Abstract
Insulin-like growth factor 1 receptor (IGF-1R) expression is augmented on T cells upon ligation of CD28, and this promotes IGF-1-mediated protection from Fas-induced cell death for up to 6 days. To determine the mechanism of action of IGF-1R in T-cell expansion, we investigated the signalling pathways activated by IGF-1 in T cells and in Jurkat cells. We found that IGF-1 transiently induces Akt, jun N-terminal kinases (JNK), and c-Jun phosphorylation in activated T cells, with JNK and c-Jun phosphorylation occurring faster than Akt phosphorylation. To mimic IGF-1R expression levels in CD28-stimulated Jurkat cells these cells were stably transfected to over-express the IGF-1R. Jurkat/IGF-1R cells exhibited enhanced constitutive Akt phosphorylation compared with mock-transfected controls, but IGF-1 induced transient phosphorylation of MKK4, JNKs, and c-Jun. Inhibition of PI-3 kinase activity and Akt phosphorylation with LY294002 totally suppressed IGF-1-mediated protection from Fas killing in activated T cells, but only partially suppressed IGF-1-mediated protection in Jurkat/IGF-1R cells. However, either dicumarol in T cells or a dominant negative JNK1 (APF) in Jurkat/IGF-1R cells greatly suppressed IGF-1-mediated protection from Fas killing. Together, these data demonstrate that IGF-1-mediated activation of JNKs and PI-3 kinase contributes to normal T-cell survival, whereas the JNK pathway may be more important in Jurkat leukaemia cells.
Introduction
Cell death mediated through the Fas death receptor is essential for the maintenance of homeostasis in the immune system1 by clearing activated T cells as the immune response declines. Soon after activation, T cells express high levels of the Fas receptor but are insensitive to death through ligation with Fas ligand.2 It is thought that this relative insensitivity to Fas ligation is conferred by powerful survival signals due to a variety of mechanisms that are transiently activated during the early stages of T-cell activation. These include expression of the caspase 8 inhibitor, c-Flip;3 and genes up-regulated by co-stimulation through the CD28 receptor, such as interleukin-2 (IL-2)4 and the antiapoptotic gene Bcl-xL.5 CD28 ligation also induces increased expression of insulin-like growth factor-1 receptor (IGF-1R) on activated T cells and this promotes IGF-1-mediated protection from Fas killing.6
The IGF-1 system has long been suggested to play an important role in T-lymphocyte development in the thymus and in the growth of mature T cells.7–11 IGF-1 also promotes growth of myelomas12 and suppresses apoptosis in response to IL-3 withdrawal in the BaF313 and in the FL5.12 B-lymphoblastic-cell lines.14 However, while the signalling pathways activated by the IGF-1R have been well studied in different cells, its function in T-cell activation is not understood nor is it known how signals from the IGF-1R interact with signals from the T-cell receptor or co-stimulatory molecules such as CD28.
Ligation of the IGF-1R by its ligands IGF-1 or IGF-2 promotes the survival, growth and differentiation of a wide variety of cell types (reviewed in refs 14,15). The IGF-1R can activate phosphatidylinositol (PI-3 kinase) and protein kinase B/Akt,16 resulting in phosphorylation of several apoptosis regulatory proteins including the pro-apoptotic Bcl-2 family member Bad,17 the Forkhead transcription factor FKHRL1,18 and apoptosis signal-regulating kinase I (Ask-1).19 Activated Akt can protect fibroblasts from Fas-induced death20 and it can mediate CD28 signals leading to expression of IL-2 and interferon-γ (IFN-γ) in activated T cells.21 Akt has also been associated with protection from Fas killing by the observation that mice deficient in the phosphatase and Tensin homolog deleted on chromosome Ten (PTEN), which negatively regulates the Akt pathway, exhibit enhanced resistance to Fas killing and are more susceptible to autoimmunity.22
