Abstract
Understanding the difference between the development of a productive T-cell response and tolerance is central to discerning how the immune system functions. Intravenous injection of soluble protein is thought to mimic the presentation of self-serum and orally introduced antigens. It is generally toleragenic. The current view is that this outcome reflects the failure of ‘immunogenic’ dendritic cells to relocate to the T-cell zone of the secondary lymphoid tissues. Here, using a peptide/I-Ek tetramer and antibodies to stain splenic sections, we showed that antigen-specific T cells were activated in the spleen within hours of injection or feeding of protein. The activated T cells were found to be located at the T–B junction, the bridging zone and the B-cell area, interacting directly with B cells. In addition, B cells gain the ability to present antigen. Our results suggest a way for T cells to be stimulated by blood-borne antigen presented by naïve B cells, a potential mechanism of tolerance induction.
Introduction
It has been suggested that the nature of the antigen-presenting cell (APC) plays a critical role in determining whether a T-cell encounter with antigen elicits tolerance or a productive T-cell response.1–4 Studies based on cell-transfer experiments using cultured, antigen-loaded cells, have suggested that resting B cells and fibroblasts can induce tolerance,5–7 whereas mature dendritic cells (DCs) can prime a T-cell response.3,8,9 More recent experiments have favoured the notion that different lineages of DCs, or different activation states of these cells, are responsible for either priming or tolerance.10 Antigen administration that fails to induce activation and/or migration of DCs leads to tolerance.2–4,11 It is assumed that the site of induction of a toleragenic or immunogenic response is the T-cell area of the secondary lymphoid organs. This is supported by the observation that when recipients of ovalbumin (OVA)-specific T-cell receptor (TCR) transgenic CD4 cells are immunized with OVA in adjuvant, the transgenic T cells in the lymph node first proliferate in the T-cell zone before migrating into the B-cell follicles 2 days later.12 In contrast, when mice are given OVA peptide in saline intravenously, antigen-specific T cells remain in the T-cell area of the lymph node when examined 3 days later.13
The introduction of soluble protein antigen through the blood has long been established as a model for studying the mechanism of T-cell tolerance induction. This system appears to mimic the presentation of self-serum proteins and orally administered antigens. This route of antigen administration allows almost immediate access of antigen to B cells, DCs and macrophages in the spleen,14 a lymphoid organ that seems dedicated to processing blood-borne antigens. However, it is not clear whether tolerance induction is caused by selective antigen presentation by a subset of APCs. An understanding of this process requires the ability to follow antigen-specific T cells to determine where they encounter antigen and what APCs they are in contact with after antigen administration.
In the past, we have administrated pigeon cytochrome c protein (cyt c) to induce peripheral T-cell tolerance15 in mice transgenic for the TCR β-chain of the 5C.C7 T-cell clone. Here, we report a histological analysis of early events in the spleen in this tolerance-induction model. Within a few hours after antigen administration, B cells acquire the ability to present antigen to T cells, and activated T cells can be found contacting B cells at the T–B cell border, the bridging zone and the B-cell area of splenic follicles. Lack of costimulatory molecules such as B7.1 or 7.2 (CD80, CD86) on naive B cells16,17 has been correlated with their inability to induce immunogenic responses.6,18 This finding suggests that early T–B cell contact may be an important step in tolerance induction by blood-borne antigens.
Materials and methods
Mice and antigen administration
B10.BR (The Jackson Laboratory, Bar Harbor, ME), 5C.C7 TCR-β19 and TCR-αβ chain20 transgenic mice (on the B10.BR background) (all 8–10 weeks of age) were injected intravenously (into the retro-orbital venous sinus) or fed by intragastric gavage (20-gauge feeding needles) with phosphate-buffered saline (PBS) containing pigeon cyt c (Sigma, St Louis, MO) or with PBS alone. The cyt c protein was size-purified by centrifugation (5 min at 23 000 g) with centricon 10 filters (Millipore, Bedford, MA), to remove peptide fragments. Animals were handled in accordance with NIH guidelines. The animal facility at Stanford University is accredited by the Association for Assessment of Accreditation of Laboratory Animal Care.
