Abstract
4-1BB(CD137) is a member of the tumour necrosis factor receptor superfamily and is expressed on activated T cells, monocytes and natural killer (NK) cells. The interaction of 4-1BB and 4-1BB ligand provides a costimulatory signal leading to T-cell activation. The expression of 4-1BB has been known to be activation dependent. Interestingly, we found that expression of 4-1BB increased in human peripheral blood mononuclear cells after exposure to mitomycin C. Thus, we tested whether the treatment with other DNA-damaging agents, such as doxorubicin, bleomycin, and γ-irradiation, could induce 4-1BB expression. The data indicated that 4-1BB expression increased dose-dependently by these agents reaching maximum at 2–3 days after the exposure. We found that the major 4-1BB-expressing population was CD3+ T cells, although a moderate number of CD14+ cells and a few NKB1+ cells also expressed 4-1BB. The levels of 4-1BB expression induced by anticancer drugs, were relatively lower than that induced by CD3 ligation. Interestingly, at subcytotoxic concentrations, doxorubicin and bleomycin considerably enhanced 4-1BB expression induced by CD3 ligation in CEM cells. The ligation of the damage-induced 4-1BB by monoclonal antibody enhanced the viability and proliferating capacity of the cells. In conclusion, the expression of 4-1BB might be one of the cellular responses of the immune cells against various genotoxic stresses.
Introduction
4-1BB (CD137) is a member of the tumour necrosis factor receptor (TNFR) superfamily1–4 and is expressed on immune cells such as activated CD4+ and CD8+ T cells,5,6 activated natural killer (NK) cells7 and murine dendritic cells.8 Although recent studies showed that 4-1BB is constitutively expressed in primary human monocytes and blood vessel endothelial cells in malignant tumours,9,10 the expression of 4-1BB mostly has been known to be dependent on various stimuli for activation. 4-1BB is inducible through the T-cell receptor (TCR) in the presence of cytokines or in combination with CD28 ligation5,11 or by stimulation with concanavalin A (Con A) or anti-TCR monoclonal antibody (mAb), or anti-CD3 mAb.5,12,13 This induction could be inhibited by cyclosporin A (Cys A)12 or cycloheximide.13
4-1BB binds to a high affinity ligand (4-1BBL) expressed on several antigen-presenting cells (APC) such as activated B cells, macrophages14–18 and splenic dendritic cells.19 The interaction of 4-1BB and its ligand provides a costimulatory signal leading to T-cell activation and growth.19,20 Signals delivered to the T cell by the 4-1BB receptor can induce T cells to produce interleukin-2 (IL-2), proliferate, and differentiate,15,16,21,22 as well as protect T cells from activation-induced cell death.23,24
For years, the ability of various anticancer drugs (e.g. cyclophosphamide, L-PAM, 1,3-bis(2-chloroethyl)-1-nitrosourea, vinblastin and bleomycin) to assist the acquisition of antitumour immunity in a variety of animal tumour models25–29 and in tumour bearers30–32 has been reported. Recently, it has been shown that genotoxic stresses by mitomycin C, γ-irradiation and melphalan exert their immunopotentiation effect by the up-regulation of B7-1 surface expression on APC through up-regulating B7-1 gene expression.33 Other studies on the mechanisms of acquiring antitumour immunity revealed that the chemotherapy induces the shift of cytokine profile from anti-inflammatory cytokines toward proinflammatory cytokines.34–41 However, no studies have shown the expression pattern of costimulatory molecules on T cells under genotoxic stresses.
In this study, we report that 4-1BB is induced by DNA-damaging agents such as anticancer drugs or γ-irradiation in normal human peripheral blood mononuclear cells (PBMC). We find that the major 4-1BB-expressing cells are CD3+ T cells and CD14+ monocytes. The DNA-damaging agents directly act on T cells to express 4-1BB in the absence of APC or other cells. The treatment with less cytotoxic concentrations of doxorubicin or bleomycin considerably enhance 4-1BB expression induced by CD3 ligation. The ligation of the damage-induced 4-1BB enhances the viability and proliferating capacity of the cells expressing 4-1BB. Taken together, this study suggests that the induction of 4-1BB expression may be one of the cellular responses of the immune cells against various genotoxic stresses.
Materials and methods
Antibodies and cells
Previously, we generated and characterized a mouse mAb, 4B4 against the human 4-1BB molecule.42 MAG56 mAb is an irrelative, isotype-matched antibody used as control.43 For flow cytometric analysis, 4B4 and MAG56 mAbs conjugated with fluorescein isothiocyanate were used. Fresh human PBMC were obtained from heparinized peripheral blood of healthy donors by Ficoll density gradient centrifugation. Isolated PBMC were washed three times with Hank's balanced salt solution. Then peripheral blood T cells were purified by two rounds of rosetting with sheep red blood cells. The purity of T cells was verified by flow cytometry (CD3+ T cell > 95%, data not shown). Cells were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 µg/ml penicillin and 100 U/ml streptomycin. All cell culture media and supplements were purchased from Biowhittaker (Walkersville, MD) unless otherwise specified. A mAb specific to human CD3 was purchased from BD Pharmingen (San Diego, CA). The human T-cell tumour line CEM was purchased from the American Type Culture Collection (Manassas, VA). For TCR stimulation, anti-CD3 mAb or control mAb was diluted in phosphate-buffered saline (PBS) at various concentrations. Each well of a 48-well plate was coated with 100 µl of diluted mAb overnight at 4°, and then washed with PBS.
