Abstract
The haematopoietic homeobox gene Hex (also called Prh) is expressed in myeloid cells and B cells but not T cells. To investigate whether Hex levels might play a role in myeloid versus T-cell development, two types of transgenic mouse lines were constructed, each with ectopic expression of Hex in T cells (CD11a/Hex and Lck/Hex). Both these types of transgenic mouse had the same defects in T-cell maturation, indicating that proper T-cell development may be dependent not just on the up-regulation of lymphoid-specific transcriptional regulators but also on the co-ordinated down-regulation of myeloid-specific transcriptional regulators such as Hex. In addition, Hex over-expression significantly increased myeloid progenitor cycling, which may explain its role in retrovirally induced murine leukaemia.
Introduction
Homeodomain proteins make up a family of conserved transcription factors that control lineage commitment and differentiation in many tissues by regulating the location and timing of gene expression.1,2 A number of homeobox genes have been found to be expressed in a lineage or stage-specific manner in haematopoietic cells.3,4 In addition, their aberrant expression can misdirect lineage development and result in leukaemia.5–8
Hex (also called PRH) is a divergent orphan homeobox gene which is a member of the Antennapedia/Ftz class.9–11Hex plays a role in the embryonic formation of the foregut and central nervous system, and of endothelial and haematopoietic cells. Whole-mount in situ hybridization studies in early chicken embryos found Hex transcripts expressed in cells of the early endoderm, and then later in both endothelial and haematopoietic cells.12 Homozygous disruption of murine Hex resulted in embryonic lethality at day 11·5 from anterior truncation of the forebrain as well as liver and thyroid hypoplasia.13,14
Studies in zebrafish found that Hex is expressed in endothelial and blood cell precursors from an early stage.15 Its forced expression leads to premature and ectopic expression of early endothelial and blood differentiation genes like Fli1, Flk1 and Gata1. Hex is expressed in developing endothelial cells in Xenopus, and its over-expression results in an increased production of endothelial cells.16
In adult mammals, Hex is preferentially expressed in myeloid haematopoietic cells. It is expressed in a range of B-cell, granulocyte, erythroid, and monocyte progenitors and is generally down-regulated during terminal differentiation.17 However, Hex expression was not detected in any T-lymphocyte subsets, including CD4 CD8 double-negative, double-positive and single-positive T cells.9–11,17 Interestingly, in AKXD13 murine T-cell leukaemia, Hex is a common retroviral insertion site.18 Hex is located near HOX11 on chromosome 10,9 and therefore may also be dysregulated in t;(10,14) acute lymphoblastic leukaemia, when HOX11 is translocated to the TCRδ locus.7,8
To investigate the role Hex may have in myeloid versus T-cell lineage commitment and stage progression, two transgenic mouse lines were constructed. One transgenic line expresses Hex in bone marrow, spleen and thymus via the CD11a promoter19 while the second transgenic line has thymus-restricted expression using the Lck promoter.20 Both types of transgenic mice have abnormalities in T-cell development, indicating that down-regulation of this myeloid homeobox gene is important in T-cell maturation. In addition, there was a significant increase in immature, but not mature, myeloid cell proliferation in the CD11a/Hex mice, providing a potential mechanism for the finding that Hex is a retroviral insertion site in murine leukaemia.18
Methods and materials
Transgenic construction
The full-length Hex cDNA was subcloned into the BamHI site of both the CD11a transgene cassette19 and the Lck transgene cassette.20 Clones were sequenced to ensure correct orientation. To generate the CD11a/Hex transgenic mice, a 4·3-kilobase (kb) HindIII/NsiI fragment containing the CD11a-Hex transgene cassette cDNA was isolated from an agarose gel, purified using the GENECLEAN II kit (Bio 101, La Jolla, CA), extracted with phenol–chloroform and ethanol-precipitated. To generate the Lck/Hex transgenic mice, the same purification scheme was applied to a 6·1-kb NotI transgene cassette. Both purified, linearized constructs were microinjected into fertilized C3HeB/FeJ mouse blastocysts to generate transgenic founders.21
Founders were screened by Southern analysis. Genomic DNA from each mouse was digested with BglII to generate a diagnostic 3·5-kb fragment for the CD11a/Hex transgenic and a 3·0-kb fragment for the Lck/Hex mice. These digests were separated on a 1% agarose gel, Southern blotted and then probed with a 1·6-kb SmaI fragment that contains both Hex cDNA and human growth hormone sequences (Figs 1 and 2). Injection of the CD11a/Hex construct produced seven transgenic founders and injection of Lck/Hex produced six founders. Founders were crossed to a C3HeB/FeJ wild-type mouse to produce individual transgenic lines.
