Abstract
Thrombomodulin is a transmembranous glycoprotein of endothelial cells. In vitro it is a marker of endothelial cell injury. In vivo the levels of serum thrombomodulin are regarded as a parameter of activity in vasculitides. The latter are pathophysiologically determined by neutrophil-derived inflammation and endothelial cell injury caused by secretion of proteases and hydrogen peroxide. It was the objective of this study to determine whether thrombomodulin is only a late marker of advanced endothelial cell injury or whether it indicates also earlier stages of cell alterations. Over 24 hr endothelial cell cultures were incubated with hydrogen peroxide or the neutrophil proteases proteinase-3, elastase and cathepsin G. The time-dependent increase of thrombomodulin in the supernatant was determined by enzyme-linked immunosorbent assay and immunoblot. In addition the viability (eosin, tetrazolium dye assay), detachment (crystal-violet assay), and apoptosis (4′,6-diamine-2′-phenylindole-dihydrochloride assay) of the respective endothelial cells were determined for adherent and non-adherent cells. A rapid thrombomodulin increase was found under all experimental conditions. The additional immunoblotting analysis showed the pattern of proteolytic cleavage caused by the protease reactivity. In case of hydrogen peroxide the thrombomodulin increase was closely correlated with the loss of cell viability and lysis. The incubation of endothelial cells with the different proteases resulted in a time-dependent detachment of primarily viable cells. In addition to cell necrosis apoptotic cell death was found in the subgroup of detached endothelial cells after prolonged incubation over 24 hr with proteinase-3 (23%), elastase (31%), and cathepsin G (19%). In contrast, still adhering cells did not show any signs of necrosis or apoptosis. In summary these studies confirm in vitro that soluble thrombomodulin is not only a parameter of advanced endothelial cell destruction itself but also in addition an early marker of initial endothelial cell membrane changes induced by neutrophil derived proteases and oxygen radicals.
Introduction
Thrombomodulin is an endothelial cell transmembranous glycoprotein acting as a receptor for thrombin.1,2 A soluble form of thrombomodulin (TM) is found after endothelial cell injury as shown by several in vitro studies including 51Cr-release assays. Therefore thrombomodulin is regarded as a reliable marker of endothelial cell injury in vitro.3–5 Furthermore, soluble serum thrombomodulin is a recently established marker of disease activity in patients with multisystemic autoimmune vasculitis including systemic lupus erythematosus (SLE), Wegener's granulomatosis, ulcerative colitis and other systemic vasculitides.6–10 However, it is still uncertain whether this serological thrombomodulin increase is an early sign of an inflammatory endothelial cell alteration or if it is mainly a late marker of an advanced endothelial cell injury with cell destruction.
The vasculitides themselves are autoimmune disorders characterized by intra- as well as perivascular inflammation. This inflammation is mediated by neutrophils and leads to endothelial cell injury. The latter occurs after activation of neutrophils by inflammatory cytokines only.11–16 Several mechanisms are involved in this inflammatory process. After activation neutrophils secrete oxygen radicals as well as proteolytic enzymes. These are stored in the azurophilic granules of the neutrophils. They include the serine proteases proteinase-3 (PR-3), elastase and cathepsin G. After secretion these different agents result in a direct toxic effect on the attacked cells as well as indirect effects leading to the detachment of cells from the surrounding matrix.17–21
In the present in vitro study endothelial cell cultures were incubated with PR-3, elastase and cathepsin G as well as hydrogen peroxide (H2O2) in order to determine the time dependence of the occurrence of thrombomodulin in comparison with the cell viability, adherence and cytolyses over 24 hr. Our intention was to determine whether these different inflammatory mediators would induce a thrombomodulin increase only as a result of an advanced endothelial cell injury or as a result of alteration of the endothelial cell membrane; this was in order to get evidence whether increased serum thrombomodulin could be regarded as an early or only as an advanced parameter of disease activity in patients with vasculitides.
Materials and Methods
Reagent and proteinases of neutrophils
Highly purified human PR-3, prepared and purified according to the methods of Kao et al.18 and Leid et al.22 was kindly provided by Prof. K. Andrassy (University of Heidelberg, Department of Nephrology). In addition, commercially available human leucocyte elastase (Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany) and cathepsin G from human neutrophils (ICN Biochemicals GmbH, Eschwege, Germany) were used. These different reagents were experimentally used in the same range of concentration as previously published by other investigators.3–5,23 However, the respective concentrations used in the present study were slightly modified because of the known variability of the biological, proteolytic activity of distinct proteinase preparations in order to reach a comparable experimental thrombomodulin releasing efficacy for all reagents. The following final concentrations were used according to several dose finding experiments (data not shown): PR-3 10 µg/ml medium (serum-free), elastase 5 µg/ml medium (serum-free), cathepsin-G 5 µg/ml medium (serum-free), and H2O2 0·5% (medium serum-free). In addition, if not otherwise stated reagents were used from Sigma (Sigma-Aldrich Chemie GmbH).
Endothelial cell culture
Human endothelial cells were isolated from umbilical veins according to the methods of Maruyama and Jaffe with minor modifications as described previously.15,24,25 In brief, human umbilical vein endothelial cells (HUVEC) were isolated by collagenase digestion (0·1% collagenase from Clostridium histolyticum; Roche/Boehringer Mannheim, Mannheim, Germany) from human umbilical cord veins, washed and cultured in tissue culture flasks precoated with fibronectin (5 µg/cm2, Roche/Boehringer Mannheim) using medium 199 (Sigma-Aldrich Chemie GmbH) supplemented with 200 mmol l-glutamine, 100 µg/ml endothelial growth factor (Sigma-Aldrich Chemie GmbH), 100 µg/ml heparin (Sigma-Alderich Chemie GmbH), 20% fetal calf serum (FCS, Seromed; Biochrom KG, Berlin, Germany), and 50 U/ml penicillin/50 µg/ml streptomycin (Sigma-Aldrich Chemie GmbH). Medium without FCS and endothelial cell growth factor was used for incubations of cells under experimental conditions if not otherwise stated.