Anticancer drug treatment and γ-irradiation
Doxorubicin, bleomycin, and mitomycin C were purchased from Sigma (St. Louis, MO). Cells were treated with doxorubicin at a dose range of 20–200 ng/ml, or with bleomycin at a dose range of 6–60 µg/ml, or with mitomycin C at a dose range of 100–1000 ng/ml for 1–4 days at 37°. The concentrations relevant for therapy are 1–20 ng/ml for doxorubicin,44 1·5–3 µg/ml for bleomycin45 and 100–1000 ng/ml for mitomycin C46 in patients' sera.
Otherwise, human PBMC were irradiated with single doses at 600, 1250, or 2500 rad by the cesium irradiator IBL 437C (CIS Biointernational, B.P.N., 32-91192 Gif-Sur-Yvette Cedex, France).
Flow cytometry
Cells were harvested at determined time points and washed with staining media (Dulbecco's modified Eagle's minimal essential medium containing 1% bovine serum albumen and 0·01% sodium azide). 2 × 105 cells were incubated with 10 µg/ml 4B4 or control MAG56 mAb labelled with fluorescein isothiocyanate for 1 hr at 4°. Stained cells were washed twice with staining media and resuspended in PBS. To exclude dead cells from the data, we added 1 µg/ml propidium iodide to the cell suspension and incubated for 5 min prior to analysis. Only the cells that were negative to propidium iodide staining were gated and further analysed for 4-1BB expression. To identify the cell population, we double stained the cells with one of CD3-, CD4-, CD8-, CD14- or NKB1-specific antibodies labelled with phycoerythrin (all purchased from BD Pharmingen) together with 4B4 or MAG56 mAb labelled with fluorescein isothiocyanate. Flow cytometry was performed using a PAS IIIi flow cytometer (Partec GmbH, Münster, Germany) and analysed using Winlist software (Verity Software House, Topsham, ME). A 488-nm excitation laser and a filter > 560 nm for propidium iodide detection were used. The percentage of the 4-1BB-expressing cells was calculated as percentage 4B4-binding cells − percentage control mAb-binding cells.
RNA isolation and reverse transcriptase–polymerase chain reaction (RT–PCR)
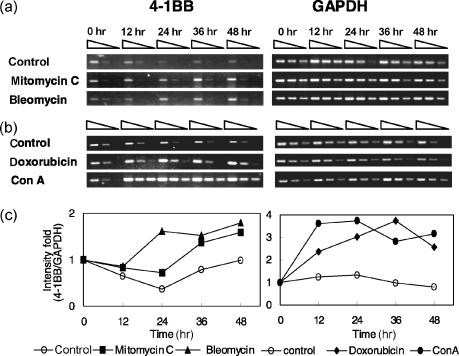
Purified human peripheral blood T cells were obtained from two healthy donors (A, B). One person's T cells were cultured with 1000 ng/ml mitomycin C or 60 µg/ml bleomycin and the other's were cultured with 200 ng/ml doxorubicin or 10 µg/ml Con A, and then harvested at 0, 12, 24, 36, and 48 hr. Total RNA was isolated from each sample using the guanidinium method that relies on a CsCl step gradient.47 Total RNA (3·3 µg) was reverse transcribed into cDNA by oligo-dT priming using superscript II RT (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's recommended method. The enzymatic amplification of the cDNA by PCR was performed as modified by Saiki et al.48 The reaction conditions yielding product in the linear range were established in a preliminary study (data not shown). Threefold serial dilutions of cDNA were subjected to PCR using 1 unit of Taq DNA polymerase (Roche Molecular Biochemicals, Mannheim, Germany), 0·2 mm of each dNTP (Promega, Madison, WI), 1·5 mm of MgCl2 and 60 mm of each specific primer for 4-1BB or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in a final volume 25 µl of 1 × PCR buffer (Roche). GAPDH was used to normalize for template amount. Sense and antisense primers used in the PCR were as follows: for 4-1BB, 5′-GCTTTGGGACATTTAACGAT-3′ and 5′-GCAGCTACAGCCATCTTCCT-3′; for GAPDH, 5′-TTAGCACCCCTGGCCAAGG-3′ and 5′-CTTACTCCTTGGAGGCCATG-3′. For each PCR reaction, the sample was first denatured at 95° for 3 min and amplified by 38 cycles (see Fig. 5a) or 36 cycles (see Fig. 5b) of PCR (94°, 30 s; 55°, 30 s; 72°, 1 min), followed by 72° 7 min for 4-1BB or by 28 cycles (see Fig. 5a) or 26 cycles (see Fig. 5b) of PCR for GAPDH. Equal aliquots of RT-PCR product for 4-1BB (368 bp) and GAPDH (500 bp) were separated on 1·5% agarose gels stained with ethidium bromide.
Figure 5.
4-1BB mRNA expression is increased by anticancer drugs. Purified peripheral blood T cells from two healthy donors were cultured with bleomycin (60 µg/ml) or mitomycin C (1000 ng/ml) (a), doxorubicin (200 ng/ml) or Con A (10 µg/ml) (b). The cells were harvested at 0, 12, 24, 36, and 48 hr. The total RNA was extracted and analysed for relative levels of 4-1BB mRNA by RT–PCR as described in Materials and Methods. GAPDH was used to normalize for template quantity. Three-fold serial dilutions of cDNA at each time were subjected to PCR and equal amount of product for 4-1BB (368 bp) and GAPDH (500 bp) were resolved on 1·5% agarose gels stained with ethidium bromide. Data shown were the representative results from 15 independent experiments with the samples from five donors. (c) The first band intensity of each time point was divided by the corresponding GAPDH band intensity and the results were represented as intensity fold when 0 hr value in each reagent group was designated as 1.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
4B4 mAb or MAG56 mAb was diluted in PBS at 10 µg/ml. Each well of 96 well plate was coated with 100 µl of diluted mAb overnight at 4°, then washed with PBS.
