Abstract
Myelin oligodendrocyte glycoprotein (MOG) is found to induce both autoreactive T-cell and antibody responses associated with demyelinating pathology and is implicated in the pathogenesis of multiple sclerosis (MS). In this study, we addressed the potential association of anti-MOG immune responses with MS by examining, comparatively, both the T-cell and antibody responses to recombinant MOG fragments in MS patients and healthy subjects. T cells recognizing MOG were detected in MS patients as well as in healthy subjects, and their precursor frequency in the blood was not increased in patients with MS. MOG-reactive T cells isolated from both MS patients and healthy subjects exhibited a similar cytokine profile, producing interleukin (IL)-4, IL-10 and tumour necrosis factor (TNF), but not interferon-γ (IFN-γ), and recognized predominantly the extracellular (residues 1–60) and the transmembrane/cytoplasmic (residues 154–218) domains of MOG. In contrast, anti-MOG antibodies derived from MS patients displayed a skewed reactivity pattern, even though the occurrence and titres of serum anti-MOG antibodies were only slightly elevated in MS patients. MS-derived autoantibodies were predominantly directed at the 1–60 region of MOG, while naturally occurring anti-MOG antibodies derived from healthy individuals reacted selectively to the 154–218 domain. These differences were statistically significant. The findings of this study are consistent with the presence of anti-MOG antibodies within demyelinating lesions of MS and their role in the induction of demyelinating pathology in animal models. The study has important implications in the understanding of the autoimmune processes in MS.
Introduction
Multiple sclerosis (MS) is a paralytic disease of the central nervous system (CNS), characterized pathologically by focal infiltration of inflammatory cells and demyelination confined to the white matter.1 Although the aetiology and the pathogenesis of MS remain elusive, there is an increasing body of evidence suggesting that autoimmune responses to myelin antigens may play an important role in the disease processes. There are several candidate myelin antigens that have been implicated as relevant myelin antigens in autoimmune processes involved in MS. They include myelin basic protein (MBP), proteolipid protein (PLP)2,3 and myelin oligodendrocyte glycoprotein (MOG),4–6 among others. These myelin antigens have been documented for their ability to induce experimental autoimmune encephalomyelitis (EAE), an animal model for MS.7,8 Substantial T-cell responses to all three myelin antigens have been reported in patients with MS.2,3,5,6,9–13 However, these myelin-reactive T cells can also be isolated from normal individuals at a relatively lower precursor frequency, representing part of the normal T-cell repertoire.9,10,14–17 Unlike MBP and PLP that have been studied extensively for many years, the nature of autoimmune responses to MOG and its potential association with MS are poorly understood.
MOG is expressed exclusively in the CNS myelin, and is preferentially incorporated into the outermost surface of the myelin sheath where a single immunoglobulin-like domain (extracellular domain) is exposed to the extracellular environment.18 Although MOG accounts for less than 0·05% of the CNS myelin protein constituent, it has a potent encephalitogenic property in the induction of EAE in rodents and primates.19–23 MOG has been found to induce both an encephalitogenic T-cell response and a significant demyelinating antibody response in several models of EAE.24–26 In both rodent and primate EAE models induced by immunization with MOG, anti-MOG antibodies are important for the induction of the CNS pathology characterized by extensive demyelination and local inflammation, closely resembling the lesions seen in MS.27 In contrast, EAE induced by MBP and PLP is mediated by encephalitogenic T cells and is generally characterized by extensive CNS inflammation with mild demyelination.19,27 The results accumulated to date suggest that MOG-reactive T cells can also be isolated from both MS patients and healthy individuals.28–33 In some reports, substantial antibody reactivity was detected in patients with MS, which was higher than that in healthy individuals.6 In all of these studies, only full-length MOG and/or a synthetic peptide(s) corresponding to the extracellular domain was used to determine the T-cell and the antibody responses to MOG. The T-cell recognition of the myelin antigens is not necessarily limited to the extracellular domain, and antigenic peptides of the transmembrane and cytoplasmic domains can be presented to the T cells after processing by antigen-presenting cells (APC), as seen in cases of MBP and PLP in which some immunodominant epitopes are located in the transmembrane and cytoplasmic domains.3,10,11,14,15,34 More importantly, it remains to be determined whether aberrant autoimmune T- and B-cell responses to a particular immunodominant region(s) of MOG are potentially associated with MS.
To address these issues in the present study, we prepared recombinant MOG and four overlapping fragments of MOG corresponding to various domains of MOG and used them in a series of experiments to detect both the T-cell and the antibody responses to MOG in MS patients and healthy subjects. The study revealed that the T-cell responses to MOG were not significantly different between MS patients and control subjects, as demonstrated by a relatively similar precursor frequency of MOG-reactive T cells and their functional properties. Both the extracellular 1–60 domain and the transmembrane/cytoplasmic 154–218 domain represented immunodominant regions for the T-cell recognition of MOG. Most importantly, the study demonstrated for the first time that serum anti-MOG antibodies derived from MS patients displayed a skewed reactivity pattern in recognition of the extracellular 1–60 domain of MOG, which differed substantially from that of naturally occurring anti-MOG antibodies present in healthy individuals. The study suggests that an autoantibody response to the extracellular 1–60 domain of MOG is associated with MS and provides important implications in our understanding of the role of anti-MOG autoimmune responses in the disease processes in MS.