The second or third passage of endothelial cell cultures was used for the respective experiments. The cells were subcultured on 96-well plates or four-chamber slides under optimal culture conditions up to near confluent cell density. Before use, the anticoagulant activity as well as the purity of the endothelial cell culture were tested as described recently.16,26 The latter was performed by indirect immunofluorescence using an anti-thrombomodulin monoclonal antibody (M617; Dako-Diagnostik, Hamburg, Germany).
ELISA (enzyme-linked immunosorbent assay)
A prototype two-site ELISA was used for the determination of TM in the supernatant of the endothelial cell cultures as described elsewhere.10 The test was performed according to the manufacturer's instructions (Thrombomodulin VarElisa, charge no. 17067; ELISA/Pharmacia & Upjohn, Freiburg, Germany). Briefly, the precoated 96-well plates were washed and incubated for one hour at room temperature with the diluted samples in duplicates (50 µl supernatant and 75 µl sample buffer) or the provided standards. After washing the plates were further incubated with the peroxidase-conjugated secondary anti-TM antibody (100 µl/well) for the next hour and finally with the substrate solution (tetramethylbenzidine; TMB) at room temperature in the dark for 10 min. With exception of the last step the plates were washed between each incubation step. Finally, the plates were stopped with 2 n H2SO4 and the optical density measured after 30 min of colour stabilisation by an automated ELISA plate reader at 450 nm (Titertek Multiscan Plus MKII; ICN/Flow, Meckenheim, Germany).
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blotting
SDS–PAGE was carried out on a slab gel according to the method of Laemmli27 with minor modifications. Five per cent acrylamide (containing 0·2% SDS 10%) were used for the staking gel and 10% acrylamide (containing 0·2% SDS 10%) for the separating gel. The respective samples were prepared by boiling (100°) in samples buffer (2% SDS, 100 mmol β-mercaptoethanol, 10% glycerol, 80 mmol Tris/HCl pH 6·8) for 5 min 10 µl of marker (Rainbow coloured protein molecular weight marker 220–14 3000 MW, Amersham Life Science, Braunschweig, Germany) or sample were loaded on the SDS gel and run with constant current intensity of 20 mA (staking gel) or 70 mA (separating gel).
Subsequently the proteins were transferred to the nitrocellulose membrane (Amersham Life Science) using the semidry method with glycine transfer buffer (1000 ml containing 80 ml Tris/glycine buffer (3·03 g Tris and 14·4 g glycine in 1000 ml) and 200 ml methanol) and three different preparation buffers (buffer 1: 300 mmol Tris/HCl; buffer 2: 25 mMol Tris/HCl; buffer 3: 40 mmol 6-amino-n-hexan-acid; Biotec-Fischer GmbH, Reiskirchen, Germany). After transfer the membrane was blocked and incubated with the specific antithrombomodulin antibody (M617, Dako Diagnostik). For detection the Western-light CSPD-chemoluminescence assay was used with an alkaline-phosphatase conjugated goat anti-mouse detection antibody (TROPIX Inc Bedford, MA, distributed by SERVA Feinbiochemica GmbH & CoKG, Heidelberg, Germany) and finally the X-rays were automatically developed.
Proliferation and viability assays
Crystal-violet assay
The cells were incubated with crystal-violet (crystal-violet 7·5 g/l, NaCl 2·5 g/l, formaldehyde 1·57%, methanol 50%) for 20 min at the end of the respective experiments in order to measure the relative number of endothelial cells in the 96-well plates. After removing the crystal-violet solution the wells were washed, the dye eluted from the cells by methanol and the extinction measured in an automated ELISA plate reader by 593 nm (Titertek Multiscan Plus MKII; ICN/Flow, Meckenheim, Germany).28–30
Tetrazolium dye assay
The endothelial cells which had been cultured in 96-well plates under optimal conditions to near confluence were coincubated according to the experimental requirements in FCS-free medium without endothelial cell growth factor. At the fixed time intervals the medium was replaced by fully supplemented medium and the adherent cells incubated for further 12 hr. Subsequently the capacity of the cells to produce formazan was measured in the modification according to Mossman using the CellTiter 96-kit (non-radioactive cell proliferation assay Promega, distributed by SERVA Feinbiochemical GmbH & CoKG).28–30 In brief, the cells were incubated with MTT-[3(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide] dye solution for 3 hr. The medium was removed and the amount of reduced MTT dye measured after lyses of cells in an automatic ELISA reader at 570 nm (Titertek Multiscan Plus MKII; ICN/Flow).
Eosin exclusion assay
The endothelial cells were grown on 4-chamber slides and incubated according to the required experimental conditions. At the fixed time intervals the number of viable endothelial cells were investigated by removing the supernatant and adding 2% eosin solution to the culture. Ten minutes later the percentage of dead cells (coloured) were determined microscopically by counting 100 cells. The number of viable cells was investigated in the supernatant in the same way after gently centrifugation and addition of the 2% eosin solution.30
Investigation of apoptosis
DNA fragmentation analysis
The DNA fragmentation was analysed according to the method of Maniatis.31 Briefly, the cells were cultured in 6-well plates and incubated as requested. At the fixed time intervals the supernatant was removed and gently centrifugated in 1·5 ml tubes. Meanwhile the remaining cells were removed from the plates by adding trypsin/ethylenediaminetetraacetic acid (EDTA) solution (Roche/Boehringer Mannheim) and separately centrifuged in 1·5 ml tubes as well. 100 µl DNA-lysis buffer (200 mmol Tris, 100 mmol EDTA, 1% SDS) supplemented with proteinase K (10 µg/106 cells; Roche/Boehringer Mannheim) was added to all cell pellets and incubated at 50°. After one hour RNase (25 µg/106 cells; Roche/Boehringer Mannheim) was added and incubated for an additional hour at 50°. Finally, the DNA samples were electrophoretically separated on an 1% agarose gel (80 V, maximum current intensity), these gels were stained with 1% ethidiumbromide solution (Sigma-Aldrich Chemie GmbH), the DNA analysed under UV-light and documented by Polaroid camera.