We cultured normal human peripheral blood T cells with doxorubicin (200 ng/ml) or bleomycin (60 µg/ml) or mitomycin C (1000 ng/ml) for 2 days. At day 2, only the viable cells were isolated by ficoll density gradient centrifugation and 4 × 105 cells were plated in each well of 96-well plates coated with 4B4 mAb or MAG56 mAb, then cultured for 4 days. At the end of culture, 10 µl of 5 mg/ml MTT (Sigma) solution was added to each well and the plates were incubated at 37°. After 4 hr, insoluble blue formazan crystals were solved in 0·04 m HCl–isopropanol, and absorbance was measured at 540 nm. Each sample was determined in triplicate. The numbers of viable cells were calculated by extrapolating absorbances into the internal standard curve.
Proliferation assay
Normal human peripheral blood T cells were cultured with doxorubicin (200 ng/ml) or bleomycin (60 µg/ml) or mitomycin C (1000 ng/ml) for 2 days. At day 2, only the viable cells were isolated and 4 × 105 cells were plated in each well of 96-well plates coated with an anti 4-1BB mAb (4B4 mAb) or an isotype control mAb (MAG56 mAb), then were incubated at 37°. At day 3, 10 µg/ml Con A was added to each well and the 96-well plates were incubated for 16 hr. At the end of culture, 1 µCi of [methyl-3H]thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) was added to each well and the plates were incubated for additional 8 hr. At day 4, the cells were harvested and the radioactivity was measured by liquid scintillation counting. Each sample was determined in triplicate.
Statistical analysis
In the MTT assay and proliferation assay, each sample was determined in triplicate wells and the data were represented as mean value ± SD. At the same time, the differences between control mAb (MAG56) and 4B4 mAb treatment samples were analysed by Student's t-test (*represents P < 0·05).
Results
Induction of 4-1BB molecules by anticancer drugs in normal human PBMC
Accidentally, we identified that mitomycin C induced human PBMC to express 4-1BB molecules. The percentage of 4-1BB-expressing cells increased according to the dose of mitomycin C (Fig. 1a). However, it was not clear that the induction of 4-1BB was a specific event for mitomycin C, or a universal phenomenon induced by the common DNA-damaging agents. Therefore, we tested whether other anticancer drugs could induce 4-1BB molecules on PBMC. Normal human PBMC obtained from healthy volunteers were cultured with one of anticancer drugs, then the cells were harvested and analysed by flow cytometry to detect 4-1BB expression at days 1, 2, 3, and 4. The percentage of 4-1BB-expressing cells peaked between 48 hr (day 2) and 72 hr (day 3) after exposure to all three anticancer drugs (doxorubicin, bleomycin, and mitomycin C), showing a dose-dependent tendency, and decreased at 96 hr (day 4). In freshly isolated PBMC (day 0), the percentage of 4-1BB-expressing cells maintained at low levels (Fig. 1b).
Figure 1.
4-1BB induction by anticancer drugs in normal human PBMC. (a) Fresh human PBMC obtained from healthy volunteers were incubated in presence of indicated concentrations of mitomycin C for 2 days. Then the cells were harvested, stained with 4B4 (filled) or control mAb (unfilled), and analysed by flow cytometry to detect 4-1BB expression. The percentage of 4-1BB-expressing cells increased according to the dose of mitomycin C. (b) Human PBMC were incubated in presence of indicated concentrations of doxorubicin, bleomycin, or mitomycin C. At days 1, 2, 3, and 4, the cells were harvested and analysed by flow cytometry to detect 4-1BB expression. The data were presented as the percentage of the 4-1BB-expressing cells in whole PBMC population, calculated as percentage 4B4-binding cells − percentage control mAb-binding cells. 4-1BB molecules were induced by doxorubicin, bleomycin, mitomycin, and the percentage of 4-1BB-expressing cells peaked at days 2–3 showing dose-dependent tendency, and decreased at day 4. These data show the representative results from six independent experiments. Each sample was determined in duplicate and the mean values were presented.
Analysis of 4-1BB-expressing cell population
It has been well known that 4-1BB molecules are mainly expressed on activated CD4+ and CD8+ T cells.5,6 However, 4-1BB expression is not limited to activated T cells. Recent reports showed that NK cells and monocytes are able to express 4-1BB at a considerable level.7,9 Thus, we analysed the 4-1BB-expressing cells to identify which cell population in PBMC was induced to express 4-1BB by anticancer drugs. Fresh human PBMC were incubated in presence of 200 ng/ml doxorubicin, 60 µg/ml bleomycin, or 1000 ng/ml mitomycin C for 48 h (2 days), then analysed by flow cytometry. The results showed that the predominant 4-1BB-expressing cell population was CD3+ cells, although some CD14+ cells also expressed 4-1BB (Fig. 2). Moderate numbers of CD14+ cells expressed 4-1BB at day 0, and the percentage of 4-1BB-expressing CD14+ cells slightly increased after the treatment with anticancer drugs. On the other hand, only a few NK cells showed 4-1BB expression.
Figure 2.
The predominant 4-1BB-expressing population is CD3+ cells and CD14+ cells. Fresh human PBMC were incubated in presence of 200 ng/ml doxorubicin, 60 µg/ml bleomycin, or 1000 ng/ml mitomycin C for 48 hr, then were double stained with one of CD3, CD14, or NKB1-specific antibodies together with an anti 4-1BB mAb or a control mAb. The numerical values in bold style represent the percentage of the 4-1BB-positive cells, and the values in parenthesis represent the background staining with isotype control mAb. The percentage of 4-1BB-expressing cells was highest in CD3+ cells, although some CD14+ cells and a few NKB1+ cells also expressed 4-1BB. Data shown were the representative results from four independent experiments.