Materials and methods
Reagents
Tetanus toxoid (TT; SVM, Bilthoven, the Netherlands) served as a control antigen for T-cell proliferation studies. Hen-egg lysozyme (HEL) was obtained from Sigma (St. Louis, MO) and served as a control antigen for antibody detection by B cells. Media used for cell culture were AIM-V serum-free medium (Gibco BRL, Grand Island, NY) and RPMI-1640 supplemented with l-glutamine, sodium pyruvate, non-essential amino acids, 10 mm HEPES (Gibco), 10% (vol/vol) fetal bovine serum (FBS) (Gibco) and 1% penicillin/streptomycin (Gibco). Recombinant human IL-2 (rIL-2) was purchased from Boehringer Mannheim (Indianapolis, IN).
Patients
Twenty MS patients were recruited to the study. All patients were characterized as having relapsing-remitting or secondary progressive MS for longer than 2 years. They were not treated with immunosuppressive agents, including steroids or immunomodulatory agents (e.g. beta-interferon or Copaxone), for at least 3 months prior to enrolling in the study and throughout the study. Informed consent was obtained from the patients after explaining the experimental procedures. The protocol was approved by the Institutional Human Subjects Committee at Baylor College of Medicine. Ten asymptomatic healthy volunteers were included as control subjects. A separate group of additional MS patients and healthy individuals was recruited, using the same selection criteria, for determination of the specificity of serum anti-MOG antibodies.
Recombinant protein and overlapping fragments of MOG
The following procedure was used to prepare recombinant human MOG protein and protein fragments using recombinant DNA technology. Human MOG RNA was isolated from human brain tissue white matter by the RNA spin column technique (Qiagen, Valencia, CA) and cDNA was synthesized with oligo(dT) primer. MOG and four overlapping fragments were amplified by the polymerase chain reaction (PCR) as follows: 25 cycles of 50 seconds at 94°, 50 seconds at 56°, 2 min at 72°, and a final extension for 10 min at 72° in a PE9700 thermal cycler (Perkin Elmer, Foster City, CA). The PCR products were cloned into the cloning vector pCR2.1 TA (Invitrogen, Carlsbad, CA) and sequenced completely. PCR was performed using specific primers for the full-length MOG and the MOG fragments (MOG-1 to -4) under universal PCR conditions (a total of 25 cycles). The expression plasmid was constructed as follows. DNA was digested from the pCR2.1 TA cloning vector using XhoI and HindIII restriction endonucleases and cloned, in-frame, into expression vector pRSET (Invitrogen), which carried the T7 promoter sequence and a sequence encoding an N-terminal histidine fusion tag. Segments were expressed in Escherichia coli BL21 (DE3) by optimal induction with 1 mm IPTG, and subsequently purified by using nickel-chelating affinity binding to the histidine tag. The purified protein and segments were passed through a Detoxi-Gel™ endotoxin-removing affinity gel (Pierce, Rockford, IL) three times to remove trace endotoxins.
Estimation of the precursor frequency of MOG-reactive T cells
Blood specimens were obtained from patients, and sera were separated for antibody detection. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood by Ficoll density-gradient separation, and washed three times with sterile Hanks' balanced salt solution (HBSS; Gibco). PBMCs were seeded at 2 × 105 cells per well in 96-well, U-bottomed plates (Costar, Cambridge, MA) in the presence of MOG fragments (30 µg/ml). TT was used as a control antigen (5 Lf/ml). Cells were incubated at 37° in a 5% CO2 atmosphere. Seven days later, each culture was examined for specific proliferation in response to the corresponding MOG fragment in a proliferation assay. Briefly, each well was split into three aliquots and cultured in duplicate with 105 irradiated (6000 rads) autologous PBMC in the presence or absence of the corresponding fragment. Culture supernatants were collected after 48 hr for analysis by cytokine enzyme-linked immunosorbent assay (ELISA). The remaining cultures were maintained for an additional 24 hr and pulsed subsequently with [3H]methylthymidine (Amersham, Arlington Heights, IL) at 1 µCi/well during the last 16 hr of culture. Cells were then harvested using an automated cell harvester (Tomtec, Orange, CT), and [3H]methylthymidine incorporation was measured in a Microbeta Trilux counter (Wallac, Turku, Finland). A T-cell line was defined as specific for a MOG fragment when the counts per minute (c.p.m.) were greater than 1000 and exceeded the reference c.p.m. (in the absence of the antigen) by at least threefold.11 The frequency of MOG-reactive T cells was then estimated by dividing the number of positive wells by the total number of PBMC seeded in the initial culture.
Cytokine measurement
The cytokine profile of the resulting MOG-specific T cells was determined quantitatively using ELISA kits (PharMingen, San Diego, CA). Microtitre plates (96 wells; NUNC Maxisorp, Rochester, NY) were coated overnight at 4° with 1 µg/well of a purified mouse capture monoclonal antibody (mAb) to the following cytokines: interleukin (IL)-4, IL-10, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) (PharMingen), in 100 µl of carbonate buffer (100 mm, pH 9·5). Plates were washed five times with phosphate-buffered saline (PBS) (pH 7·0) containing 0·05% Tween-20 (PBS-T). Non-specific binding sites were saturated with 10% (wt/vol) FBS in PBS (FBS-PBS) for 1 hr and washed subsequently with PBS-T. Supernatants and cytokine standards were diluted with PBS and added, in duplicate, to wells. Plates were incubated at 4° overnight and subsequently washed five times with PBS-T. One hundred microlitres of the matched biotinylated detecting antibody was added to each well and incubated at room temperature for 2 hr. After washing, avidin-conjugated horseradish peroxidase (diluted 1 : 5000; Vector Laboratories, Burlingame, CA) was added and the plates were incubated for 1 hr. Plates were then washed and 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma) was used as a substrate for colour development. The reaction was stopped by adding 1 N HCl. The optical density was measured at 450 nm using an ELISA reader (Bio-Rad Laboratories, Hercules, CA).
