Abstract
Recent studies have indicated that cells undergoing apoptosis are the source of autoantigens which drive autoimmune responses in systemic lupus erythematosus (SLE). It has been recognized for many years that in vitro stimulation of T cells with irradiated major histocompatibility complex (MHC) class II-bearing autologous cells results in T-cell proliferation with immunological specificity and memory, namely the autologous mixed lymphocyte reaction (AMLR). The nature of the major stimulants in the AMLR is still unclear. We investigated whether apoptotic fragments from irradiated cells act as antigenic stimulators for AMLR or nucleohistone-primed T cells. T-cell proliferation in the primary AMLR was significantly suppressed by the presence of a caspase inhibitor Z-Val-Ala-Asp-CH2F (Z-VAD.fmk), indicating that apoptotic antigens released from irradiated autologous feeder cells act as stimulators of AMLR T cells. This inhibitory effect of Z-VAD was not caused by toxic effects, because the T-cell response to the mitogen phytohaemagglutinin (PHA) was not inhibited by Z-VAD. A nucleohistone preparation was shown to contain antigens that are important in the AMLR, as culture with nucleohistone (but not with thyroglobulin or hen-egg lysozyme) primed T cells to respond with secondary kinetics in a subsequent AMLR that was also suppressed by Z-VAD. Our data provide evidence that the AMLR constitutes a model for the evaluation of cellular and molecular mechanisms that may be relevant to the pathogenesis of SLE and similar autoimmune diseases.
Introduction
Systemic lupus erythematosus (SLE) belongs to a genetically complex, heterogeneous group of diseases in which it is proposed that the immune system targets apoptotic cell antigens, which are a highly complex group of cell-surface and intracellular autoantigens. In recent years, disturbances in the process of apoptosis and the clearance of apoptotic cells, resulting in increased levels of apoptotic antigens in the circulation of SLE patients,1–5 have been proposed as fundamental to the development of SLE. The accumulated apoptotic cells in SLE may arise from an increased production of apoptotic cells,1,6 a decreased clearance of apoptotic cells,5,7,8 or from the combination of both. In this regard, nucleosomes, the fundamental unit of chromatin and ubiquitous product of cell apoptosis, are suggested as major T- and B-cell autoantigens in SLE.9–12
Since its identification over 30 years ago,13 the autologous mixed lymphocyte reaction (AMLR) has become an integral tool in immunology. Following structural definition of the major histocompatibility complex (MHC) and T-cell receptor proteins, it became clear that the basis of T-cell autoreactivity in the AMLR was essentially similar to the recognition of foreign peptides presented by self-MHC molecules.14 Thus, the AMLR represents a self-recognition mechanism, indicating that self-tolerance in the periphery is not an entirely passive process, but rather an active, dynamic state. Although it has been suggested that the AMLR is a proliferative response of T cells to MHC antigens or xenogeneic antigens,15,16 the nature of the major stimulus in the AMLR has not been clarified. Here we present evidence that apoptotic antigens, including nucleohistone (NH), released from irradiated autologous feeder cells might be major stimulators of AMLR T cells. These data support the hypothesis that the AMLR could be an in vitro model for evaluation of immune responses in SLE.
Materials and methods
Peripheral blood mononuclear cell (PBMC) preparation
PBMC were isolated from heparinized whole blood of normal healthy donors by standard gradient centrifugation using Histopaque 1077 (Sigma, Poole, UK). PBMC were harvested from the interface, then washed once with phosphate-buffered saline (PBS) and once with RPMI-1640 (Invitrogen, Paisley, UK), by centrifugation at 400 g for 5 min.
Monocyte-depleted cell fraction (responder cells)
The PBMC were suspended at 2 × 106 cells/ml in RPMI-1640 supplemented with 10% human AB serum, 10 mm HEPES (Sigma), 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mm l-glutamine (all from Invitrogen). Heat-inactivated human AB serum (PAA, Yeovil, UK), rather than fetal calf serum (FCS), was used to avoid the presence of potentially stimulatory xenogeneic antigens. The PBMC (maximum 12 ml) were then incubated in 25-cm2 tissue culture flasks (Costar, Cambridge, UK) for 60 min at 37°. The non-adherent cells were recovered and washed once with medium, centrifuging at 300 g for 5 min. This fraction, depleted of adherent monocytes and containing mainly T cells and B cells, was called monocyte-depleted PBMC, or responder cells. In total, 90–95% of CD14+ monocytes were eliminated from PBMC, as determined by flow cytometry.
T-cell-depleted cell fraction (feeder cells)
A T-cell-depleted cell fraction was used as feeder cells in the AMLR. Briefly, PBMC were resuspended in PBS supplemented with 2 mm EDTA and 0·5% human AB serum and then incubated with paramagnetic MACS Beads conjugated with anti-human CD3 monoclonal antibody (mAb) (Miltenyi Biotec, Surrey, UK) for 15 min at 4°. Thereafter, they were passed through a column in a strong magnetic field. The negatively selected cells were washed once in medium and resuspended in 10 ml RPMI-1640 supplemented with 10% human AB serum. The percentage of CD3+ cells eliminated from PBMC was 85–90%.