4-1BB expression was induced directly by anticancer drugs in purified T cells
To identify whether the DNA-damaging stimuli directly act on T cells to induce 4-1BB or indirectly effect via other cells in PBMC, purified T cells were cultured with anticancer drugs, then analysed by flow cytometry to detect 4-1BB expression. Anticancer drugs induced 4-1BB expression in the purified T cells (Fig. 3) and both CD4+ and CD8+ T cells expressed 4-1BB (data not shown). The percentage of 4-1BB-expressing cells increased with a dose-dependent tendency (Fig. 3). This result indicated that the DNA-damaging stimuli directly act on T cells in the absence of APCs or other cells.
Figure 3.
Direct 4-1BB expression by anticancer drugs in purified T cells. Peripheral blood T cells were isolated from PBMC by two rounds of rosetting with sheep red blood cells. The purity of T cells was verified by flow cytometry (CD3+ T cell > 95%, data not shown). The purified T cells were cultured with anticancer drugs and harvested at indicated time points, then analysed by flow cytometry to detect 4-1BB expression. The percentages of the cells expressing 4-1BB were presented as a function of treatment time and concentration of each anticancer drug. Each sample was determined in duplicate and the mean values were presented. Anticancer drugs directly induced 4-1BB expression in the purified T cells in the absence of APC or other cells. These data show the representative results from three independent experiments.
Induction of 4-1BB molecules by γ-irradiation
To investigate whether 4-1BB is induced by another kind of DNA-damaging stimulations other than the anticancer drugs, normal human PBMC were exposed to γ-irradiation. The results showed that γ-irradiation induced high expression of 4-1BB in both CD4+ and CD8+ cells at day 1 (Fig. 4). The percentage of 4-1BB-expressing cells was higher in CD4+ cells than in CD8+ cells, and it increased at day 2 in both CD4+ and CD8+ T cells (Fig. 4). These results suggest that 4-1BB molecules are generally induced by various DNA-damaging stimuli, not only by anticancer drugs.
Figure 4.
4-1BB molecules are induced by γ-irradiation both in CD4+ and CD8+ cells. Freshly isolated PBMC were irradiated with single doses at indicated dosed by caesium irradiator. The irradiated cells were cultured and harvested at indicated time points and analysed by flow cytometry to detect 4-1BB expression. Cells were double stained with CD4- (a and c) or CD8-specific (b and d) antibodies together with an anti 4-1BB mAb or a control mAb. Each sample was determined in duplicate and the mean values were presented as a function of time and irradiation dose (a and b), or the representative results were presented in flow cytometry histograms (c and d). Data shows that γ-irradiation induced considerable expression of 4-1BB in both CD4+ and CD8+ cells.
Induction of 4-1BB mRNA by anticancer drugs in T cells
The amount of 4-1BB mRNA was measured in purified T cells cultured with anticancer drugs. RT–PCR analysis (Fig. 5) showed the 4-1BB mRNA expression level by mitomycin C (1000 ng/ml), bleomycin (60 µg/ml), and doxorubicin (200 ng/ml). Figure 5 consists of the results of two different donors' samples (A and B) and the RT–PCR conditions are described in Materials and Methods. The reaction conditions yielding product in the linear range were established in a preliminary study (data not shown) and GAPDH was used to normalize for the template amount. 4-1BB mRNA expression was observed at 0 hr, but any increase was not detected afterwards in both T cells cultured without drug. However, the increase of 4-1BB mRNA amount was obviously detected at 36 hr and 48 hr by mitomycin C (Fig. 5a). In the case of bleomycin, 4-1BB mRNA expression was increased at 24 hr and the elevated amount was maintained until 48 hr (Fig. 5a). For doxorubicin, the induction of 4-1BB mRNA expression was detected at 12 hr, and the pattern was continued until 48 hr (Fig. 5b). We also cultured the purified human peripheral blood T cells with Con A (10 µg/ml), and 4-1BB mRNA expression levels at 12, 24, 36, and 48 hr were much higher than those of other anticancer drugs (Fig. 5b).
Comparative analysis of 4-1BB expression by DNA damaging agents and by TCR stimulation on the T-cell line
To compare the features of 4-1BB expression by anticancer drugs with that by TCR stimulation, we utilized CEM cells, a T-cell tumour line which highly express 4-1BB molecules in response to mitogenic stimulation.49 CEM cells were treated with various concentrations of anticancer drugs for 2 hr at 37°. Then stimulated CEM cells were transferred to 48-well plates coated with various concentrations of anti-CD3 mAbs or control mAbs. After 24 hr, the CEM cells were harvested and the 4-1BB expression was measured by flow cytometry. As shown in Fig. 6, the level of 4-1BB expression induced by anticancer drugs, at even higher concentrations, was relatively lower than that of 4-1BB induction by CD3 ligation. Interestingly, the lower (less cytotoxic) concentrations of doxorubicin considerably enhanced 4-1BB expression induced by CD3 ligation. At a concentration of 80 ng/ml, the level of 4-1BB expression induced by doxorubicin plus 10 µg/ml CD3 antibody (42·41%) was higher than a simple sum of those induced by doxorubicin or CD3 antibody only (9·35% and 17·10%, respectively). However, by 2000 ng/ml doxorubicin, the 4-1BB expression remarkably decreased regardless of the intensity of CD3 ligation. Bleomycin also showed similar effects of enhancing 4-1BB expression at lower concentrations, while mitomycin C did not.