Reactivity of MOG-specific T-cell lines
A total of 20 000 cells of each MOG-specific T-cell line were cultured in duplicate with irradiated autologous PBMC (100 000 cells/well) as a source of APC in the presence and absence of the MOG fragments (30 µg/ml), respectively. Cells were cultured for 72 hr and the cell proliferation was measured in [3H]thymidine-incorporation assays, as described above.
Generation of antibody-producing B-cell lines by Epstein–Barr virus (EBV) transformation
PBMCs were seeded in 20% FBS-RPMI at 4 × 104 cells per well in 96-well, flat bottomed plates (Costar) in the presence of cell-free supernatant of the EBV-producing cell line, B95.8 (ATCC, Bethesda, MD). Cyclosporin A (1 µg/ml) (Sigma) was added in the initial culture to selectively inhibit T-cell growth. The total number of wells for each patient was 60. Cells were cultured at 37° in a 5% CO2 atmosphere for 14 days with changes of medium every 3–4 days. On day 14, the growth-positive wells were visualized and the culture supernatants harvested and stored at −80° until analysis. The presence of anti-MOG antibodies was determined in the supernatants by ELISA. Briefly, microtitre plates were coated overnight at 4° with 5 µg/ml of a purified full-length MOG or HEL protein (1 µg/ml), used as a control antigen, respectively, in carbonate buffer (100 mm, pH 9·5). The same ELISA procedure as described above was used to detect anti-MOG antibodies. Wells were defined as positive when the net absorbance (A) was greater than the background A and the control A (HEL) by at least twofold. The precursor frequency of B cells producing anti-MOG antibodies was estimated by dividing the number of positive wells by the total number of PBMC plated.
Detection of serum titres and specificity of anti-MOG antibodies
Antibody titres were determined in serum specimens using ELISA. Microtitre plates were coated overnight at 4° with 5 µg/ml of a purified full-length MOG protein, 10 µg/ml of the MOG fragments (MOG-1 to -4), or MBP as a control myelin antigen for comparison, respectively, in carbonate buffer (100 mm, pH 9·5). Non-specific binding sites were saturated with FBS-PBS for 1 hr and washed subsequently with PBS-T. Serum samples were prepared in serial dilutions (1 : 50, 1 : 100, 1 : 250, 1 : 500 and 1 : 1000) with FBS-PBS, and 100 µl of each sample was added in duplicate wells. The same ELISA procedure as described above was used to detect serum antibody titres.
Statistical analysis
A Student's t-test for normally distributed variables, and the Mann–Whitney rank-sum test for non-normally distributed variables, were used for data analysis. A P-value of < 0·05 was considered statistically significant.
Results
The T-cell responses to recombinant MOG fragments in patients with MS and healthy individuals
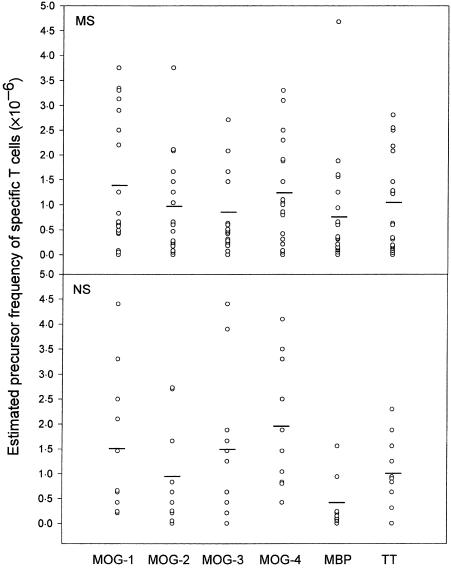
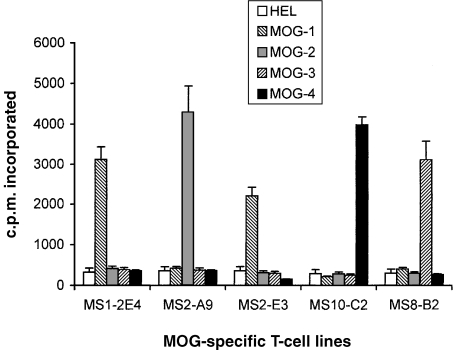
A group of patients with relapsing-remitting MS (n = 14) and chronic progressive MS (n = 6) were included in the study. Ten healthy volunteers were examined in parallel as control subjects. The clinical characteristics of the MS patients and healthy subjects are summarized in Table 1. It should be noted that MS patients included in this study did not match exactly with the control subjects in age and gender. We first examined the T-cell reactivity to recombinant fragments encompassing the four regions of human MOG (with an overlap of 10 amino acids), in MS patients and control subjects, by estimating the precursor frequency of specific T cells. As shown in Fig. 1, the precursor frequency of T cells recognizing the MOG fragments ranged from 0·8 × 10−6 to 1·4 × 10−6, respectively, in patients with MS. The highest T-cell responses were directed towards the extracellular (residues 1–60; MOG-1) and the transmembrane/cytoplasmic (residues 154–218; MOG-4) domains of MOG. With the exception of the T cells recognizing the 102–164 region (MOG-3), which was higher in control subjects than in MS patients, the estimated precursor frequency of MOG-specific T cells did not differ significantly between the two groups. MBP-reactive T cells occurred at a relatively higher precursor frequency in the same group of MS patients compared to the control subjects, while the precursor frequency of T cells recognizing TT, a recall antigen, was the same for both groups. A panel of 30 resulting T-cell lines derived from MS patients, as well as from healthy controls, were examined and found to express exclusively the CD4 phenotype (data not shown). As shown in Fig. 2, representative T-cell lines raised against various MOG fragments exhibited specific reactivity to the original fragment, but not to other MOG fragments.
