigated in presence of inhibitors of several well-known signal transduction pathways. The inhibitors used were: 1 µm SB203580 (specific for the p38 MAP kinase pathway), 1 µg/µl CsA, specific for the calcineurin-dependent pathway), 10 µm PD98059 (specific for the MEK-1 pathway) and 10 µm LY294002 (specific for the PI3-kinase pathway).21–23 As depicted in Fig. 5(a), all inhibitors reduced the secretion of IL-5, indicating that the pathways that are blocked by these inhibitors are essential for optimal IL-5 production. Furthermore, in the presence of SB203580 we did not observe a significant up-regulation of IL-5 production in response to IL-6 (fold induction 1·1 ± 0·2, NS), indicating that the up-regulatory effect of IL-6 is dependent on p38 activity. Similarly, in the presence of CsA no significant increase in IL-5 secretion could be induced by IL-6. In contrast, when inhibitors of the MEK-1 pathway (PD98059) and the PI3-kinase pathway (LY294002) were added, IL-6 was still able to induce an increase in the IL-5 protein levels when compared to the levels in the absence of IL-6 (fold induction 1·5 ± 0·1 and 1·6 ± 0·3, respectively, P < 0·05). This indicates that the PI3-K and MEK-1 pathways are not crucial for the up-regulatory effect of IL-6.
Figure 5.
The up-regulatory effect of IL-6 on IL-5 production is blocked by inhibition of the p38 MAPk pathway. (a) Effect of IL-6 preincubation (5 min) in absence and presence of inhibitors of the p38, calcineurin-dependent, ERK-1/2 and PI3-kinase pathway (SB203850, CsA, PD98059 and LY294002, respectively) on α-CD3/α-CD28 stimulated IL-5 production by freshly isolated T lymphocytes. After preincubation with IL-6, T cells were stimulated with α-CD3/α-CD28 during 8 hr and IL-5 protein was measured in supernatants. Protein levels are expressed as a percentage (x ± SEM, n = 6) of the production after stimulation with α-CD3/α-CD28 alone. *, P < 0·05 between relative IL-5 protein in the presence and absence of IL-6. (b) Effect of IL-6 on α-CD3/α-CD28 induced p38 phosphorylation, in the presence and absence of CsA. T cells were cultured overnight in RPMI-1640 medium containing 0·5% FCS and subsequently stimulated with α-CD3/α-CD28 for 60 s in the presence and absence of IL-6 and CsA. Total cell lysates were prepared. Phosphorylated p38 is depicted in the upper panel and total p38 is shown in the lower panel (marked by arrows). Results shown are representative of three independent experiments.
Because the p38 pathway seems to be involved in the effects of IL-6, Western blot analysis was performed using phospho-specific p38 antibodies. Antibodies for total p38 were used as a control for equal loading. Preincubation with IL-6 followed by α-CD3/α-CD28 stimulation resulted in an increase in p38 phosphorylation compared to the effect of α-CD3/α-CD28 alone (fold up-regulation ± 1·75). This promotive effect of IL-6 could be inhibited by CsA (Fig. 5b). In contrast, no effect of IL-6 was observed on JNK and ERK-1/2 phosphorylation (data not shown).