Figure 6.
Comparative analysis of 4-1BB expression by DNA damaging agents and by TCR stimulation. CEM cells were treated with various concentrations of anticancer drugs for 2 hr at 37°, then were transferred to 48-well plates coated with various concentrations of anti-CD3 mAb or control mAb. After 24 hr, The CEM cells were harvested and the 4-1BB expression was measured by flow cytometry. The levels of 4-1BB expression induced by anticancer drugs were relatively lower than that induced by CD3 ligation. At the lower concentrations, doxorubicin and bleomycin considerably enhanced 4-1BB expression induced by CD3 ligation, while mitomycin C did not. Data shown were the representative results from three independent experiments. Each sample was determined in duplicate and the mean values were presented.
The enhancement of the cell viability and proliferating capacity by ligation of the damage-induced 4-1BB
To elucidate the functional role of the damage-induced 4-1BB molecules, we cultured normal human peripheral blood T cells with anticancer drugs for 2 days. At day 2, 4-1BB expression levels of the cells were measured by flow cytometry (data not shown). Then only the viable cells were isolated and plated in 96 wells coated with an anti-4-1BB mAb or an isotype control mAb, and cultured for 4 days. At day 4, the viability of the cells was measured by MTT assay. Also, the abilities of the cells to proliferate in response to Con A, mitogenic stimulus were evaluated. As shown in Fig. 7(a), the anticancer drug-treated cells plated in the wells coated with an anti-4-1BB mAb showed higher viabilities compared to the cells in wells coated with a control mAb (P < 0·05 in all three treatment groups). The enhanced viabilities by 4-1BB ligation were appeared in all three treatment groups. Moreover, the cells in the wells coated with an anti-4-1BB mAb showed higher proliferating abilities than the cells in wells with a control mAb (Fig. 7b). Although the enhancement effects of proliferation by 4-1BB ligation showed the variation among the treatment groups, the effects appeared obvious (P < 0·05 in all three-treatment groups).
Figure 7.
The ligation of DNA damage-induced 4-1BB enhances the viability and proliferating capacity of the cells treated with anticancer drugs. Freshly isolated peripheral blood T cells were cultured with doxorubicin (200 ng/ml) or bleomycin (60 µg/ml) or mitomycin C (1000 ng/ml) for 2 days. At day 2, only the viable cells were isolated by Ficoll density gradient centrifugation and 3 × 105 cells were plated in each well of 96-well plates coated with an anti-4-1BB mAb or an isotype control mAb, then cultured for 4 days. At the end of culture, the viability of the cells was measured by MTT assay (a). At the same time, the abilities of the cells to proliferate in response to mitogenic signal (10 µg/ml Con A) were evaluated by [methyl-3H]thymidine uptake (b). Data shown were the representative results from four independent experiments. Each sample was determined in triplicate wells and the data were represented as mean value ± SD. The differences between control mAb (MAG56) and 4B4 mAb treatment samples were analysed by Student's t-test. *P < 0·05.
Discussion
In this study, we report that 4-1BB is induced by DNA-damaging agents such as anticancer drugs or γ-irradiation in normal human PBMC. We found that the major 4-1BB-expressing cells are CD3+ T cells and CD14+ monocytes. The DNA-damaging agents directly acted on T cells to express 4-1BB in the absence of APC or other cells. The levels of 4-1BB expression induced by anticancer drugs, were relatively lower than that induced by CD3 ligation. At subcytotoxic concentrations, doxorubicin and bleomycin considerably enhanced 4-1BB expression induced by CD3 ligation in CEM cells. The ligation of the damage-induced 4-1BB enhanced the viability and proliferating capacity of the cells.
So far, the expression of 4-1BB has been known to be dependent on various stimuli for activation, such as TCR ligation or mitogen(s). 4-1BB mRNA is detectable by 3 hr and its surface expression peaks at 40–64 hr and declines again by 110 hr after stimulation.50 4-1BB is expressed on immune cells such as activated CD4+ and CD8+ T cells, activated NK cells, monocytes and murine dendritic cells. In contrast to these known properties of the ‘activation-induced’ expression of 4-1BB, we found that genotoxic stress by anticancer drugs or γ-irradiation could induce 4-1BB expression, even though the concentrations of anticancer drugs with which we treated human PBMC are clinically relevant or somewhat higher. Each anticancer drug used in this study showed a different time period of ‘damage-induced’ 4-1BB mRNA induction (at 36–48 hr by mitomycin C, 24–48 hr by bleomycin, 12–48 hr by doxorubicin; Fig. 5a,b). In addition, the surface expression of 4-1BB peaked at day 2–3, then decreased at day 4 (Fig. 1b). These results indicate that the expression of ‘damage-induced’ 4-1BB mRNA or protein is slightly later than that of ‘activation-induced’ 4-1BB. Furthermore, the expression levels of ‘damage-induced’ 4-1BB mRNA (Fig. 5a,b) or protein (Fig. 6) were relatively lower compared to that of ‘activation-induced’ expression by mitogenic or TCR stimulation.
According to fluorescence-activated cell sorting analysis of 4-1BB-expressing cell population, the percentage of 4-1BB-expressing cells was the highest in CD3+ T cells, although some CD14+ cells and a few NK cells also expressed 4-1BB (Fig. 2). The CD14+ monocytes showed moderate and relatively constitutive level of 4-1BB expression compared to that of CD3+ T cells. However, although the percentages of monocytes that expressed 4-1BB increased in response to anticancer drugs, this 4-1BB induction might be less significant than that of CD3+ T cells, considering the evidence that 4-1BB is constitutively expressed on human monocytes without any specific stimulation.9 Thus, the major cell population in PBMC that express 4-1BB in response to anticancer drugs seems to be CD3+ T cells.