IGF-1 can activate a PI-3 kinase-independent pathway involving c-Jun N-terminal kinases (JNKs), which was recently found to be associated with IGF-1-mediated survival in FL5.12 cells.23 JNKs belong to a family of stress-activated protein kinases (SAPKs) that include p38.24 Activation of SAPKs occurs in response to cellular stress stimuli, but they also have important roles in normal T-cell activation and function (reviewed in refs 25,26). Interestingly, activation of JNK in response to T-cell receptor ligation is dependent on CD28 co-stimulation,27 and this leads to increased activity at the IL-2 gene promoter.28,29 Studies from JNK knockout mice indicate that JNKs are not required for T-cell activation, but are required for subsequent differentiation into the T helper type 1 (Th1) and type 2 (Th2) subsets.30,31 Transient activation of JNK by cytokines such as tumour necrosis factor (TNF)32 or transforming growth factor-β,33 or by adhesion signals such as those from fibronectin in fibroblasts,34 have been associated with promoting cell survival. Transient JNK activity can prolong cell survival upon cytokine withdrawal through its ability to phosphorylate and enhance Bcl-2 anti-apoptotic activity.35 In contrast, prolonged JNK activation is associated with apoptosis and JNKs are required for UV-induced death in fibroblasts.36 Furthermore JNK3 is required for transcription-dependent apoptosis in neurons.37,38
CD28 stimulation in T cells leads to augmented IGF-1R expression and activation of both Akt and JNK. Furthermore, inhibition of IGF-1R signalling induces apoptosis in T-cell cultures. These observations raise the possibility that CD28-induced IGF-1R expression serves to enhance T-cell survival through IGF-1 stimulation of the Akt or JNK pathways. To investigate this, we examined IGF-1 signalling in both primary activated T cells and in Jurkat cells. Here we show that IGF-1 induced Akt and JNK phosphorylation in activated T cells, and JNK activation occurred more rapidly than Akt phosphorylation. Inhibition of PI-3 kinase activity abrogated IGF-1-mediated protection from Fas killing in T cells, but only partially abrogated protection in Jurkat cells. However, dominant negative JNK1 (APF) blocked IGF-1 survival activity in Jurkat cells. Taken together these data demonstrate that enhanced IGF-1R expression in T cells facilitates IGF-1-mediated activation of Akt and JNK that are active in promoting T-cell survival and that transient activation of JNKs by IGF-1 plays a role in the survival of transformed T lymphocytes.
Materials and methods
Antibodies
The anti-IGF-1R antibody Ab-1 (IR-3) (Calbiochem, Nottingham, UK) and the anti-CD3 antibody T3RW24B6 (kindly provided by Immunogen Inc., Cambridge, MA) were used for immunofluoresence experiments. The anti-Fas immunoglobulin M (IgM) monoclonal antibody (mAb) 7C1139 was provided by Dr J. Ritz (Dana Farber Cancer Institute, Boston, MA) and the fluorescein isothiocyanate (FITC)-conjugated anti-IgG secondary antibodies were obtained from Sigma-Aldrich (Dorset, UK). For analysis of intracellular signalling pathways anti-phospho-Akt, anti-Akt, anti-phospho-c-Jun (Ser73), anti-JNK, anti-phospho-p38 and anti-p38 antibodies were all purchased from New England Biolabs Inc. The non-radioactive assay kit for JNK activity was also obtained from New England Biolabs (UK) LTD (Herts, UK). Anti-Bcl-xL antibody was purchased from Transduction Laboratories (Lexington, KY) and anti-Flag and anti-Actin antibodies were obtained from Sigma.
Cell culture
Peripheral blood mononuclear cells were isolated from whole blood using Ficoll–Hypaque (Pharmacia, Uppsala, Sweden). Monocytes were removed by adherence and B cells were removed with anti-CD19-coated magnetic beads (Dynal UK, Wirral, UK). The remaining enriched T-cell population, which was routinely 95% CD3 positive as detected by immunofluoresence, was cultured in complete medium, which consisted of RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 2 mm penicillin–streptomycin and 100 U/ml l-glutamine (all from Gibco/BRL, Paisley, UK). T cells were activated by the addition of 5 μg/ml concanavalin A (ConA; Sigma) in the presence of recombinant IL-2 (rIL-2; PeproTech Inc, Rocky Hill, NJ) at 30 ng/ml. Jurkat cells were maintained in RPMI-1640 supplemented with 10% FBS, 2 mm penicillin–streptomycin and 100 U/ml l-glutamine.
