Abstract
Exposure to Mycobacterium tuberculosis results in clinical tuberculosis only in a small percentage of healthy individuals. In most instances the bacilli are controlled by the immune system and survive in a latent state within granuloma. Immunosuppression, however, may result in reactivation of infection, resulting in clinical disease. Using a low-dose aerosol infection (30 colony-forming units) in mice, we describe a short-duration model for studying spontaneous and drug-induced reactivation of anti-tuberculous drug-treated, latent tuberculosis infection. Although a 4-week treatment with rifampicin and isoniazid reduced the number of bacilli to undetectable levels, the infection spontaneously reactivated following therapy. By contrast, an 8-week treatment period induced a state of latent infection, requiring immunosuppression to reactivate infection. Finally, a 12-week treatment period eliminated the bacilli completely and aminoguanidine did not induce reactivation of infection. In view of the fact that therapy in the selected protocol reduces the mycobacterial load to undetectable levels, the data suggest that an 8-week treatment period is necessary and sufficient to mount protective immunity in mice.
Introduction
In the majority of cases, exposure to Mycobacterium tuberculosis (M. tuberculosis) leads to a clinically asymptomatic controlled infection in a latent state, and it is estimated that one-third of the world population is latently infected with M. tuberculosis.1,2 Reactivation of latent tuberculosis accounts for a substantial proportion of active tuberculosis cases.3 In recent years, co-infection with human immunodeficiency virus (HIV) has probably been the most important cause and greatest risk factor for reactivation of tuberculosis.4–6 As a result of the high incidence of HIV infection in southern Africa, tuberculosis in this area has reached epidemic proportions.
Little is understood of the underlying immune interaction between the host and the bacillus in its evasion of the host immune system, and the mechanism of reactivation of tuberculosis is difficult to investigate in humans. Animal models present an opportunity for elucidating factors of the immune system involved in latent and reactivated tuberculosis. A more detailed understanding of these mechanisms will facilitate improved treatment and better prevention of tuberculosis.
Several models have been developed to study the latency and reactivation of tuberculosis, of which the Cornell model was the first reported. In the Cornell model, mice were intravenously infected with M. tuberculosis H37Rv and treated with pyrazinamide and isoniazid (INH) in their diet for 12 weeks. No viable bacilli were detected in organs from mice during the subsequent period, a so-called ‘sterile state’. However, after several months, a substantial proportion of mice spontaneously reactivated with active tuberculosis.7,8
Most of the latency and reactivation animal models described since have defined latency as stable, but low, bacterial numbers in organs and use chronic infection to attain this state.9–12 Reactivation of latent tuberculosis in the Cornell model was induced with cortisone, a broad-spectrum immunosuppressant.7,8 Several more methods of reactivation have since been employed, including restraining of mice resulting in activation of the hypothalamic–pituitary–adrenal axis,9 administration of corticosterone,13 aminoguanidine,11 dexamethasone and glucocorticoid hydrocortisone acetate (HCA), and neutralization of interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α).14
An aerosol-infection model described by Orme involves the delivery of a relatively low dose of M. tuberculosis Erdman strain [5–10 colony-forming units (CFU)] to C57BL/6 mice. The infection is controlled solely by the host's immune response and remained stable, although with a relatively high bacterial burden in the lungs (103−104 CFU). Mice spontaneously reactivated ≈ 20 months later, presumably owing to natural immunosuppression associated with ageing.10 Most of these animal models have both benefits and shortfalls relating to the parallels in human tuberculosis.
In this study we aimed to provide a short-duration murine model of latency and reactivation of tuberculosis. We present an aerosol model using M. tuberculosis H37Rv as infectious agent and an anti-tuberculous drug regimen consisting of rifampicin (RMP) and INH to attain dormancy of bacilli. In addition, we showed that reactivation of this latent state can be achieved by administration of aminoguanidine, an inhibitor of inducible nitric oxide synthase (iNOS), as first demonstrated by Flynn et al.11
Materials and methods
Animals
C57BL/6 male mice (University of Cape Town breeding stock), 8–12 weeks of age, as well as homozygous TNF-deficient mice,15 were used in this study. The genotypes of gene-deficient mice were confirmed by polymerase chain reaction (PCR) analysis of DNA from tail biopsies. Mice were housed under specific pathogen-free conditions at the University of Cape Town biosafety level 3 animal unit. The University of Cape Town Animal Ethics Committee approved all protocols employed in this study.
