Abstract
T helper type 2 (Th2) -polarized immune responses are characteristically dominant in helminth infections. Two murine models that show a Th1 to Th2 polarization with infection progression are those of Schistosoma mansoni and Taenia crassiceps. In both, an early Th1 response is replaced by a late Th2 response. We report that the nucleic acid-, protein- and lipid-free carbohydrate fraction of T. crassiceps metacestodes (denoted T-CHO) possesses Th2-like immunomodulatory activity. Immunization of two strains of rats (Dark Agouti and Albino Oxford) and BALB/c mice with chicken albumin in the presence of T-CHO resulted in selective enhancement of immunoglobulin G1 (IgG1) antibodies, considered to be associated with Th2 responses in both rats and mice. Interleukin-6 (IL-6) followed by IL-10 were the dominant cytokines detected in in vitro cultures of mouse spleen cells stimulated with T-CHO. IL-4 and IL-5 were not detected in these culture supernates. Furthermore, Taenia carbohydrates were mitogenic to spleen cells, activated serine phosphorylation of proteins and up-regulated the expression of the anti-apoptotic protein, Bcl-2. When mouse spleen cells were cultured in the presence of Taenia carbohydrates, a concentration-dependent down-regulation of IL-2 and an overlapping up-regulation of IL-6 secretion were seen.
Introduction
T helper type 2 (Th2) -polarized immune responses are characteristically dominant in chronic human helminth infections1 and determination of antiparasite immunoglobulin G4 (IgG4) antibody (associated with human Th2 responses) is the basis of serodiagnostic test development (e.g. lymphatic filariasis,2 Schistosomiasis3). Similar Th2 dominance is seen in murine models of Schistosoma mansoni4–6 and Taenia crassiceps,7 progressing from an early Th1 type to a late Th2 type. These murine models also show that cytokines of the innate immune system play a major role in directing the adaptive immune response to a Th2 type. While interleukin-12 (IL-12) is considered the key cytokine in initiating a Th1 response, IL-4 is said to be the counterpart in a Th2 response. However, the demonstration that effective and strong Th2 responses can be generated in the absence of IL-4 (and IL-13) suggests alternative mechanisms of Th2 response induction.8–12
Equally important in understanding the mechanisms underlying the initiation and maintenance of a dominant Th2 response is the identity of the parasite molecules responsible. In the case of S. mansoni, considerable evidence points to egg glycoproteins or carbohydrates.4–6,13–15 The nature of Th2 driving molecules in the T. crassiceps model7 is not known.
In order to determine whether the Th2 dominance in T. crassiceps infections is also mediated via carbohydrates, the adjuvant effects of lipid-, nucleic acid- and protein-free extracts of metacestodes (denoted T-CHO) were determined in two strains of rats and in BALB/c mice. Immunization of animals with albumin in the presence of T-CHO resulted in enhanced IgG1 antibodies, IL-6 and IL-10 production. We conclude that Taenia carbohydrates possess Th2-like immunomodulatory activity, probably mediated via IL-6 and IL-10.
Materials and methods
Carbohydrate extracts from T. crassiceps metacestodes
An infective stock of T. crassiceps metacestodes was a kind gift from Dr Abraham Landa, Department of Immunology, National University of Mexico, Mexico. These were maintained in BALB/c and CBA mice according to the method of Terrazas et al.7 Mice were infected with 10–15 metacestodes by intraperitoneal injection. Approximately 10–12 weeks post-infection, infected animals were killed by cervical dislocation and metacestode-stage parasites in the peritoneal cavity were harvested, washed in endotoxin-free tissue-culture-grade phosphate-buffered saline (PBS; Gibco BRL Life Technologies Ltd, Paisley, UK) and stored at −40°.
These metacestode larvae were re-suspended in an equal volume of endotoxin-free PBS, sonicated at 300 W for 10 seconds and subjected to sequential digestion with DNase I and RNase (D 4263, R 7003, Sigma Chemicals, St. Louis, MO, for 3 hr at 37°), Proteinase K (Sigma P6556, 0·1 mg/ml, for 16–18 hr at 37°) followed by digestion with agarose-linked protease (Sigma P8790) for 12–18 hr at 37°. The digest was then centrifuged at 30 000 g for 30 min and the supernate was dialysed against PBS (MW cut-off 12 000) and repeatedly extracted with chloroform. The aqueous phase from chloroform extraction was lyophilized, re-dissolved in de-ionized water, and sterilized by filtration (0·22 μm). The concentration was adjusted to 1 OD260 U/ml and then aliquots were stored at − 40°. This preparation was denoted as Taenia carbohydrates (T-CHO).
Acid hydrolysis of T-CHO was carried out by the method of Ip et al.16 Ten millilitres of T-CHO (1 OD260 U/ml) was treated with hydrofluoric acid (2 m) at 65° for 2 hr followed by overnight incubation at 98° in the presence of 2 m trifluoroacetic acid. After hydrolysis, the solution was diluted with 2 ml of 10× PBS (Gibco) and dialysed against several changes of PBS, lyophilized and re-dissolved in 10 ml of PBS. This preparation was denoted as acid hydrolysed T-CHO and was used in in vitro stimulation of spleen cells.