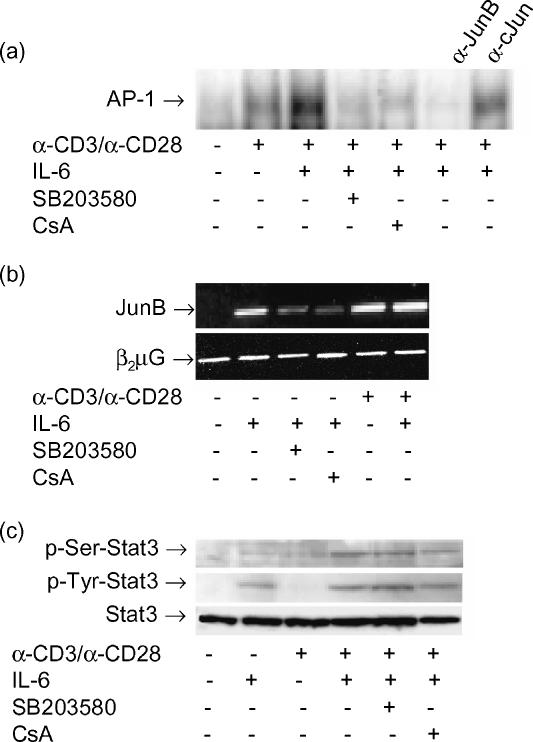
CsA is an immunosuppressive agent that affects the Ca2+-mediated intracellular signalling pathways, including MAP kinases, stress activated protein (SAP) kinases and the calcineurin-dependent activation of nuclear factor of activated T cells (NF-AT) transcriptional activity. The nuclear component of the NF-AT complex is composed of AP-1 (Fos/Jun) and non-AP-1 components, which may bind cooperatively.24 We carried out electrophoretic mobility shift assays (EMSAs) to reveal DNA-binding activitiy to the AP-1 oligonucleotide induced by IL-6. IL-6 enhanced DNA-binding of an α-CD3/α-CD28 induced AP-1 complex to its specific oligonucleotide (fold induction ± ·0, Fig. 6a), which was inhibited by CsA and SB203580. Supershift experiments with monoclonal antibodies against JunB demonstrated that the IL-6 induced AP-1 binding activity was strongly inhibited by treatment with excessive α-JunB, but not α-c-Jun, indicating that JunB is the major component of the IL-6 induced AP-1 binding complex. In accordance with these data, RT–PCR showed that IL-6 induces expression of JunB mRNA in unstimulated T cells (fold induction ± 4, Fig. 6b) and 5 min of preincubation with IL-6 also increased JunB mRNA expression in α-CD3/α-CD28 stimulated T cells (fold up-regulation ± 1·5, Fig. 6b). Furthermore, these studies revealed that blocking of the p38 pathway with SB203580 or CsA also strongly reduced IL-6 induced JunB expression (Fig. 6b). Together, these results indicate that the p38 dependent up-regulation of JunB may be involved in the up-regulatory effect of IL-6 on IL-5 production, since IL-6 enhances the α-CD3/α-CD28 stimulated JunB mRNA expression, AP-1 binding activity and IL-5 production in a p38-dependent manner.
Figure 6.
The effect of IL-6 on AP-1 binding activity, but not STAT3 phosphorylation, is inhibited by SB203580 and CsA. (a) Effect of IL-6 on DNA binding activity of AP-1. Freshly isolated T cells were stimulated for 4 hr with α-CD3/α-CD28, in the presence and absence of IL-6, SB203580 and CsA. Supershifts were carried out using 1 µg of polyclonal α-JunB or α-c-Jun antibody. Results shown are representative of three independent experiments. (b) JunB RNA expression is up-regulated by IL-6. Freshly isolated T cells were incubated with IL-6 (10 ng/ml) for 1 hr, in presence and absence of SB203580 or CsA, or preincubated with IL-6 during 5 min and subsequently stimulated with α-CD3/α-CD28 during 1 hr. In the upper panel the JunB signal is shown. In the lower panel the β2µG signal shows that comparable amounts of product were amplified for each condition. Results shown are representative of three independent experiments. (c) Effect of IL-6 on STAT3 serine and tyrosine phosphorylation. T cells were cultured over night in RPMI containing 0·5% FCS and subsequently stimulated with IL-6, α-CD3/α-CD28 or their combination in presence and absence of SB203580 and CsA. Total cell lysates were prepared. Serine phosphorylated STAT3 is depicted in the upper panel, tyrosine phosphorylated STAT3 is shown in the middle panel and total STAT3 is depicted in the lower panel (marked by arrows). Results shown are representative of two independent experiments.