Figure 1.
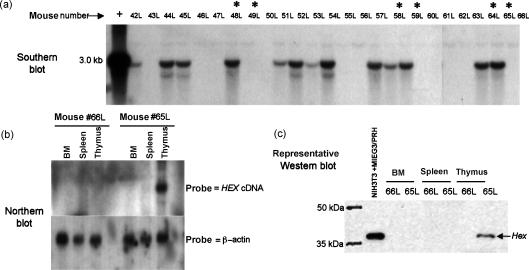
Genotype and Hex protein expression in the CD11a/Hex transgenic mice. (a) Southern blot analysis to determine whether an individual mouse carries the CD11a/Hex transgene. Transgenic mice and their wild-type littermates selected for the flow cytometry in this study are marked with an asterisk. The transgenic line from which each analysed mouse came is shown below the blot along with a legend indicating the founders crossed. (b) A representative Western blot showing Hex transgenic expression. NIH3T3 cells transiently transfected with a Hex expression vector serves as the positive control. Upon longer exposure endogenous Hex expression can be seen in wild-type mice in the marrow and to a lesser extent the spleen, but not the thymus.
Figure 2.
Genotype and Hex expression in the Lck/Hex transgenic mice. (a) Southern blot analysis on Lck/Hex transgenic mice. Mice chosen for flow cytometric analysis are indicated by an asterisk. (b) Northern blot analysis to demonstrate that Hex expression under the control of the Lck proximal promoter was thymus-specific and different from the CD11a expression pattern. Transgenic Hex mRNA is only expressed in the thymus. However, on a much longer exposure, endogenous Hex expression can be detected in the bone marrow and to a lesser extent in the spleen, but not the thymus, as expected. (c) A representative Western blot is shown here, confirming that Hex protein is only expressed in the thymus of transgenic positive mice.
Southern, Northern and Western blots were used to analyse transgene insertion and expression (Figs 1 and 2). For Western blots an affinity-purified rabbit polyclonal anti-Hex antibody was raised against a unique peptide corresponding to residues 118–135, just upstream of the Hex homeodomain.
Flow cytometry analysis
The following conjugated antibodies were purchased from BD PharMingen (San Diego, CA): Cyc-anti-CD3e (145-2C11), fluorescein isothiocyanate (FITC) -anti-CD3e (145-2C11), phycoerythrin (PE) -anti-CD4 (RM4-5), biotin-anti-CD4 (RM4-5), FITC-anti-CD8a (53-6.7), FITC-anti-CD19 (1D3), FITC-anti-Gr-1 (RB6-8C5), PE-anti-Sca-1 (D7), FITC-anti-CD41 (MWReg30), PE-anti-CD31 (MEC13.3), and PE-anti-TER-119 (TER-119). An allophycocyanin–streptavidin secondary antibody (#S-868; Molecular Probes, Eugene, OR) was used to reveal staining by biotin-conjugated antibodies. After erythrocyte lysis, 105 cells from bone marrow, spleen, thymus and erythrocyte-lysed peripheral blood were stained with 1 μg of conjugated antibody for 30 min in the dark at 4° in 50 μl of phosphate-buffered saline (PBS) with 1% albumin. After washing twice in PBS/1% albumin, data were collected on 2 × 104 events using a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA) and analysed using both cell quest (Becton Dickinson) and flowjo (Tree Star, Inc., San Carlos, CA).
Haematopoietic progenitor cell assays
Bone marrow and spleen cells from transgenic mice and their wild-type littermates were assessed for granulocyte–macrophage [colony-forming unit-granulocyte–macrophage (CFU-GM)], erythroid [burst-forming unit-erythroid (BFU-E)] and multipotential [colony-forming unit granulocyte/erythroid/monocyte/megakaryocyte (CFU-GEMM)] progenitor cells as has been described elsewhere.22 The percentage of progenitors in S-phase of the cell cycle was assessed by use of the [3H]thymidine kill technique as described previously.22 Statistical significance was determined using the Student's t-test.
TUNEL assay
Terminal deoxytransferase duTP nick and labelling assay (TUNEL) assays were performed as we previously described.23
Immunohistology
Peroxidase immunohistology was performed as previously described on thymic sections from transgenic and wild-type mice.24
Results
Tissue-specific transgenic expression of Hex
Hex is not normally expressed in T cells at any stage of differentiation.9–11,17 These experiments sought to address whether down-regulation of Hex is important for proper T-cell development from haematopoietic progenitors, which express Hex. The CD11a and Lck promoter constructs used to generate the transgenic mice were chosen because both will direct ectopic expression of Hex in the T cells. As expected, Western blot analysis in this study found that the CD11a promoter drove Hex expression in bone marrow, spleen and thymus (Fig. 1). Western blot analysis of the Lck/Hex transgenic mice found that Hex is only expressed in the transgenic thymus (Fig. 2).