Mycobacteria
M. tuberculosis H37Rv (obtained from G. Kaplan, Rockefeller University, New York, NY) was grown in Middlebrook 7H9 medium (Difco Laboratories, Detroit, MI), supplemented with 10% oleic acid albumin dextrose catalase (OADC) (State Vaccine Institute, Pinelands, South Africa) and 1% glycerol (Merck, München, Germany), in 5% CO2 at 37°, and frozen in aliquots. Prior to use, an aliquot was thawed, vortexed briefly, diluted in sterile saline containing 0·04% Tween-80 (Merck) and clumping disrupted by aspiration through a 29-gauge needle (Omnican®; Braun, Melsungen, Germany) 20 times.
Infection and antibiotic treatment of mice
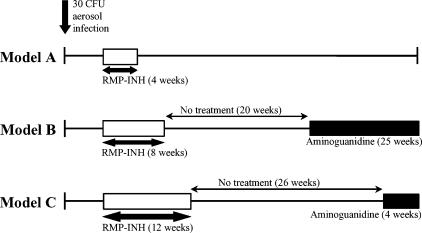
Mice were infected with 30 viable CFU M. tuberculosis via the aerosol route using an inhalation exposure system (Glas-Col, Terre Haute, IN). Mice were exposed to an aerosol produced by nebulizing 5 ml of a bacterial suspension in saline containing 0·04% Tween at a concentration of 106 bacilli/ml. Two weeks postinfection, groups of mice were treated with 0·1 g/l RMP and 0·1 g/l INH (Sigma, St. Louis, MO), delivered ad libitum in drinking water (changed weekly) for 4, 8 and 12 weeks (presented in this work as models A, B and C, respectively). M. tuberculosis-infected control mice received plain drinking water ad libitum. The experimental design, indicating the variations in treatment and reactivation of models A, B and C, is depicted in Fig. 1.
Figure 1.
Schematic diagram of the experimental design for establishing the optimal duration of rifampicin and isoniazid (RMP-INH) treatment in mice to achieve latency of tuberculosis. Unfilled bars depict RMP-INH therapy, while black bars indicate periods of aminoguanidine administration. CFU, colony-forming units.
Immunosuppression
In models B and C, mice were given 2·5% (wt/vol) aminoguanidine (Sigma), containing 10% glucose (wt/vol) (BDH Limited, Poole, UK), ad libitum in drinking water (changed weekly) in order to inhibit iNOS and cause reactivation of tuberculosis.11 Volumes of drinking water containing drugs were monitored regularly to verify that mice consumed the correct dosages. Glucose was added to the water containing aminoguanidine to ensure that the mice drank the required amount. M. tuberculosis-infected control mice received plain drinking water ad libitum.
Quantification of viable mycobacteria in organs
The initial infective dose was verified by killing ten mice 24 hr after exposure to aerosol. Lungs were removed aseptically, weighed, homogenized in saline containing 0·04% Tween-80, and 10-fold serial dilutions were plated in duplicate onto 7H10 agar (Difco), supplemented with 10% OADC and 0·5% glycerol. Plates were incubated at 37° and the number of CFU counted after 21 days. Thereafter, at specific time-points, mice were killed and their lungs, livers and spleens removed aseptically and weighed. Two-thirds of each organ was homogenized in saline containing 0·04% Tween-80 for CFU enumeration, as described above. Data are presented as means of log10 CFU per organ and standard error of the mean (SEM), indicated by error bars (n = 3–4 mice per group per time-point).
Histopathology
One-third of lungs, livers and spleens of mice were prepared by fixing in 10% buffered formalin (BDH) before paraffin embedment. Sections were stained with haematoxylin and eosin (H & E) and Ziehl–Neelsen acid-fast stain, for evaluation of pathological changes and bacillary load, respectively.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections were deparaffinized and rehydrated through graded alcohol series. Sections were incubated for 16 hr at 4° with a 1 : 2000 dilution of rabbit anti-mouse antibody (obtained from J. Pfeilschifter, University of Frankfurt, Germany), specific for iNOS, then rinsed in phosphate-buffered saline (PBS). Sections were then incubated with a rat anti-rabbit secondary antibody for 30 min at room temperature, rinsed again in PBS, and incubated with ABC Vector (Vector Laboratories, Burlingame, CA) for 30 min at room temperature. Subsequently, sections were rinsed in PBS, incubated with 3,3′-diaminobenzidene tetrahydrochoride (DAB) substrate (Vector Laboratories) for 10 min at room temperature, washed in running water, counterstained in haematoxylin and mounted in Entellen (Merck).