An additional transcription factor relevant for IL-6 mediated signalling is STAT3. The role of STAT3 in transcriptional activation of Th2 cytokine genes is still unclear, but STAT3 activation might be involved in the induction of JunB expression25 thereby enhancing AP-1 binding activity. Western blotting showed that IL-6 alone induces STAT3 tyrosine phosphorylation in T cells. For optimal serine phosphorylation of STAT3 the presence of α-CD3/α-CD28 is required (Fig. 6c). In contrast to p38 phosphorylation and AP-1 binding activity, STAT3 phosphorylation – both serine and tyrosine – was not susceptible to CsA and SB203580 inhibition. This indicates that the abrogation of the IL-6-induced effects by CsA and SB203580 is not caused by inhibition of the STAT3 pathway.
Discussion
Effector Th2 cells play an important role in the development of atopic diseases. Although it is known that cytokines are crucial for the differentiation towards a Th1 or Th2 phenotype, the mechanisms by which gene transcription and differentiation are regulated remain largely unclear. In our present report we demonstrate that IL-6 primes for enhanced production of IL-4 and IL-5 in T cells. In atopic asthma, peripheral T cells are primed to produce a two-fold higher level of Th2 cytokines compared to healthy controls, which is further enhanced after allergen challenge.8 Our data show that IL-6 may be involved in this enhanced capacity to produce Th2 cytokines and that exposure to IL-6 may promote a Th2-like immune response. Short preincubation with IL-6 up-regulated the α-CD3/α-CD28 induced production of both IL-4 and IL-5 protein, whereas the production of IFN-γ was not affected. After 24 hr of preincubation with IL-6, the up-regulatory effect on IL-5 secretion became most pronounced. At that time, a slight but significant inhibitory effect was found on IFN-γ secretion, implicating a negative feedback mechanism on the Th1-type immune response by IL-6.
The immediate up-regulatory effect of IL-6 found for both IL-4 and IL-5 production suggests a direct effect of IL-6. Our results indicate that the p38 dependent up-regulation of JunB is one of the early events in IL-6 signalling resulting in the elevation of IL-5 production. The up-regulatory effect of IL-6 was blocked by specific p38 MAPK inhibitor SB203580 and in addition by CsA. The latter effect is at least partly mediated by the p38 pathway, because CsA inhibited the IL-6-induced p38 phosphorylation. We further show that IL-6-induced signalling results in enhanced binding of the AP-1 complex to its binding site in the promoter region of Th2 cytokines, thereby possibly enhancing transcriptional activity of these cytokine genes. In agreement with our data, it has been reported that binding of AP-1 was stronger in IL-5-producing cells from asthmatic patients than in non-IL-5-producing cells from control subjects26 implicating that AP-1 is involved in expression of IL-5. Furthermore, it has been shown that transcriptional activity of AP-1 is enhanced in Th2 cells compared to Th1 cells.27 The AP-1 complex in Th2 cells was demonstrated to be composed of JunB dimers, which do not accumulate in Th1 cells.27 The inducible AP-1 moiety of the conserved lymphokine element 0 (CLE0) in the IL-5 promoter has been demonstrated to consist of JunB and c-Fos.28 In addition, JunB was selectively induced in Th2 cells, but not in Th1 cells and able to activate IL-4 promoter activity.29 Thus, expression of JunB might contribute to a Th2 profile. In accordance, our data demonstrate that JunB expression is up-regulated by IL-6. It has also been reported that STAT3 may enhance AP-1 activity by induction of JunB.24 In contrast to AP-1 binding, our results demonstrate that STAT3 phosphorylation is not inhibited by SB203580 and CsA, in accordance with recent findings demonstrating that STAT3 serine phosphorylation is dependent on Vav, Rac and protein kinase Cδ (PKCδ) activation.30 This implicates that the reduced AP-1 binding activity as a result of incubation with SB203580 and CsA is caused by inhibition of other pathways than the STAT3 pathway. Because both CsA and SB203580 block p38 activity, the p38 MAP kinase pathway might be involved in this effect and be required for optimal binding activity of the AP-1 complex. Accordingly, it has been described that p38 MAPK activity is required for the induction of JunB expression in primary murine T cells and that JunB is phosphorylated by p38 MAPK.31