Primary AMLR
Feeder cells were inactivated by irradiation with 1500 rads from a 137Cs source and washed with medium, centrifuging at 300 g for 5 min. Responder cells (100 µl/well of 1 × 106 cells/ml) were cultured with feeder cells (100 µl/well of 1–5 × 106 cells/ml) in 96-well flat-bottomed tissue culture plates (Nunc, Roskilde, Denmark) in triplicate wells. The cell mixture was incubated for up to 9 days at 37° in a 5% CO2-humidified atmosphere, and the cultures were pulsed with 1·0 µCi of [3H]thymidine ([3H]TdR) (Amersham Life Science, Bucks., UK) for 24 hr before harvesting. [3H]TdR incorporation was measured as counts per minute (c.p.m.) per culture using a liquid scintillation system (Microplate Scintillation Counter, Packard), and the data were expressed as mean c.p.m. per culture.
Secondary AMLR
The viable cells from day-10 primary AMLR were separated using the Dead Cell Removal Kit (Miltenyi Biotec) or by standard gradient centrifugation with Histopaque 1077. The results of secondary AMLR were the same by either method (data not shown). Briefly, the day-10 AMLR cell suspension was centrifuged at 300 g and then the supernatant was removed completely. The cells were resuspended in 100 µl of Dead Cell Removal MicroBeads per 107 total cells and incubated for 15 min at room temperature. Binding buffer (0·5 ml) was added to the cell suspension and this was passed through an MS+ column (Miltenyi Biotec). The column was rinsed with 4 × 500 µl of 1 × binding buffer. The eluate was collected as the live cell fraction. The secondary AMLR cultures were established by co-culturing 100 µl/well of 1 × 106 cells/ml viable primary AMLR cells with 100 µl/well of 0·5–6 × 106 irradiated autologous non-T cells (data with 1 : 0·5 ratio not shown) in 96-well flat-bottomed tissue culture plates in triplicate wells. The cell-culture conditions and [3H]TdR-incorporation measurements were similar to those of the primary AMLR.
Co-culture of AMLR and phytohaemagglutinin (PHA)-stimulated PBMC with Z-VAD
One milligram of stock caspase inhibitor Z-VAD.fmk (Z-Val-Ala-Asp-CH2F) (CN Biological, Nottingham, UK) was dissolved in 40 µl of dimethylsulphoxide (DMSO) and diluted subsequently in medium. It was added at the initiation of primary AMLR cultures, at concentrations of 5, 10 and 20 µm. Controls were AMLR co-cultured with equivalent amounts of DMSO diluted in medium at concentrations of 7, 15 and 30 µm. The cell-culture conditions and [3H]TdR-incorporation measurements were similar to those of the primary AMLR.
PBMC suspensions in RPMI-1640 containing 10% AB serum (1 × 106 cells/ml) were stimulated with 0·5, 1, 2 and 4 µg/ml PHA-P (Sigma) (suboptimal concentration), in the presence or absence of 10 or 30 µm Z-VAD, for 5 days in 96-well flat-bottomed tissue culture plates at 37° in a 5% CO2-humidified atmosphere. The cultures were pulsed with 1·0 µCi of [3H]TdR for 24 hr before harvesting, and then [3H]TdR incorporation was measured using a liquid scintillation system and the data were expressed as mean c.p.m. per culture.
Detection of apoptotic feeder cells based on their fractional DNA content
Non-irradiated and gamma-irradiated feeder cells, the latter treated with 5, 10, 20 or 30 µm Z-VAD, or medium only, were cultured in RPMI-1640 supplemented with 10% human AB serum (2 × 105 cells/ml) for 48 hr in 96-well flat-bottomed tissue culture plates (Nunc, Roskilde, Denmark). The cells were then dispensed into 5-ml fluorescence-activated cell sorter (FACS) tubes (Elkay, Hampshire, UK) at 1 × 105 cells per tube. After removal of the supernatant by centrifugation, 1 ml of ice-cold 70% ethanol was added to each tube and mixed well. Cells were washed twice with PBA (PBS containing 0·5% bovine serum albumin and 0·1% sodium azide), at 300 g for 5 min at 4°, and resuspended in 1 ml PBA. RNAse (0·1 mg/ml) (Sigma, Poole, UK) and 50 µg/ml propidium iodide (PI) (Sigma) were added to each tube and the incubation was continued for a further 20 min at 37° in a water bath. Cells were analysed using an Epics Profile XL flow cytometer (Beckman Coulter, Luton, UK). PI DNA fluorescence was emitted at 600–700 nm, so it was detectable in an FL3 detector. Cell debris was excluded from the analysis by appropriate gating on forward scatter and side scatter, and a second gate was created in the dot-plot of the FL3 area versus the FL3 peak to identify single cells.