Results
Optimizing anti-tuberculous therapy
In an attempt to establish a relatively short-duration model to study spontaneous and drug-induced reactivation of latent infection, mice were infected with a low aerosol exposure of mycobacteria (30 CFU), and RMP-INH anti-tuberculous therapy was commenced 2 weeks postinfection. The duration of anti-tuberculous therapy was varied between 4 and 12 weeks (Fig. 1).
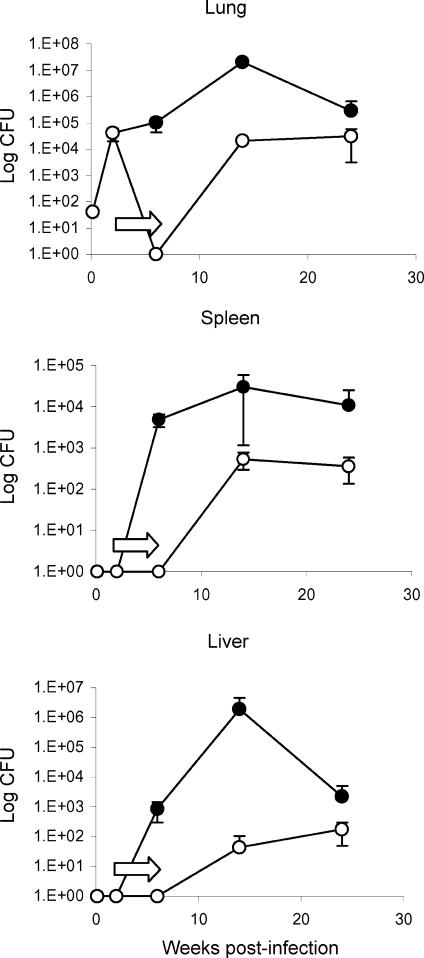
First, aerosol-infected mice were treated for 4 weeks (model A). The combined administration of RMP and INH for 4 weeks eliminated the bacilli completely, and no viable colonies were detected in the lungs, liver or spleen (Fig. 2). This finding is consistent with effective therapy. In contrast, the bacterial load in the lungs of untreated mice was ≈ 105 CFU, and in the spleen and liver ≈ 104 CFU and ≈ 103 CFU, respectively.
Figure 2.
Model A. Bacterial burden of C57BL/6 mice treated with rifampicin and isoniazid (RMP-INH) for 4 weeks after aerogenic infection with 30 colony-forming units (CFU) of Mycobacterium tuberculosis H37Rv. Two weeks postinfection, one group of mice (○) received 0·1 g/l RMP-INH, delivered in drinking water ad libitum (indicated by arrows) for a period of 4 weeks, whereas infected control mice (•) received plain drinking water ad libitum. At specific time-intervals up to 24 weeks postinfection, the number of viable bacilli were determined in the lung, spleen and liver by plating organ homogenates and enumerating CFU. No viable bacilli were detected in organs of mice upon cessation of anti-tuberculous therapy; however, mice spontaneously reactivated and bacilli were cultured 8 weeks later (open circles). Both infected controls and reactivated mice controlled the infection (100% survival). Results are expressed as means ± SEM and are representative of three independent experiments with three to four mice per group per time-point.
However, 8 weeks after following cessation of drug treatment, bacilli were found in the lungs, spleen and liver (≈ 104, ≈ 103 and ≈ 102 CFU) and persisted over the 24-week experimental period. Therefore, all mice reactivated the mycobacterial infection spontaneously. The mycobacterial load, however, remained lower as compared to the untreated control mice. As expected, all mice survived the infection during the experimental period.
In conclusion, 4 weeks of chemotherapy was insufficient to eliminate mycobacteria following an initial infective dose of 30 CFU. Although no viable bacilli could be detected with conventional plating methods following drug treatment, spontaneous reactivation of infection occurred in all mice.