Determination of the adjuvant effects of Taenia carbohydrates
Adjuvant activity of T-CHO was determined against purified chicken albumin (A2512, Sigma) in two strains of rats (Dark Agouti, DA, and Albino Oxford, AO) and in BALB/c mice. Rats were immunized subcutaneously in the dorsal neck region with chicken albumin (100 μg per immunization per rat) mixed with PBS (no adjuvant control), aluminium hydroxide (Alum, from Intergen, New York, NY), or 0·5 OD260 U T-CHO in a total volume of ∼500 μl. Two booster immunizations were given by the same route and dosage at 3-weekly intervals. Test bleeds were obtained by the retro-orbital method at the time of first immunization and 10 days after the booster immunizations. All sera were stored at − 40° until analysed.
BALB/c mice were similarly immunized with chicken albumin in PBS, Alum, or T-CHO. Each mouse received 50 μg of albumin in 200 μl mixed with 500 μl of PBS, Alum, or T-CHO [0·25–1 OD260 U, given intraperitoneally (i.p.)] at two sites. Mice were bled at approximately 10–12 days, after the primary and booster immunizations.
Antibody levels in test sera were determined by standard enzyme-linked immunosorbent assay (ELISA) using peroxidase-conjugated anti-rat IgG subclass-specific antibodies (anti-rat IgG1, IgG2a, IgG2b, IgG2c; Biosource International, Camarillo, CA). Mouse IgG1, IgG2a and IgG2b antibody levels were determined with biotinylated anti-mouse subclass-specific antibodies (61-0240, 61-0140, 61-0340, Zymed, San Francisco, CA) and streptavidin peroxidase (E2882, Sigma). Rat sera were tested at a single dilution of 1 : 50 and the mouse sera were titrated.
Antibody ELISAs were performed in 96-well ELISA plates (Maxisorp Immunoplates, Nunc A/S, Roskilde, Denmark) in 50-μl reactions, with antigen coated at 5 μg/ml, conjugates were used at 1 : 2000 dilution, incubation was 45 min for primary antibody and 30 min for the conjugate. Both test sera and conjugates were diluted in PBS/5% bovine serum albumin/0·05% Tween-20 and contained 5% normal goat serum (G9023, Sigma) for goat antibodies and 5% normal rabbit serum for rabbit antibodies. All incubations were carried out at room temperature.
In vitro culture of spleen cells for cytokine analysis and proliferation
Spleen cells were obtained from mice immunized with T-CHO by i.p. injection of 0·25 OD260 U/mouse or from control mice injected with PBS. Spleen cells were harvested at days 1, 3 and 5 post i.p. injection. Standard 96-well plate cultures were set up with 0·5 million cells per well in a volume of 200 μl or in 24-well plates in a total volume of 1 ml. For in vitro stimulation with T-CHO, serial dilutions were made from a stock of 1 OD260 U/ml in a volume of 100 μl prior to addition of cells (100 μl, 5 million per ml). Acid-hydrolysed T-CHO was used as the control. For proliferation assays, 0·5 μCi of [3H]thymidine in 20 μl was added to each well and cells were harvested 6 hr later. Proliferation was expressed as total counts per minute per million cells.
Cytokine ELISA
Cytokines in culture supernates were determined by ELISA using purified anti-IL-2, -IL-4, -IL-5, -IL-6, -IL-10 and -IL-12 as capture antibodies and the corresponding biotinylated antibodies as reporter antibodies according to supplier recommendations (PharMingen, San Diego, CA). Primary capture antibodies and biotinylated reporter antibodies were used at 2 and 1 μg/ml, respectively. The enzyme substrate used was TMB microwell peroxidase (Kirkgaard & Perry Labs. Inc, Gaithersberg, MD). Purified recombinant IL-2, IL-6 and IL-10 (PharMingen) were used as standards.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis of T-CHO
For SDS–PAGE, aliquots of total Taenia extract and T-CHO were lyophilized and re-dissolved in SDS–PAGE sample buffer to result in a 100-fold excess of T-CHO concentration over that of the total extract. Twenty-five-microlitre aliquots were loaded onto 1-cm width lanes on SDS–PAGE gels. Gels were electrophoresed at 150 V/25 mA for 2 hr, stained for 24 hr with 1% Coomassie Brilliant Blue R-250 and destained in 10% acetic acid and 15% methanol in water.
Western blot analysis of in vitro cultured spleen cell extracts
Spleen cells cultured in vitro were purified by Histopaque® (Sigma 1077) density gradient centrifugation to remove dead cells, washed in cold PBS and re-suspended in PBS at a cell density of 1 million/ml. Cells were lysed by addition of five volumes of cold acetone and freezing at −70° for 6 hr. The residue and precipitated proteins were collected by centrifugation, re-dissolved in 0·2 ml of SDS–PAGE sample buffer, boiled for 5 min in a water bath and re-precipitated with five volumes of cold acetone at −70° for 6 hr. The precipitate was then dissolved in 0·1 ml of SDS–PAGE sample buffer and loaded on to SDS–PAGE gels. Each lane received the protein equivalent of 0·1 million cells. SDS–PAGE fractionated proteins were electrophoretically transferred to nitrocellulose membranes (BioRad Laboratories, Hercules, CA), blocked with purified bovine serum albumin (Sigma) and used for Western blot analysis. For Bcl-2 determination, a polyclonal antibody reactive with human and mouse Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, sc-783) was used. Serine and tyrosine phosphorylated proteins were detected with biotinylated anti-phosphoserine (B7911, Sigma Chemicals) and anti-phosphotyrosine (B1531, Sigma Chemicals) antibodies. Membranes were developed with appropriate alkaline phosphatase conjugates and 5-bormo-4 chloro-3 indoyl phosphate/introblue tetrazolium BCIP/NBT one component phosphatase substrate (Kirkgarrd and Perry, 50-81-18).