4′,6-diamine-2′-phenylindole-dihydrochloride (DAPI) assay
The endothelial cells were grown on four-chamber slides as for the eosin exclusion assay. At the end of the respective culture and/or coincubation period the supernatant was removed, the cells washed and finally fixed with acetone and methanol. The cells in the supernatant were prepared in the same way after centrifugation on a slide using the cytospin technique. Next the slides were incubated with the DAPI solution (storing solution: 0·1 mg/ml DAPI in 180 mmol Tris/HCl, pH 7·5; solution ready for use: 0·5 µl/ml DAPI storing solution in 180 mmol Tris/HCl). Finally, the slides were washed, mounted and the percentage of apoptotic cells determined by fluorescence microscopy counting 100 cells.32
Statistical analysis
If not otherwise stated, the mean and standard deviation are given. For the assessment of significance between test and control values the Wilcoxon paired difference test was used considering all single values which were measured (P<0·05).
Results
Release of endothelial thrombomodulin
Thrombomodulin is very rapidly found in the supernatant of endothelial cell cultures after incubation with PR-3, elastase, cathepsin-G, or H2O2 under serum-free culture conditions. A very marked release of endothelial thrombomodulin is already detected by the TM-specific ELISA as soon as 30 min after start of incubation (Fig. 1). The majority of the thrombomodulin is released in the first four hours of incubation (Fig. 1; Table 1). The culture medium itself contains no detectable amounts of thrombomodulin. The control culture shows only minor amounts of soluble thrombomodulin because of physiological cell turnover.
Figure 1.
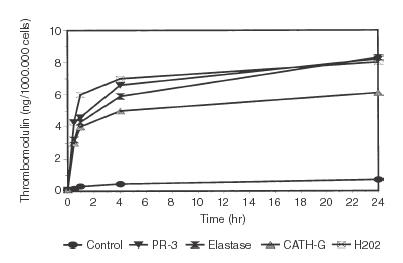
The increase of thrombomodulin in the supernatant of endothelial cell cultures is shown after 0·5, 1, 4, and 24 hr of incubation. 10 µg/ml proteinase-3, 5 µg/ml elastase, and 5 µg cathepsin G as well as 0·5% hydrogen peroxide in serum free medium was used. The thrombomodulin values were determined by ELISA. Standard cultures were used as controls. The mean of three experiments is shown. For more details see Table 1.
Table 1. Time-dependent endothelial cell viability, detachment and thrombomodulin release after incubation with neutrophil derived proteases and hydrogen peroxide.
| Adherent cells | ||||
|---|---|---|---|---|
| Assay | Time | Crystal-violet (optical density) | Tetrazolium dye (optical density) | Supernatant thrombomodulin (ng/106 cells) |
| Starting conditions | 0 hr | 100±23 | 134±19 | 0·1±0·2 |
| Proteinase 3 | 0·5 hr | 80±17 | 108±27* | 4·3±0·5* |
| 1 hr | 68±27* | 100±25* | 4·6±0·4* | |
| 4 hr | 51±14* | 94±38* | 6·6±0·8* | |
| 6 hr | 45±16* | 81±35* | 6·9±0·7* | |
| 24 hr | 15±9* | 54±37* | 8·2±0·9* | |
| Elastase | 0·5 hr | 69±19* | 93±41* | 3·2±0·5* |
| 1 hr | 52±28* | 88±31* | 4·3±0·7* | |
| 4 hr | 26±13* | 37±19* | 5·9±0·7* | |
| 6 hr | 21±11* | 47±25* | 6·3±0·9* | |
| 24 hr | 14±8* | 34±29* | 8·3±1·0* | |
| Cathepsin G | 0·5 hr | 56±19* | 93±55* | 3·0±0·5* |
| 1 hr | 51±20* | 83±37* | 4·0±0·7* | |
| 4 hr | 36±15* | 41±23* | 5·1±0·7* | |
| 6 hr | 31±14* | 55±31* | 5·3±0·8* | |
| 24 hr | 13±7* | 35±22* | 6·1±0·8* | |
| H2O2 | 0·5 hr | 22±11* | 17±14* | 4·3±0·6* |
| 1 hr | 12±9* | 10±6* | 6·1±0·8* | |
| 4 hr | 6±6* | 6±6* | 7·2±0·9* | |
| 6 hr | 5±7* | 9±5* | 7·3±0·8* | |
| 24 hr | 2±3* | 9±5* | 8·0±0·9* | |
| Control | 0·5 hr | 100±9 | 135±25 | 0·3±0·1 |
| 1 hr | 100±23 | 133±29 | 0·3±0·1 | |
| 4 hr | 97±22 | 131±23 | 0·4±0·2* | |
| 6 hr | 98±25 | 131±37 | 0·5±0·1* | |
| 24 hr | 101±27 | 159±51 | 0·7±0·2* | |
Mean and standard deviation of three experiments (*P<0·05.).