The priming effect of IL-6 on IL-4 (but not IL-5) secretion gradually disappeared after 12 hr of preincubation, suggesting that nuclear components responsible for increased IL-4 production are down-regulated after long-term incubation with IL-6. Furthermore, these data indicate that signalling events crucial for IL-5 but not IL-4 expression are involved in the long-term preincubation effect of IL-6. Although IL-4 and IL-5 expression are regulated by similar nuclear components (AP-1, NF-ATc)31,32 expression of these cytokines is regulated differentially. For example, c-Maf was demonstrated to be critical for IL-4 but not IL-5 expression33 whereas GATA-3 seems to be more important for antigen-induced IL-5 production.20,34 Thus, this transcription factor might be involved in the long-term priming effect on IL-5 production, which was found in our study. In contrast to the short-term effect of IL-6, the long-term effect on IL-5 production was shown to be dependent on the presence of IL-2. Indeed, IL-6 has been reported to be capable of increasing IL-2 production and IL-2 receptor expression.35,36 However, an additional effect of IL-6 was found in the presence of high levels of IL-2, suggesting that the effect of IL-6 is mediated in conjunction with activation of the IL-2-dependent signalling pathway. Our study shows that up-regulation of GATA-3 mRNA expression is one of events in IL-2 signalling that may lead to enhanced IL-5 secretion. This observation has clinical relevance, as enhanced GATA-3 mRNA expression has been observed in the airways of asthmatic subjects compared to healthy controls.37 In addition, increased IL-2 mRNA expression was found in bronchial mucosa from asthmatic patients5 indicating that IL-2 is produced at the site of allergen-induced reactions. It is remains unclear whether the effect of IL-6 is dependent on the levels of GATA-3 expression. One might speculate that IL-6 is able to up-regulate transcription factors that cooperatively bind GATA-3 and thereby enhance activity of the IL-5 promoter, which has been described for the AP-1 complex and Ets.38,39 This needs further investigation, however.
In addition to our findings concerning IL-6, IL-4 has been widely described to induce the differentiation towards a Th2 phenotype. However, the source of IL-4 in vivo often remains elusive, while T cells have ample opportunity to encounter IL-6 secreting cells. It has been demonstrated that alveolar macrophages from atopic asthmatics produce higher levels of IL-6 than alveolar macrophages from healthy controls.10,11 IL-6 produced by macrophages or epithelial cells at sites of tissue inflammation may prime local lymphocytes for enhanced production of Th2 cytokines. While 24 h of preincubation with IL-4 did not enhance the levels of Th2 cytokines in α-CD3/α-CD28 stimulated T cells (data not shown), the priming effect of IL-6 on Th2 cytokines was observed after short preincubation periods. The rapid priming for enhanced production of Th2-like cytokine IL-4 by IL-6 may be sufficient to lead to stable Th2 differentiation under local circumstances. The results from our study seem to be in accordance with the in vivo findings in atopic asthmatic subjects after house dust mite induced obstructive reactions; 24 hr after allergen challenge the capacity of peripheral T cells to produce IL-5 is enhanced.40 In the same setting, the capacity of T cells to produce IFN-γ was not significantly affected, but tended to be decreased 24 hr after allergen challenge. In addition, it has been demonstrated that monocytes from newborns with a high risk to develop allergy show increased capacity to produce IL-6, which is associated with a decreased capacity of cord blood mononuclear cells to produce IFN-γ.11 This is compatible with our findings demonstrating that IL-6 inhibits secretion of Th1-like cytokine IFN-γ. This effect may be mediated by up-regulation of SOCS1 and subsequent inhibition of STAT1 induced IFN-γ production. Together, these and our findings indicate that IL-6 may contribute to the development of allergic reactions such as atopic asthma, by inducing a shift in the immune response towards a Th2 type.