Tetramer staining of splenic sections
Spleen from 5C.C7 TCR-αβ transgenic mice injected intravenously with cyt c in PBS was embedded in warm (65°) 4% (wt/vol) agarose (Invitrogen, Carlsbad, CA) in PBS and cooled at 4° for 5 min. Sections of 400 µm were cut in PBS using a vibrating microtome (microtome series 1000). Sections were layered onto glass slides (SuperFrost plus; Fisher Scientific, Nepean, Ontario, Canada) and blocked for 30 min with 0·1% bovine serum albumin (BSA) in PBS. Phycoerythrin (PE)-conjugated moth cytochrome c (MCC) 88-103/I-Ek tetramer was generated according to our previously published protocol.15 Sections were stained with PE-conjugated MCC 88-103/I-Ek tetramer and fluorescein isothiocyanate (FITC)-conjugated anti-B220 (RA3-6B2; Pharmingen, San Diego, CA) in 0·1% BSA/PBS for 60 min at room temperature. Slides were viewed and analysed using a Multiprobe 2010 laser-scanning confocal microscope, ImageSpace (Molecular Dynamics, Sunnyvale, CA) and photoshop (Adobe).
In addition, spleens were embedded in Tissue-tek OCT and snap-frozen in a bath of 2-methylbutane immersed in liquid nitrogen. Cryosections (of 6 µm) were cut within 1 hr of freezing and fixed with 2% paraformaldehyde for 90 seconds on Superfrost plus (Fisher Scientific) glass slides. Sections were incubated with PE-conjugated rat anti-mouse B220 (Pharmingen) at 2·5 µg/ml and horseradish peroxidase (HRP) conjugated MCC 88-103/I-Ek tetramer for 1 hr at room temperature, then developed using the tyramine signal amplification (TSA)-direct green signal amplification (NEN Life Science Products, Boston, MA) for 3 min and analysed using a Nikon (Melville, NY) laser-scanning confocal microscope and Bio-Rad (Hercules, CA) Lasarsharp 3·1.
While the cyt c-specific T cells can be identified using the tetramer reagent in the 400-µm, ‘live’, unfixed spleen sections as well as in cryosections fixed by a brief treatment with paraformaldehyde, no signal can be detected on spleen sections fixed with acetone, formaldehyde, or glutaraldehyde (data not shown).
Immunohistochemical analysis of cryosections
Air-dried spleen cryosections (6–7 µm) were acetone-fixed twice, dried overnight and blocked with 0·1% BSA/10% normal mouse serum/1 : 100 Fc block (CD16/CD32; 2·4G2; Pharmingen) for 90 min. They were stained with PE-conjugated anti-B220 (RA3-6B2; Pharmingen) and biotinylated anti-CD69 (H1·2F3) in PBS/0·1% BSA for 2 hr and then counterstained with avidin–HRP for 30 min. Slides were developed using the TSA-direct green signal amplification (NEN Life Science Products) for 3 min and analysed using an BX60 microscope (Olympus, Melville, NY).
Flow cytometry
A total of 1 × 106 cells from the spleen or lymph node were stained with avidin–PE-coupled MCC 88-103/I-Ek tetramer, as described previously.15 In addition, antibodies against CD4 (PE-conjugated GK1·5), B220 (cy-chrome conjugated RA3-6B2; Pharmingen), CD69 (FITC-conjugated H1·2F3; Pharmingen), CD86 (PE-conjugated GL1; Pharmingen) and Vα chain of the 5C.C7 TCR-Vα11 (PE-conjugated RR8-1), were used. Propidium iodide (PI) at 1 µg/ml was added in the last wash. Cells were analysed on a Becton-Dickinson (San Diego, CA) fluorescence-activated cell sorter (FACScan) apparatus (Stanford University FACS Facility). A total of 50 000 events was collected. PI-positive cells were excluded from the analysis.