Interestingly, only two of the six Lck/Hex founder mice survived to 16 months while all wild-type littermates survived. The founders that died showed the highest transgenic copy numbers as determined by densitometry of Southern blots. These mice did not breed. Post-mortem analysis of these dead founders did not reveal any pathology, including malignancy, that would explain their untimely deaths.
Since Hex is a common retroviral insertion site in some acute leukaemias in the AKXD13 murine model,18 20 CD11a/Hex and 20 Lck/Hex transgenic mice were observed for the development of neoplasia for 18 months. Only one mouse developed a malignancy over that time period. One Lck/Hex mouse developed a CD3+ CD4+ CD8− B220− T-cell lymphoma at 13 months that had involvement of all nodes, thymus, and spleen, but not the peripheral blood or marrow.
Although all transgenic lines that expressed Hex protein had the same phenotype, the analysis presented here represents data from multiple mice from two CD11a/Hex lines and one Lck/Hex line each analysed individually and compared with same sex, non-transgenic littermates (wild-type). There was no pooling of mouse tissues for analysis, but each mouse was analysed separately, and the data were then averaged and compared statistically. For all experiments Western blots were used to ensure that each mouse tested had continuous tissue-specific expression of transgenic Hex. All experiments were performed on mice not less than 3 months old and not more than 7 months old.
Thymic T-cell development in Hex transgenic mice
There were differences in weights or total cell numbers between transgenic and wild-type thymus (wild-type mice averaged 2·9 × 107 total cells, CD11a/Hex 2·4 × 107, and Lck/Hex 3·6 × 107, P = NS).
Thymocytes from transgenic and wild-type mice were concurrently stained for CD4 and CD8 expression (Fig. 3). Evaluation of CD4+ versus CD8+ T cells in the thymus of either type of Hex transgenic mice revealed a marked increase in CD8 single-positive cells. CD4 CD8 double-positive were also decreased in transgenic mice (Fig. 3). There was a six-fold increase in CD8 single-positive total thymocytes (P < 0·001), and a 1·5-fold decrease in double-positive thymocytes in CD11a/Hex transgenic mice (n = 6, P < 0·001). There was a 3·7-fold increase in CD8 single-positive thymocytes (P = 0·001) and a 1·4-fold decrease in double-positive thymocytes in the Lck/Hex transgenic mice (n = 3, P = 0·001).
Figure 3.
Flow cytometric analysis on CD3+ thymocytes from CD11a/Hex and control mice. Six CD11a/Hex with six wild-type littermate controls and three Lck/Hex with three wild-type littermate controls were analysed. The average percentage of thymocytes that fell within the indicated gates are placed adjacent to each gate. Each mouse was analysed separately, and then the distinct histograms were computer-merged to generate the individual histograms in this figure.
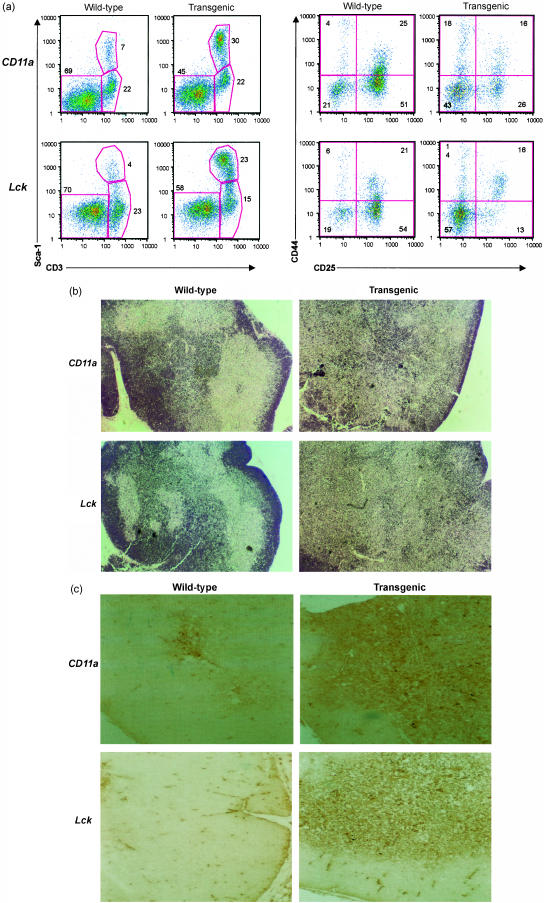
To analyse the effect of Hex on the earliest T-cell progenitors, the relative percentage of CD25- and CD44-positive, CD4 CD8 double-negative thymocytes was analysed. In the CD4 CD8 double-negative population, there was a two-fold decrease in the relative percentage of CD25+ CD44− thymocytes in the CD11a/Hex mice (Fig. 4, n = 3, P = 0·001) and a 4·1-fold decrease of the CD25+ CD44+ thymocytes in the Lck/Hex mice as compared to wild-type controls (Fig. 4, n = 4, P = 0·001). There was a concomitant two-fold increase in CD4 CD8 double-negative cells that were CD25− CD44− in the CD11a/Hex mice, and a three-fold increase in these cells in Lck/Hex mice (Fig. 4).