Challenge of antigen-primed PBMC with autologous feeder cells in the presence or absence of Z-VAD
PBMC from healthy donors were resuspended, at a concentration of 1 × 106 cells per ml, in RPMI-1640 supplemented with 10% heat-inactivated human AB serum. Then, cell suspensions were dispensed (2-ml aliquots) into a 24-well plate (Costar) and cultured with 50 µg/ml bovine NH, 10 µg/ml hen-egg lysozyme (HEL), 10 µg/ml bovine thyroglobulin (Tg) (all from Sigma), or without antigen, at 37° in a 5% CO2-humidified atmosphere for up to 11 days. NH were dissolved, with constant slow stirring, for up to 1 week in sterile water. Any insoluble material from the water-dissolved NH was removed by centrifugation at 2000 g for 10 min. [3H]TdR-incorporation measurements were carried out on these cultures from days 4–11. Day-10 cells (with 94% viability) from the above cultures were washed once with RPMI-1640, by centrifugation at 300 g for 5 min, and resuspended in RPMI-1640 supplemented with 10% heat-inactivated human AB serum at a concentration of 1 × 106 cells per ml. These cells (100 µl/well of 1 × 106 cells/ml) were cultured with 100 µl/well irradiated autologous feeder cells (1 × 106 cells/ml), in the presence or absence of NH or Tg, in 96-well flat-bottomed tissue culture plates in triplicate wells. In some of the above cultures, Z-VAD was added (at concentrations of 10, 20 and 30 µm) at the initiation of the secondary cultures using day-10 NH-primed cells. Cultures with equivalent amounts of DMSO, diluted at concentrations of 15, 30 and 45 µm in medium, served as controls. The cell mixture was incubated for up to 9 days at 37° in a 5% CO2-humidified atmosphere, and the cultures were pulsed with 1·0 µCi of [3H]TdR for 24 hr before harvesting. [3H]TdR-incorporation measurements were performed as for the primary AMLR.
Gel electrophoresis of NH
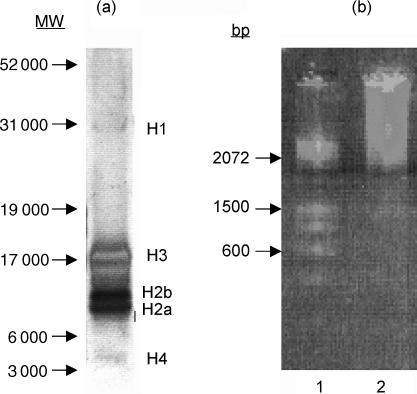
Aliquots (7·5 µl) of NH preparations, at a concentration of 1 mg/ml, were mixed 3 : 1 with the NuPage sample buffer (4×) containing lithium dodecyl sulphate (Invitrogen) and 10% reducing agent [0·5 m dithiothreitol (DTT)]. The samples were heated at 70° for 10 min and then centrifuged for 1 min at 10 000 g. The samples (10 µl/lane) were loaded onto a 10% Bis-Tris polyacrylamide gel (NuPage; Invitrogen) and 5 µl of colour marker was also used. The dissolved antigens were separated in an electrophoretic cell at 200 V for 35 min in MES running buffer (Invitrogen). The gels were then silver stained according to the method described by Blum et al.17 The DNA content of NH was revealed by ethidium bromide staining following electrophoresis through a 1% agarose gel. As shown in Fig. 1, the NH used comprised high-molecular-weight DNA and all histone subfractions (H1, H2a, H2b, H3 and H4), but mainly H3, H2a, and H2b. Little contamination with other proteins was evident.
Figure 1.
(a) Gel electrophoresis of nucleohistone (NH). A 7·5-µg sample of NH preparation was loaded onto gels, as described in the text. Arrows indicate molecular-weight markers, and histone positions are presented on the right of the gel. (b) Ethidium bromide-stained agarose-gel electrophoresis of NH. Lane 1, DNA base pair (bp) marker; lane 2, NH sample.
Statistical analysis
The paired Student's t-test was used to compare the levels of [3H]TdR uptake (c.p.m.) obtained from either Z-VAD-treated or non-treated cultures. P-values of less than 0·05 were considered significant.
Results
The characteristics of an immune response in AMLR
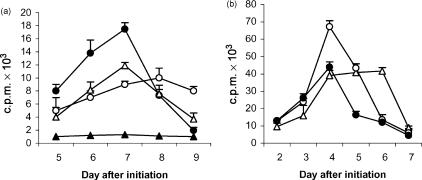
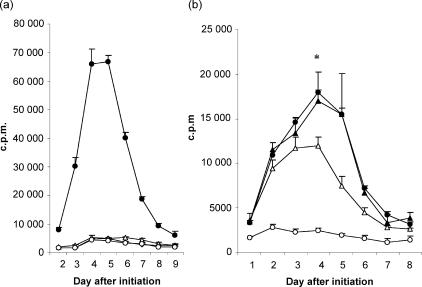
In the primary AMLR, [3H]TdR incorporation peaked on day 7, at a ratio of one responder cell to four irradiated stimulator cells. In the secondary AMLR, cultures established with cells from day-10 primary AMLR cultures that had returned to a non-proliferating state, maximum [3H]TdR uptake occurred on days 3–4, at a ratio of one responder cell to one irradiated feeder cell (Fig. 2). The accelerated response following rechallenge with a lower dose of autologous cells confirmed that ‘memory’ had developed in the AMLR T-cell population.18
Figure 2.