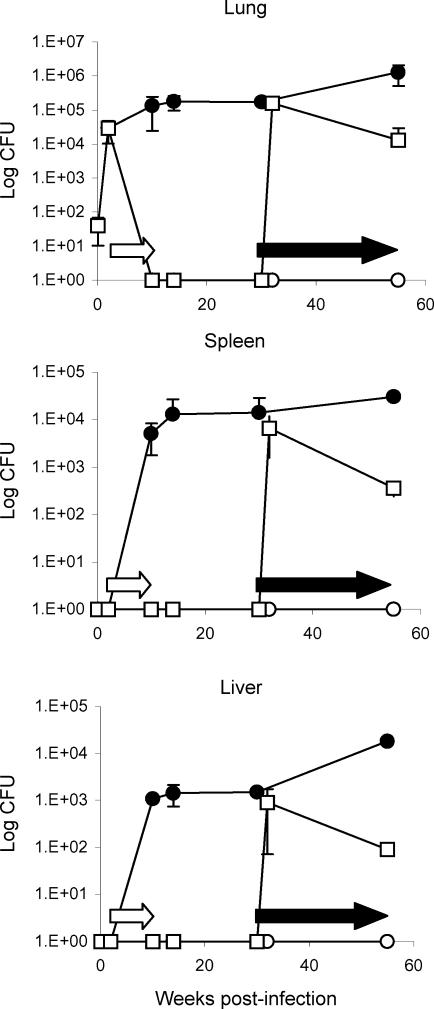
Second, in order to have a long-lasting effect on the infection, RMP-INH chemotherapy was given for 8 weeks (model B). The bacilli, as assessed by CFU counts, were completely eliminated from the lungs and remained undetectable in spleen and liver tissue after an 8-week course of anti-tuberculous therapy, while untreated controls had ≈ 105 CFU in the lungs and spread of infection to the spleen and liver at ≈ 104 and ≈ 103 CFU, respectively (Fig. 3). Interestingly, no spontaneous reactivation was detected over the 55-week duration of the experiment, suggesting effective elimination of bacilli from the host.
Figure 3.
Model B. Bacterial burden of C57BL/6 mice treated with rifampicin and isoniazid (RMP-INH) for 8 weeks after aerogenic infection with 30 colony-forming units (CFU) of Mycobacterium tuberculosis H37Rv. Two weeks postinfection, two groups of mice (○ and □) received 0·1 g/l RMP-INH, delivered in drinking water ad libitum (open arrows) for a period of 8 weeks, whereas the control group (•) received plain drinking water ad libitum. At specific time-points up to 55 weeks postinfection, the number of viable bacilli in the lung, spleen and liver were determined by plating organ homogenates and enumerating CFU. No viable bacilli were detected in the organs of mice upon completion of chemotherapy. After administration of 2·5% aminoguanidine in drinking water containing 10% glucose (solid arrows) from weeks 30–55, reactivated infection was observed in 67% of mice (□, representative of reactivated mice only), although they survived (100% survival) and seemed to control the infection. The group of mice with latent infection (○) remained culture negative, with no viable CFU in organs detected for the duration of the experiment (55 weeks). Infected control mice (•) showed a rapid initial increase in the CFU in lung, liver and spleen, although these mice contained the infection successfully for the duration of the experiment (100% survival). Results are expressed as means ± SEM and are representative of three independent experiments with three to four mice per group per time-point.
However, administration of aminoguanidine, an inhibitor of iNOS, 20 weeks after RMP-INH chemotherapy, resulted in a rapid reactivation of M. tuberculosis infection. As early as 2 weeks following reactivation, the mycobacterial load had increased to ≈ 105 CFU in the lungs, with lower levels in spleen and liver (≈ 104 and ≈ 103 CFU), which was comparable to that of untreated controls.
Therefore, treatment for 8 weeks resulted in the long-term absence of infection. As aminoguanidine-induced inhibition of iNOS allowed rapid reactivation, viable mycobacteria were still present in the host during the latent phase, although not detectable by culture.