The additional Western-blot analysis confirmed the rapid release of TM-degradation products into the supernatant of the endothelial cell cultures under the different experimental conditions tested. The relative amount of TM indicated by the staining intensity only slightly increased during later incubation periods comparing the four different time points of each experimental setting. The control cultures showed background amounts of TM only. Nearly the same TM degradation products occurred in all three cultures treated with the different proteases. The major molecular weight was 56 000 using the antithrombomodulin antibody DAKO M617 for detection (Fig. 2). Undegraded thrombomodulin (105 000 MW) was not found under any of the experimental conditions.
Figure 2.
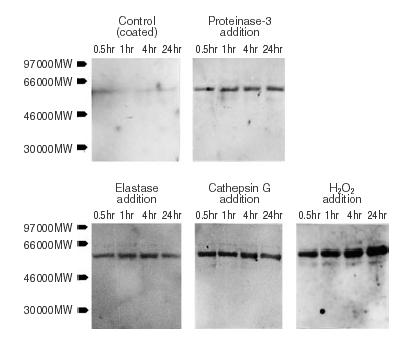
The immunoblot analysis of the respective culture supernatants confirms the rapid loss of thrombomodulin from the endothelial cells. The different blots show the typical pattern of proteolytic thrombomodulin cleavage for the experiments with the three neutrophil proteases proteinase-3, elastase, and cathepsin G. Background staining is found in the standard control culture only. A representative blot is shown.
Viability of endothelial cells
As expected, the control cultures showed a slightly increasing number of adherent cells per well because of cell growth over 24 hr of incubation using the crystal-violet assay for detection. In contrast, nearly all cells rapidly detached after incubation with hydrogen radicals. In addition, a rapidly decreasing number of adherent cells was found after incubation with the proteases. The latter was particularly marked for PR-3 (Table 1).
So far, these results together with the thrombomodulin kinetics presented first could be explained as an increasing endothelial cell damage due to a direct toxic effect of the incubation with proteases or hydrogen radicals. However, the additionally performed eosin exclusion assay documents a preserved viability of adherent endothelial cells as well as cells in the supernatant over the first hours of incubation for all experimental settings with one exception. Only the incubation with hydrogen radicals led to a rapid cell damage with loss of adherence and release of thrombomodulin (Table 2).
Table 2. Time-dependent endothelial cell viablility and apoptotic cell death of adherent and non-adherent cells.
| Adherent cells | Non-adherent cells | ||||
|---|---|---|---|---|---|
| Assay | Time | Eosin exclusion (% viability) | DAPI (apoptosis) | Eosin exclusion (% viability) | DAPI (apoptosis) |
| Starting conditions | 0 hr | 99±2% | <1% | 1±2% | <1% |
| Proteinase 3 | 0·5 hr | 99±1% | <1% | 98±1%* | <1% |
| 1 hr | 98±1% | <1% | 97±1%* | <1% | |
| 4 hr | 89±7%* | <1% | 89±5%* | 1±1% | |
| 24 hr | 68±8%* | <1% | 21±5%* | 23±5%* | |
| Elastase | 0·5 hr | 99±1% | <1% | 97±2%* | <1% |
| 1 hr | 97±2% | <1% | 97±2%* | 2±1% | |
| 4 hr | 89±4%* | <1% | 39±6%* | 5±3%* | |
| 24 hr | 54±8%* | <1% | 6±3%* | 31±6%* | |
| Cathepsin G | 0·5 hr | 98±1% | <1% | 97±2%* | <1% |
| 1 hr | 69±9%* | <1% | 86±4%* | 1±1% | |
| 4 hr | 30±7%* | <1% | 66±5%* | 3±2%* | |
| 24 hr | 21±8%* | <1% | 6±4%* | 19±5%* | |
| H2O2 | 0·5 hr | 4±3%* | <1% | 4±3% | <1% |
| 1 hr | 1±2%* | <1% | 2±1% | <1% | |
| 4 hr | <1%* | <1% | 1±2% | <1% | |
| 24 hr | <1%* | <1% | 1±1% | <1% | |
| Control | 0·5 hr | 99±1% | <1% | 1±1% | <1% |
| 1 hr | 98±2% | <1% | 1±1% | <1% | |
| 4 hr | 96±3% | <1% | 3±2% | <1% | |
| 24 hr | 96±4% | <1% | 3±1% | 4±3%* | |
Mean and standard deviation of three experiments (*P<0·05.).
These results were confirmed by the tetrazolium dye assay. Hereby, the cells were cultured and incubated with proteases or hydrogen radicals as for the other experiments. After the respective time intervals of incubation the supernatant was removed, the cells washed with medium and the remaining cells cultured under optimal conditions using serum supplemented medium (for details please see the method section) for a further 12 hr in order to evaluate endothelial cells without progressive injury of cells. Thereafter the tetrazolium dye assay was performed. This test showed an enzymatic activity of the remaining adherent cells as expected because of the estimated number of cells by the crystal-violet assay. In contrast, the incubation of endothelial cells with hydrogen radicals lead to a very quick cell damage without enzymatic activity in all cultures (Table 1).
Apoptosis of endothelial cells
Finally we investigated the occurrence of apoptosis as one potential pathophysiological mechanism for the sustained endothelial cell death after incubation of endothelial cells with proteases. First an agarose gel electrophoresis of the DNA was performed investigating the different subgroups of adherent endothelial cells as well as the cells in the supernatant. In summary, DNA degradation was only detectable in the group of non-adherent cells after incubation for 24 hr with all three different proteases (Fig. 3). Moreover, indications of a typical DNA ladder pattern were additionally found in these protease related DNA degradation smears when these were directly viewed under the ultraviolet lamp. Therefore, the question of the occurrence of apoptosis was further investigated with the DAPI assay using short times for fixation and washing in order to prevent diffusion of DNA.33 Likewise this DAPI assay revealed no detectable apoptosis of adherent cells under all of the distinct culture conditions tested. However, after 24 hr of incubation a significant amount of apoptosis occurred among the detached endothelial cells of the supernatant in the proteases treated groups showing the typical pattern of dense chromatin granules because of DNA condensation23,33,34 (Fig. 4). The incubation with H2O2 did not result in a detectable apoptosis under any culture conditions (Fig. 3, Table 2).