In vitro antigen-presentation assay
Mice were injected intravenously with either PBS or a single 70-µg dose of cyt c protein in PBS, and killed 4 hr later. Splenic B cells were purified by MACS sorting to >96% purity using anti-B220, anti-rat immunoglobulin beads and MS columns (Miltenyi Biotec, Auburn, CA). A total of 2 × 105 total spleen cells or 2 × 105 purified splenic B cells were incubated with 1 × 105 of 5C.C7 αβ TCR transgenic lymph node cells in 200 µl of complete RPMI medium. After 12 hr, cells were stained with antibodies against CD69 (coupled with FITC), CD4 (coupled with PE), and B220 (coupled with cy-chrome). B220- and PI-positive cells were excluded from the analysis.
Results
Intravenous cyt c administration induces rapid antigen-specific T-cell activation and T–B cell contact
Previously, we have fed cyt c to 5C.C7 TCR-β-chain transgenic mice to study the mechanism of oral tolerance induction. These mice have normal T-cell development and T-cell compartments, as well as small intestine that is indistinguishable from that of normal mice on histological analysis (data not shown). The 5C.C7 TCR-β-chain transgenic mice have elevated frequencies of cyt c-specific T cells (3–5% of total CD4+ T cells as compared to <0·01% in normal B10.BR mice). This frequency of antigen-specific T cells is comparable to the frequency of autoreactive cells seen in some autoimmune disease models. Using an MCC 88-103/I-Ek tetrameric staining reagent, we showed that multiple feedings of ‘low dose’ (0·5 mg) cyt c protein to these mice reduced the number of antigen-specific T cells in the peripheral lymphoid organs, as well as T-cell antigen-specific interleukin-2 (IL-2) production and proliferation, as assayed in vitro. Furthermore, our results showed that the orally administered cyt c protein is rapidly distributed to, and recognized in, the peripheral lymphoid organs. Within 6 hr of cyt c administration, CD69 expression is found on antigen-specific T cells in the Peyer's patches, mesenteric lymph nodes and spleen. In addition, total spleen cells from mice fed 6 hr earlier gained the ability to stimulate cyt c-specific T cells.15 These observations suggest that orally introduced cyt c can pass directly from the gut lumen into the bloodstream, and that T-cell tolerance induction in this experimental system is similar to that induced by administration of protein antigens systemically in the absence of adjuvant.
To identify the APCs in the spleen that cyt c-specific T cells were encountering shortly after antigen administration, we sought to localize antigen-specific T cells in histological sections using an MCC 88-103/I-Ek tetramer. To establish the staining conditions, we used spleen sections from mice transgenic for the αβ TCR of the 5C.C7 T-cell clone, which has a much higher frequency of MCC 88-103/I-Ek-specific T cells (≈ 75% of the CD4+ cells). Surprisingly, we found that in splenic sections from mice that had been injected with cyt c protein 4 hr earlier, the boundary between the T- and B-cell zones had largely disappeared and the antigen-specific T cells intermingled with B cells (Fig. 1). In contrast, the T- and B-cell zones were clearly discernable in splenic sections from mice injected with PBS (Fig. 1a, 2a). In fact, as early as 1 hr after cyt c administration, T cells were observed to be in close contact with B cells in the B-cell area (Fig. 2), and the majority of these T cells expressed the activation marker CD69 (Fig. 3A).
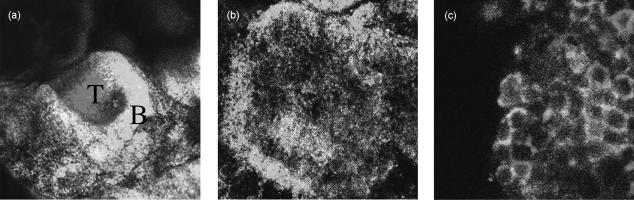
Figure 1.