Figure 4.
(a) Flow cytometric analysis of transgenic T-cell progenitors. Thymocytes from six transgenic mice and six wild-type littermates were stained with Sca1(Ly-6A/E) and CD3, or CD4/8 and CD25/44. Numbers (means ± standard deviations) indicate the percentage of total thymocytes that fall within the indicated gates. Each mouse was analysed separately, and then the distinct histograms were computer-merged to generate this figure. (a) In the four panels on the left, thymocytes from CD11a or Lck/Hex transgenic mice were analysed for expression of Sca1, a myeloid marker. The transgenic CD3+ thymocytes had retention of the Sca1 myeloid marker. In the four panels on the right CD4 CD8 double-negative thymocytes were analysed for expression of CD25 and CD44, the earliest markers of T-cell progenitors. Transgenic mice had decreases in the CD25+ T-cell progenitors and an increase in CD25− CD44− cells. The average percentage of thymocytes that fell within the indicated gates are placed adjacent to each gate. Each mouse was analysed separately, and then the distinct histograms were computer-merged to generate the individual histograms in this figure. (b) There was disruption of the thymic architecture in Hex transgenic mice. H & E stains of wild-type and Hex transgenic thymus showing the disruption of the medullary–cortical boundaries in the transgenic mice. (c) Immunohistological staining of wild-type and transgenic thymus with Sca1 indicating a marked increase in Sca1+ cells.
We next tested whether myeloid markers remained present in the developing transgenic T cells. Sca1 is a marker of myeloid haematopoietic progenitors that remains present on early T-cell progenitors, but then generally decreases in expression as the T cells mature. There was a relative increase in Sca1+ CD3+ T cells in the thymus of both types of transgenic mouse by flow cytometry (Fig. 4). In CD11a/Hex transgenic mice there was a 4·3-fold increase in the Sca1+ CD3+ cells as compared to wild-type littermate controls (n = 6, P < 0·015), while Lck/Hex mice had a 6·1-fold increase (n = 3, P < 0·009). However, these Sca1+ cells were greater then 90% CD4 or CD8 single-positive, indicating that rather than an increase of T-cell progenitors, the transgenic mature T cells had retention of this myeloid marker.
Transgenic mice had significant changes in the morphology of the thymus as compared to wild-type littermate controls. Transgenic mice had lost any histological demarcation between the medulla and cortex in the thymus. There was an increased cellularity in the central section of both types of Hex transgenic thymi that obliterated the thymic medulla (Fig. 4). Consistent with the Sca1+ flow cytometry, the vast majority of T cells that obliterated the medulla were Sca1+.
Since there was a phenotypic distinction in thymocyte development between the Hex and wild-type mice, TUNEL assays were performed to test whether this difference was due to a difference in apoptosis between the wild-type and transgenic lines.23 There was no difference between transgenic and wild-type thymocytes in their incidence of apoptosis (not shown).
Effect on peripheral blood lymphocytes of transgenic Hex expression
The effects of transgenic Hex expression on the ability of the thymus to produce normal numbers of peripheral blood T cells was examined. Hex transgenic expression was found to produce a general leukopenia that was due to decreased T cells in the peripheral blood. CD11a/Hex transgenic mice had 1·9 ± 0·8 × 106 leucocytes/ml while wild-type littermates had 2·5 ± 0·7 × 106/ml (n = 4). The Lck/Hex transgenic mice had 1·8 ± 0·2 × 106 leucocytes/ml of peripheral blood while wild-type littermate controls had 2·5 ± 0·7 × 106/ml (n = 4). There was no absolute or relative increase in the granulocytes or monocyte populations. The leukopenia in the Hex transgenic mice was explained by a decrease in absolute lymphocytes. The number of total leucocytes obtained by haemacytometer counting was multiplied by the percentage of cells within the lymphocyte gate on flow cytometry to obtain absolute lymphocyte numbers. The CD11a transgenic mice had 0·54 ± 0·08 × 106 lymphocytes/ml as assessed by flow cytometry while wild-type littermates had 1·0 ± 0·16 × 106 lymphocytes/ml. The Lck transgenic mice had 0·58 ± 0·08 × 106 lymphocytes/ml while their wild-type littermates had 1·0 ± 0·15 × 106 lymphocytes/ml.