Transfection of Jurkat cells
Jurkat cells were transfected with pcDNA3 encoding full-length IGF-1R, or with pcDNA3 vector alone by electroporation. To do this cells (107 per transfection) were washed once in RPMI-1640 (no additions) and resuspended in 0·4 ml RPMI-1640 (no additions), then transferred to electroporation cuvettes (Biorad, Herts, UK), and 20 μg of the relevant plasmid DNA was added. Cells were allowed to rest at room temperature for 10 min after which they were electroporated in a Gene Pulse electroporator (Biorad) at 0·26 V and a capacitance of 960 μF with no resistance. Immediately afterwards the cuvettes were placed on ice for 10 min. The cells were then allowed to recover for 24 hr at 37° before addition of 1·5 mg/ml G418. After 14 days culture in the presence of G418 growing cells were assayed for over-expression of the IGF-1R by immunofluoresence and flow cytometry, and then subcloned by limiting dilution in 96-well plates to isolate clones stably expressing the IGF-1R.
Jurkat cells were transiently transfected by electroporation with identical conditions to those outlined above, with the exception that 1·5 × 107 cell were transfected in 600 μl of medium per sample. The pcDNA3 JNK1 (APF), kindly provided by R. J. Davis, University of Massachusetts (Worcester, MA), and pcDNA3 Bcl-xL were transfected into Jurkat cells which were examined for over-expression of transfected proteins after recovery in culture for 20 hr at 37°.
Immunofluoresence assays
Cells (5 × 105 per sample) were removed from culture and resuspended in 100 μl RPMI-1640 or Iscove' modified Dulbecco's medium containing 25 mm HEPES, 10% horse serum and 0·01% azide (FACS buffer) containing the indicated primary antibodies at a concentration of 2 μg/ml. Following 1 hr incubation at room temperature, cells were washed and incubated for 30 min at 4° in darkness with FITC-labelled secondary antibodies specific for mouse IgG. Cells were again washed with FACS buffer, and the cell-associated fluorescence was quantified with a FACScan flow cytometer (Becton Dickinson, Oxford, UK).
Western blotting
Primary T cells and Jurkat cells, which had been stimulated as indicated, were harvested from culture and washed with sterile phosphate-buffered saline. Cells were then lysed in one of two separate lysis buffers depending on the proteins being analysed. For the analysis of cell surface and cytoplasmic proteins, cell lysis buffer contained 150 mm NaCl, 5 mm ethylenediaminetetraacetic acid, 50 mm Tris–HCl pH 7·4, 1 mm phenylmethylsulphonyl fluoride, 1 mm NaVO4, 1% Triton X-100 and 0·1% aprotinin. For the analysis of cellular proteins which are also found in the nucleus, the cell lysis buffer used was identical to that outlined above, with the exception that Triton X-100 was replaced with 1% nonidet P-40 and 0·1% sodium dodecyl sulphate (SDS). Irrespective of buffer used, lysis was carried out at 4° for 20 min. To examine levels of protein expression and activation of intracellular signalling pathways, equal amounts of protein per sample (50 μg/well) as determined by Bradford assay, were resolved by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) on either 12% polyacrylamide gels or 4–20% polyacrylamide gradient gels. Proteins were then electrophoretically transferred to nitrocellulose membranes (Schleicher-Schüll, Dassel, Germany). Membranes were incubated in the primary antibodies indicated either overnight at 4° or for 1·5 hr at room temperature. Phospho-antibodies were used at a dilution of 1 in 700 in Tris-buffeled saline (TBS)–Tween (TBS-T) with 7% goat serum. All other primary antibodies were used at a 1 in 1000 dilution in TBS-T 5% milk with the exception of the anti-Flag antibody, which was used at a dilution of 1 in 300 in TBS-T 7% goat serum, and the anti-β-actin antibody, which was used at a dilution of 1 in 4000 in TBS-T 5% milk. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Dako, Hamburg, Germany) were used for detection with the Enhanced ECL™ system (Amersham International, Little Chalfont, UK).