Activation and migration of splenic T and B cells in response to blood-borne antigen. Sections (400 µm) of spleens from 5C.C7 αβ T-cell receptor (TCR) transgenic mice injected intravenously, 4 hr previously, with phosphate-buffered saline (PBS) alone (a), or with PBS containing 70 µg of cytochrome c protein (cyt c) (b), were double-stained with phycoerythrin (PE)-conjugated MCC 88-103/I-Ek tetramer and fluorescein isothiocyanate (FITC)-conjugated anti-B220 and viewed using a laser-scanning confocal microscope with a 10× objective (panels a and b) or a 60× objective (panel c). Antigen-specific T cells are viewed as red and B cells are green. The T-cell area is marked by ‘T’ and the B-cell area is marked by ‘B’ in panel (a). Similar results were obtained from three PBS- and three cyt c-injected animals.
Figure 2.
Intravenous cytochrome c protein (cyt c) administration induces rapid contact between activated antigen-specific T cells and B cells. Splenic cryosections from 5C.C7 αβ T-cell receptor (TCR) transgenic mice, injected 1 hr previously with phosphate-buffered saline (PBS) (a) or 70 µg cyt c in PBS (panels b and c), were co-stained with phycoerythrin (PE)-conjugated anti-B220 and horseradish peroxidase (HRP)-conjugated MCC 88-103/I-Ek tetramer (panels a and b) or with PE-conjugated anti-B220 and biotinylated anti-CD69 (c), followed by avidin HRP, and developed using the tyramine signal amplification (TSA)-direct green signal amplification kit. B cells are red and activated T cells are green. Yellow interfaces between green and red cells indicate close T–B contact. Images are representative of tissue sections from 20 PBS-injected and four cyt c-injected mice at 1 hr. A 20× objective with confocal microscopy (panels a and b) or a 60× objective with standard fluorescence microscopy (panel c) were used.
Figure 3.
Blood-borne cytochrome c protein (cyt c) induces rapid T-cell activation in the spleen. (A) Spleen cells from 5C.C7 T-cell receptor (TCR) αβ-chain transgenic mice (left panels) and β-chain transgenic mice (right panels), which had been injected intravenously with 70 µg of cyt c 1 hr (a and e), 2.5 hr (b and f), 4 hr (c and g) and 72 hr (d and h) previously, were isolated and stained with antibodies against Vα11, CD4, CD69, B220 and propidium iodide (PI). Histograms represent CD69 expression on T cells (Vα11 positive, B220 and PI negative). The dotted lines represent CD69 expression on T cells from animals injected with phosphate-buffered saline (PBS), 4 hr previously. Similar results were obtained from 20 (0 hr), three (1 hr), four (2.5 hr), eight (4 hr), and six (72 hr) 5C.C7 αβ-transgenic mice and from 19 (0 hr), four (1 hr), four (2.5 hr), six (4 hr), and four (72 hr) 5C.C7 β-transgenic mice. (B) Spleen cells from 5C.C7 TCR β-chain transgenic mice injected intravenously 4 hr previously with PBS alone (a), or with PBS containing 70 µg of cyt c (b), were stained with MCC 88-103/I-Ek tetramer, anti-CD69 and anti-B220 antibodies. PI- and B220-positive cells were excluded from the analysis.
This antigen-induced T-cell activation and movement can also be readily observed in TCR β-chain transgenic mice. Within 1–4 hr of injecting a single 70-µg dose of cyt c protein, all of the MCC 88-103/I-Ek tetramer-positive T cells, but no other cell population in the spleen, were CD69 positive in the TCR β-chain transgenic mice (Fig. 3B and data not shown). This allowed us to use CD69 staining to identify cyt c-specific T cells in the spleen at early time-points after antigen administration. We found that within 1–4 hr of injecting cyt c protein, the majority of activated T cells were at the T–B interzone, in close contact with B cells (Fig. 4 and data not shown). Similarly, feeding 0·5 mg of cyt c protein also induced antigen-specific T-cell movement towards B-cell areas within 4 hr (Fig. 4c).