Results
Characteristics of Taenia carbohydrates
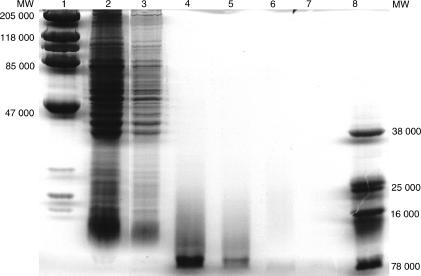
T-CHO had an UV absorption maxima at 260 nm. The average yield of T-CHO was ∼0·1 OD260 U/ml of metacestodes suspended in an equal volume of PBS. Total protein analysis by Khjedhal method showed less than 5% total nitrogen in T-CHO. SDS–PAGE analysis of T-CHO did not show proteins by Coomassie Blue R250 staining in heavily overloaded gels (Fig. 1). Proteins were not detected in a 100-fold concentrated T-CHO, equivalent of the highest amount of Taenia total extract loaded on to gel, whereas a 100-fold dilution of the total extract showed proteins on SDS–PAGE gels. Formal proof that the high molecular weight Taenia extract used in these studies was carbohydrate was by enzymatic release of N-linked glycans and chemical reductive elimination of O-linked glycans, followed by chemical derivatization and fast atom bombardment mass spectrometric analysis (S. Haslem and A. Dell, Imperial College, London, personal communication). Endotoxin was not detected in T-CHO by Limulus Amoebocyte Lysate assay (E-Toxate®, Sigma Chemicals).
Figure 1.
SDS–PAGE analysis of Taenia carbohydrate extract T-CHO. Lanes 2 and 3, Taenia total extract (lane 3 is a 100-fold dilution of lane 2). Lanes 4 and 5 contain the equivalent of 10- and 100-fold concentrated T-CHO prior to chloroform extraction. Lanes 6 and 7 are the equivalent of lanes 4 and 5, after chloroform extraction. Lanes 1 and 8 are markers.
T-CHO selectively enhanced IgG1 antibody responses to chicken albumin in AO and DA rats
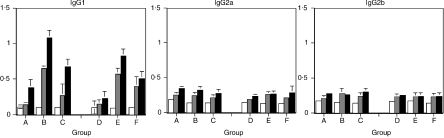
In both AO and DA strain rats immunized with chicken albumin in the presence of T-CHO and Alum as adjuvant, the dominant antibody response was IgG1, compared to IgG2a and IgG2b (Fig. 2). Rat IgG2c antibodies were not detected. In two different experiments, the mean T-CHO/PBS and Alum/PBS ratios of the anti:albumin IgG1 antibody responses ranged between 1·8 and 2·5 and 2·9 and 4·8, respectively. The same ratios for total IgG antibodies were 1·5–2·3 (T-CHO/PBS) and 1·9–2·7 (Alum/PBS). Overall, the T-CHO-induced antibody level was approximately two-fold higher than that of the PBS control and approximately half that of the Alum control.
Figure 2.
Histogram showing IgG1, IgG2a and IgG2b antibody levels to chicken albumin in AO (Groups A–C) and DA (Groups D–F) rats immunized with albumin in PBS (Groups A and D), Alum (Groups B and E) and T-CHO (Groups C and F). Bld 1, sera from preimmunization bleed; Bld 2, sera after first boost; and Bld 3, sera at 10 days post second boost. Bld 1-open bars; Bld 2-grey bars; Bld 3-closed bars. There were six animals in each group, sera were tested in duplicate at 1 : 50 dilution. Error bars are SD.
T-CHO selectively enhanced IgG1 antibody responses to albumin in BALB/c mice
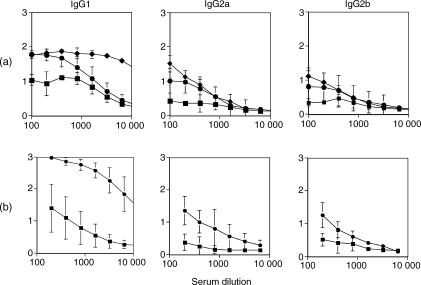
As shown in Fig. 3, T-CHO enhanced IgG1 responses to chicken albumin in BALB/c mice. As in the case of rats immunized with albumin in PBS, T-CHO and Alum, the highest IgG1 anti-albumin antibody responses were seen in alum-immunized animals, the lowest in PBS-immunized animals and they were intermediate in T-CHO-immunized animals (Fig. 3a). Compared to IgG1 antibodies, the IgG2a and IgG2b responses were relatively weaker in all three situations. Selective enhancement of IgG1 antibodies was much more prominent when two booster immunizations were given (Fig. 3b).
Figure 3.
(a) Antibody levels to chicken albumin in BALB/c mice immunized with 50 μg albumin in PBS (▪), Alum (♦) and T-CHO (• 0·5 OD260 U/mouse) as adjuvant. Test sera were collected 10 days after a single booster immunization. (b) Antibody levels to chicken albumin in BALB/c mice immunized with 50 μg albumin in PBS (▪) and T-CHO (• 0·25 OD260 U/mouse) as adjuvant. Sera were collected after two booster immunizations. There were eight animals in both experiments and in each group, sera were tested in duplicate in serial dilutions. Mean + SD of ELISA OD are shown on y axes.