Primary and secondary immune responses in a human autologous mixed lymphocyte reaction (AMLR). (a) Primary AMLR. Responder cells (1 × 105) were cultured in the absence of feeder cells (closed triangles) or in the presence of 1 × 105 (open circles), 4 × 105 (closed circles) or 6 × 105 (open triangles) autologous-irradiated feeder cells, in triplicate wells for 9 days. (b) Secondary AMLR. Viable cells (1 × 105) from the day-10 primary AMLR (panel a) were cultured with 0·5 × 105 (data not shown), 1 × 105 (open circles), 4 × 105 (closed circles), or 6 × 105 (open triangles) autologous-irradiated feeder cells, for 7 days. T-cell proliferation assays were carried out with these cultured cells. Each point represents the mean value of triplicate wells, and error bars indicate the standard deviation (SD) (representative of five experiments). c.p.m., counts per minute.
γ-Radiation-induced apoptosis of feeder cells is inhibited by the caspase inhibitor Z-VAD.fmk
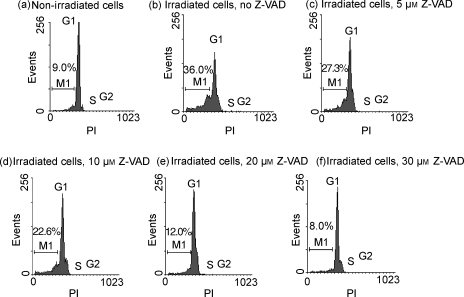
We investigated the effect of Z-VAD on γ-irradiated feeder-cell apoptosis based on their fractional DNA content. Feeder cells that were exposed to 1500 rads were cultured for 48 hr in medium alone or in medium containing increasing concentrations of Z-VAD. As shown in Figs 3(b)–3(f), Z-VAD displayed a dose-dependent effect, and almost 40% and 80% inhibition of apoptosis occurred at a concentration of 10 µm and 30 µm Z-VAD, respectively (Fig. 3), but not with DMSO alone (data not shown).
Figure 3.
Detection of apoptotic cells with fractional DNA content based on cellular DNA content analysis. Non-irradiated (a) and gamma-irradiated (b) feeder cells were cultured for 48 hr. Some of the latter cells were cultured with 5 µm (c), 10 µm (d), 20 µm (e), or 30 µm (f) caspase inhibitor Z-Val-Ala-Asp-CH2F (Z-VAD.fmk) from the beginning of culture. The cells were then fixed in 70% ethanol, permeabilized and stained with propidium iodide (PI), and their fluorescence was measured by flow cytometry. The population of early and late apoptotic cells is represented by the M1 fraction.
T-cell proliferation in the primary AMLR is inhibited by the caspase inhibitor Z-VAD.fmk
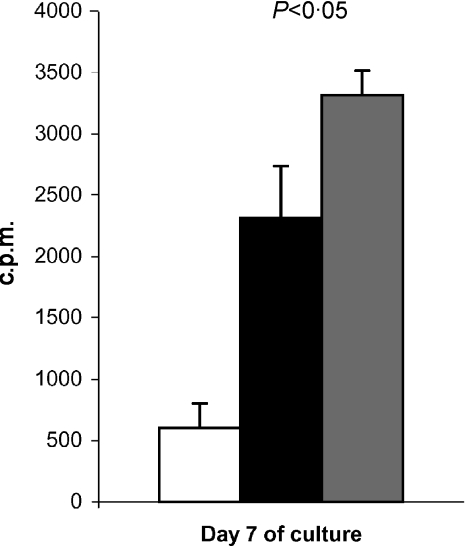
Z-VAD is a highly specific, cell-permeable and irreversible inhibitor of caspases-1, -3, -6 and -7.19 We have shown that Z-VAD.fmk effectively inhibits DNA fragmentation of irradiated feeder cells as a result of apoptosis inhibition. To study the possible stimulatory effect of apoptotic antigens on AMLR T cells, we investigated the effect of Z-VAD on T-cell proliferation in the AMLR system of normal individuals. Z-VAD (10 µm) was added at the initiation of primary AMLR cultures, and T-cell proliferation was assessed by [3H]TdR uptake on day 7 (the peak of cell proliferation). As Z-VAD was dissolved in DMSO, AMLR cultures with an equivalent concentration of DMSO alone (15 µm) were used as controls. T-cell proliferation was significantly inhibited by 10 µm Z-VAD compared to the equivalent concentration of DMSO (15 µm) (Fig. 4). Higher concentrations of DMSO were toxic to primary AMLR cultures, and therefore higher Z-VAD concentrations were not employed.
Figure 4.
The effect of the caspase inhibitor Z-Val-Ala-Asp-CH2F (Z-VAD.fmk) on the autologous mixed lymphocyte reaction (AMLR) of normal individuals (representative of four experiments). The day of the AMLR culture is designated on the x-axis, and proliferative responses, in counts per minute (c.p.m.), designated by vertical bars. The results represent the mean of triplicate wells (1 × 105 responding cells and 4 × 105 stimulating cells/well), and error bars indicate the standard deviation (SD). The AMLR proliferative response in the presence of 10 µm Z-VAD (black bar) is shown compared to that in the presence of 15 µm dimethylsulphoxide (DMSO) (grey bar) (control). The white bar corresponds to the c.p.m. measured in the absence of stimulating cells. The P-value is shown above the bars.