Figure 3.
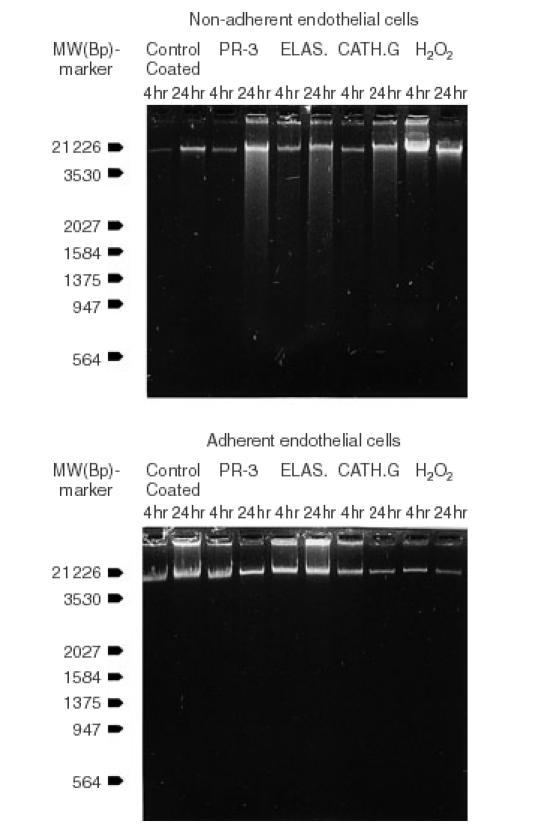
The DNA agarose gel electrophoresis shows no signs of DNA degradation for the subsets of adherent endothelial cells under all experimental condition. However, DNA degradation occurs time-dependent in the subgroups of these nonadherent endothelial cells incubated with one the three proteases which were tested; proteinase-3, elastase, and cathepsin G. In addition, indications of a typical DNA ladder pattern were additionally found in these three protease related DNA degradation smears when these were directly viewed under the ultra violet lamp.
Figure 4.
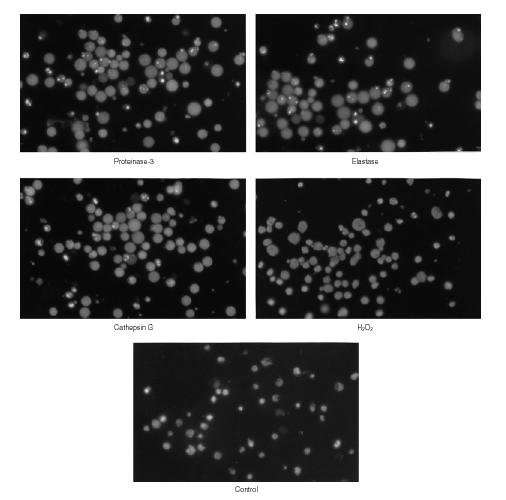
After 24 hr of incubation the fluorescence microscopy of detached endothelial cells in the supernatant shows the typical apoptotic pattern of dense chromatin granules after staining with DAPI (preparation on slides using cytospin technique; for more details see the method section). This pattern is due to DNA condensation. The apoptotic pattern is found in the subgroups of endothelial cells treated with proteases (proteinase-3, elastase, cathepsin G) but not with hydrogen radicals. No detectable apoptotic pattern occurred under any culture conditions among the endothelial cell, which still remained adherent (not shown; more results in Table 2) (magnification ×40).
Discussion
Vasculitides are an inhomogeneous group of autoimmune disorders involving a variety of organ systems. Up to today reliable serological markers of disease activity are still missing. Pathophysiologically vasculitides are characterized by inflammatory vascular damage whereby the endothelial cells are affected, during the first phase in particular. The vascular inflammatory process includes local secretion of proinflammatory cytokines like tumour necrosis factor-α or interleukin-1, cytokine activation of endothelial cells as well as leucocytes, and increasing expression of adhesion molecules. This inflammatory process leads to augmented cell adhesion with consecutive cellular cytotoxicity of activated neutrophils. Finally the vascular inflammatory cascade results in a local endothelial cell injury.11–16,35 In vitro thrombomodulin – a transmembranous glycoprotein of endothelial cells – is regarded as reliable marker of endothelial cell injury.3–5 Soluble thrombomodulin is not released in vitro into the supernatant but internalized and degraded after activation under normal culture conditions.36 In addition, serum thrombomodulin concentrations show in vivo a promising correlation to the disease activity in a variety of autoimmune vasculitides and diseases with substantial vascular injury. These include SLE, Wegener's granulomatosis, ulcerative colitis, Takayasu's arteritis, Behçet's disease, giant cell arteritis, sepsis, disseminated intravascular coagulation and malaria.6–10,37–39 However, it is still unknown whether or not the respective serological thrombomodulin increase is only a late sign of advanced endothelial cell destruction.