The costimulatory capacity of ‘activation-induced’ 4-1BB has been well demonstrated. When the T cells are stimulated by TCR signals, 4-1BB is able to facilitate both T-cell proliferation and high-level production of IL-2 by resting T cells simultaneously.19,22,51 Also, cross-linking 4-1BB with mAb resulted in the 2–10-fold enhancement of T-cell proliferation.5 Here we show that ligation of the ‘damage-induced’ 4-1BB with mAb resulted in enhanced survival rate (Fig. 7a) and higher proliferation capacity of the cells in response to stimulation by Con A (Fig. 7b). These results indicate that the ‘damage-induced’ 4-1BB molecules are functionally active and the biological functions might be similar to that of the ‘activation-induced’ 4-1BB. In this regard, the augmented expression of 4-1BB by TCR stimulation plus DNA damage (Fig. 6) suggest that mild DNA damage could work as a danger signal that stimulate the immune cells to provoke immune response more effectively.
Many investigators have reported the ability of anticancer drugs to facilitate the acquisition of antitumour immunity by tumour bearers.25–32 To elucidate the mechanism of genotoxic stress-induced immunopotentiating effect, previous studies tends to focus on professional APC or the change of cytokine-secreting pattern from anti-inflammatory cytokines (e.g. transforming growth factor-β and IL-10) with inhibitory activity for cytotoxic T lymphocyte generation toward proinflammatory cytockines (such as TNF-β, interferon-γ, and granulocyte–macrophage colony-stimulating factor) that favour the development of antitumour cell-mediated immunity.34–41 Sojka et al. have shown that administration of low-dose melphalan to mice bearing a large MOPC-315 plasmacytoma led to a rapid up-regulation of B7-1 expression of the surface of MOPC-315 tumour cells. In addition, in vitro treatment of γ-irradiation and mitomycin C to MOPC-315 and P815 tumour cells led to the same result as that of the melphalan-treated case.33 Moreover, Seo et al. reported that in vitro exposure of the A20.2 J lymphoma to γ-irradiation resulted in the up-regulation of B7-1 surface expression, in turn leading to irradiation-induced enhancement of APC function.52 On the other hand, several studies have reported that 4-1BBL, a counterpart of 4-1BB, contributes to potentiate antitumour and host immunity with co-operation of B7-1 and B7-2 molecules.53–55 In this regard, DNA-damage induced 4-1BB may contribute to the enhancement of antitumour immunity by a low dose of bleomycin, cyclophosphamide, L-PAM, and vinblastin.
It seems possible many molecules other than 4-1BB could be induced by DNA damage in normal human cells. Although most part of the properties and biological roles of these molecules still remain undefined, there is no doubt that the investigations into the function of these molecules have considerable value to understand the mechanism of antitumour immunity induced by various genotoxic stress.
Acknowledgments
We thank Dr R. Ward, US Environmental Protection Agency, for discussions. This work was supported by Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University and in part by SRC fund to IRC from the KOSEF and by BK21 project for Medicine, Dentistry and Pharmacy.
Abbreviations
- NK
natural killer
- Con A
concanavalin A
- mAb
monoclonal antibody
- PBMC
peripheral blood mononuclear cell
- APC
antigen-presenting cells
- TCR
T-cell receptor
References
- 1.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci USA. 1989;86:1963–7. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallett S, Barclay AN. A new superfamily of cell surface proteins related to the nerve growth factor receptor. Immunol Today. 1991;12:220–2. doi: 10.1016/0167-5699(91)90033-P. [DOI] [PubMed] [Google Scholar]
- 3.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins. Activation, Costimulation, Death Cell. 1994;76:959–62. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 4.Armitage RJ. Tumor necrosis factor receptor superfamily members and their ligands. Curr Opin Immunol. 1994;6:407–13. doi: 10.1016/0952-7915(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 5.Pollok KE, Kim YH, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS. Inducible T-cell antigen 4–1BB. An analysis of expression and function. J Immunol. 1993;150:771–81. [PubMed] [Google Scholar]
- 6.Shuford WW, Klussman K, Tritchler DD, et al. 4–1BB co-stimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification of in vivo cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CD137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–72. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 8.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. Expression and function of 4-1BB and 4–1BBL on murine dendritic cells. Int Immunol. 2002;14:275–86. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 9.Kienzle G, von Kempis J. CD137 (ILA/4-1BB), expressed by primary human monocytes, induces monocyte activation and apoptosis of B lymphocytes. Int Immunol. 2000;12:73–82. doi: 10.1093/intimm/12.1.73. [DOI] [PubMed] [Google Scholar]
- 10.Broll K, Richter G, Pauly S, Hofstaedter F, Schwarz H. CD137 expression in tumor vessel walls. High correlation with malignant tumors. Am J Clin Pathol. 2001;115:543–9. doi: 10.1309/e343-kmyx-w3y2-10ky. [DOI] [PubMed] [Google Scholar]