The inhibitory effect of Z-VAD is the result of blocking of apoptosis rather than a toxic effect of Z-VAD
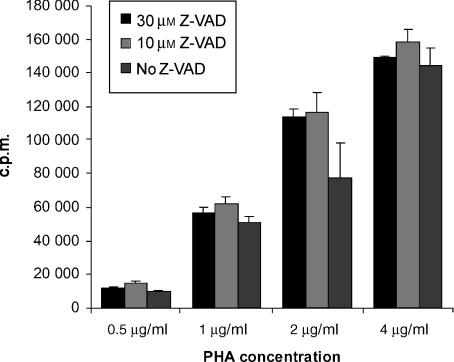
PBMC from normal subjects were stimulated with suboptimal concentrations of PHA (0·5, 1, 2 and 4 µg/ml) in the presence or absence of 10 µm and 30 µm Z-VAD. [3H]TdR uptake was measured on day 3 of cell proliferation. Interestingly, Z-VAD-treated cells tended to show a higher [3H]TdR uptake than cells not treated with Z-VAD (Fig. 5). This indicates that the inhibitory effect of Z-VAD on T-cell proliferation in the primary AMLR is the result of blocking of apoptosis, rather than a toxic effect of Z-VAD on T cells. It also suggests that inhibition of apoptosis in Z-VAD-treated T cells enables them to continue proliferating, rather than dying by activation-induced apoptosis, in response to PHA stimulation.
Figure 5.
Lack of inhibitory effect of the caspase inhibitor Z-Val-Ala-Asp-CH2F (Z-VAD.fmk) on T-cell proliferation of phytohaemagglutinin (PHA)-stimulated cells. T-cell proliferation assays were carried out for 3 days with peripheral blood mononuclear cells (PBMC) from healthy normal donors stimulated with PHA (0·5, 1, 2 or 4 µg/ml) in the absence of Z-VAD, or in the presence of 10 or 30 µm Z-VAD. Each bar represents the mean value of triplicate wells (2 × 105 cells per well), and error bars indicate the standard deviation (SD). Data are representative of five experiments. c.p.m., counts per minute.
NH-primed PBMC show a secondary response in an AMLR
PBMC from normal individuals were cultured in optimum conditions with 50 µg/ml NH, 10 µg/ml HEL, 10 µg/ml Tg, or in medium alone. The peak primary response of PBMC to NH occurred on day 9, and on day 8 to HEL or Tg; by days 10–11 of culture, the [3H]TdR uptake had returned to baseline levels. Day-11 cells from all cultures were rechallenged with autologous irradiated feeder cells at a ratio of one antigen-primed PBMC to one autologous irradiated feeder cell. As demonstrated in Fig. 6(a), NH-primed PBMC from normal healthy donors gave a very strong proliferative response to autologous apoptotic cells compared to HEL- or Tg-primed PBMC, or to PBMC previously cultured with medium alone.
Figure 6.
(a) Peripheral blood mononuclear cells (PBMC) from normal individuals were initially stimulated with 50 µg/ml bovine nucleohistone (NH) (closed circles), 10 µg/ml hen-egg lysozyme (HEL) (open circles), 10 µg/ml bovine thyroglobulin (Tg) (open triangles) or medium alone (open diamonds), for 11 days. T-cell proliferation assays were carried out with these cells stimulated with an equivalent number of irradiated autologous feeder cells. (b) Day-11 NH-primed cells were challenged with feeder cells (closed circles) and day-11 Tg-primed cells were stimulated with 10 µg/ml bovine Tg alone (open circles), feeder cells alone (open triangles), or Tg plus feeder cells (closed triangles). Each point represents the mean value of triplicate wells (1 × 105 primed cells and 1 × 105 feeder cells per well), and error bars indicate the standard deviation (SD). The data are representative of five experiments. *P < 0·05 comparing Tg-primed cells stimulated with feeder cells alone or with Tg plus feeder cells. c.p.m., counts per minute.
To confirm that the response to NH and apoptotic cells is comparable to that of other antigens, the secondary immune responses of Tg-primed cells to Tg alone, to feeder cells alone, or to Tg plus feeder cells, were also evaluated (Fig. 6b). Tg-primed cells responded more strongly to Tg plus feeder cells than to feeder cells alone, whereas NH-primed cells gave a stronger response to feeder cells alone than to NH alone or to NH plus feeder cells. In each case the peak response occurred on day 4 of stimulation.