The cellular toxicity of activated neutrophils is mainly due to the secretion of oxygen radicals and proteolytic enzymes.11,12,17 Oxygen radicals are known to rapidly induce direct toxic effects on endothelial cells (cytolysis) in vitro in high concentrations (in µmolar to mmolar range) using the Cr51-release assay. This endothelial cell cytotoxicity is closely correlated with a thrombomodulin increase in the culture supernatant.3–5,19 In contrast the incubation with very low concentrations of oxygen radicals (in µmolar to nmolar range) does not result in direct endothelial cell toxicity or thrombomodulin release. However, these low concentrations of oxygen radicals lead to endothelial cell lysis by inducing apoptosis.5,40
High concentrations of oxygen radicals pathophysiologically occur in the intracellular microenvironment leading to a direct cytotoxicity, e.g. after cell to cell contact. In contrast low concentrations of oxygen radicals are found in the extracellular fluid of the inflammatory environment and are involved in the neutralization of a protective antiproteinase shield at the inflammation site.12 Furthermore, recently published results point to an additional possibility, that low concentrations of oxygen radicals can increase the activity of neutrophil derived proteases.5,19 Nevertheless, the present study was not especially designed to further investigate the ability of low oxygen radical concentrations to induce apoptosis or to determine additional synergistic effects of oxygen radicals on the activity of proteases. In contrast, high concentrations of oxygen radicals were used in this present study because of their known, constantly occurring, effects of cytotoxicity, with the intention to compare these results with the alterations induced on endothelial cells by the proteinases. Therefore, the present experiments confirmed the direct endothelial cell toxicity of high concentrations of hydrogen peroxide as well as their capacity to induce a rapid and marked increase of thrombomodulin in the supernatant. Likewise, no signs were detected of the induction of an indirect cell toxicity like apoptotic cell death.
Proteolytic enzymes are the second source of activated neutrophils to cause cell injury. They include the serin proteases PR-3, elastase and cathepsin G, which are stored in the azurophilic granules.17–21 The concentrations (per ml) of all proteases that were used experimentally were in the pathophysiological relevant range, as 1×106 neutrophils contain in general a few micrograms of these proteinases, with minor variability concerning the distinct proteases.12,18
In vitro these proteases are able to degrade proteins of the endothelial matrix including fibronectin, laminin, collagen and proteoglycans. Therefore, they can induce detachment of endothelial cells from the surrounding matrix.18,41–44 The induction of emphysematous lesions as well as the detachment of glomerular endothelial cells has been described.18–21In vitro these proteases lead to a rapid loss of the thrombomodulin activity of the endothelial cells themselves as judged by the protein C activation capacity.5 In addition a rapid increase of thrombomodulin occurs in the supernatant of the protease treated endothelial cell cultures.4,5 This was confirmed in the present experiments. These effects of the proteases on the endothelial cells could be fully inhibited by the addition of α-1-proteinase inhibitor (data not shown) as already known.5,21
The immunoblotting analysis of the released thrombomodulin showed the pattern of proteolytic cleavage, as recently described for purified protein45 and not the pattern of thrombomodulin degradation as found in vivo which is characterized by the occurrence of multiple fragments.46,47 The latter was only found in cell cultures incubated under non-adhering unphysiological conditions (data not shown). These results confirm, that thrombomodulin is rapidly released from endothelial cells by proteases as well as oxygen radicals. However, the difference in the pattern of the degradation fragments under in vitro in contrast to in vivo conditions might be a result of the different influences and multiplicity of various simultaneously occurring pathophysiological interactions during the inflammatory process. In contrast, the similarity of the detected degradation fragments in vitro under non-adherent, unphysiological culture conditions and in vivo indicate the occurrence of similar regulatory mechanism of cell death after detachment as under pathophysiological conditions in vivo.
In addition, the results of the endothelial cell viability assay documented that this increase of thrombomodulin in the supernatant because of protease treatment was not directly correlated with an immediate cell destruction and lysis as for hydrogen peroxide. Furthermore, the tetrazolium dye assay confirmed the remaining viability of adherent endothelial cells after a short incubation time with the proteases used in the experiments. However the protease-treated endothelial cell cultures showed a marked and time-dependent detachment of the endothelial cells in connection with the release of thrombomodulin in the supernatant. This is most likely caused by the ability of neutrophil-derived proteases to degrade matrix proteins leading to endothelial cell detachment, as discussed above. Recently a dose-dependent cytolysis and detachment of endothelial cells has been shown for PR-3 as well as elastase after incubation of 3 hr. However in this study this is not distinguished between adherent and non-adherent cells.21 In addition, it is known that the cultivation of endothelial cells under adhesion preventing conditions results in rapid loss of cell viability and triggering of apoptosis.48 Furthermore, in vitro apoptosis of endothelial cells can be induced by prolonged incubation of endothelial cells with proteinase-3 and elastase. However, a mixed culture of adherent and detached cells is measured in this investigation.23 Corresponding to these results we found a significant percentage of endothelial cell apoptosis beside necrotic cell death occurring after prolonged incubation with all three proteases used. However, both kinds of cell death occurred only in the subgroups of detached cells. The occurrence of necrosis as well as apoptosis in the subgroups of proteinase-treated cells in contrast to oxygen-radical injured cells indicate the existence of various mechanisms responsible for endothelial cell destruction. These include toxic as well as environmental or regulatory factors. In vivo, apoptotic endothelial cell death might be of pathophysiological relevance for the deletion of detached or injured endothelial cells, e.g. in the circulation or locally, respectively. In contrast, direct, toxic necrotic tissue damage mediated by invading neutrophils is the primary leading, pathophysiological feature of inflammation12 whereby apoptosis of endothelial cells is an additionally occurring event under these conditions only.