In summary, our data demonstrate that IL-6 promotes the production of Th2-like cytokines, but not Th1-like cytokines. Two different mechanisms appear to mediate the short-term and the long-term effect. The p38-dependent up-regulation of JunB expression and AP-1 binding activity seem to be involved in the short-term effect, whereas the long-term effect is dependent on the endogenous production of IL-2.
Acknowledgments
This work was supported by grants from the Groningen University Institute for Drug Exploration (GUIDE) and the ‘Stichting Astma Bestrijding’ (SAB).We thank the department of Tumor Immunology for providing the α-CD3/α-CD28 antibodies.
Abbreviations
- AP-1
activator protein
- BAL
bronchoalveolar lavage
- CsA
cyclosporin A
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-related kinase
- IL
interleukin
- IFN
interferon
- MAP
mitogen activated protein
- JNK
c-Jun N-terminal kinase
- MEK
MAPK/ERK kinase
- NF-AT
nuclear factor of activated T cells
- PI3-kinase
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- rh
recombinant human
- RT—PCR
reverse transcriptase–polymerase chain reaction
- SOCS
suppressor of cytokine signalling
- STAT
signal transducer and activator of transcription
- TBE
TRIS boric acid EDTA
- Th
T helper
References
- 1.Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 2.Tang C, Rolland JM, Ward C, Quan B, Waters EH. IL-5 production by bronchoalveolar lavage and peripheral blood mononuclear cells in asthma and atopy. Eur Respir J. 1997;10:624–32. [PubMed] [Google Scholar]
- 3.Robinson DS, Hamid Q, Bentley AM, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells, increased Th2-type mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993;92:313–24. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 4.Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991;87:1541–6. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ying S, Durham SR, Corrigan CJ, Hamid Q, Kay AB. Phenotype of cells expressing mRNA for Th2-type (interleukin-4 and interleukin-5) and Th1-type (interleukin-2 and interferon-gamma) cytokines in bronchoalveolar lavage and bronchial biopsies from atopic asthmatic and normal control subjects. Am J Respir Cell Mol Biol. 1995;12:477–87. doi: 10.1165/ajrcmb.12.5.7742012. [DOI] [PubMed] [Google Scholar]
- 6.Ying S, Durham SR, Jacobson MR, Rak S, Masuyama K, Lowhagen O. T lympocytes and mast cells express messenger RNA for interleukin-4 in the nasal mucosa in allergen-induced rhinitis. Immunology. 1994;82:200–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Ying S, Durham SR, Barkans J, Masuyama K, Jacobson MR, Rak S. T cells are the principal source of interleukin-5 mRNA in allergen-induced rhinitis. Am J Respir Cell Mol Biol. 1993;4:356–60. doi: 10.1165/ajrcmb/9.4.356. [DOI] [PubMed] [Google Scholar]
- 8.Borger P, Ten Hacken NTH, Vellenga E, Kauffman HF, Postma DS. Peripheral blood lymphocytes from asthmatic patients are primed for enhanced expression of Interleukin (IL)-4 and IL-5 mRNA. association with lung function and serum IgE. Clin Exp Allergy. 1998;29:772–9. doi: 10.1046/j.1365-2222.1999.00478.x. [DOI] [PubMed] [Google Scholar]
- 9.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang C, Rolland JM, Ward C, Li X, Bish R, Thien F, Walters EH. Modulatory effects of alveolar macrophages on CD4+ T-cell IL-5 responses correlate with IL-1beta, IL-6, and IL-12 production. Eur Respir J. 1999;14:106–12. doi: 10.1034/j.1399-3003.1999.14a18.x. [DOI] [PubMed] [Google Scholar]