The response of NH-primed PBMC to autologous feeder cells is inhibited dose dependently by Z-VAD.fmk
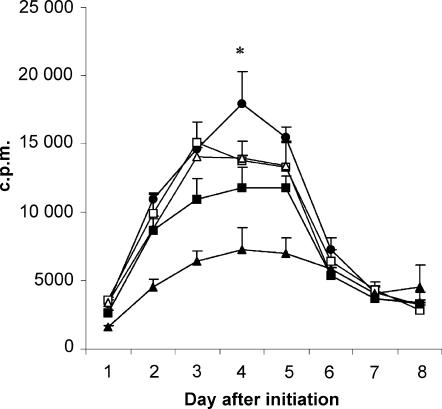
Although T-cell proliferation in a primary AMLR culture was significantly inhibited by Z-VAD, the inhibitory effect was relatively weak, at about 20% (Fig. 4). However, the effect of Z-VAD on the primary AMLR was restricted by the high sensitivity of primary AMLR cells to the toxic effect of DMSO: concentrations higher than 15 µm DMSO (= 10 µm Z-VAD) inhibited AMLR responses, and 30 µm DMSO completely inhibited primary AMLR responses (data not shown). Moreover, it is notable that only 40% of the apoptosis of gamma-irradiated feeder cells was inhibited at a concentration of 10 µm Z-VAD (Fig. 3), which was used in the experiment shown in Fig. 4. As DMSO showed less toxicity on NH-primed PBMC, the inhibitory effect of higher concentrations of Z-VAD was investigated on the secondary AMLR response following NH priming (Fig. 7). As shown in Fig. 7, Z-VAD caused a significant dose-dependent inhibition: almost 50% inhibition of proliferation was observed in the response of NH-primed cells to irradiated autologous feeder cells in the presence of 30 µm Z-VAD, compared to DMSO-treated cultures, on day 4. Interestingly, there was a positive correlation between the amount of feeder cell apoptosis inhibition (Fig. 3) and inhibition of the NH-primed cell response to feeder cells by Z-VAD (Fig. 7). A similar result was obtained using another caspase inhibitor, D-FMK (caspase inhibitor III; CN Biological, Nottingham, UK) (data not shown).
Figure 7.
The effects of caspase inhibitor Z-Val-Ala-Asp-CH2F (Z-VAD.fmk) on the secondary autologous mixed lymphocyte reaction (AMLR) response following nucleohistone (NH) priming. Peripheral blood mononuclear cells (PBMC) were initially stimulated with 50 µg/ml bovine NH for 11 days. T-cell proliferation assays were carried out with these cells stimulated with equivalent numbers of irradiated autologous feeder cells in the presence of 10 µm (closed squares) or 30 µm (closed triangles) Z-VAD, or medium alone (closed circles). Cultures with 15 µm (open squares) or 45 µm (open triangles) dimethylsulphoxide (DMSO) were used as controls for 10 µm and 30 µm Z-VAD, respectively. Each point represents the mean value of triplicate wells (1 × 105 primed-cells and 1 × 105 feeder cells per well), and error bars indicate the standard deviation (SD). *P < 0·001 comparing NH-primed cells cultured in the presence of 30 µm Z-VAD with DMSO controls.
Discussion
Twenty years ago it was suggested that xenogenic antigens,15 or MHC class II molecules per se,16 might be the source of stimulation in the AMLR. However at that time, techniques for cell isolation and culture were less sophisticated and much less was understood about the functions of MHC molecules and the nature of apoptosis. In this study, we have focused on the role of apoptosis in AMLR cultures and hypothesized that the apoptotic antigens released from irradiated feeder cells are presented by MHC class II to stimulate AMLR T cells. This hypothesis is based on the findings of Casciola-Rosen et al.,20 who showed that nuclear and cytoplasmic antigens (which are characteristic targets for autoantibodies) are clustered in surface membrane blebs of cells in the late stages of apoptosis. Furthermore, alteration of cellular constituents during apoptosis, occurring by post-translational modifications21 or proteolytic cleavage,22,23 could induce immunogenicity.
The responder cell fraction used in this study probably contains dendritic cells (DCs), as the depletion was not complete, and the feeder cell fraction as a source of apoptotic cells contained about 10–15% CD3+ cells. However, γ-radiation induces apoptosis in CD3+ cells and this fraction could be considered as a source of autologous apoptotic antigens. Moreover, the non-irradiated DCs amongst the responder cells could enhance AMLR responses, as preactivation of DCs (but not of monocytes) with granulocyte–macrophage colony-stimulating factor (GM–CSF) leads to a 113-fold increase in the stimulatory capacity of DCs in an AMLR, which occurs through the up-regulation of costimulatory molecules and MHC class I and II on DCs.24
Feeder cells were irradiated to prevent their proliferation, but this also induces apoptosis. Therefore, in our AMLR system, depletion of monocytes/macrophages from responding cells, and the high level of apoptotic material released from feeder cells, may mirror the in vivo conditions thought to be manifest in patients with SLE. In fact, it has been shown that the [3H]TdR uptake in an AMLR is significantly decreased when stimulating cells are enriched for monocytes/macrophages,25 which may reflect the role of macrophages in the clearance of apoptotic antigens in an AMLR. Data from patients with lupus provide evidence that the level of apoptotic lymphocytes in SLE patients in vitro as well as in vivo are higher than in healthy controls.1,6 Furthermore, Hermann et al.5 showed that phagocytosis of apoptotic cells by in vitro-differentiated macrophages obtained from SLE patients was impaired compared to phagocytosis by macrophages obtained from healthy controls. These findings might explain the increased levels of early apoptotic cells, DNA and nucleosomes reported in the circulation of patients with SLE.1–3
Radiation-induced DNA damage may initiate apoptosis via p53-dependent mechanisms, by regulating the expression of Bcl-2 family members.26 Moreover, p53 may also up-regulate CD95 receptor–ligand interactions.27 As the final common execution phase of apoptosis involves activation of effector caspases, the caspase inhibitor Z-VAD.fmk can block radiation-induced apoptosis in the B cells of normal subjects, e.g. 40% apoptosis inhibition at a concentration of 10 µm Z-VAD.28 Here we have shown that T-cell proliferation was significantly inhibited in the primary AMLR by Z-VAD (Fig. 4), indicating that the apoptotic antigens released from irradiated autologous feeder cells act as a stimulator for AMLR T cells. We confirmed that this effect was caused by the blocking of apoptosis rather than by a toxic effect of Z-VAD on T cells because Z-VAD by itself enhanced human T-cell proliferation in response to suboptimal concentrations of PHA (Fig. 5). Hence, it is, perhaps, surprising that it has been repeatedly reported that the in vitro AMLR of patients with SLE is decreased compared to that of healthy individuals. However, this could be because the relevant T cells are sequestered from the circulation into tissues in SLE, or because these T cells readily undergo apoptosis themselves as a consequence of chronic activation in vivo.