Taking these presented and recently published results into consideration the neutrophil-derived proteases induce rapid loss of thrombomodulin from the endothelial cell membrane caused by proteolytic cleavage without immediate cell lysis. However, the proteolytic activity of these proteases additionally induce the detachment of the endothelial cells from the underlying matrix, subsequently resulting in sustained cell injury and cell death including necrosis as well as apoptosis. In contrast, oxygen radicals in higher concentrations lead to rapid thrombomodulin release connected with necrotic endothelial cell lysis. In this respect all three neutrophil-derived proteases tested are equally effective. In addition, we showed that the increase of soluble thrombomodulin is not only an early event but is also independent of the finally occurring kind of endothelial cell death with regard to necrosis or apoptosis.
In summary the presented study confirms that soluble thrombomodulin is not only a parameter of endothelial cell destruction itself but also in particular an early marker of initial endothelial cell membrane changes induced by neutrophil-derived proteases and oxygen radicals. Therefore, these in vitro data provide evidence for soluble thrombomodulin as an early indirect parameter of disease activity in vasculitides and vascular diseases with endothelial cell injury.
Acknowledgments
The authors would like to thank Professor Dr K. Andrassy (University of Heidelberg, Department of Nephrology) for kindly providing the highly purified human proteinase-3 as well as for several helpful comments.
References
- 1.Esmon CT, Owen WG. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci USA. 1981;78:2249–52. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dittman WA, Majerus PW. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990;75:329–36. [PubMed] [Google Scholar]
- 3.Ishii H, Yama H, Kazama M. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. Thromb Haemost. 1991;65:618–23. [PubMed] [Google Scholar]
- 4.Sawada K, Yamamoto H, Yago H, Suehiro S. A simply assay to detect endothelial cell injury: measurement of released thrombomodulin from cells. Exp Mol Pathol. 1992;57:116–23. doi: 10.1016/0014-4800(92)90003-t. [DOI] [PubMed] [Google Scholar]
- 5.Abe H, Okajima K, Okabe H, Takatsuki K, Binder BR. Granulocyte proteases and hydrogen peroxide synergistically inactivate thrombomodulin of endothelial cells in vitro. J Lab Clin Med. 1994;123:874–81. [PubMed] [Google Scholar]
- 6.Takaya M, Ichikawa Y, Kobayashi N, et al. Serum thrombomodulin and anticardiolipin antibodies in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 1991;9:495–9. [PubMed] [Google Scholar]
- 7.Boehme MWJ, Nawroth PP, Kling E, Amiral J, Riedesel J, Raeth U, Scherbaum WA. Serum thrombomodulin – a novel marker of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1994;37:572–7. doi: 10.1002/art.1780370419. [DOI] [PubMed] [Google Scholar]
- 8.Boehme MWJ, Autschbach F, Zuna I, Scherbaum WA, Stange E, Raeth U, Sieg A, Stremmel W. Elevated serum levels and reduced immunohistochemical expression of thrombomodulin in active ulcerative colitis. Gastroenterology. 1997;113:107–17. doi: 10.1016/s0016-5085(97)70086-6. [DOI] [PubMed] [Google Scholar]
- 9.Boehme MWJ, Schmitt WH, Youinou P, Stremmel WR, Gross WL. Clinical relevance of elevated serum thrombomodulin and soluble E-selectin in patients with Wegener's granulomatosis and other systemic vasculitides. Am J Med. 1996;101:387–94. doi: 10.1016/S0002-9343(96)00230-6. [DOI] [PubMed] [Google Scholar]
- 10.Boehme MWJ, Raeth U, Galle PR, Stremmel W, Scherbaum WA. Serum thrombomodulin – a reliable marker of disease activity in systemic lupus erythematosus (SLE): advantage over established serological parameters to indicate disease activity. Clin Exp Immunol. 2000;119:189–95. doi: 10.1046/j.1365-2249.2000.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pall AA, Savage COS. Mechanisms of endothelial cell injury in vasculitis. Springer Semin Immunopathol. 1994;16:23–37. doi: 10.1007/BF00196711. [DOI] [PubMed] [Google Scholar]
- 12.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 13.Yamada O, Moldow CF, Sacks T, Craddock PR, Boogaerts MA, Jacob HS. Deleterious effects of endotoxin on cultured endothelial cells: an in vitro model of vascular injury. Inflammation. 1981;5:115–26. doi: 10.1007/BF00914201. [DOI] [PubMed] [Google Scholar]
- 14.Zeck-Kapp G, Kapp A, Busse P, Riede UN. Interaction of granulocytes and endothelial cells upon stimulation with tumor necrosis factor-α: an ultrastructural study. Immunbiology. 1990;181:267–75. doi: 10.1016/s0171-2985(11)80518-8. [DOI] [PubMed] [Google Scholar]
- 15.Boehme MWJ, Deng Y, Raeth U, Bierhaus A, Ziegler R, Stremmel W, Nawroth PP. Release of thrombomodulin from endothelial cells by concerted action of TNF-α and neutrophils: in vivo and in vitro studies. Immunology. 1996;87:134–40. [PMC free article] [PubMed] [Google Scholar]