- 11.Liao SY, Liao TN, Chang BL, Huang MS, Chen CC, Chou CC, Hsieh KH. Decreased production of IFNγ and increased production of IL-6 by cord blood mononuclear cells of newborns with high risk of allergy. Clin Exp Allergy. 1996;26:397–405. [PubMed] [Google Scholar]
- 12.Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincon M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–15. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 13.La Flamme C, MacDonald A, Pearce EJ. Role of IL-6 in directing the initial immune response to schistosome eggs. J Immunol. 2000;164:2419–26. doi: 10.4049/jimmunol.164.5.2419. [DOI] [PubMed] [Google Scholar]
- 14.Roozendaal R, Vellenga E, de Jong MA, Traanberg KF, Postma DS, de Monchy JGR, Kauffman HF. Resistance of activated human Th2 cells to NO-induced apoptosis is mediated by γ-glutamyltranspeptidase. Int Immunol. 2001;13:519–28. doi: 10.1093/intimm/13.4.519. [DOI] [PubMed] [Google Scholar]
- 15.Hoekstra MO, Hoekstra Y, de Reus D, Rutgers B, Gerritsen J, Kauffman HF. Interleukin-4 (IL-4) and Interferon-γ and interleukin-5 (IL-5) in peripheral blood of children with moderate atopic asthma. Clin Exp Allergy. 1997;27:1254–60. [PubMed] [Google Scholar]
- 16.Schreiber E, Matthias P, Müller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’prepared from small number of cells. Nucl Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford MMA. Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Bird JJ, Brown DR, Mullen AC, Moskowitz MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:232–7. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 19.Mori A, Suko M, Kaminuma O, et al. A critical role of IL-2 for the production and gene transcription of IL-5 in allergen-specific human T cell clones. Int Immunol. 1996;8:1889–95. doi: 10.1093/intimm/8.12.1889. [DOI] [PubMed] [Google Scholar]
- 20.Zhang DH, Yang L, Ray A. Differential responsiveness of the IL-5 and IL-4 genes to transcription factor GATA-3. J Immunol. 1998;161:3817–21. [PubMed] [Google Scholar]
- 21.Gringhuis SI, de Leij LFMH, Wayman GA, Tokumitsu H, Vellenga E. The Ca2+/calmodulin-dependent kinase type IV is involved in the CD5-mediated signaling pathway in human T lymphocytes. J Biol Chem. 1998;272:31809–20. doi: 10.1074/jbc.272.50.31809. [DOI] [PubMed] [Google Scholar]
- 22.Gringhuis SI, de Leij LFMH, Coffer PJ, Vellenga E. Signaling through CD5 activates a pathway involving phosphatidylinositol 3-kinase, Vav, and Rac1 in humane mature T lymphocytes. Mol Cell Biol. 1998;18:1725–35. doi: 10.1128/mcb.18.3.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gringhuis SI, de Leij LFMH, Verschuren EW, Borger P, Vellenga E. Interleukin-7 upregulates the interleukin-2 gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both NFAT and AP-1. Blood. 1997;90:2690–700. [PubMed] [Google Scholar]
- 24.Rincon M, Flavell RA. Reprogramming transcription during the differentiation of precursor CD4+ T cells into effector Th1 and Th2 cells. Microbes Infection. 1999;1:43–50. doi: 10.1016/s1286-4579(99)80013-6. [DOI] [PubMed] [Google Scholar]
- 25.Sjin RM, Lord KA, Abdollahi A, Hoffman B, Liebermann DA. Interleukin-6 and leukemia inhibitory factor induction of JunB is regulated by distinct cell type-specific cis-acting elements. J Biol Chem. 1999;274:28697–707. doi: 10.1074/jbc.274.40.28697. [DOI] [PubMed] [Google Scholar]
- 26.Mori A, Kaminuma O, Ogawa K, Okudaira H, Akiyama K. Transcriptional regulation of IL-5 gene by nontransformed human T cells through the proximal promoter element. Intern Med. 2000;398:618–25. doi: 10.2169/internalmedicine.39.618. [DOI] [PubMed] [Google Scholar]