JNK kinase assay
JNK kinase assays were carried out using a specific JNK assay kit (New England Biolabs Inc.). Freshly isolated T cells were activated for 4 days with ConA before being washed three times in serum-free medium, resuspended in RPMI-1640 2% fetal calf serum and re-stimulated with IGF-1 (100 ng/ml). Cells were harvested at 0, 10, 20, 40 and 60-min intervals and lysed by sonication in the buffer provided before addition of 2 μg of c-Jun fusion protein beads to each sample and gentle agitation overnight at 4°. Samples were centrifuged, allowing beads and associated proteins to be washed in lysis and kinase buffers provided. Samples were then suspended in kinase buffer supplemented with 100 μm ATP and incubated at 30° for 45 min to allow kinase reactions to occur. The reaction was terminated by the addition of SDS protein sample buffer. This reaction allowed activated JNK to phosphorylate the c-Jun substrate specifically on serine 63. Phosphorylated c-Jun (Ser 63) was subsequently detected by Western blotting with the specific anti-Phospho-Jun Ser 63 antibody provided. To determine equal protein loading between samples the membrane was stripped of bound antibody and re-probed for expression of the JNK protein.
Fas killing and IGF-1 survival assays
To examine protection of primary activated T cells and Jurkat/IGF-1R cells from Fas-mediated apoptosis by stimulation with IGF-1, freshly isolated T cells were activated with ConA for 96 hr. Activated T cells and Jurkat/IGF-1R cells were washed in serum-free medium to reduce background levels of IGF-1 and IGF-2. Cells were re-suspended in medium supplemented with 2% FCS (low serum medium) and treated with the anti-Fas 7C11 mAb at a dilution of 1 in 300 in the presence or absence of IGF-1. To inhibit various intracellular signalling pathways, LY294002 (20 μm) or SB203580 (15 μm) (Calbiochem, Nottingham, UK), were added to cells 20 min prior to IGF-1 stimulation. Dicumarol (75 μm) (Sigma) was added 5 min and PD89059 (50 μm) (Calbiochem) was added 30 min prior to IGF-1. Dicumarol was dissolved at 200 mg in 8 ml pyridine, to give a stock concentration of 74·34 mm. After a 5-hr incubation cell viability was determined by the trypan blue method or by propidium iodide incorporation. To measure propidium iodide incorporation cells were suspended in 0·5 ml HEPES buffer (10 mm HEPES–NaOH pH 7·4, 150 mm NaCl, 5 mm KCl, 1 mm MgCl2, 1·8 mm CaCl2) and propidium iodide (10 μg/ml). Cell-associated fluorescence was immediately analysed by flow cytometry. All experiments were carried out in triplicate using T cells extracted from at least five different donors.
To examine IGF-1-mediated activation of intracellular signalling pathways, Jurkat/IGF-1R cells and T cells activated for 96 hr were stimulated with IGF-1 in low serum medium. At 0, 10, 20, 40 and 60 min following stimulation 5 × 106 cells were removed from culture and lysed as described above. To block intracellular signalling pathways, inhibitors were used as indicated above.
Jurkat/IGF-1R cells, transiently transfected to express either DN-JNK1 (APF) or Bcl-xL, were washed three times in serum-free medium 20 hr after transfection and re-suspended at a density of 1 × 106/ml in RPMI-1640/2% fetal calf serum. Cells were then incubated with the anti-Fas mAb in the presence or absence of IGF-1 (100 ng/ml) in a 24-well plate. After a 5-hr incubation at 37° and 7% CO2, live and apoptotic cell numbers were determined by trypan blue exclusion and propidium iodide incorporation.
Results
IGF-1 protects activated T cells from Fas-mediated apoptosis
We have previously reported that expression of the IGF-1R is transiently increased in response to CD28 stimulation alone, on T cells and Jurkat cells, or in response to activation of T cells with lectins. IGF-1R inhibitory antibodies abrogated the survival of activated T cells and enhanced their susceptibility to Fas-induced death.6 To determine how IGF-1 protects T cells from Fas killing we now investigated IGF-1 signalling in activated T cells that have augmented IGF-1R expression and in Jurkat cells that over-express the IGF-1R.