This absolute lymphopenia in Hex transgenic mice was due to a significant decrease in T cells, especially CD8+ cells, as measured by flow cytometry (Fig. 5). These data indicated a 5·5-fold decrease in peripheral CD8 counts in CD11a mice (n = 4, P < 0·001) and a 1·7-fold decrease in Lck transgenic mice (n = 4, P = 0·06). In addition, there was a slight decrease in CD4+ T cells in CD11a transgenic mice (not significant) and in Lck transgenic mice. Consistent with this, there was no difference in the percentages of B220+ or CD19+ cells between either CD11a or Lck transgenic mice and their wild-type controls.
Figure 5.
Hex transgenic mice have an absolute lymphopenia due to decreased peripheral blood T cells, especially CD8+ cells. CD11a mice are shown in the top two panels and Lck are shown in the bottom two panels. Numbers indicate the average percentage of total T cells that fall within the indicated gates. Four transgenic mice of each line and four wild-type mice were analysed separately, and then the distinct histograms were computer-merged to generate this figure.
Analysis of haematopoietic progenitors in CD11a/Hex transgenic mice
The CD11a/Hex transgenic mice over-express Hex in all leucocyte lineages. The effect of this over-expression on haematopoiesis was also analysed. Transgenic bone marrow cells showed no morphological differences in the marrow or spleen when compared to their wild-type counterparts by histological staining. Flow cytometric analysis of the relative percentages of mature myeloid cells (Gr-1), B cells (CD19), T cells (CD3) and erythrocytes (TER-119) in the marrow did not reveal any differences between CD11a/Hex transgenic mouse and wild-type littermate controls. There were no differences between total nucleated cell counts per femur or spleen between CD11a/Hex transgenic mice and wild-type controls (Table 1).
Table 1. Analysis of haematopoiesis in CD11/Hex transgenic mice; absolute numbers and cycling status of myeloid and erythroid progenitor cells in CD11a/Hex transgenic mice.
| Absolute numbers of myeloid progenitor cells × 106/organ | Cycling rates of myeloid progenitor progenitor cells (% in S-Phase) | ||||||
|---|---|---|---|---|---|---|---|
| Nucleated cellularity × 106/organ | CFU-GM | BFU-E | CFU-GEMM | CFU-GM | BFU-E | CFU-GEMM | |
| Femoral bone marrow | |||||||
| Wild-type | 15·0 ± 1 | 16·1 ± 0·9 | 2·0 ± 0·1 | 1·4 ± 0·1 | 15 ± 2 | 13 ± 4 | 14 ± 4 |
| Transgenic | 16·0 ± 1 | 20·0 ± 1·5 | 3·1 ± 0·3 | 1·8 ± 0·2 | 36 ± 7 | 40 ± 7 | 38 ± 7 |
| Fold change | 1·06 | 1·24 | 1·55 | 1·29 | 2·40 | 3·08 | 2·71 |
| P-value | >0·05 | <0·02 | <0·001 | <0·002 | <0·004 | <0·002 | <0·005 |
| Spleen | |||||||
| Wild-type | 138 ± 11 | 10·0 ± 1·1 | 1·7 ± 0·2 | 1·0 ± 0·1 | 2 ± 1 | 2 ± 1 | 1 ± 1 |
| Transgenic | 122 ± 9 | 14·7 ± 2·3 | 3·3 ± 0·7 | 1·8 ± 0·04 | 37 ± 8 | 46 ± 8 | 40 ± 8 |
| Fold change | 0·88 | 1·47 | 1·94 | 1·80 | 18·5 | 23·0 | 40·0 |
| P-value | >0·05 | <0·04 | <0·02 | <0·02 | <0·001 | <0·001 | <0·001 |
Absolute haematopoietic progenitor numbers were quantified by colony formation assays, and progenitor proliferation was measured by thymidine suicide assays. The data represent the mean ± SEM obtained from 10 CD11a/Hex transgenic and 10 wild-type mice analysed individually in two separate experiments.
Colony-forming assays were performed on bone marrow and spleen cells from the two CD11a/Hex transgenic lines to assess absolute numbers and cycling status of granulocyte-macrophage (CFU-GM), erythroid (BFU-E), and multipotential (CFU-GEMM) progenitor cells (Table 1).