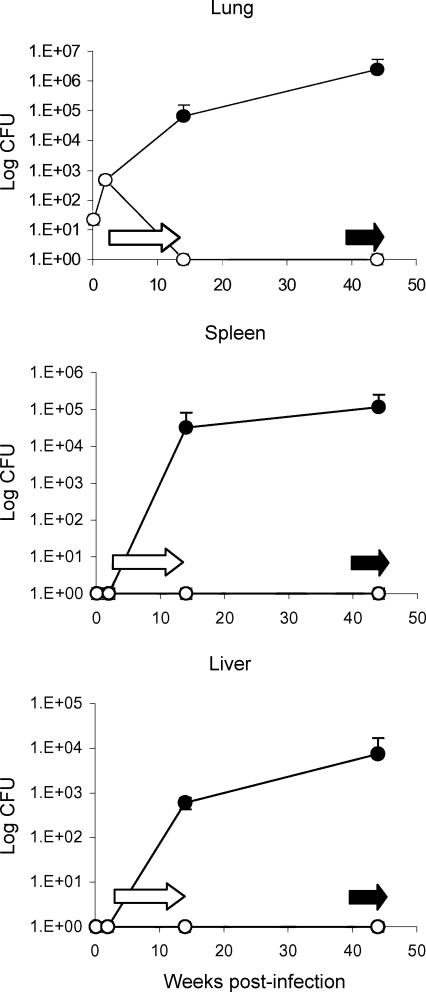
Third, we extended the RMP-INH anti-tuberculous therapy for 12 weeks to achieve a cure in infected mice. The bacilli were eliminated from the lung tissue after therapy. Furthermore, no CFU were detectable from any organ during the 44-week duration of the experiment, while the untreated controls developed chronic tuberculosis (Fig. 4). In order to ascertain whether mycobacteria had indeed been eradicated, aminoguanidine was administered at week 40, 26 weeks after RMP-INH treatment. No reactivation was observed within the subsequent 4 weeks or at any time-point thereafter (data not shown).
Figure 4.
Model C. Bacterial burden of C57BL/6 mice treated with rifampicin and isoniazid (RMP-INH) for 12 weeks after aerogenic infection with 30 colony-forming units (CFU) of Mycobacterium tuberculosis H37Rv. Two weeks postinfection, one group of mice (○) received 0·1 g/l RMP-INH, delivered in drinking water ad libitum (open arrows) for a period of 12 weeks, whereas the control group (•) received plain drinking water ad libitum. At specific time-points up to 44 weeks postinfection, the number of viable bacilli in the lung, spleen and liver were determined by plating organ homogenates and enumerating CFU. No viable bacilli were detected in the organs of mice after completion of chemotherapy. Mice treated with RMP-INH for 12 weeks and subsequently with aminoguanidine for 4 weeks (solid arrows) failed to reactivate infection. Results are expressed as means ± SEM and are representative of three independent experiments with three to four mice per group per time-point.
In conclusion, RMP-INH drug therapy over 12 weeks under these experimental conditions cured M. tuberculosis infection, while 8 weeks of therapy induced a latent infection.
The pathology of latent and chronic infection
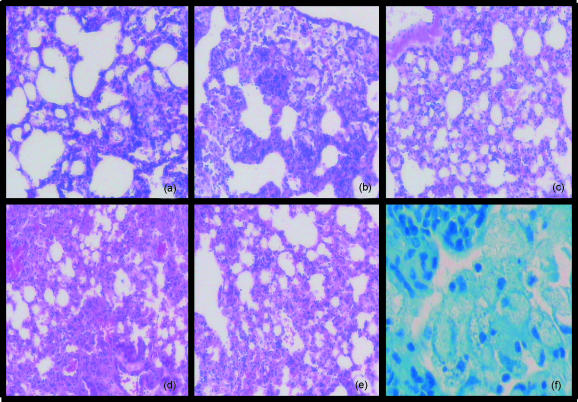
Examination of the H & E-stained tissue sections of the lungs at 2 weeks postinfection revealed recruitment of lymphocytes and macrophages with an incipient granuloma formation (Fig. 5a). At 10 weeks postinfection, differences between anti-tuberculous treated and unreated mice were evident. Untreated controls had a well-developed granulomatous response with lymphoid aggregates, while the treated mice had only small granulomata (Fig. 5b, 5c). At 55 weeks, untreated mice displayed chronic tuberculosis with abundant granulomata containing lipid-loaded macrophages and lymphoid cell aggregates (Fig. 5d). Mice immunosuppressed with aminoguanidine revealed increased recruitment of mononuclear cells and loss of granuloma structures (Fig. 5e).
Figure 5.