Table 1. Clinical characteristics of controls and patients with multiple sclerosis (MS) in the study.
| Subjects | n | Average age (years) | Gender | RR/SP | Mean disease duration | Mean EDSS |
|---|---|---|---|---|---|---|
| Original group | ||||||
| ″MS | 20 | 44·1 ± 8·4 | 18F/2M | 14/6 | 5·3 ± 4·5 | 3·7 ± 2·2 |
| ″Control | 10 | 34·2 ± 3·0 | 5F/5M | – | – | – |
| Extended group* | ||||||
| ″MS | 39 | 45·2 ± 11·8 | 33F/6M | 22/17 | 9·3 ± 9·2 | 4·5 ± 2·27 |
| ″Control | 26 | 33·1 ± 7·0 | 19F/7M | – | – | – |
An extended group of additional patients with MS and healthy individuals was included for serum antibody studies.
EDSS, expanded disability scale score; RR, relapsing-remitting MS; SP, secondary progressive MS.
Figure 1.
The T-cell responses to the myelin oligodendrocyte glycoprotein (MOG) fragments in patients with multiple sclerosis (MS) and healthy controls. The precursor frequency of T cells recognizing the overlapping fragments of MOG was estimated in peripheral blood mononuclear cell (PBMC) specimens from 20 patients with MS and 10 normal subjects (NS). The precursor frequency of T cells recognizing myelin basic protein (MBP) and tetanus toxoid (TT) was analysed in parallel under the same experimental conditions. The open circles represent the individual precursor frequency and the bars indicate the mean precursor frequency of specific T cells within the group. MOG-1, residues 1–60; MOG-2, residues 50–92; MOG-3, residues 102–164; MOG-4, residues 154–218;
Figure 2.
The reactivity of representative T-cell lines to the myelin oligodendrocyte glycoprotein (MOG) fragments. A panel of five representative MOG-specific T-cell lines that exhibited a high stimulation index were tested for their recognition of the MOG fragments. The T-cell lines were stimulated with the MOG fragments, respectively, in the presence of irradiated autologous peripheral blood mononuclear cell (PBMC) as a source of antigen-presenting cells (APC). The proliferation of the T-cell lines was measured after 72 hr in a [3H]thymidine-uptake assay. The results are expressed as counts per minute (c.p.m.) ± standard deviation (SD).
The cytokine profile of short-term MOG-reactive T-cell lines derived from MS patients and healthy individuals
The resulting short-term MOG-reactive T-cell lines derived from MS patients, as well as healthy individuals, were examined for their cytokine profile, including IL-4, IL-10, TNF-α and IFN-γ. As shown in Table 2, both panels of the MOG-reactive T-cell lines produced large amounts of the T helper 2 (Th2) cytokines (IL-4, IL-10 and TNF-α), but not of IFN-γ, a typical T helper 1 (Th1) cytokine. Although there were no significant differences in the cytokine profile between T-cell lines recognizing various MOG fragments, MOG-reactive T-cell lines derived from MS patients varied in concentrations of the cytokines from those obtained from control subjects. With one exception, MOG-reactive T-cell lines derived from control subjects appeared to produce significantly more TNF-α and IL-4 than that of the T-cell lines generated from MS patients.
Table 2. Cytokine profile and the concentrations produced by myelin oligodendrocyte glycoprotein (MOG)-reactive T-cell lines (pg/ml).
| Subjects | Antigen | Number of T-cell lines | IL-10 | IL-4 | TNF-α | IFN-γ |
|---|---|---|---|---|---|---|
| MS | MOG-1 | 50 | 362 ± 36 | 307 ± 64 | 297 ± 5* | 59 ± 10 |
| MOG-2 | 43 | 372 ± 26 | 185 ± 36* | 957 ± 155 | 37 ± 5 | |
| MOG-3 | 46 | 238 ± 34 | 309 ± 63* | 409 ± 89 | 40 ± 9 | |
| MOG-4 | 42 | 299 ± 37 | 157 ± 44* | 453 ± 54* | 33 ± 5 | |
| NS | MOG-1 | 48 | 338 ± 21 | 219 ± 68 | 974 ± 113 | 31 ± 6* |
| MOG-2 | 50 | 294 ± 15 | 420 ± 100 | 952 ± 102 | 23 ± 5 | |
| MOG-3 | 41 | 341 ± 20 | 141 ± 43 | 518 ± 71 | 16 ± 4 | |
| MOG-4 | 46 | 397 ± 23 | 436 ± 80 | 1346 ± 11 | 37 ± 7 |
MOG-reactive T-cell lines were challenged with the corresponding MOG fragments, and the supernatants were collected after 48 hr. The absolute cytokine concentrations were measured by enzyme-linked immunosorbent assay (ELISA). The detection limits for all cytokines studied were between 15 and 35 pg/ml.
Significant differences in the cytokine production between multiple sclerosis (MS)-derived T-cell lines and normal subjects (NS)-derived T-cell lines specific for the same MOG fragments.
IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumour necrosis factor-α.