The percentage of CD11a/Hex bone marrow CFU-GM in S-phase was increased 2·4-fold over wild-type, BFU-E was increased 3·1-fold, and CFU-GEMM was increased 2·7-fold. In the spleens of CD11a/Hex transgenic mice, the percentage of CFU-GM in S-phase went up 18·5-fold, BFU-E increased 23-fold and CFU-GEMM was up 40-fold. This increased cycling of progenitors was associated with respective increases of 24%, 55% and 29% in absolute numbers of CFU-GM, BFU-E, and CFU-GEMM in marrow and respective increases of 47%, 94%, and 80% in absolute numbers of these progenitors in the spleen of CD11a transgenic mice compared to wild-type littermates. The increase in myeloid progenitors did not translate into increased mature myeloid cells in the peripheral blood of CD11a/Hex transgenic mice, as described in the previous section. Because of a report that described retroviral insertion at the Hex gene locus and subsequent leukaemognesis,18 we bred and set aside 20 CD11a/Hex and the same number of Lck/Hex mice for observation, as mentioned above. These CD11a/Hex mice have been observed for 18 months, with none developing malignancy. However, as previously mentioned, only the one Lck/Hex mouse developed a malignancy.
Discussion
While the homeobox protein Hex is expressed in most myeloid and B cells, it is not expressed in any T-cell subset.9,11,17 Hex has been previously reported to not be expressed in CD4/8 DN, CD4/8 DP, or CD4 or 8SP T cells.17 Thus, the down-regulation of Hex is thought to be a very early event in T-cell development, shortly after the marrow common lymphoid progenitor differentiates into the B- and T-lineages.17
Therefore, it was hypothesized that down-regulation of Hex was necessary for proper T-cell maturation. This study tested that hypothesis by analysing the over-expression of Hex in the T cells of these transgenic models had multiple deleterious effects on T-cell development. Specifically, expression of Hex in thymic T cells in either transgenic model, where it is not normally expressed, leads to a decrease of CD25+ T-cell precursors, decreased double-positive cells, with increased CD8+ single-positive and Sca1+ cells. Hex over-expression also produced a significant decrease in peripheral blood T cells, especially CD8+ cells, while B cells, erythrocytes, granulocytes and monocytes were unaffected. There appears to be a major block in T-cell progenitor maturation between CD25− CD44− (increased) and CD4+ CD8+ double-positive T-cell (decreased) progenitors. The loss of double-positive T-cell progenitors, which normally make up much of the cortex, and the increase in CD8 single-positive and Sca1+ medullary cells, could explain the abnormal thymic architecture. However, it is unlikely that the expansion in marrow myeloid progenitors could explain the peripheral leukopenia as the absolute number of marrow and peripheral monocytes and granulocytes were not different between wild-type and transgenic mice. It is also not likely that the increase in transgenic Sca1+ thymocytes represents an infiltration of the increased marrow myeloid progenitors, as those increased Sca1+ thymocytes were all CD3+. Rather, it seems more likely that the developing Hex transgenic T cells maintain some myeloid characteristics, since Sca1 is most commonly found on myeloid progenitors, but the increase in Sca1 expression was found only in CD3+ cells.
These results taken together indicate that forced expression of Hex in the thymus interferes with both T-cell progenitor maturation to double-positive cells, and CD8+ single-positive exit from the thymus. There are two possible mechanisms for this finding. First, it is possible that Hex directly transcriptionally regulates CD4 CD8 lineage phenotypes. In this situation CD8 single-positive accumulation could be a result of a repressed CD4 single-positive phenotype, where the double-positive cells that exist are shunted to CD8 from CD4 production. In this mechanism it is also possible that Hex over-expression could drive double-positive T cells to the CD8 lineage, by directly activating CD8 lineage phenotypes, alone or in concert with other transcription factors. However, this mechanism does not explain the decrease in double-negative T-cell progenitors in the transgenic mice.
The second possibility is that the increase in CD8 single-positive cells seen here may be due to an early general inhibition of the pre-T-cell, with the CD8 single-positive T cells emerging from default maturation. These CD8 single-positive cells still lack essential developmental characteristics because of the Hex over-expression, and therefore fail to mature to circulating CD8+ T cells. This is consistent with an instructional model of CD4 CD8 co-receptor maturation, where the critical signal for further differentiation or apoptosis is transmitted through the co-receptor itself after major histocompatibility complex/T-cell receptor engagement.25 In the instructional model CD8 is a default pathway for T-cell maturation when developmental signals are below threshold.26,27
The phenotypic findings with these Hex transgenic mice are similar to those seen when the signalling protein Notch1 is over-expressed in T-cells. Notch1 was first characterized because of its involvement in the t (7;9) in human T-cell acute lymphoblastoid leukaemia.28 Transgenic expression of Notch1 in thymocytes can result in arrested early T-cell development.29,30 If Notch1 is homozygously deleted, the result is a block in the development of the most immature CD4− CD8− thymocytes.31 It should be noted that the CD8+ single-positive thymocytes induced by Notch1 do not survive to populate the peripheral blood, similar to the data here. Thus, it is possible that Hex when aberrantly expressed subverts the Notch signalling pathway, either as an end target or as a co-regulator with Notch-regulated transcription factors.