The stimulatory effect of NH was investigated in our AMLR model. NH used in this experiment contain high-molecular-weight DNA plus all subfractions of histones (H1, H2a, H2b, H3 and H4), but mainly H3, H2a, and H2b; little contamination with other proteins was observed (Fig. 1). It should be noted that the NH employed was an enriched preparation, rather than a pure preparation, and therefore may contain other cellular constituents. However, this remains consistent with a range of components derived from apoptotic cells (in addition to NH) acting as T-cell stimulants in the AMLR. We found that NH-primed human PBMC showed a very strong secondary immune response to autologous feeder cells compared to PBMC primed with antigens unrelated to apoptosis (Fig. 6). This suggests that nuclear constituents (nucleosomes/NH) released from feeder cells have a significant immunoproliferative effect on AMLR T cells. Interestingly, there was a positive correlation between the amount of feeder cell apoptosis inhibition and inhibition of NH-primed cell secondary responses to feeder cells by Z-VAD. Evidence is accumulating that the nucleosomes, the fundamental units of chromatin and ubiquitous product of cell apoptosis, are major T- and B-cell autoantigens in SLE.9 Nucleosome-specific CD4+ T cells are detected long before lupus mice produce pathogenic autoantibodies, suggesting that these cells play a role in triggering the disease.10 The presence of NH-specific (or histone-specific) pathogenic autoantibody-inducing T helper (Th) cells has also been demonstrated in human lupus.11 Although the critical peptide epitopes in SLE have not been identified, several regions in histone molecules (core histones, H2B, and H4) have been found to contain peptide epitopes recognized by pathogenic autoantibody-inducing Th cell clones derived from nephritic (NZB × SWR)F1 lupus mice.12 These findings strongly suggest that nucleosomes may be the immunogen leading to the production of anti-double-stranded DNA-, anti-histone-, and nucleosome-specific antibodies. Furthermore, Bell et al.18,29 showed that a potential source of nucleosomes are apoptotic cells, and that nucleosomes released spontaneously in short-term culture from both human and murine lymphocytes have a significant immunoproliferative (polyclonal B-cell activation) effect in vitro. In some circumstances, nucleosomes may induce cell death in culture; however, in our experiments NH clearly prime cells to give a secondary AMLR response.
It is possible that a component of the response to NH and/or apoptotic cells was polyclonal, but the secondary response to apoptotic feeder cells of NH-primed T cells was very similar to the kinetics of the secondary response to Tg of Tg-primed cells.
We have demonstrated that the AMLR can be inhibited by preventing apoptosis, and that the key apoptotic antigens (NH) can drive the AMLR response. Taken together, our data provide evidence that the AMLR could be a model for the evaluation of cellular and molecular mechanisms that are responsible for the pathogenesis of SLE and similar autoimmune diseases.
Acknowledgments
Financial support for this work was provided by The Jones Charitable Trust and Lupus UK. M. R. Amel Kashipaz was supported by a scholarship from the Iranian Ministry of Health and Medical Education.
Abbreviations
- AMLR
autologous mixed lymphocyte reaction
- c.p.m.