Morphology of the lungs of mice during active, latent and reactivated tuberculosis infection. Lungs were examined at 2 weeks (a), 10 weeks (b) and (c), and 55 weeks (d)–(f), postinfection. Panel (a) represents a C57BL/6 mouse 2 weeks postinfection, prior to chemotherapy; panels (b) and (c) 10 weeks postinfection – an infected control (b) and a latently infected mouse after 8 weeks of rifampicin and isoniazid (RMP-INH) chemotherapy (c). Panels (d), (e) and (f) represent mice 55 weeks postinfection – an infected control [(d) and (f)] and a mouse reactivated after aminoguanidine administration (e). Sections were stained with haematoxylin and eosin [(a) to (e)] or with Ziehl–Neelsen (f). Magnification: panels (a)–(e) × 100; panel (f) × 400.
The lung sections stained with Ziehl–Neelsen were analysed for the presence of bacilli during latent infection. No bacilli were detected in RMP-INH treated mice, while abundant acid-fast bacilli were observed in untreated control lungs (Fig. 5f).
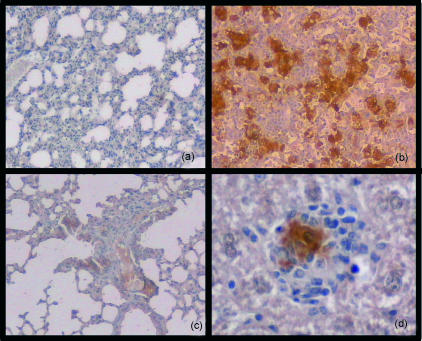
We next examined the expression of iNOS on lung sections from mice with latent infection. While lungs from chronically infected untreated mice strongly expressed iNOS (Fig. 6b), it was not detectable during latent infection in RMP-INH treated mice (Fig. 6a). Aminoguanidine-induced reactivation of infection augmented the iNOS expression in the lungs (Fig. 6c).
Figure 6.
Inducible nitric oxide synthase (iNOS) expression in lung tissue in mice during active, latent and reactivated tuberculosis infection. Mice were aerogenically infected with 30 colony-forming units (CFU) of Mycobacterium tuberculosis H37Rv, after which a group was treated with rifampicin and isoniazid (RMP-INH) for 8 weeks to induce latency of infection. Lung tissue of C57BL/6 mice was examined at 32 weeks postinfection. No iNOS staining was detected in mice during latent infection (a), whereas intense iNOS expression was observed in infected control mice (b). An augmented iNOS staining pattern was observed in mice 2 weeks after aminoguanidine-induced reactivation (c). Panel (d) shows a granuloma in the liver of an infected control mouse to illustrate the origin of iNOS protein from activated macrophages. Magnification: panels (a), (b) and (c) × 100; (d) × 400.
Reactivation of latent infection
Although no bacteria were detectable by culture following 4 weeks of RMP-INH drug treatment, viable bacilli were still present in the host. This explains the spontaneous reactivation of infection after discontinuing drug administration, demonstrated by a dramatic increase of bacilli (104−105 CFU) in the lung, which stabilized over the following 10 weeks (Fig. 2).
Eight weeks of treatment with RMP-INH controlled the infection and no spontaneous reactivation was observed. However, interfering with the immune defence by inhibiting iNOS activity with aminoguanidine resulted in reactivation of infection, with a similar rapid increase in mycobacterial loads (Fig. 3).
Sixty seven percent of mice showed reactivation of infection upon aminoguanidine administration, and this percentage is represented in Fig. 3. Two weeks following reactivation, 100% of mice were culture-positive in lung, liver and spleen. However, this decreased to 50% in all organs 4 weeks after reactivation, culture positivity. Therefore, it would be optimal to further characterize the reactivated state 2 weeks after aminoguanidine administration, as at this time-point a peak in the CFU of all organs was observed following reactivation.
A 12-week RMP-INH anti-tuberculous course, however, eradicated the infection completely, and immunosuppression with aminoguanidine did not result in reactivation (Fig. 4).
These results suggest that a 4-week course of RMP-INH treatment is insufficient to mount protective immunity, while extended treatment regimens allow for immunological control by the host.
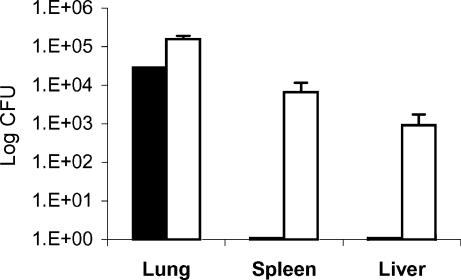
Aminoguanidine-induced reactivation of latent tuberculosis infection resulted in a rapid increase in the number of bacilli in the lungs of mice (from undetectable levels to ≈ 105 CFU within 2 weeks), comparable to levels found at a similar time-point following primary infection (Fig. 7). In contrast with primary infection, however, reactivation in the spleen and liver of mice is the result of haematogenous spread.