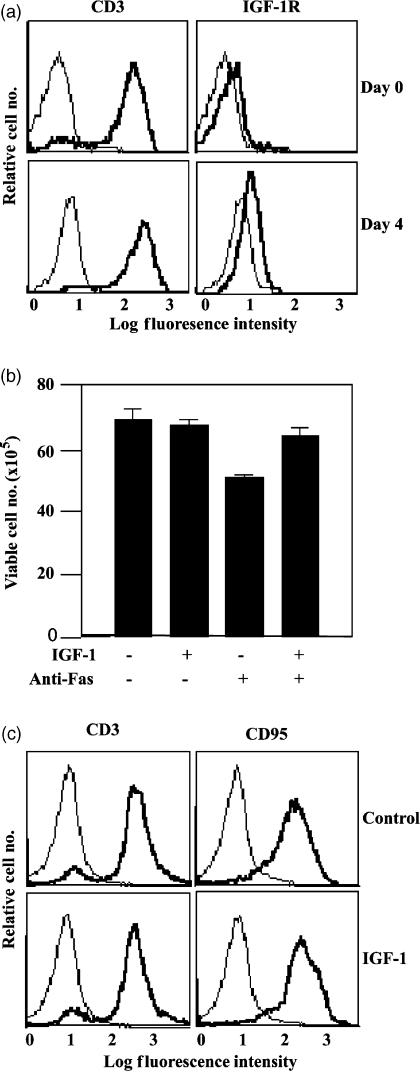
Freshly isolated T cells were activated with ConA for 96 hr and IGF-1R expression levels were measured by immunofluorescence. As shown in Fig. 1(a), and in agreement with previous studies, expression levels of the IGF-1R were enhanced at 96 hr compared with naïve T cells (Day 0). The increase in IGF-1R protein expression in these cells was accompanied by an increase in IGF-1R mRNA (not shown). To investigate IGF-1 protection from Fas killing, cells were cultured in medium containing reduced serum and incubated for 6 hr with a Fas cross-linking antibody in the presence or absence of IGF-1. Under these conditions Fas ligation resulted in a 1·5-fold decrease in viable cell number and this could be suppressed by IGF-1 to levels similar to those in the absence of Fas ligation (Fig. 1b). This decrease in viable cell number was not due to IGF-1 repressing Fas, which was expressed at levels similar to those found in the absence of IGF-1 (Fig. 1c).
Figure 1.
IGF-1R expression is enhanced on activated T cells and IGF-1 protects these cells from Fas-induced cell death. A T-cell-enriched population was activated by culture in the presence of ConA (5 μg/ml) and rIL-2 (30 ng/ml) for 96 hr. CD3 expression and IGF-1R expression were measured 0 and 96 hr after activation by immunofluorescence. The thin line represents staining with the secondary antibody alone and the bold line represents staining with the anti-CD3 or anti-IGF-1R antibody. To measure IGF-1-mediated protection from Fas killing, cells were washed extensively in serum-free medium and incubated either with or without the anti-Fas IgM 7C11 antibody in the presence or absence of IGF-1 (100 ng/ml) for 6 hr. Cell viability was assessed by trypan blue exclusion and morphological analysis and the data are presented as the mean and standard deviation of the apoptotic/dead cell number from triplicate cultures. To ensure that the IGF-1 mediated protection from Fas killing was not the result of Fas repression by IGF-1, CD95 expression was determined on activated cells which were stimulated with IGF-1 or which were left unstimulated.
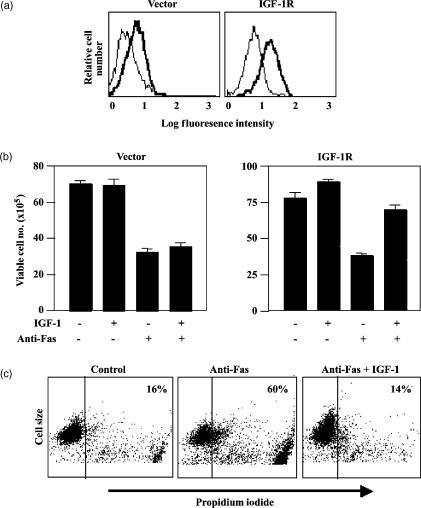
Jurkat cells express low levels of IGF-1R and this is enhanced upon stimulation with CD28. To facilitate investigation of IGF-1R function in Jurkat cells we generated a Jurkat cell line that stably over-expresses the IGF-1R. Several clones with similar levels of IGF-1R expression and function were isolated and one of these was chosen for further studies. IGF-1R expression levels on these Jurkat/IGF-1R cells compared with vector-transfected control cells are shown in Fig. 2(a). Ligation of Fas resulted in a similar level of cell killing in Jurkat/IGF-1R and vector cells as shown by a reduction in the number of viable cells in the cultures (Fig. 2b). However, the Jurkat/IGF-1R cells could be substantially rescued from Fas-mediated apoptosis by IGF-1, whereas the vector cells, which express lower endogenous levels of the IGF-1R, were not rescued by IGF-1 from Fas-mediated apoptosis (Fig. 2b). IGF-1-mediated protection was also measured by flow cytometric analysis of propidium iodide incorporation into dead cells (Fig. 2c). Anti-Fas treatment increased the percentage of cells that had propidium iodide incorporation from 16 to 60%, and the numbers of cells taking up propidium iodide was reduced back to 14% by addition of IGF-1. Altogether these data demonstrate that IGF-1 can protect both activated T cells and Jurkat/IGF1-R cells from Fas killing. Expression levels of the Fas receptor and c-Flip (not shown) did not change in T cells or Jurkat cells in response to IGF-1 stimulation. This suggests that IGF-1 protects T cells from Fas by activation of intracellular survival signalling pathways.