The precursor frequency of B cells producing anti-MOG antibodies in MS patients and control subjects
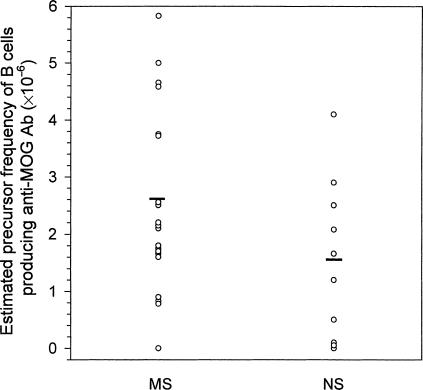
We then examined whether B cells producing anti-MOG antibodies occurred at a different precursor frequency in MS patients and control subjects. To achieve this, we immortalized antibody-producing B cells from PBMC obtained from MS patients and control subjects using a cell culture-based technique combining EBV transformation with limiting dilution.35 Supernatants of the resulting B-cell lines (60 cell lines from each patient/individual) were tested for the presence of antibodies to MOG by ELISA. As shown in Fig. 3, B cells committed to producing anti-MOG antibodies occurred at a slightly increased precursor frequency in MS patients compared to that in control subjects (2·7 × 10−6 versus 1·6 × 10−6). The difference was not statistically significant (P = 0·2).
Figure 3.
The estimated precursor frequency of B cells producing anti-myelin oligodendrocyte glycoprotein (MOG) antibodies in peripheral blood mononuclear cells (PBMCs) of patients with multiple sclerosis (MS) and healthy controls. PBMCs were obtained from 20 patients with MS and from 10 normal subjects (NS). B cells were transformed using culture supernatant derived from an Epstein–Barr virus (EBV)-producing cell line (B95.8). The T-cell growth was selectively inhibited by cyclosporin A. All growth-positive wells of B cells were screened for the presence of anti-MOG antibodies by enzyme-linked immunosorbent assay (ELISA). The precursor frequency of B cells producing anti-MOG antibodies was estimated by dividing the number of positive wells by the total number of PBMCs plated initially. The circles indicate the individual precursor frequencies of B cells producing anti-MOG antibodies, and the bars represent the mean precursor frequency within the group.
Titres and the specificity of serum anti-MOG antibodies in MS patients and control subjects
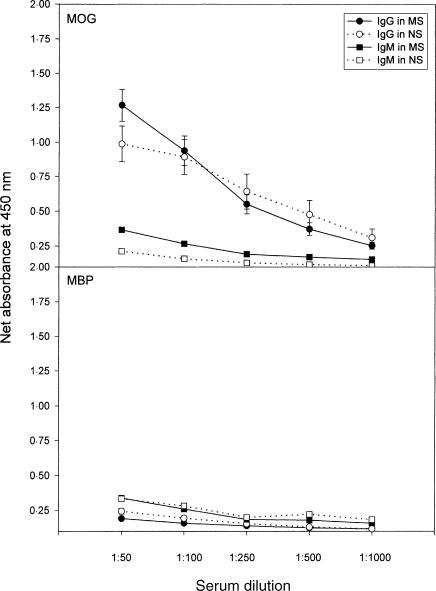
Next, we examined the titres and the specificity of serum anti-MOG antibodies derived from MS patients and control subjects to address whether an aberrant autoantibody response to MOG is associated with MS. Serum anti-MOG antibodies were present in both MS patients and control subjects at a comparable level (80% in MS versus 70% in the control group). The immunoglobulin G (IgG) fraction represented the dominant antibody component specific for MOG. The reactivity of IgG antibodies to MOG was slightly elevated, at a dilution of 1 : 50, in serum specimens obtained from MS patients compared to those of control subjects (Fig. 4). However, the differences in the antibody titres were not statistically significant between MS patients and control subjects (P = 0·177). No differences in the antibody reactivity to MOG were seen between the MS group and the control group at high serum dilutions. In parallel experiments, the reactivity of serum antibodies of both IgG and immunoglobulin M (IgM) to MBP did not differ between the same study groups.
Figure 4.
Detection of serum immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies specific for myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein (MBP) in patients with multiple sclerosis (MS) and in healthy controls. The titres of IgG and IgM antibodies to MOG and MBP were determined in serum specimens derived from 20 patients with MS and from 10 normal subjects (NS) by enzyme-linked immunosorbent assay (ELISA). The net absorbance was calculated as experimental absorbance, subtracting background absorbance in the same experimental setting. The background absorbance was below 0·1. Data are presented as the mean value ± SEM.
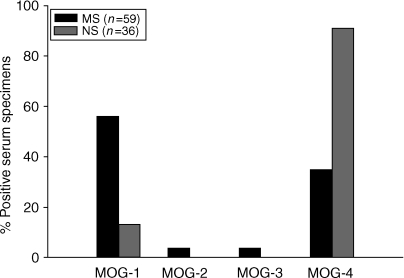
To confirm the initial finding, we repeated and expanded the analysis to include an extended group of 39 MS patients and 26 healthy individuals, in addition to the initial group of 20 MS patients and 10 healthy controls. The analysis of the combined groups revealed that 46/59 (78%) specimens derived from MS patients and 23/36 (64%) control specimens were positive for anti-MOG IgG antibodies. All resulting positive specimens were further characterized for reactivity to the overlapping MOG fragments. Serum IgG antibodies derived from MS patients reacted preferentially to the extracellular domain (residues 1–60) and, to a lesser extent, the transmembrane/cytoplasmic domain (residues 154–218), while anti-MOG antibodies from control subjects recognized selectively the 154–218 domain of MOG. The results, summarized in Fig. 5, indicate that MS-derived autoantibodies exhibited a skewed pattern of reactivity to the extracellular 1–60 domain of MOG (56% in the MS group versus 13% in the control group, P < 0·05). In contrast, naturally occurring anti-MOG antibodies present in healthy individuals were directed preferentially at the transmembrane/cytoplasmic 154–218 domain of MOG (13% in the MS group versus 91% in the control group, P < 0·05). Thus, the findings confirm that the reactivity of MS-derived anti-MOG autoantibodies is skewed from those within the normal repertoire of the immune system.