Figure 4.
Injection of cytochrome c protein (cyt c) induces rapid T- and B-cell contact in the spleen of T-cell receptor (TCR) β-chain transgenic mice. Splenic cryosections from 5C.C7 TCR β-transgenic mice injected with phosphate-buffered saline (PBS) (a), or with PBS containing 70 µg of cyt c, 2.5 hr (b) and 4 hr (d) previously, or fed intragastrically with 0·5 mg of cyt c 4 hr previously (c), were stained with phycoerythrin (PE)-conjugated anti-B220 and biotinylated anti-CD69 followed by avidin horseradish peroxidase (HRP), and developed using the tyramine signal amplification (TSA)-direct green signal amplification kit. Activated T cells appear green and B cells appear red. Yellow interfaces between green and red cells indicate close T–B contact. ‘T’ indicates T-cell areas and ‘B’ indicates B-cell areas. A 10× objective (panels a, b and c) or a 60× objective (panel d) were used with standard fluorescence microscopy. Two additional experiments showed similar results. No staining was observed on spleen sections where only the TSA-direct green reagent was used (data not shown).
Splenic B cells are able to present intravenously administered cyt c
Our results suggested that T cells have the opportunity to be stimulated by B cells after antigen administration in our experimental system. To test whether B cells were in fact able to present antigen to T cells, B10.BR or 5C.C7 β-chain TCR transgenic mice were injected with a single 70-µg dose of cyt c protein and killed 4 hr later. As shown in Fig. 5, purified splenic B cells (> 96% B220+) were able to stimulate cyt c-specific T-cell responses in vitro as well as, if not better than, total spleen cells. This is consistent with our previous observation that splenic cells from mice fed intragastrically 6 hr earlier with a single dose of 5 mg of cyt c protein can stimulate cyt c-specific transgenic T cells in vitro.15 This is despite the fact that there is no increase in the number of cyt c-specific B cells in either TCR-β or -αβ transgenic mice, as compared to normal mice (data not shown).
Figure 5.
Splenic B cells from mice injected with cytochrome c protein (cyt c) stimulate cyt c-specific T cells in vitro. A total of 1 × 105 5C.C7 αβ T-cell receptor (TCR) transgenic lymph node cells were cocultured for 12 hr with 2 × 105 total splenic cells (panels a and b) or with 2 × 105 B220-enriched splenic cells (panels d and e) isolated from B10.BR mice injected with phosphate-buffered saline (PBS) (a and d) or with a single dose of 70 µg of cyt c protein (b and e) 4 hr previously or 5C.C7 αβ TCR transgenic lymph node cells cultured in media alone (c). Cells were stained with antibodies against CD69 [coupled to fluorescein isothiocyanate (FITC)], CD4 [coupled to phycoerythrin (PE)] and B220 (coupled with cy-chrome), to analyse for CD69 expression on CD4-positive T cells. Three experiments showed similar results.
Discussion
Previously, we have shown that oral as well as intravenous administration of cyt c protein induces rapid T-cell activation and tolerance by systemic presentation of this antigen.15 Here, we show that within 1 to 4 hr of antigen administration, activated T cells are found in close proximity to B cells at the T–B junction of splenic follicles. A productive T-cell response generally requires the engagement of both the TCR and costimulation molecules. Naïve B cells do not express the costimulation molecules B7.1 or 7.2, and cyt c injection does not induce B7 expression on splenic B cells (data not shown). Thus, a toleragenic signal will probably result from early contacts observed between newly activated T cells and naïve B cells.