Figure 2.
Jurkat/IGF-1R cells have enhanced IGF-1-mediated protection from Fas killing. Jurkat cells were transfected with a pcDNA3 vector encoding the full-length IGF-1R or with the empty vector. (a) Expression levels of the IGF-1R on vector-expressing cells and on a Jurkat/IGF-1R clone were measured by immunofluorescence with the anti-IGF-1R antibody. The thin line represents staining with the FITC-labelled secondary antibody alone and the bold line represents staining with anti-IGF-1R mAb. (b) IGF-1-mediated protection from Fas killing was measured in vector- or IGF-1R-expressing Jurkat cells by washing the cells in serum-free medium and incubation ± the anti-Fas cross-linking antibody 7C11, for 6 hr, ± IGF-1. Cell viability was monitored by trypan blue exclusion and the data are presented as the mean viable cell number from triplicate cultures. Standard deviations are represented by error bars. (c) In a separate experiment carried out as described in (b) above, IGF-1-mediated protection from Fas killing was assessed by measurement of propidium iodide incorporation by flow cytometry.
Stimulation of activated T cells with IGF-1 leads to transient activation of Akt and JNK signalling pathways
We next investigated the signalling pathways induced by the IGF-1R in activated T cells and Jurkat/IGF-1R cells. We were particularly interested to determine if JNK was activated by IGF-1 stimulation of T cells because JNK activation by engagement of the T-cell receptor requires co-stimulation through CD28.40 Our previous observations that CD28 regulates IGF-1R expression, and that IGF-1-induced JNK activity is associated with cell survival in FL5.12 cells23 also suggest that IGF-1 may be one of the mediators of JNK activation in response to CD28 co-stimulation of T cells.
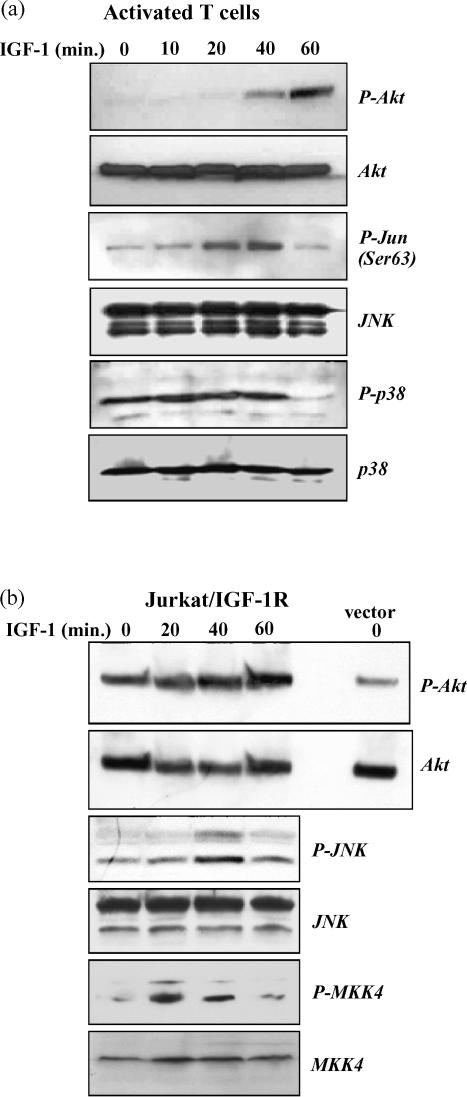
To investigate this, T cells were activated for 96 hr, washed, then re-suspended in reduced serum medium, and stimulated with IGF-1 for the indicated times (Fig. 3a). IGF-1 induced phosphorylation of Akt by 40 min and this was further increased at 60 min. However there was no Akt phosphorylation evident at 10 or 20 min as generally occurs in response to IGF-1 stimulation in different cell lines.41,42 This slow kinetics of Akt phosphorylation may be due to a difference in culture conditions. In our experiments in order to preserve cell viability the primary T cells were not subjected to a period of serum/growth factor starvation that is routinely used with cell lines, and this may result in a slower activation of the PI-3 kinase pathway by exogenously added IGF-1.