- 11.Pollok KE, Kim SH, Kwon BS. Regulation of 4-1BB expression by cell–cell interactions and cytokines, interlukin-2 and interlukin-4. Eur J Immunol. 1995;25:488–94. doi: 10.1002/eji.1830250227. [DOI] [PubMed] [Google Scholar]
- 12.Kwon BS, Kestler DP, Eshhar Z, Oh KO, Wakulchik M. Expression characteristics of two potential T cell mediator genes. Cell Immunol. 1989;121:414–22. doi: 10.1016/0008-8749(89)90040-3. [DOI] [PubMed] [Google Scholar]
- 13.Kwon BS, Kim GS, Prystowsky MB, Lancki DW, Sabath DE, Pan JL, Weissman SM. Isolation and initial characterization of multiple species of T-lymphocyte subset cDNA clones. Proc Natl Acad Sci USA. 1987;84:2896–900. doi: 10.1073/pnas.84.9.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin RG, Din WS, Davis-Smith T, et al. Molecular cloning of a ligand for the inducible T-cell gene 4-1BB. a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–41. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 15.Alderson MR, Smith CA, Tough TW, et al. Molecular and biological characterization of human 4-1BB and its ligand. Eur J Immunol. 1994;24:2219–27. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- 16.DeBenedette MA, Chu NR, Pollok KE, Hurtado J, Wade WF, Kwon BS, Watts TH. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its upregulation on M12 B lymphoma by cAMP. J Exp Med. 1995;181:985–92. doi: 10.1084/jem.181.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollok KE, Kim YJ, Hurtado J, Zhou Z, Kim KK, Kwon BS. 4-1BB T-cell antigen binds to mature B-cells and macrophages and costimulates anti-µ-primed splenic B cells. Eur J Immunol. 1994;24:367–74. doi: 10.1002/eji.1830240215. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz H, Valbracht J, Tuckwell J, Von Kempis J, Lotz M. ILA, The human 4-1BB homologue, is inducible in lymphoid and other cell lineages. Blood. 1995;85:1043–52. [PubMed] [Google Scholar]
- 19.DeBenedette MA, Shahinian A, Mak TW, Watts T. Costimulation of CD28– T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–9. [PubMed] [Google Scholar]
- 20.Schwarz H, Blanco FJ, Von Kempis J, Valbracht J, Lotz M. ILA, a member of the human nerve growth factor/ tumor necrosis factor receptor family, regulates T-lymphocyte proliferation and survival. Blood. 1996;87:2839–45. [PubMed] [Google Scholar]
- 21.Hurtado JC, Kim SH, Pollok KE, Lee ZH, Kwon BS. Potential role of 4-1BB in T cell activation: comparison with the costimulatory molecule CD28. J Immunol. 1995;155:3360–7. [PubMed] [Google Scholar]
- 22.Saoulli K, Lee SY, Cannons JL, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187:1849–62. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol. 1997;158:2600–9. [PubMed] [Google Scholar]
- 24.Takahashi C, Mittler RS, Vella AT. 4-1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999;162:5037–40. [PubMed] [Google Scholar]
- 25.Takesue BY, Pyle JM, Mokyr MB. Importance of tumor-specific cytotoxic CD8+ T-cells in eradication of a large subcutaneous MOPC-315 tumor following low-dose melophalan therapy. Cancer Res. 1990;50:7641–9. [PubMed] [Google Scholar]
- 26.Mokyr MB, Barker E, Weikirch L, Takesue By Pyle JM. Importance of Lyt2+ T-cells in the curative effectiveness of a low dose of melphalan for mice bearing a large MOPC-315 tumor. Cancer Res. 1989;49:4597–606. [PubMed] [Google Scholar]
- 27.Yuan L, Kuramitsu Y, Li Y, Kobayashi M, Hosokawa M. Restoration of interleukin-2 production in tumor-bearing rats through reduction of tumor-derived transforming growth factor β by treatment with bleomycin. Cancer Immunol Immunother. 1995;41:355–62. doi: 10.1007/BF01526555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagarkatti M, Toney DM, Nagarkatti PS. Immunomodulation by various nitrosoureas and its effect on the survival of the murine host bearing a syngenic tumor. Cancer Res. 1989;49:6587–92. [PubMed] [Google Scholar]
- 29.North RJ, Awwad M. Elimination of cycling CD4+ suppressor T-cells with anti-mitotic drug releases non-cycling CD8+ T-cells to cause regression of an advanced lymphoma. Immunology. 1990;71:90–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Sahasrabudhe DM, deKernion JB, Pontes JE, Ryan DM, O'donnell RW, Marquis DM, Mudholkar GS, McCune CS. Specific immunotherapy with suppressor function inhibition for metastatic renal cell carcinoma. J Biol Response Modif. 1986;5:581–94. [PubMed] [Google Scholar]
- 31.Berd D, Mastrangelo MJ. Active immunotherapy of human melanoma exploiting the immunopotentiating effects of cyclophosphamide. Cancer Invest. 1988;6:337–49. doi: 10.3109/07357908809080657. [DOI] [PubMed] [Google Scholar]
- 32.Bass K, Mastrangelo MJ. Immunopotentiation with low-dose cyclophosphamide in the active specific immunotherapy of cancer. Cancer Immunol Immunother. 1998;47:1–12. doi: 10.1007/s002620050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sojka DK, Donepudi M, Bluestone JA, Mokyr MB. Melphalan and other anticancer modalities up-regulate B7-1 gene expression in tumor cells. J Immunol. 2000;164:6230–6. doi: 10.4049/jimmunol.164.12.6230. [DOI] [PubMed] [Google Scholar]