Retroviral insertional mutagenesis studies in mice identified Hex as a candidate oncogene producing acute leukaemia when its normal expression is altered.18 The finding that the haematopoietic progenitors in CD11a/Hex mice had an increased proliferation lends some insight into the mechanism of those leukaemias. However, we did not see any induction of leukaemia in the 20 CD11a/Hex mice observed for 18 months. We did find one Lck/Hex mouse that developed a T-cell lymphoma that may indeed be due to the transgenic over-expression of Hex based on the involvement of the thymus with the malignancy. However, in general, the over-expression of Hex was not in itself sufficient for oncogenesis in these transgenic mice. These findings indicate that there are probably additional cryptic genetic lesions necessary for the previously reported retroviral leukaemias. This is not surprising, as these retroviral leukaemias have multiple additional insertions.18
In summary, these data imply that down-regulation of myeloid transcription factors may be as important for proper T-cell development as the up-regulation of lymphoid-specific transcriptional regulators. In this study progression past the T-cell progenitor stage was difficult when the myeloid homeobox protein Hex was over-expressed in T-cells.
Acknowledgments
R. H. is supported by NIH grants HL48914 and HL 66308, and by a Leukemia and Lymphoma Society Translational Research Award. H.E.B. is supported by NIH grants HL56416 and DK53674. The CD11a transgenic cassette was the generous gift of Dr Dennis Hickstein of the NIH, Bethesda, MD. The Lck transgenic cassette was the generous gift of Dr Roger Perlmutter, Merck Research Laboratories, NJ.
References
- 1.Gehring WJ, Affolter M, Burglin T. Homeodomain proteins. Annu Rev Biochem. 1994;63:487–526. doi: 10.1146/annurev.bi.63.070194.002415. [DOI] [PubMed] [Google Scholar]
- 2.Kornberg TB. Understanding the homeodomain. J Biol Chem. 1993;268:26813–26. [PubMed] [Google Scholar]
- 3.Magli MC, Barba P, Celetti A, DeVita G, Cillo C, Boncinelli E. Coordinate regulation of HOX genes in human hematopoietic cells. Proc Natl Acad Sci USA. 1991;88:6348–52. doi: 10.1073/pnas.88.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vieille-Grosjean I, Roullo V, Courtois G. Lineage and stage specific expression of HOX 1 genes in the human hematopoietic system. Biochem Biophys Res Commun. 1992;183:1124–30. doi: 10.1016/s0006-291x(05)80307-9. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence HJ, Sauvageau G, Humphries RK, Largman C. The role of HOX homeobox genes in normal and leukemic hematopoiesis. Stem Cells. 1996;14:281–91. doi: 10.1002/stem.140281. [DOI] [PubMed] [Google Scholar]
- 6.Hunger SP, Galili N, Carroll AJ, Crist WM, Lin MP, Cleary ML. The t (1; 19) (q23; p13) results in consistent fusion of E2A and PBX1 coding sequences in acute lymphoblastic leukemias. Blood. 1991;77:687–93. [PubMed] [Google Scholar]
- 7.Hatano M, Roberts CW, Minden M, Crist WM, Korsmeyer SJ. Deregulation of a homeobox gene, HOX11, by the t (10; 14) in T cell leukemia. Science. 1991;253:79–82. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy MA, Gonzalez-Sarmiento R, Kees UR, Lampert F, Dear N, Boehm T, Rabbitts TH. HOX11, a homeobox-containing T-cell oncogene on human chromosome 10q24. Proc Natl Acad Sci USA. 1991;88:8900–4. doi: 10.1073/pnas.88.20.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hromas R, Radich J, Collins SJ. PCR cloning of an orphan homeobox gene (PRH) preferentially expressed in myeloid and liver cells. Biochem Biophys Res Commun. 1993;195:976–83. doi: 10.1006/bbrc.1993.2140. [DOI] [PubMed] [Google Scholar]