While it is recognized that naïve T cells responding to antigen presented by naïve B cells do not result in a productive response from either cell type, the significance of this interaction to in vivo tolerance induction is not clear. It has been assumed that T cells do not come into contact with B cells in the early phase of the response. Even so, if T cells were to come into contact with B cells, non-antibody-directed antigen uptake by B cells has been reported to be very inefficient21 and thus antigen presentation would be poor. In contrast, we have shown that after protein injection, antigen-specific T cells move rapidly into the B-cell zones.
Our data also show that naïve splenic B cells, which do not have a higher-than-normal frequency of anti-cyt c specificity, can efficiently process and present blood-borne cyt c protein. This finding is consistent with the observation that nearly all B cells in the spleen of CBA/J mice have substantial levels of hen egg lysozyme (HEL) peptide/I-Ak complex on their surface as early as 1–2 hr after intravenous administration of HEL. Interestingly, after HEL injection, peptide–major histocompatibility complex (MHC) complexes can be detected on naïve B cells earlier than on DCs.14,22 Our observation, that antigen-specific T cells are in fact encountering naïve B cells, is a significant step towards recognizing naïve B cells as the cell population with the greatest potential to establish tolerance to self-serum proteins. However, this observation does not imply that B cells are the only APCs that are capable of inducing tolerance. On the contrary, it adds to the large body of literature showing that antigen targeted to a variety of different APCs lacking appropriate costimulation can induce peripheral tolerance.23–25
Recently, it has been reported that commercially available stocks of proteins, such as HEL, BSA, OVA and collagens are contaminated with lipopolysaccharide (LPS). This has raised the issue of the appropriateness of using these proteins to mimic the induction of tolerance by self-serum proteins. While the display of processed HEL/I-Ak complexes on splenic DCs after intravenous injection of HEL protein was not observed when HEL protein with low endotoxin levels or LPS hypo-responsive mice were used, substantial levels of HEL/I-Ak were found on all B cells, irrespective of the LPS effect.14
Previously, it has been observed that when peptide is injected intravenously, B cells and DCs in the spleen have similar levels of peptide loaded on their surface.14 Thus, a previous report showing that adoptively transferred T cells remain in the T-cell zone after peptide injection13 may be because peptide rather than protein antigen was administered. Also, there may be a fundamental difference between our system, using TCR single-chain transgenic mice, and a system where T cells are first purified and then adoptively transferred into hosts.
The early and rapid migration of activated T cells into the B-cell zone after oral or intravenous administration of antigen in our 5C.C7 TCR β-chain transgenic mice is very different from the T- and B-cell movement observed after immunization, where T cells first proliferate in the T-cell zone in the lymph nodes before migrating into the B-cell follicles 2 days later.12 Recently, chemokines have been identified that are important for the induction of T- and B-cell movement after immunization regimens.26–28 Based on the findings presented here, we speculate that a unique set of chemokines and/or their receptors might be induced in response to soluble antigen administration, accounting for this T-cell movement.
Acknowledgments
We thank Jason Cyster and Rodolfo Chaparro, for advice and critical reading of the manuscript, Pete Savage, Cenk Sumen, Lawrence Wu for helpful advice in preparing reagents, Nelida Prado for animal care and Kevin Montegrande for graphics assistance. This work was supported by grants from the NIH (Y.C), NIH-DK07056 (J.M.D) and HHMI (M.M.D).