T-CHO was mitogenic to spleen cells, inhibited IL-2 secretion and induced IL-6 and IL-10 secretion in cultured spleen cells
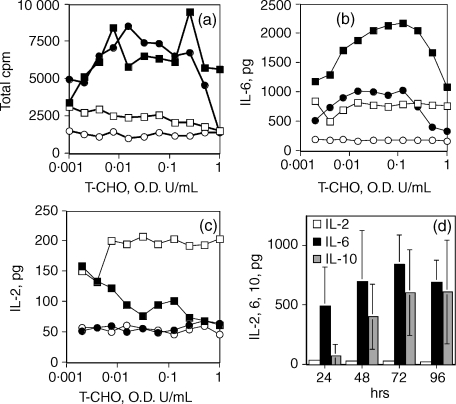
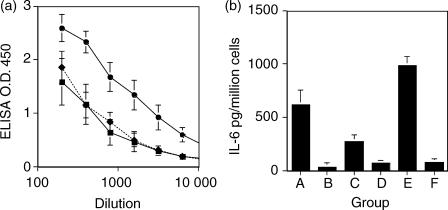
T-CHO was mitogenic (72 hr cultures) to both naive and Taenia carbohydrate-sensitized mouse spleen cells in a concentration-dependent manner (Fig. 4a). Maximal proliferation was seen at the concentration range of 0·005–0·1 OD260 U/ml. Of the cytokines tested (IL-2, IL-4, IL-5, IL-6, IL-10 and IL-12), IL-6 was the major cytokine detected in the T-CHO-stimulated culture supernates for up to 48 hr in culture and showed a similar concentration dependence to the mitogenic response (Fig. 4b). Both naive and T-CHO-stimulated cells secreted IL-6 and IL-10 in response to T-CHO in culture, but the effect was more pronounced in spleen cells presensitized with T-CHO. IL-10 was detected at and after 48 hr in culture. In contrast, quantitatively lower levels of IL-2 (< 250 pg/million cells) were detected in cultures of spleen cells from presensitized mice in the absence of T-CHO, which appeared to be inhibited by the presence of T-CHO in culture (Fig. 4c). Acid-hydrolysed T-CHO did not have the IL-2 inhibitory activity or the IL-6 and IL-10 stimulatory activity.
Figure 4.
(a) Mitogenic and (b–d) cytokine stimulatory activity of T-CHO. (a–c) Spleen cells from BALB/c mice sensitized with T-CHO (i.p. injection of 0·5 OD260 U) or from naive mice were used. (▪) Spleen cells from T-CHO pre-exposed mice + T-CHO in culture; (□) spleen cells from T-CHO exposed mice + acid-hydrolysed T-CHO in culture; (•) spleen cells from naive mice + T-CHO in culture; (cir;) spleen cells from naive mice + acid hydrolysed T-CHO in culture. Data shown are mean values for pooled spleen cells from two mice, performed in triplicate from two independent experiments. (d) Time–course determination of IL-2, IL-6 and IL-10 secretion by spleen cells from mice exposed to T-CHO, 0·25 OD260 U, 3 days post i.p. injection. Spleen cells from three animals were cultured separately, in triplicate. Error bars are SD. IL-4 and IL-5 were not detected. Representative experiment shown.
In time–course assays, cultures were maintained in 24-well plates in a volume of 1 ml with 5 million cells per well. For stimulation with T-CHO, 0·1 OD260 U/ml were added. Control cultures received an equal volume of acid-treated T-CHO. For cytokine analysis, 200 μl aliquots were withdrawn at 24, 48, 72 and 96 hr of culture. As shown in Fig. 5, high IL-6 expression was seen as early as 24 hr in culture and was maintained at increased levels for up to 72–96 hr. Moderate levels of IL-10 were seen after 48 hr of culture while IL-4 was not detected.
Figure 5.
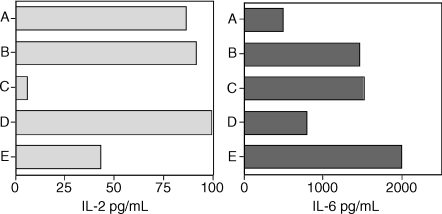
Effect of pre-exposure duration to T-CHO and staurosporine in culture on IL-2 (left panel) and of IL-6 (right panel) secretion by spleen cells. Group A, cells from 1-day pre-exposed mice cultured in the absence of T-CHO; group B, same as group A, but cultured in the presence of T-CHO; group C, same as group B, in the presence of staurosporine; group D, cells from 5-day pre-exposed mice cultured in the absence of T-CHO; group E, same as group D but cultured in the presence of T-CHO. Spleen cells from two animals were cultured separately in triplicate. Values shown are the mean for each group. Representative experiment shown.
Figure 5 shows that prior in vivo sensitization for 24 hr was sufficient for stimulation of in vitro secretion of IL-6 by spleen cells in response to T-CHO, whereas a minimum of 3–5 days of sensitization was required for the inhibitory effect of T-CHO on IL-2 secretion by spleen cells cultured in vitro. Prior sensitization was not a requirement for the mitogenic activity of T-CHO (Fig. 4a) or for IL-6 secretion as naive cells produced IL-6 in response to T-CHO (Fig. 4b). The protein kinase inhibitor staurosporine (S4400, Sigma Chemicals, used at 0·1–1 μg/ml) did not inhibit the IL-6 secretion, while it almost completely eliminated the base level IL-2 secretion by T-CHO presensitized spleen cells.