counts per minute
- HEL
hen-egg lysozyme
- NH
nucleohistone
- PBMC
peripheral blood mononuclear cells
- PHA
phytohaemagglutinin
- SLE
systemic lupus erythematosus
- Tg
thyroglobulin
- Z-VAD.fmk
caspase inhibitor Z-Val-Ala-Asp-CH2F
References
- 1.Courtney PA, Crockard AD, Williamson K, Irvine AE, Kennedy RJ, Bell AL. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann Rheum Dis. 1999;58:309–14. doi: 10.1136/ard.58.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amoura Z, Piette JC, Chabre H, Cacoub P, Papo T, Wechsler B, Bach JF, Koutouzov S. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus: correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis Rheum. 1997;40:2217–25. doi: 10.1002/art.1780401217. [DOI] [PubMed] [Google Scholar]
- 3.Steinman CR. Circulating DNA in systemic lupus erythematosus. Isolation and characterisation. J Clin Invest. 1984;73:832–41. doi: 10.1172/JCI111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine JS, Koh JS. The role of apoptosis in autoimmunity: immunogen, antigen, and accelerant. Semin Nephrol. 1999;19:34–7. [PubMed] [Google Scholar]
- 5.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–50. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 6.Emlen W, Niebur J, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosis. J Immunol. 1994;152:3685–92. [PubMed] [Google Scholar]
- 7.Rosen A, Casciola Rosen L. Clearing the way to mechanisms of autoimmunity. Nat Med. 2001;7:664–5. doi: 10.1038/89034. [DOI] [PubMed] [Google Scholar]
- 8.Walport MJ. Lupus, DNAse and defective disposal of cellular debris. Nat Genet. 2000;25:135–6. doi: 10.1038/75963. [DOI] [PubMed] [Google Scholar]
- 9.Bruns A, Blass S, Hausdorf G, Burmester GR, Hiepe F. Nucleosomea are major T and B cell autoantigens in systemic lupus erythematosus. Arthritis Rheum. 2000;43:2307–15. doi: 10.1002/1529-0131(200010)43:10<2307::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;183:1367–81. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voll RE, Roth EA, Girkontaite I, Fehr H, Herrmann M, Lorenz HM, Kalden JR. Histone-specific Th0 and Th1 clones derived from systemic lupus erythematosus patients induce double-stranded DNA antibody production. Arthritis Rheum. 1997;40:2162–71. doi: 10.1002/art.1780401210. [DOI] [PubMed] [Google Scholar]
- 12.Kaliyaperumal A, Mohan C, Wu W, Datta SK. Nucleosomal peptide epitopes for nephritis-inducing T helper cells of murine lupus. J Exp Med. 1996;183:2459–69. doi: 10.1084/jem.183.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bach ML, Bach FH, Joo P. Leukemia-associated antigens in the mixed leukocyte culture test. Science. 1969;166:1520–5. doi: 10.1126/science.166.3912.1520. [DOI] [PubMed] [Google Scholar]
- 14.Romain PL, Schlossman SF, Reinherz EL. Surface molecules involved in self-recognition and T cell activation in the autologous mixed lymphocyte reaction. J Immunol. 1984;133:1093–100. [PubMed] [Google Scholar]
- 15.Kagan J, Choi YS. Failure of the human autologous mixed lymphocyte reaction in the absence of foreign antigens. Eur J Immunol. 1983;13:1031–6. doi: 10.1002/eji.1830131215. [DOI] [PubMed] [Google Scholar]
- 16.Damle NK, Gupta S. Autologous mixed lymphocyte reaction in man. V. Functionally and phenotypically distinct human T-cell subpopulations respond to non-T and activated T cells in AMLR. Scand J Immunol. 1982;16:59–68. doi: 10.1111/j.1365-3083.1982.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 17.Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA, and DNA in polyacrylamide gel. Electrophoresis. 1987;8:93–9. [Google Scholar]
- 18.Opelz G, Kiuchi M, Takasugi M, Terasaki PI. Autologous stimulation of human lymphocyte subpopulation. J Exp Med. 1975;142:1327–33. doi: 10.1084/jem.142.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterhouse NJ, Finucane DM, Green DR, et al. Calpain activation is upstream of caspases in radiation-induce apoptosis. Cell Death Differ. 1998;5:1051–61. doi: 10.1038/sj.cdd.4400425. [DOI] [PubMed] [Google Scholar]
- 20.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utz PJ, Anderson P. Posttranslational protein modifications, apoptosis, and the bypass of tolerance to autoantigens. Arthritis Rheum. 1998;33:1152–60. doi: 10.1002/1529-0131(199807)41:7<1152::AID-ART3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med. 1995;182:1625–34. doi: 10.1084/jem.182.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casciola Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implication for initiation of autoimmunity. J Exp Med. 1999;190:815–26. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheinecker C, Machold KP, Majdic O, Hocker P, Knapp W, Smolen JS. Initiation of the autologous mixed lymphocyte reaction requires the expression of costimulatory molecules B7-1 and B7-2 on human peripheral blood dendritic cells. J Immunol. 1998;161:3966–73. [PubMed] [Google Scholar]
- 25.Fernandez LA, Macsween JM. The suppressive effects of monocytes in the autologous mixed lymphocyte reaction. Immunology. 1981;44:653–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Miyashita T, Kralewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–805. [PubMed] [Google Scholar]
- 27.Kastan M. On the TRAIL from p53 to apoptosis? Nat Genet. 1997;17:1317–22. doi: 10.1038/ng1097-130. [DOI] [PubMed] [Google Scholar]
- 28.Hallan E, Blomhoff HK, Smeland EB, Lomo J. Involvement of ICE (caspase) family in γ-radiation-induced apoptosis of normal B lymphocytes. Scand J Immunol. 1997;46:601–8. doi: 10.1046/j.1365-3083.1997.d01-173.x. [DOI] [PubMed] [Google Scholar]
- 29.Bell DA, Morrison B, VandenBygaart B. Immunogenic DNA-related factors: nucleosomes spontaneously released from normal murine lymphoid cells stimulate proliferation and immunoglobulin synthesis of normal mouse lymphocytes. J Clin Invest. 1990;85:1487–96. doi: 10.1172/JCI114595. [DOI] [PMC free article] [PubMed] [Google Scholar]