Figure 5.
The specific reactivity of serum anti-myelin oligodendrocyte glycoprotein (MOG) immunoglobulin G (IgG) antibodies to the MOG fragments. The combined analysis was performed with serum specimens obtained from the initial group of 20 patients with multiple sclerosis (MS) and in 10 normal subjects (NS), and from an extended group of 39 patients with MS and 26 normal subjects. Forty-six of 59 (78%) specimens derived from MS patients and 23 of 36 (68%) control specimens were positive for anti-MOG antibodies, respectively. All positive specimens were examined for their reactivity to the MOG fragments. Specific reactivity to a given MOG fragment was determined by enzyme-linked immunosorbent assay (ELISA) when mean the mean absorbance (A) of serum antibodies to the MOG fragment exceeded the mean A of other fragments and background A by at least twofold. In some cases, the sum of the percentage of positive specimens exceeded 100% because the specimens contained anti-MOG antibodies that reacted with more than one fragment. The secondary antibody used in all the experiments was a conjugated monoclonal antibody specific for human IgG. The data are presented as: (positive specimens to MOG fragments ÷ total specimens within a group) × 100%.
Discussion
The study represents a detailed demonstration of both the autoreactive T-cell and autoantibody responses to MOG in MS as compared to the normal repertoire of the immune system, taking advantage of using recombinant overlapping fragments corresponding to the various domains of MOG. It has yielded several important findings that bear particular relevance to the understanding of the role of the autoimmune responses to MOG in the pathogenesis of MS. First, the findings described here indicate that the T-cell responses to MOG in patients with MS are not significantly different from the normal T-cell repertoire, with respect to levels of the precursor frequency of MOG-reactive T cells in the blood, their cytokine profile and the recognition pattern to the MOG fragments. The results are in agreement with a recent study by Lindert and colleagues.6 In that study, the authors demonstrated that there was no difference in the T-cell proliferative responses to the extracellular domain of MOG between MS patients and control subjects.
This observation does not rule out an ongoing T-cell sensitization to MOG in MS patients. It is probable that a significant proportion of these MOG-reactive T cells may undergo in vivo activation in MS patients, which may not be reflected by their overall precursor frequency in the blood, as previously demonstrated in MBP-reactive T cells in MS patients.11 The T-cell responses to MOG may correlate with the clinical activity in MS. For example, we demonstrated recently that T cells recognizing a synthetic peptide corresponding to the extracellular domain of MOG occurred at significantly increased precursor frequency only at the time of clinical exacerbation in patients with MS.13 It should be mentioned that the method employed for T-cell frequency analysis is not sufficiently accurate and sensitive to measure the true T-cell precursor frequencies. However, various studies published previously have shown that the method is appropriate, when used consistently in the same experimental setting, to compare the precursor frequency of specific T cells between individual patients or groups.3,10–14 The potential difference in the precursor frequency of MOG-reactive T cells between MS patients and control subjects may be more discernable using a methodology with greater sensitivity.32
The study demonstrated that in both MS patients and healthy controls, the T-cell responses are directed preferentially at the extracellular 1–60 domain and the transmembrane/cytoplasmic 154–218 domain of MOG. The T cells recognizing the 154–218 domain of MOG occur at a relatively low precursor frequency in the blood of MS patients. The pattern of the T-cell recognition is consistent with the reactivity of anti-MOG antibodies derived from the same cohort. The finding suggests that the predominant T-cell reactivity of the extracellular 1–60 and the transmembrane/cytoplasmic 154–218 domains of MOG in both MS patients and healthy individuals reflect an intrinsic property of the normal T-cell repertoire. Similarly, T cells recognizing other myelin proteins, such as MBP, are also present as part of the normal T-cell repertoire and their recognition patterns are largely similar between MS patients and healthy individuals.10,11,14,36 Furthermore, in contrast to T cells recognizing MBP and PLP, which generally produce Th1 cytokines but little IL-4 and IL-10,13,37 MOG-reactive T cells secrete large amounts of IL-4 and IL-10 but not IFN-γ, a typical Th1 cytokine. It is probable that the ability of MOG-reactive T cells to produce large amounts of IL-4 and IL-10 may relate to the unique property of MOG to induce autoantibody responses, as Th2 cells and cytokines are known to act as helpers to the differentiation and antibody production of B cells.
One of the most important findings from the study is related to the aberrant antibody responses to MOG in MS patients. First, both the occurrence and the titres of serum antibodies to the full-length MOG were relatively elevated in MS patients, which is consistent with an increased precursor frequency of B cells producing anti-MOG antibodies in the same MS patients. However, the findings are not statistically significant between MS patients and healthy subjects, probably owing to variation among individual data points and the small sample size. It should be noted that the precursor frequency of B cells producing autoantibodies to another candidate myelin antigen (MBP) was in the range of 0·3 × 10−6 in MS patients and 0·15 × 10−6 in healthy individuals when analysed under the same experimental conditions.35 By comparison, the range of B cells producing anti-MOG antibodies described here is nearly 10 times higher, further confirming the association of MOG with predominant autoantibody responses. More interestingly, anti-MOG antibodies derived from MS patients exhibit a skewed pattern of reactivity to MOG, which is distinct from that of the naturally occurring anti-MOG antibodies found in healthy individuals. It should be noted that MOG is a highly glycosylated protein that contains B-cell epitopes related to carbohydrate moieties. Therefore, the use of non-glycosylated fragments and peptides of MOG may have limitations as a result of the possibility that some of the B-cell epitopes are not detected. Furthermore, it can be argued that most of the autoantibody is produced within the central nervous compartment. Further investigations to analyse the reactivity pattern of cerebrospinal fluid-derived antibodies are needed to delineate the binding properties and the potential role of anti-MOG antibodies in MS.