Adjuvant effect of T-CHO was extraction and concentration dependent
The in vivo IgG1 enhancing effect and in vitro IL-6 stimulatory effect of T-CHO were dependent on the method of extraction and the concentration of T-CHO used. The IgG1 enhancing effect was concentration dependent, with doses higher than 0·5 OD260 U/mouse having no effect (Fig. 6a). When metacestode carbohydrates were extracted with PBS followed by chloroform removal of lipids, the resultant product was approximately pH 5 and showed both properties when used at concentrations below 0·5 OD260 U/mouse. However, if extracted in water and with no lipid removal, the product was of neutral pH and was of much reduced activity. Chloroform extraction of the water-soluble extract restored IL-6 stimulatory activity (Fig. 6b).
Figure 6.
(a) Effect of T-CHO concentration on the IgG1 response. Mice were immunized with 50 μg albumin in PBS (▪), in T-CHO, 1 OD260 U/mouse (♦) or in T-CHO, 0·25 OD260 U/mouse (•). There were five mice in each group and the mean OD is shown. Error bars are SD. Sera were collected 12 days after the first booster immunization. (b) Effect of extraction procedure on in vitro IL-6 stimulatory effect of T-CHO. Groups A–D, spleen cells from mice presensitized (24 hr) with chloroform-extracted T-CHO were stimulated with PBS and chloroform-extracted T-CHO (group A), water-extracted but not chloroform-extracted T-CHO (group B), water- and chloroform-extracted T-CHO (group C) and no stimulus (group D). Groups E and F, naive cells stimulated in culture with PBS–chloroform-extracted T-CHO (group E) and naive cells with no stimulus in culture (group F). Mean values from two experiments with three mice in each group are shown. Error bars are SD.
T-CHO induced Bcl-2 expression and serine phosphorylation of proteins
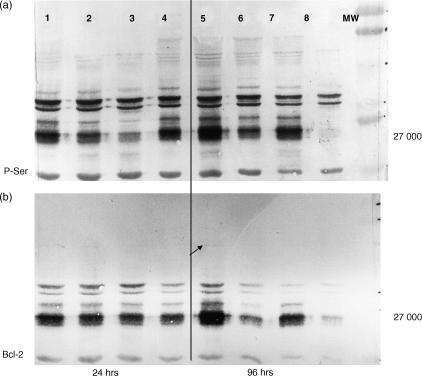
Culture of spleen cells from both pre-exposed and naive mice induced up-regulation of Bcl-2 expression and serine phosphorylation of a number of proteins, including a broad band at 27 000 MW, tentatively identified as either Bcl-2 or the high-mobility group-2 (HMG-2) or overlapping bands of both (Fig. 7). In 24-hr cultures, the levels of serine phosphorylated proteins and Bcl-2 were comparable in all four groups of cultures, i.e. in naive and T-CHO presensitized spleen cells cultured in the presence and absence of T-CHO. After 96 hr of culture, both serine phosphorylated proteins and Bcl-2 were practically absent in the naive cells cultured in the absence of T-CHO (Fig. 7, lane 8), while both were qualitatively much higher in presensitized spleen cells cultured in the presence of T-CHO (Fig. 7, lane 5), and even in comparison to 24 hr cultures (Fig. 7, lanes 1–4).
Figure 7.
Western blot analysis. Serine phosphorylation and Bcl-2 expression in spleen cells cultured in the presence and absence of T-CHO. (a) Serine phosphorylation; (b) Bcl-2 expression after 24 hr in culture and 96 hr in culture. Lanes 1 and 5, spleen cells from pre-exposed mice, cultured in the presence of T-CHO; lanes 2 and 4, cells from T-CHO pre-exposed mice, cultured in the absence T-CHO; lanes 3 and 7, cells from naive mice cultured in the presence of T-CHO and lanes 4 and 8, cells from naive mice cultured in the absence of T-CHO.
Discussion
The conclusion that the active components in T-CHO were carbohydrate was based on the method of preparation and mass spectroscopic data. The extraction procedure for T-CHO involved extensive digestion with DNase I, RNase and with two strong proteases. Absence of proteins in the extract was confirmed by Kjeldhal analysis and SDS–PAGE. Lipids were removed by repeated extractions with chloroform and the smaller molecular weight (< 12 000) material by dialysis. Therefore, it was logical to conclude that the remainder in the extract was larger molecular weight (> 12 000) carbohydrates. The abrogation of in vitro stimulatory activity of T-CHO by an acid hydrolysis procedure recommended for complex bacterial carbohydrates is indirect evidence that the activity resided in the polysaccharide fraction of the extract. Formal proof that T-CHO was carbohydrate was by enzymatic release of N-linked glycans and chemical reductive elimination of O-linked glycans followed by chemical derivatization and fast atom bombardment mass spectrometric analysis, which clearly showed that the Taenia high molecular weight material was both N- and O-glycosylated (S. Haslem and A. Dell, Imperial College, London, personal communication). However, further chemical characterization is needed to identify the active component(s) in order to optimize the immunization protocols.
In summary, our findings show that non-nucleic acid, non-protein, non-lipid components (putatively identified as N- and O-linked glycans) in T. crassiceps metacestodes possess immunological adjuvant properties. This finding parallels other studies showing adjuvant activities for complex carbohydrates from Schistosoma mansoni eggs13,15,17 and fungal cell walls.18 Second, the adjuvant effect of Taenia carbohydrates favoured a dominant IgG1 response, considered to be associated with Th2 type in both rats19–21 and mice,22 also in agreement with schistosomal and fungal glycans.13,15,17,18 Third, IL-6 was the dominant cytokine detected in culture supernates of spleen cells from mice pre-exposed to T-CHO by i.p. injection and stimulated in culture with T-CHO. This finding is also in agreement with studies showing up-regulation of IL-6 by complex carbohydrates.23,24 This effect was seen as early as 18 hr of culture (data not shown). As naive cells also produced IL-6 in response to T-CHO, the effect of T-CHO on IL-6 and IL-10 production was clearly an adjuvant effect. Most importantly, this adjuvant effect was seen within 24 hr of exposure to T-CHO in culture.