- 34.Weiskirch LM, Bar-Dagan Y, Mokyr MB. Transforming growth factor-β-mediated down-regulation of antitumor cytotoxicity of spleen cells from MOPC-315 tumor bearing mice engaged in tumor eradication following low-dose melphalan-therapy. Cancer Immunol Immunother. 1994;38:215–24. doi: 10.1007/BF01533512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelik L, Prokhorova A, Mokyr MB. Low-dose melphalan-induced shift in the productin of a Th2-type cytokine to a Th1-type cytokine in mice bearing a large MOPC-315 tumor. Cancer Immunol Immunother. 1994;39:117–26. [PubMed] [Google Scholar]
- 36.Lattime EC, Mastrangelo MJ, Bagasra O, Li W, Berd D. Expression of cytokine mRNA in human melanoma tissue. Cancer Immunol Immunother. 1995;41:151–6. doi: 10.1007/BF01521340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorelik L, Rubin M, Prokhorova A, Mokyr MB. Importance of TNF production for the curative effectiveness of low-dose melphalan therapy for mice bearing a large MOPC-315 tumor. J Immunol. 1995;154:3941–51. [PubMed] [Google Scholar]
- 38.Gorelik L, Mokyr MB. Low-dose melphalan-induced up-regulation of type-1 cytokine expression in the s.c. tumor nodule of MOPC-315 tumor bearers and the role of interferon-γ in the therapeutic outcome. Cancer Immunol Immunother. 1995;41:363–74. doi: 10.1007/BF01526556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mokyr MB, Kalinichenko TV, Gorelik L. Potentiation of antitumor CTL response by GM-CSF involves a B7-dependent mechanism. Cell Immunol. 1997;178:152–61. doi: 10.1006/cimm.1997.1130. [DOI] [PubMed] [Google Scholar]
- 40.Matar P, Rozados VR, Ginzalez AD, Dlugovitzky DG, Bonfil RD, Scharovsky OG. Mechanism of antimetastatic immunopotentiation by low-dose cyclophosphamide. Eur J Cancer. 2000;36:1060–6. doi: 10.1016/s0959-8049(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 41.Vladimir MJ, Mokyr MB. Melphalan-induced expression of IFN-β in MOPC-315 tumor-bearing mice and its importance for the up-regulation of TNF-β expression. J Immunol. 2001;167:4895–901. doi: 10.4049/jimmunol.167.9.4895. [DOI] [PubMed] [Google Scholar]
- 42.Lee SH, Lee NG, Suh SY, Kim JG, Choi EC, Kang CY. Characterization of immunological and molecular properties of an anti-human 4-1BB monoclonal antibody. Mol Cells. 1996;6:161–8. [Google Scholar]
- 43.Kang CY, Hariharan K, Nara PL, Sodroski J, Moore JP. Immunization with a soluble CD4-gp120 complex preferentially induces neutralizing anti-human immunodeficiency virus type 1 antibodies directed to conformation-dependent epitopes of gp120. J Virol. 1994;68:5854–62. doi: 10.1128/jvi.68.9.5854-5862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller C, Chatelut E, Gualano V, De Forni M, Huguet F, Attal M, Canal P, Laurent G. Cellular pharmacokinetics of doxorubicin in patients with chronic lymphocytic leukemia: comparison of bolus administration and continuous infusion. Cancer Chemother Pharmacol. 1993;32:379–84. doi: 10.1007/BF00735923. [DOI] [PubMed] [Google Scholar]
- 45.Hall SW, Strong JE, Broughton A, Frazier ML, Benjamin RS. Bleomycin clinical pharmacology by radioimmunoassay. Cancer Chemother Pharmacol. 1982;9:22–5. doi: 10.1007/BF00296756. [DOI] [PubMed] [Google Scholar]
- 46.Muller M, Wilder S, Bannasch D, et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033–45. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glisin V, Crkvenjakov R, Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1973;13:2633–7. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- 48.Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–91. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 49.Garni-Wagner BA, Lee ZH, Kim YJ, Wilde C, Kang CY, Kwon BS. 4-1BB is expressed on CD45RAhiROhi transitional T cell in humans. Cell Immunol. 1996;169:91–8. doi: 10.1006/cimm.1996.0095. [DOI] [PubMed] [Google Scholar]
- 50.Vinay DS, Kwon BS. Role of 4–1BB in immune response. Sem Immunol. 1998;10:481–9. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 51.Chu NR, DeBenedette MA, Stiernholm BJ, Barber BH, Watts TH. Role of IL-12 and 4–1BB ligand in cytokine production by CD28+ and CD28− T cells. J Immunol. 1997;158:3081–9. [PubMed] [Google Scholar]
- 52.Seo A, Ishikawa F, Nakano H, Nakazaki H, Kobayashi K, Kakiuchi T. Enhancement of B7-1 (CD80) expression on B-lymphoma cells by irradiation. Immunology. 1999;96:642–8. doi: 10.1046/j.1365-2567.1999.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang J. Expression of co-stimulatory 4-1BB ligand induces significant tumor regression and protective immunity. Cancer Biother Radiopharm. 1999;14:353–61. doi: 10.1089/cbr.1999.14.353. [DOI] [PubMed] [Google Scholar]
- 54.Mogi S, Sakurai J, Kohsaka T, Enomoto S, Yagita H, Okumura K, Azuma M. Tumour rejection by gene transfer of 4-1BB ligand into a CD80 (+) murine squamous cell carcinoma and the requirements of co-stimulatory molecules on tumour and host cells. Immunology. 2000;101:541–7. doi: 10.1046/j.1365-2567.2000.t01-1-00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guinn BA, Bertram EM, DeBenedette MA, Berinstein NL, Watts TH. 4-1BBL enhances anti-tumor responses in the presence or absence of CD28 but CD28 is required for protective immunity against parental tumors. Cell Immunol. 2001;210:56–65. doi: 10.1006/cimm.2001.1804. [DOI] [PubMed] [Google Scholar]