Abbreviations
- APC
antigen-presenting cell
- BSA
bovine serum albumin
- cyt c
cytochrome c
- DC
dendritic cell
- FITC
fluorescein isothiocyanate
- HEL
hen-egg lysozyme
- HRP
horseradish peroxidase
- IL
interleukin
- LPS
lipopolysaccharide
- MCC
moth cytochrome c peptide
- OVA
ovalbumin
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PI
propidium iodine
- TCR
T-cell receptor
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 3.Fazekas de St. Groth BF. The evolution of self-tolerance: a new cell arises to meet the challenge of self-reactivity. Immunol Today. 1998;19:448–54. doi: 10.1016/s0167-5699(98)01328-0. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation [comment] J Exp Med. 1999;189:611–4. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–9. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 6.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–8. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croft M, Joseph SB, Miner KT. Partial activation of naive CD4 T cells and tolerance induction in response to peptide presented by resting B cells. J Immunol. 1997;159:3257–65. [PubMed] [Google Scholar]
- 8.Levin D, Constant S, Pasqualini T, Flavell R, Bottomly K. Role of dendritic cells in the priming of CD4+ T lymphocytes to peptide antigen in vivo. J Immunol. 1993;151:6742–50. [PubMed] [Google Scholar]
- 9.Sousa CR, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas [see comments] J Exp Med. 1997;186:1819–29. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shortman K, Heath WR. Immunity or tolerance? That is the question for dendritic cells. Nat Immunol. 2001;2:988–9. doi: 10.1038/ni1101-988. [DOI] [PubMed] [Google Scholar]
- 11.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM. High levels of a major histocompatibility complex II-self peptide complex on dendritic cells from the T cell areas of lymph nodes. J Exp Med. 1997;186:665–72. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–9. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 13.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–39. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 14.Reis e Sousa C, Germain RN. Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J Immunol. 1999;162:6552–61. [PubMed] [Google Scholar]
- 15.Gutgemann I, Fahrer AM, Altman JD, Davis MM, Chien YH. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity. 1998;8:667–73. doi: 10.1016/s1074-7613(00)80571-3. [DOI] [PubMed] [Google Scholar]
- 16.Inaba K, Witmer-Pack M, Inaba M, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–60. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace PM, Rodgers JN, Leytze GM, Johnson JS, Linsley PS. Induction and reversal of long-lived specific unresponsiveness to a T-dependent antigen following CTLA4Ig treatment. J Immunol. 1995;154:5885–95. [PubMed] [Google Scholar]
- 18.Boussiotis VA, Gribben JG, Freeman GJ, Nadler LM. Blockade of the CD28 co-stimulatory pathway: a means to induce tolerance. Curr Opin Immunol. 1994;6:797–07. doi: 10.1016/0952-7915(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen JL, Esser U, Fazekas de St. Groth B, Reay PA, Davis MM. Mapping T-cell receptor–peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992;355:224–30. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- 20.Fazekas de St Groth B, Patten PA, Ho WY, Rock EP, Davis MM. An analysis of T-cell receptor–ligand interactions using a transgenic antigen model for T-cell tolerance and T-cell receptor mutagenesis. In: Alt FW, Vogel AH, editors. Molecular Mechanisms of Immunological Self-Recognition. San Diego, CA: Academic Press; 1992. [Google Scholar]
- 21.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 22.Zhong G, Sousa CR, Germain RN. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen–major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro. J Exp Med. 1997;186:673–82. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JF, Horahan G, Allison J, Bhathal PS, Cox K. T-cell tolerance in transgenic mice express major histocompatibility class I molecules in defined tissue. Immunol Rev. 1989;107:109–23. doi: 10.1111/j.1600-065x.1989.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 24.Schonrich G, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, Arnold B. Distinct mechanisms of extrathymic T cell tolerance due to differential expression of self antigen. Int Immunol. 1992;4:581–90. doi: 10.1093/intimm/4.5.581. [DOI] [PubMed] [Google Scholar]
- 25.Ferber I, Schonrich G, Schenkel J, Mellor AL, Hammerling GJ, Arnold B. Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science. 1994;263:674–6. doi: 10.1126/science.8303275. [DOI] [PubMed] [Google Scholar]
- 26.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 27.Tang HL, Cyster JG. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–22. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- 28.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines [In Process Citation] J Exp Med. 1999;190:1123–34. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]