As we were unable to detect IL-4 in T-CHO-stimulated cultures, our findings are in apparent disagreement with the generally held view that IL-4 is the major inducer of, or is required for, Th-2 responses.22,25 Although we do not rule out the remote possibility that we failed to detect low levels of IL-4 in cultures due to technical reasons, we propose that IL-6 plays a major role in the initiation and maintenance of Th-2 responses in T-CHO immunized mice. This conclusion is compatible with reports demonstrating the induction of Th-2 responses in the absence of IL-4, including IL-4−/− knockout mice.8–12
Our data support and extend reports that show IL-6 as an important early response agent that determines the type and nature of the adaptive response. In murine T. crassiceps infections, IL-6 was the major cytokine during the early stages of infection (up to 4 weeks) and fluctuated inversely with IL-10, while above normal IL-4 levels were seen only at or after 4 weeks of infection.7 Rincon et al.26 have shown that IL-6 is able to initiate an early Th2 response by polarization of naive CD4+ T cells to effector Th2 cells via induction of endogenous IL-4 production and simultaneous antagonism of IL-12-mediated differentiation of Th1 cells. However, endogenous IL-4 cannot account for Th2 responses in IL-4−/− knockout mice.9,12 Although IL-6 does not appear to be essential for Th2 responses,27 it may function either as an alternative to IL-4 or as a down-modulator of Th1 responses14,28 or both. In mice immunized with S. mansoni eggs, the down-regulatory effect of IL-6 on Th1 responses was attributed to induction of IL-6 by IL-12, but the ability of IL-12 knockout mice to produce > 50% of the IL-6 produced by wild-type mice14 suggests alternative mechanisms of IL-6 induction by S. mansoni eggs.
We are of the opinion that the role of IL-6 in parasitic infections is more general and important than hitherto recognized. Supporting this view are studies that show high levels of IL-6 in T. crassiceps infections,29,30 the increase in the number of gut cells expressing IL-6 mRNA in sheep immunized with Trichosirongylus colubrofonizis,31 the association between IL-6 and host immunity in mice infected with Nippostrongylus brasiliensis,32 S. mansoni-induced synthesis of IL-6 in pulmonary microvascular endothelial cells,33 the requirement of IL-6 in host resistance to Trypanosoma cruzi infections,34 and the correlation between local and systemic IL-6 levels and inflammatory responses in experimental35 and in human schistosomiasis.36
Our observation that Taenia carbohydrates had Th2-like adjuvant activity against an unrelated antigen is analogous to the report showing schistosome egg antigens down-regulating Th1 responses and elevating Th2 responses to an unrelated foreign antigen.37 Okano et al.13,15 also found that carbohydrates of soluble egg antigens of S. mansoni induced strong IgE and IgG1 responses to human serum albumin. We propose that adjuvant activity of complex carbohydrates is a more general property, not limited to parasite carbohydrates, as demonstrated by the Th2 adjuvant activity of fungal cell wall β1-3 glucans.18
The molecular mechanism(s) of parasite carbohydrate-induced Th2 responses in parasitic infections remains largely unknown, except for the emphasis placed on the balance between key Th1/Th2 cytokines.4–7,14 In the schistosome model, we have postulated that the nuclear high-mobility group-2 protein (HMG-2) may function as a putative nuclear receptor for parasite carbohydrates and may up-regulate cytokine expression at transcription level.17 We have extended these studies to T-CHO and found that T-CHO indeed altered transcription of HMG-2 protein in cultured spleen cells (data not shown). This is also in agreement with the reported up-regulation of IL-6 (with IL-1, tumour necrosis factor-α, IL-8, but not IL-10) synthesis in human monocytes by the related high mobility group-1 (HMG-1) protein38 and by fungal glucans in human fibroblasts.23 Our data also show a role for serine phosphorylation and Bcl-2 up-regulation in parasite carbohydrate induced Th2 responses. In agreement with reports that show IL-6 functioning in association with Bcl-2,39–44 we would like to speculate that selective proliferation or longer survival of Th2 cells could contribute to the Th2 effect of parasite carbohydrates, mediated via IL-6, in addition to its Th1 down-modulatory function. Our observations that T-CHO and staurosporine had opposite effects on IL-2 and IL-6 secretion in T-CHO-stimulated spleen cells, and that T-CHO induced serine phosphorylation and up-regulated Bcl-2 expression, implicate multiple cell types and signalling mechanisms in Taenia carbohydrate-induced Th2-like responses.
Acknowledgments
This study was supported by a grant (NP/2000/09 to S.D.) from the Faculty of Medicine and Health Sciences, UAE.