- 27.Rincon M, Derijard B, Chow CW, Davis RJ, Flavell RA. Reprogramming the signalling requirement for AP-1 (activator protein-1) of precursors CD4+ T-cells into effector Th1 and Th2 cells. Genes Function. 1997;1:51–68. doi: 10.1046/j.1365-4624.1997.00007.x. [DOI] [PubMed] [Google Scholar]
- 28.Karlen S, D'Ercole M, Sanderson CJ. Two pathways can activate the interleukin-5 gene and induce binding to the conserved lymphokine element. Blood. 1996;88:211–21. [PubMed] [Google Scholar]
- 29.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J. 1999;18:420–32. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuringa JJ, Dekker LV, Vellenga E, Kruijer W. Sequential activation of Rac-1, SEK-1/MKK-4 and PKCdelta is required for interleukin-6 induced STAT3 Ser-727 phosphorylation and transactivation. J Biol Chem. 2001;29:27709–15. doi: 10.1074/jbc.M009821200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Salojin KV, Delovitch TL. CD28 co-stimulation restores T cell responsiveness in NOD mice by overcoming deficiencies in Rac-1/p38 mitogen-activated protein kinase signaling and IL2 and IL-4 gene transcription. Int Immunol. 2001;13:377–84. doi: 10.1093/intimm/13.3.377. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida H, Nishina H, Takimoto H, et al. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–24. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 33.Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;106:745–51. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- 34.Mori A, Kaminuma O, Mikami T, Inoue S, Okumura Y, Akiyama K, Okudaira HJ. Transcriptional control of the IL-5 gene by human helper T cells: IL-5 synthesis is regulated independently from IL-2 or IL-4 synthesis. Allergy Clin Immunol. 1999;103:S429–36. doi: 10.1016/s0091-6749(99)70158-2. [DOI] [PubMed] [Google Scholar]
- 35.Joseph SB, Miner KT, Croft M. Augmentation of naive, Th1 and Th2 effector CD4 responses by IL-6, IL-1 and TNF. Eur J Immunol. 1998;28:277–89. doi: 10.1002/(SICI)1521-4141(199801)28:01<277::AID-IMMU277>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 36.La Flamme AC, Pearce EJ. The absence of IL-6 does not affect Th2 cell development in vivo, but does lead to impaired proliferation, IL-2 receptor expression, and B cell responses. J Immunol. 1999;162:5829–37. [PubMed] [Google Scholar]
- 37.Nakamura Y, Ghaffar O, Olivenstein MD, Taha RA, Soussi-Gounni A, Zhang DH, Ray R, Hamid Q. Gene expression of the GATA-3 transcription factor in increased in atopic asthma. J Allergy Clin Immunol. 1999;103:215–22. doi: 10.1016/s0091-6749(99)70493-8. [DOI] [PubMed] [Google Scholar]
- 38.Kawana M, Lee ME, Quertermous EE, Quertermous T. Cooperative binding of GATA-2 and AP1 regulates transcription of the endothelin-1 gene. Mol Cell Biol. 1995;8:4225–31. doi: 10.1128/mcb.15.8.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blumenthal SG, Aichele G, Wirth T, Czernilofsky AP, Nordheim A, Dittmer J. Regulation of the human Interleukin-5 promoter by Ets transcription factors. J Biol Chem. 1999;274:12910–6. doi: 10.1074/jbc.274.18.12910. [DOI] [PubMed] [Google Scholar]
- 40.Borger P, Jonker GJ, Vellenga E, Postma DS, de Monchy JGR, Kauffman HF. Allergen challenge primes for IL-5 mRNA production and abrogates β-adrenergic function in peripheral blood T lymphocytes from asthmatics. Clin Exp Allergy. 1999;29:933–40. doi: 10.1046/j.1365-2222.1999.00552.x. [DOI] [PubMed] [Google Scholar]