It is probable that the extracellular 1–60 domain of MOG may contain a pathologically important epitope(s) for anti-myelin autoantibody responses in MS. In this regard, the finding described here is particularly intriguing in the context of a recent study demonstrating the presence of anti-MOG antibodies to the extracellular domain, which specifically bound to the disintegrating myelin sheaths in lesions of MS and a primate model of EAE.38 The aberrant reactivity of anti-MOG antibodies in MS may be attributable to sensitization of the immune system to the surface, exposed portion of MOG during the disease processes. However, it remains unclear whether B-cell sensitization to the extracellular domain of MOG results from a primary event, e.g. through molecular mimicry during a course of infection, or whether it is secondary to an ongoing CNS demyelinating process in MS. In conclusion, the study has provided important insights into the understanding of the role of anti-MOG immune responses in the disease processes in MS.
Acknowledgments
We thank Mrs Sufang Li for excellent technical assistance and Mrs Jeanene De La Rosa for coordination of patients. This work was supported, in part, by the National Institutes of Health and the Richardson Foundation.
References
- 1.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–87. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 2.Trotter JL, Pelfrey CM, Trotter AL, Selvidge JA, Gushleff KC, Mohanakumar T, McFarland H. T cell recognition of myelin proteolipid protein and myelin proteolipid protein peptides in the peripheral blood of multiple sclerosis and control subjects. J Neuroimmunol. 1998;84:172–8. doi: 10.1016/s0165-5728(97)00260-9. [DOI] [PubMed] [Google Scholar]
- 3.Markovic-Plese S, Fukaura H, Zhang J, Al-Sabbagh A, Southwood S, Sette A, Kuchroo VK, Hafler D. T cell recognition of immunodominant and cryptic proteolipid protein epitopes in humans. J Immunol. 1995;155:982–92. [PubMed] [Google Scholar]
- 4.Kerlero de Rosbo N, Milo R, Lees MB, Burger D, Bernard CCA, Ben-Nun A. Reactivity to myelin antigens in multiple sclerosis: peripheral blood lymphocytes respond predominantly to myelin oligodendrocyte glycoprotein. J Clin Invest. 1993;92:2602–8. doi: 10.1172/JCI116875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storch MK, Stefferl A, Brehm U, et al. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681–94. doi: 10.1111/j.1750-3639.1998.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindert RB, Haase CG, Brehm U, Linington C, Wekerle H, Hohlfeld R. Multiple sclerosis: B- and T-cell responses to the extracellular domain of the myelin oligodendrocyte glycoprotein. Brain. 1999;122:2089–99. doi: 10.1093/brain/122.11.2089. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–9. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- 8.Zamvil SS, Nelson P, Trotter J, Mitchell D, Knobler R, Fritz R, Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 1985;317:355–8. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- 9.Chou YK, Vainiene M, Whithman R, Bourdette D, Chou CH, Hashim G, Offner H, Vandenbark AA. Response of human T lymphocyte lines to myelin basic protein: association of dominant epitopes with HLA class II restriction molecules. J Neurosci Res. 1989;23:207–16. doi: 10.1002/jnr.490230211. [DOI] [PubMed] [Google Scholar]
- 10.Ota K, Matsui M, Milford EL. T cell recognition of an immunodominant MBP epitope in multiple sclerosis. Nature. 1990;346:183–7. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner H, Hafler D. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–84. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trotter JL, Damico CA, Cross AH, Pelfrey CM, Karr RW, Fu X-T, McFarland H. HPRT mutant T-cell lines from multiple sclerosis patients recognize myelin proteolipid protein peptides. J Neuroimmunol. 1997;75:95–103. doi: 10.1016/s0165-5728(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 13.Tejada-Simon MV, Zang YCQ, Yang D, et al. Aberrant T cells responses to myelin antigens during exacerbation in patients with multiple sclerosis. Int Immunol. 2000;12:1629–39. doi: 10.1093/intimm/12.12.1641. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Medaer R, Hashim G, Chin Y, van den Berg-Loonen E, Raus J. Myelin basic protein-specific T lymphocytes in multiple sclerosis and controls: precursor frequency, fine specificity and cytotoxicity. Ann Neurol. 1992;32:330–8. doi: 10.1002/ana.410320305. [DOI] [PubMed] [Google Scholar]
- 15.Martin R, Howell ND, Jaraquemada D, et al. A MBP peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J Exp Med. 1991;173:19–24. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellings N, Gelin G, Medaer R, Bruckers L, Palmers Y, Raus J, Stinissen P. Longitudinal study of antimyelin T-cell reactivity in relapsing-remitting multiple sclerosis: association with clinical and MRI activity. J Neuroimmunol. 2002;126:143–60. doi: 10.1016/s0165-5728(02)00052-8. [DOI] [PubMed] [Google Scholar]