References
- 1.Urban JF, Finkelman FD, Shea-Donohue T, Gause WC. Cytokine immunomodulation of infectious diseases. In: Kresina TF, editor. Immunomodulatory Agents. New York, Basel, Hong Kong: Marcel Dekker Inc; 1998. pp. 169–86. [Google Scholar]
- 2.Dissanayake S, Zheng HJ, Dreyer G, et al. Evaluation of a recombinant filarial antigen for diagnosis of lymphatic filariasis. Am J Hyg Trop Med. 1994;50:727–34. doi: 10.4269/ajtmh.1994.50.727. [DOI] [PubMed] [Google Scholar]
- 3.Hakangard C, Deelder AM, Gabone RM, Nilsson LA, Ouchterlony O. A comparative study on specific antibodies and circulating antigen (CAA) in serum and parasitological findings for diagnosis of Schistosomiasis mansoni in an endemic area in Tanzania. Acta Tropica. 1996;61:213–22. doi: 10.1016/0001-706x(95)00146-6. [DOI] [PubMed] [Google Scholar]
- 4.Gryzch JM, Pearce EJ, Cheever A, Caulada P, Caspar S, Hieny S, Lewis F, Sher A. Egg deposition is a major stimulus for the production of Th-2 cytokines in murine Schistosoma mansoni. J Immunol. 1991;146:1322–7. [PubMed] [Google Scholar]
- 5.Vella AT, Pearce EJ. CD+ Th-2 response induced by Schistosoma mansoni eggs develops rapidly through an early transient Th0 like stage. J Immunol. 1992;148:2283–90. [PubMed] [Google Scholar]
- 6.Wynn TA, Eltoum I, Cheever AW, Lewis FA, Gause WC, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–40. [PubMed] [Google Scholar]
- 7.Terrazas LI, Bojalil R, Govezensky T, Larralde C. Shift from an early protective Th-1 immune response to a late permissive Th-2 type response in murine cysticercosis (Taenia crassiceps) J Parasitol. 1998;84:74–81. [PubMed] [Google Scholar]
- 8.Brewer JM, Conacher A, Satoskar A, Bluethmann H, Alexander J. In interleukin-4 deficient mice, alum not only generates T-helper 1 responses equivalent to Freund's adjuvant, but continues to induce T helper-2 cytokine production. Eur J Immunol. 1996;26:2062–6. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 9.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombaccher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen specificTh-2 responses in the absence of IL-4 or IL-13 signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 10.Jankovic D, Kulberg MC, Noben-Trauth N, Caspar P, Ward JM, Cheever AW, Paul WE, Sher A. Schistosome infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol. 1999;163:337–42. [PubMed] [Google Scholar]
- 11.Kropf P, Schopf LR, Chung CL, Xu D, Liew FY, Sypek JP, Muller L. Expression of Th-2 cytokines and the stable Th-2 marker ST2L in the absence of IL-4 during Leishmania major infection. Eur J Immunol. 1999;29:3621–8. doi: 10.1002/(SICI)1521-4141(199911)29:11<3621::AID-IMMU3621>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Okano M, Satoskar AR, Abe M, et al. Interleukin 4 independent production of Th-2 cytokines by nasal lymphocytes and nasal eosinophilia in murine allergic rhinitis. Allergy. 2000;55:723–31. doi: 10.1034/j.1398-9995.2000.00429.x. [DOI] [PubMed] [Google Scholar]
- 13.Okano M, Satoskar AR, Nishizaki K, Abe M, Harn DA. Induction of Th-2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J Immunol. 1999;163:6712–17. [PubMed] [Google Scholar]
- 14.La Flamme AC, MacDonald AS, Pearce EJ. Role of IL-6 in directing the initial immune response to schistosome eggs. J Immunol. 2000;164:2419–26. doi: 10.4049/jimmunol.164.5.2419. [DOI] [PubMed] [Google Scholar]
- 15.Okano M, Satoskar AR, Nishizaki K, Harn DA. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th-2 type response. J Immunol. 2001;167:442–50. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- 16.Ip CC, Manam V, Hepler R, Hennessey JP., Jr Carbohydrate composition analysis of bacterial polysaccharides: optimized acid hydrolysis conditions for HPAEC-PAD analysis. Anal Biochem. 1992;201:343–9. doi: 10.1016/0003-2697(92)90349-c. [DOI] [PubMed] [Google Scholar]
- 17.Dissanayake S. Immunomodulation by parasites: High Mobility Group 2 (HMG-2) protein is a putative intracellular mediator for fucosylated sugars of Schistosoma mansoni. Mol Immunol. 2002;38:911–19. doi: 10.1016/s0161-5890(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 18.Ormstad H, Groeng EC, Lovik M, Hetland G. The fungal cell wall component beta 1-3 glucan has an adjuvant effect on the allergic response to ovalbumin in mice. J Toxicol Environ Health A. 2000;61:55–67. doi: 10.1080/00984100050116780. [DOI] [PubMed] [Google Scholar]
- 19.Cetre C, Pierrot C, Cocude C, Lafitte S, Capron A, Capron M, Khalife J. Profiles of Th-1 and Th-2 cytokines after primary and secondary infection by Schistosoma mansoni in the semipermissive rat. Infect Immunity. 1999;67:2713–19. doi: 10.1128/iai.67.6.2713-2719.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binder J, Graser E, Hancock WW, Wasowska B, Sayegh MH, Volk HD, Kupiec-Weglinski JWB. Downregulation of intragraft IFN-gamma expression correlates with increased IgG1 alloantibody response following intrathymic immunomodulation of sensitized rat recipients. Transplantation. 1995;60:1516–24. doi: 10.1097/00007890-199560120-00025. [DOI] [PubMed] [Google Scholar]
- 21.Gracie JA, Bradley JA. Interleukin-12 induces interferon-gamma-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26:1217–21. doi: 10.1002/eji.1830260605. [DOI] [PubMed] [Google Scholar]