- 16.Boehme MWJ, Raeth U, Scherbaum WA, Galle PR, Stremmel W. Interaction of endothelial cells and neutrophils in vitro. Kinetics of thrombomodulin, intercellular adhesion molecule-1 (ICAM-1), E-selectin, and vascular cell adhesion molecule-1 (VCAM-1): implications for the relevance as serological disease activity markers in vasculitides. Clin Exp Immunol. 2000;119:250–4. doi: 10.1046/j.1365-2249.2000.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan CF. Neutrophil activation on biological surfaces. J Clin Invest. 1987;80:1550–60. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao RC, Wehner NG, Skubitz KM, Gray BH, Hoidal JR. Proteinase 3: a distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest. 1988;82:1963–73. doi: 10.1172/JCI113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varani J, Ginsburg I, Schuger L, Gibbs DF, Bromberg J, Johnson KJ, Ryan US, Ward PA. Endothelial cell killing by neutrophils. Am J Pathol. 1989;135:435–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Westlin WF, Gimbrone MA. Neutrophil-mediated damage to human vascular endothelium: role of cytokine activation. Am J Pathol. 1993;142:117–28. [PMC free article] [PubMed] [Google Scholar]
- 21.Ballieux BEPB, Hiemstra PS, Klar-Mohamad N, Hagen EC, van Es LA, van der Woude FJ, Daha MR. Detachment and cytolysis of human endothelial cells by proteinase 3. Eur J Immunol. 1994;24:3211–5. doi: 10.1002/eji.1830241245. [DOI] [PubMed] [Google Scholar]
- 22.Leid RW, Ballieux PEPB, van der Heijden I, et al. Cleavage and inactivation of human C1 inhibitor by the human leukocyte proteinase, proteinase 3. Eur J Immunol. 1993;23:2939–44. doi: 10.1002/eji.1830231132. [DOI] [PubMed] [Google Scholar]
- 23.Yang JJ, Kettritz R, Falk RJ, Jennette JCh, Gaido ML. Apoptosis of endothelial cells induced by the neutrophil serin proteases proteinase 3 and elastase. Am J Pathol. 1996;149:1617–26. [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama Y. The human endothelial cell tissue culture. Z Zellforsch Mikrosk Anat. 1963;69:69–79. doi: 10.1007/BF00329383. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163:740–5. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Flick DA, Gifford GE. Comparision of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984;68:167–75. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- 29.Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 30.Martin A, Clynes M. Comparision of 5 microplate colorimetric assays for in vitro cytotoxicity testing and cell proliferation assays. Cytotechnology. 1993;11:49–58. doi: 10.1007/BF00749057. [DOI] [PubMed] [Google Scholar]
- 31.Maniatis T, Jeffrey A, van de Sande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoreses. Biochemistry. 1975;14:3787–94. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- 32.Rotello RJ, Lieberman RC, Purchio AF, Gerschenson LE. Co-ordinated regulation of apoptosis and cell proliferation by transforming growth factor beta 1 in cultured uterine epithelial cells. Proc Natl Acad Sci USA. 1991;88:3412–5. doi: 10.1073/pnas.88.8.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczynca W, Hotz MA, Lassota P, Traganos F. Feature of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 34.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 35.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 36.Hirokawa K, Aoki N. Regulatory mechanisms for thrombomodulin expression in human umbilical vein endothelial cells in vitro. J Cell Physiol. 1991;147:157–65. doi: 10.1002/jcp.1041470120. [DOI] [PubMed] [Google Scholar]
- 37.Takakuwa T, Endo S, Nakae H, Kikichi M, Suzuki T, Inada K, Yoshida M. Plasma levels of TNF-α, endothelin-1 and thrombomodulin in patients with disseminated intravascular coagulation and sepsis. Pharmacology. 1994;84:261–9. [PubMed] [Google Scholar]
- 38.Boehme MWJ, Werle E, Kommerell B, Raeth U. Serum levels of adhesion molecules and thrombomodulin as indicator of vascular injury in severe Plasmodium falciparum malaria. Clin Invest. 1994;72:598–603. doi: 10.1007/BF00227452. [DOI] [PubMed] [Google Scholar]
- 39.Hemmer CJ, Bierhaus A, Riedesel J, et al. Elevated thrombomodulin plasma levels as a result of endothelial involvement in Plasmodium falciparum malaria. Thromb Haemost. 1994;72:457–64. [PubMed] [Google Scholar]
- 40.De-Bono DP, Yang WD. Exposure to low concentrations of hydrogen peroxide causes delayed endothelial cell death and inhibits proliferation of surviving cells. Atherosclerosis. 1995;114:235–45. doi: 10.1016/0021-9150(94)05488-5. [DOI] [PubMed] [Google Scholar]
- 41.Rao NV, Wehner NG, Marshall BC, Gray WR, Gray BH, Hoidal JR. Characterisation of proteinase-3 (PR-3), a neutrophil serin proteinase. Structural and functional properties. J Biol Chem. 1991;266:9540–8. [PubMed] [Google Scholar]
- 42.Abrahamson DR, Irwin MH, Blackburn WD, Heck LW. Degradation of basement membrane laminin by human neutrophil elastase and cathepsin G. Am J Pathol. 1990;136:1267–74. [PMC free article] [PubMed] [Google Scholar]
- 43.Wight TN, Kinsella MG, Klebanoff SJ. Degradation of endothelial cell matrix heparan sulfate proteoglycan by elastase and the myeloperoxidase–H2O2-chloride system. Am J Pathol. 1993;143:907–17. [PMC free article] [PubMed] [Google Scholar]
- 44.Vercellotti GM, Platt JL, Key NS. Vascular endothelial cell proteoglycans are susceptible to cleavage by neutrophils. Arterioscler Thromb. 1992;12:836–42. doi: 10.1161/01.atv.12.7.836. [DOI] [PubMed] [Google Scholar]
- 45.Kurosawa S, Galvin JB, Esmon NL, Esmon CT. Proteolytic formation and properties of functional domains of thrombomodulin. J Biol Chem. 1987;262:2206–12. [PubMed] [Google Scholar]
- 46.Takano S, Kimura S, Ohdama S, Aoki N. Plasma thrombomodulin in health and diseases. Blood. 1990;76:2024–9. [PubMed] [Google Scholar]
- 47.Ishii H, Nakano M, Tsubouchi J, et al. Establishment of enzyme immunoassay of human thrombomodulin in plasma and urine using monoclonal antibodies. Thromb Haemost. 1990;63:157–62. [PubMed] [Google Scholar]
- 48.Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol. 1994;127:537–46. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]