- 10.Crompton MR, Bartlett TJ, MacGregor AD, Manfioletti G, Buratti E, Giancotti V, Goodwin GH. Identification of a novel vertebrate homeobox gene expressed in haematopoietic cells. Nucl Acids Res. 1992;20:5661–7. doi: 10.1093/nar/20.21.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedford FK, Ashworth A, Enver T, Wiedemann LMHEX. a novel homeobox gene expressed during haematopoiesis and conserved between mouse and human. Nucl Acids Res. 1993;21:1245–9. doi: 10.1093/nar/21.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yatskievych TA, Pascoe S, Antin PB. Expression of the homebox gene Hex during early stages of chick embryo development. Mech Dev. 1999;80:107–9. doi: 10.1016/s0925-4773(98)00204-4. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RS. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–45. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- 14.Keng VW, Yagi H, Ikawa M, et al. Homeobox gene Hex is essential for onset of mouse embryonic liver development and differentiation of the monocyte lineage. Biochem Biophys Res Commun. 2000;276:1155–61. doi: 10.1006/bbrc.2000.3548. [DOI] [PubMed] [Google Scholar]
- 15.Liao W, Ho C, Yan YL, Postlethwait J, Stainier DY. Hhex and scl function in parallel to regulate early endothelial and blood differentiation in zebrafish. Development. 2000;127:4303–13. doi: 10.1242/dev.127.20.4303. [DOI] [PubMed] [Google Scholar]
- 16.Newman CS, Chia F, Krieg PA. The XHex homeobox gene is expressed during development of the vascular endothelium: over-expression leads to an increase in vascular endothelial cell number. Mech Dev. 1997;66:83–93. doi: 10.1016/s0925-4773(97)00092-0. [DOI] [PubMed] [Google Scholar]
- 17.Manfioletti G, Gattei V, Buratti E, et al. Differential expression of a novel proline-rich homeobox gene (Prh) in human hematolymphopoietic cells. Blood. 1995;85:1237–45. [PubMed] [Google Scholar]
- 18.Li J, Shen H, Himmel KL, et al. Leukaemia disease genes: large-scale cloning and pathway predictions. Nat Genet. 1999;23:348–53. doi: 10.1038/15531. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie KA, Aprikian A, Gollahon KA, Hickstein DD. The human leukocyte integrin CD11a promoter directs expression in leukocytes of transgenic mice. Blood. 1995;86:147–55. [PubMed] [Google Scholar]
- 20.Chaffin KE, Beals CR, Wilkie TM, Forbush KA, Simon MI, Perlmutter R. Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. Embo J. 1990;9:3821–9. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field LJ. Transgenic mice in cardiovascular research. Annu Rev Physiol. 1993;55:97–114. doi: 10.1146/annurev.ph.55.030193.000525. [DOI] [PubMed] [Google Scholar]
- 22.Cooper S, Mantel C, Broxmeyer HE. Myelosuppressive effects in vivo with very low dosages of monomeric recombinant murine macrophage inflammatory protein-1 alpha. Exp Hematol. 1994;22:186–93. [PubMed] [Google Scholar]
- 23.Robertson K, Hill D, Kelley M, et al. The myeloid zinc finger gene MZF-1 delays retinoic acid-induced apoptosis and differentiation in myeloid cells. Leukemia. 1998;12:690–8. doi: 10.1038/sj.leu.2401005. [DOI] [PubMed] [Google Scholar]
- 24.Hromas R, Orazi O, Neiman R, et al. Hematopoietic lineage and stage-restricted expression of the ETS oncogene family member PU.1. Blood. 1993;82:2998–3004. [PubMed] [Google Scholar]
- 25.Zuniga-Pflucker JC, Lenardo MJ. Regulation of thymocyte development from immature progenitors. Curr Opin Immunol. 1996;8:215–24. doi: 10.1016/s0952-7915(96)80060-4. [DOI] [PubMed] [Google Scholar]
- 26.Matechak EO, Killeen N, Hedrick SM, Fowlkes BJ. MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity. 1996;4:337–47. doi: 10.1016/s1074-7613(00)80247-2. [DOI] [PubMed] [Google Scholar]
- 27.Basson MA, Bommhardt U, Cole MS, Tso JY, Zamoyska R. CD3 ligation on immature thymocytes generates antagonist-like signals appropriate for CD8 lineage commitment, independently of T cell receptor specificity. J Exp Med. 1998;187:1249–60. doi: 10.1084/jem.187.8.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellisen LW, Bird J, West DC, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–61. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 29.Robey E, Chang D, Itano A, et al. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–92. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- 30.Izon DJ, Punt JA, Xu L, et al. Notch1 regulates maturation of CD4 (+) and CD8 (+) thymocytes by modulating TCR signal strength. Immunity. 2001;14:253–64. doi: 10.1016/s1074-7613(01)00107-8. [DOI] [PubMed] [Google Scholar]
- 31.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–58. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]