- 22.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes (review) Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 23.Kougias P, Wei D, Rice PJ, Ensley HE, Kalbfleish J, Williams DL, Browder WI. Normal human fibroblasts express pattern recognition receptors for fungal (1-3)-β-d glucans. Infect Immun. 2001;69:3933–8. doi: 10.1128/IAI.69.6.3933-3938.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van der Kleij D, Remotortere V, Schuitemaker JH, Kapsenberg ML, Deelder AM, Tielens AG, Hokke CH, Yazdanbakhsh M. Triggering of innate immune responses by schistosome egg glycolipids and their carbohydrate epitope GalNAc beta 14 (Fuc alpha 1-2Fuc alpha 1-3) GlcNAc. J Infect Dis. 2002;185:531–09. doi: 10.1086/338574. [DOI] [PubMed] [Google Scholar]
- 25.Noben-Trauth N, Hu-Li J, Paul WE. Conventional naïve CD4+ T cells provide an initial source of IL-4 during Th-2 differentiation. J Immunol. 2000;165:3620–5. doi: 10.4049/jimmunol.165.7.3620. [DOI] [PubMed] [Google Scholar]
- 26.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin 6 (IL-6) directs the differentiation of IL-4 producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Flamme AC, Pearce EJ. The absence of IL-6 does not affect Th-2 cell development in vivo, but does lead to impaired proliferation, IL2 receptor expression and B cell responses. J Immunol. 1999;162:5829–37. [PubMed] [Google Scholar]
- 28.Diehl S, Anguita J, Hoffmeyer A, Zaptoon T, Ihle JN, Fikrig E, Rincon M. Inhibition of Th-1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–15. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 29.Terrazas LI, Bojalil R, Rodriguez-Sosa M, Govezensky T, Larralde C. Taenia crassiceps cysticercosis; a role for prostaglandin E2 in susceptibility. Parasitol Res. 1999;85:1025–31. doi: 10.1007/s004360050676. [DOI] [PubMed] [Google Scholar]
- 30.Morales–Montor J, Baig S, Mitchell R, Deway K, Hallal-Calleros C, Damian RT. Immunoendocrine interactions during chronic cysticercosis determine male mouse feminization: role of IL-6. J Immunol. 2001;167:4527–33. doi: 10.4049/jimmunol.167.8.4527. [DOI] [PubMed] [Google Scholar]
- 31.Shen J, Bao SJ, McClure SJ, Emery DL, Husband AJ. Interleukin 6 expression in gut of parasite challenged sheep. Vet Immunol Immuopathol. 2000;76:163–8. doi: 10.1016/s0165-2427(00)00201-4. [DOI] [PubMed] [Google Scholar]
- 32.Bao S, Cole N, Wilcox M, Beagley K, Zhou Y, Husband AJ. Differential interleukin 6 mRNA expression in Nippostongylus brasiliensis infection of susceptible and resistant strains of mice. Immunol Cell Biol. 2000;78:646–8. doi: 10.1046/j.1440-1711.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- 33.Angeli V, Faveeuw C, Delerive P, et al. Schistosoma mansoni induces the synthesis of IL-6 in pulmonary microvascular endothelial cells: role of IL-6 in the control of lung eosinophilia during infection. Eur J Immunol. 2001;31:2751–61. doi: 10.1002/1521-4141(200109)31:9<2751::aid-immu2751>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Gao W, Pereira MA. Interleukin-6 is required for parasite specific response and resistance to Trypanosoma cruzi. Int J Parasitol. 2002;32:167–70. doi: 10.1016/s0020-7519(01)00322-8. [DOI] [PubMed] [Google Scholar]
- 35.Khalil RM, Hultner I, Mailhammer R, Luz A, Moeller J, Mohamed AA, Omran S, Dormer P. Kinetics of interleukin-6 production after experimental infection of mice with Schistosoma mansoni. Immunology. 1996;89:256–61. doi: 10.1046/j.1365-2567.1996.d01-737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Jesus AR, Silva A, Santana LB, et al. Clinical and immunologic evaluation of 31 patients with acute Schistosomasis mansoni. J Infect Dis. 2002;185:98–105. doi: 10.1086/324668. [DOI] [PubMed] [Google Scholar]
- 37.Kullberg MC, Pearce EJ, Hieny SE, Sher A, Berzofsky JA. Infection with Schistosoma mansoni alters Th-1/Th-2 cytokine responses to a non-parasite antigen. J Immunol. 1992;148:3264–70. [PubMed] [Google Scholar]
- 38.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. J Immunol. 1997;158:5791–6. [PubMed] [Google Scholar]
- 40.Rollawagen FM, Yu ZY, Li YY, Pacheco ND. IL-6 rescues enterocytes from hemorrhage induced apoptosis in vivo and in vitro by a bcl-2 mediated mechanism. Clin Immunol Immunopathol. 1998;89:205–13. doi: 10.1006/clin.1998.4600. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka H, Matsumur I, Nakajima K, et al. GATA-1 blocks IL-6 induced macrophage differentiation and apoptosis through the sustained expression of Cyclin D1 and Bcl-2 in a murine myeloid cell line M1. Blood. 2000;95:1264–73. [PubMed] [Google Scholar]
- 42.Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, Shen X, Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x (L) proteins. Oncogene. 2002;21:32–43. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto Y, Hosotani R, Doi R, et al. Interleukin-6 inhibits radiation induced apoptosis in pancreatic cells. Anti Cancer Res. 2001;21:2449–56. [PubMed] [Google Scholar]
- 44.Kovalovich K, DeAngelis R, Greenbaum LE, Ciliberto G, Taub R. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2 and Bcl-xL. J Biol Chem. 2001;276:26605–13. doi: 10.1074/jbc.M100740200. [DOI] [PubMed] [Google Scholar]