Figure 7.
Comparison of bacterial burden in lung, spleen and liver of mice following reactivation versus primary infection. C57BL/6 mice were infected with 30 colony-forming units (CFU) of Mycobacterium tuberculosis H37Rv and the number of viable CFU was determined 2 weeks postinfection (solid bars). Mice were then treated with rifampicin and isoniazid (RMP-INH) for 8 weeks to achieve latency (with no viable bacilli detected in all organs). After 20 weeks in this latent state, a group of mice received 2·5% aminoguanidine containing 10% glucose in drinking water ad libitum to induce reactivation. The increases in bacterial count following reactivated infection are shown 2 weeks after aminoguanidine administration (open bars). Results are expressed as means ± SEM and are representative of three independent experiments with four mice per group per time-point.
Reactivation of latent infection in the absence of TNF
In order to corroborate the notion that the immune system plays an important role in reactivation, we used TNF-deficient mice, which are unable control mycobacterial infection. Although no bacilli were detected after 4 weeks of RMP-INH chemotherapy, the TNF-deficient mice reactivated faster than C57BL/6 wild-type control mice and succumbed to infection within 5 weeks. After 8 weeks of chemotherapy, the TNF-deficient mice reactivated spontaneously and succumbed to infection, unlike the wild-type control mice (data not shown).
By contrast, no reactivation was observed in TNF-deficient mice treated with RMP-INH for 12 weeks following cessation of treatment. Therefore, this extensive treatment apparently had eliminated all viable bacilli.
Discussion
The results of this study emphasize the fine equilibrium that exists between the initial bacterial burden (30 CFU) and length of RMP-INH anti-tuberculous chemotherapy, as short-term treatment (4 weeks) results in spontaneous reactivation of tuberculosis in mice, while long-term treatment (12 weeks) eradicates all bacilli. Eight weeks of anti-tuberculous therapy seemed to be optimal in this model under these experimental conditions, whereby treatment induces stable latency in mice and can be reactivated with aminoguanidine, which functions through inhibition of macrophage iNOS, preventing production of bactericidal reactive nitrogen intermediates.
The optimal model (model B in this study) proceeded over an experimental period of 55 weeks in order to verify sustained latency and no spontaneous reactivation. It was, however, ascertained that following 8 weeks of RMP-INH chemotherapy and a rest period of 4 weeks, aminoguanidine induced reactivation of infection (data not shown). This reduces the experimental period to 18 weeks, hence it can be considered a short-duration model.
To date, two types of murine tuberculosis models have been described: the low-dose (or chronic infection) model; and the drug model. These two models show different patterns of disease progression and reactivation. Aminoguanidine-induced reactivation in C57BL/6 mice chronically infected with M. tuberculosis resulted in a rapidly fatal infection, whereas aminoguanidine-induced reactivation in drug-treated latent mice could be controlled and mice could stably maintain the bacillary burden.11 We have also made a similar observation with aminoguanidine-induced reactivation in our model B. After a sharp initial increase in the number of CFU in lung, spleen and liver, bacterial numbers decreased to levels that could be contained and ensured survival of mice.
Few other animal models have been described in which latency is induced with anti-tuberculous drugs;7,8,11,14 however, pyrazinamide and INH were used to control and contain M. tuberculosis in these models. We opted for chemotherapeutic treatment of tuberculosis with a combination of RMP and INH because of their current use as first-line drugs in both the ‘intensive phase’ and ‘continuation phase’ of chemotherapeutic patient treatment.16 In the mouse model described by Grosset (1978), RMP-INH concentration therapy was used for 12 months following intravenous infection with M. tuberculosis, but did not result in mycobacterial elimination. Sixty percent of mice reactivated with tuberculosis after cortisone administration.17 In contrast to this study, we have shown that it is indeed possible to eliminate mycobacteria using this drug regime for 12 weeks following low dose aerosol challenge, even in TNF gene deficient mice.