- 17.Pette M, Fujita K, Wilkinson D, et al. Myelin autoreactivity in multiple sclerosis: recognition of MBP in the context of HLA-DR2 products by T lymphocytes of multiple sclerosis patients and healthy donors. Proc Natl Acad Sci USA. 1990;87:79–2. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroepfl JF, Viise LR, Charron AJ, Linington C, Gardinier MV. Investigation of myelin/oligodendrocyte glycoprotein membrane topology. J Neurochem. 1996;67:2219–22. doi: 10.1046/j.1471-4159.1996.67052219.x. [DOI] [PubMed] [Google Scholar]
- 19.Linington C, Lassmann H. Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin oligodendrocyte glycoprotein (MOG) J Neuroimmunol. 1987;17:61–9. doi: 10.1016/0165-5728(87)90031-2. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–76. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 21.Genain CP, Nguyen M-H, Letvin NL, et al. Antibody facilitation of multiple sclerosis-like lesions in a nonhuman primate. J Clin Invest. 1995;96:2966–74. doi: 10.1172/JCI118368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genain CP, Gritz L, Joshi N, Panicali D, Davis RL, Whitaker JN, Letvin NL, Hauser SL. Inhibition of allergic encephalomyelitis in marmosets by vaccination with recombinant vaccinia virus encoding for myelin basic protein. J Neuroimmunol. 1997;79:119–28. doi: 10.1016/s0165-5728(97)00109-4. [DOI] [PubMed] [Google Scholar]
- 23.Weissert R, Wallstrom E, Storch MK, Stefferl A, Lorentzen J, Lassmann H, Linington C, Olsson T. MHC haplotype-dependent regulation of MOG-induced EAE in rats. J Clin Invest. 1998;102:1265–73. doi: 10.1172/JCI3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linington C, Berger T, Perry L, et al. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol. 1993;23:1364–72. doi: 10.1002/eji.1830230627. [DOI] [PubMed] [Google Scholar]
- 25.Amor S, Groome N, Linington C, Morris MM, Dornmair K, Gardinier MV, Matthieu JM, Baker D. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol. 1994;153:4349–56. [PubMed] [Google Scholar]
- 26.Genain CP, Hauser SL. Allergic encephalomyelitis in common marmosets: pathogenesis of a multiple sclerosis-like lesion. Methods. 1996;10:420–34. doi: 10.1006/meth.1996.0120. [DOI] [PubMed] [Google Scholar]
- 27.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulation mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130:443–54. [PMC free article] [PubMed] [Google Scholar]
- 28.Cross AH, Trotter JL. B cells and antibodies in CNS demyelinating disease. J Neuroimmunol. 2001;112:1–14. doi: 10.1016/s0165-5728(00)00409-4. [DOI] [PubMed] [Google Scholar]
- 29.Archelos J, Storch M, Hartung H-P. The role of B cells and autoantibodies in multiple sclerosis. Ann Neurol. 2000;47:694–706. [PubMed] [Google Scholar]
- 30.Sun J, Olsson T, Wang W-Z, Xiao B-G, Kostulas V, Fredrikson S, Ekre H-P, Link H. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur J Immunol. 1991;21:1461–8. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- 31.Kerlero de Rosbo N, Hoffman M, Mendel I, et al. A predominance of the autoimmune response to myelin oligodendrocyte glycoprotein (MOG) in multiple sclerosis: reactivity to the extracellular domain of MOG is directed against three main regions. Eur J Immunol. 1997;27:3059–69. doi: 10.1002/eji.1830271144. [DOI] [PubMed] [Google Scholar]
- 32.Wallström E, Khademi M, Andersson M, Weissert R, Linington C, Olsson T. Increased reactivity to myelin oligodendrocyte glycoprotein peptides and epitope mapping in HLA DR2(15)+ multiple sclerosis. Eur J Immunol. 1998;28:3329–35. doi: 10.1002/(SICI)1521-4141(199810)28:10<3329::AID-IMMU3329>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Hasse CG, Guggenmos J, Brehm U, et al. The fine specificity of the myelin oligodendrocyte glycoprotein autoantibody response in patients with multiple sclerosis and normal healthy controls. J Neuroimmunol. 2001;114:220–5. doi: 10.1016/s0165-5728(00)00462-8. [DOI] [PubMed] [Google Scholar]
- 34.Tejada-Simon MV, Hong J, Rivera VM, Zhang JZ. Reactivity pattern and cytokine profile of T cells primed by myelin peptides in multiple sclerosis and healthy individuals. Eur J Immunol. 2001;31:907–17. doi: 10.1002/1521-4141(200103)31:3<907::aid-immu907>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Chin Y, Henderikx P, Medaer R, Chou C-H, Raus JCM. Antibodies to myelin basic protein and measles virus in multiple sclerosis: precursor frequency analysis of the antibody producing B cells. Autoimmunity. 1991;11:27–34. doi: 10.3109/08916939108994705. [DOI] [PubMed] [Google Scholar]
- 36.Martin R, Jaraquemada D, Flerlage M, Richert J, Whitaker J, Long EO, McFarlin DE, McFarland HF. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J Immunol. 1990;145:540–8. [PubMed] [Google Scholar]
- 37.Hermans G, Stinissen P, Hauben L, van den Berg-Loonen E, Raus J, Zhang J. Cytokine profile of myelin basic protein-reactive T cells in multiple sclerosis and healthy individuals. Ann Neurol. 1997;42:18–27. doi: 10.1002/ana.410420106. [DOI] [PubMed] [Google Scholar]
- 38.Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1990;5:170–5. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]