The present model of latency and reactivation of tuberculosis is relatively easy to perform, with all drugs delivered in the drinking water of mice. Stable latent infection is attainable, which can be reactivaed with high efficiency, and no spontaneous reactivation occurs. Infection with M. tuberculosis H37Rv is considered the strain of choice in a murine model, as this strain maintains virulence through multiple passages in the mouse.16 Additionally, infection via the aerosol route is the most relevant route of infection (mimicking human tuberculosis) and shows a different bacillary distribution amongst the mouse organs as compared to intravenous infection (data not shown). Therefore, the present model is an ideal one for further elucidation of immune functioning during latency and reactivation of tuberculosis.
Moreover, owing to an increased resistance of M. tuberculosis to drugs, poor adherence by patients to lengthy therapy and the unpleasant side-effects of current anti-tuberculous drugs, research towards improved therapy has taken high priority. In addition to a new generation of anti-tuberculous drugs, novel vaccines will have to be developed in order to combat the most prevalent form of tuberculosis, namely latent tuberculosis. We believe that our model will be a beneficial tool for assisting with these vital investigations.
Acknowledgments
This work was supported by the Wellcome Trust, National Research Foundation and Medical Research Council, Cape Town, South Africa.
References
- 1.Sudre P, Ten Dam G, Kochi A. Tuberculosis: a global overview of the situation today. Bull WHO. 1992;70:149–59. [PMC free article] [PubMed] [Google Scholar]
- 2.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 3.Stead WW. Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection? Am Rev Resp Dis. 1967;95:729–45. doi: 10.1164/arrd.1967.95.5.729. [DOI] [PubMed] [Google Scholar]
- 4.Raviglione MC, Snider DE, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a global epidemic. JAMA. 1995;273:220–6. [PubMed] [Google Scholar]
- 5.Antonucci G, Girardi E, Raviglione MC, Ippolito G. Risk factors for tuberculosis in HIV-infected persons: a prospective cohort study. JAMA. 1995;274:143–8. doi: 10.1001/jama.274.2.143. [DOI] [PubMed] [Google Scholar]
- 6.Narain JP, Raviglione MC, Kochi A. HIV-associated tuberculosis in developing countries: epidemiology and strategies for prevention. Tuber Lung Dis. 1992;73:311–21. doi: 10.1016/0962-8479(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 7.McCune RM, Feldmann FM, McDermott W. Microbial persistence II. Characteristics of the sterile state of tubercle bacilli. J Exp Med. 1966;123:469–86. doi: 10.1084/jem.123.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCune RM, Feldmann FM, Lambert HP, McDermott W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123:445–68. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown DH, Miles BA, Zwilling BS. Growth of Mycobacterium tuberculosis in BCG-resistant and -susceptible mice: establishment of latency and reactivation. Infect Immunol. 1995;63:2243–7. doi: 10.1128/iai.63.6.2243-2247.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orme IM. A mouse model of the recrudescence of latent tuberculosis in the elderly. Am Rev Respir Dis. 1988;137:716–8. doi: 10.1164/ajrccm/137.3.716. [DOI] [PubMed] [Google Scholar]
- 11.Flynn JL, Scanga CA, Tanaka KE, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998;160:1796–803. [PubMed] [Google Scholar]
- 12.Mustafa T, Phyu S, Nilsen R, Johnson Bjune G. A mouse model for slowly progressive primary tuberculosis. Scand J Immunol. 1999;50:127–36. doi: 10.1046/j.1365-3083.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- 13.Phyu S, Mustafa T, Hofstad T, Nilsen R, Fosse R, Bjune G. A mouse model for latent tuberculosis. Scand J Infect Dis. 1998;30:59–68. doi: 10.1080/003655498750002321. [DOI] [PubMed] [Google Scholar]
- 14.Scanga CA, Mohan VP, Joseph H, Yu K, Chan J, Flynn JL. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect Immun. 1999;67:4531–8. doi: 10.1128/iai.67.9.4531-4538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marino MW, Dunn A, Grail D, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry C, Cole S, Fourie B, et al. Scientific blueprint for TB drug development. Tuberculosis. 2001;81(Suppl. 1):1–52. doi: 10.1054/tube.2001.0288. [DOI] [PubMed] [Google Scholar]
- 17.Grosset J. The sterilizing value of rifampicin and pyrazinamide in experimental short-course chemotherapy. Bull Int Union Tuberc. 1978;53:5–12. [1(Intanbul number)] [PubMed] [Google Scholar]