Figure 3.
IGF-1 stimulates phosphorylation of Akt, JNK, and c-Jun in T cells and in Jurkat/IGF-1R cells. (a) T cells were activated with ConA (5 μg/ml) for 96 hr and washed extensively in serum-free medium to reduce background levels of IGF-1 and IGF-11. Cells were then cultured in medium containing 2% FCS and stimulated with IGF-1 (100 ng/ml) for the indicated times before lysis and preparation for Western blotting. An anti-phospho-Akt antibody was used to examine activation of Akt in response to IGF-1 stimulation and the blot was re-probed with anti-Akt antibody to demonstrate equal loading. JNK activity in T cells was measured using a JNK assay kit that uses a c-Jun fusion protein pull down to detect specific phosphorylation of the JNK substrate, c-Jun, on serine 63. This was subsequently detected by Western blotting with an anti-phospho-Jun (ser 63) antibody. Levels of JNK protein in the same lysates were detected by probing with an anti-JNK antibody. An anti-phospho-p38 antibody was used to measure p38 activation in response to IGF-1, and the blot was re-probed with an anti-p38 antibody to control for equal loading. (b) Jurkat IGF-1R cells were stimulated with IGF-1 for the indicated times and Akt phosphorylation and Akt levels were determined as above. Levels of basal Akt and phospho-Akt in vector-transfected cells without stimulation are also shown. Phosphorylation of JNK and MKK 4 were detected in the same cell lysates using anti-phospho JNK and anti-phospho MKK4 antibodies. The blot was reprobed with anti-JNK and anti-MKK4 antibodies to control for loading.
In the same cells using a JNK activity assay kit we found that there was a transient phosphorylation of c-Jun on serine 63, which peaked within 20–40 min after addition of IGF-1, and receded to basal levels by 60 min after stimulation (Fig. 2). Interestingly, phosphorylation of c-Jun in response to IGF-1 occurred faster than phosphorylation of Akt in T cells. This suggests that JNK activation precedes Akt activation in response to IGF-1 stimulation in T cells. IGF-1 did not induce activation of p38 MAP kinase above basal levels in activated T cells, but almost completely abolished basal levels of p38 phosphorylation at 60 min stimulation. This effect on p38 activity in response to IGF-1 stimulation has also been observed in the FL5.12 cells line (data not shown).
Akt and JNK phosphorylation by IGF-1 was also investigated in Jurkat/IGF-1R cells. As demonstrated in Fig. 3(b), IGF-1 stimulation did not significantly enhance Akt phosphorylation in Jurkat/IGF-1R cells. However, basal levels of Akt activity were higher in Jurkat/IGF-1R cells compared with empty vector cells, which also had a substantial degree of basal phosphorylated Akt. This indicates that increased levels of IGF-1R resulted in a higher level of basal Akt phosphorylation, probably due to IGFs present in FBS, which is sustained due to lack of PTEN activity.43 In contrast, IGF-1 stimulation led to transient activation of the JNK in Jurkat/IGF-1R cells (Fig. 3b), and this was accompanied by a transient phosphorylation of the upstream JNK activator kinase MKK4, which peaked at 20 min after addition of IGF-1 and returned to basal levels by 60 min stimulation. Overall, the data demonstrate that IGF-1 activates both Akt and the JNK in activated T cells and in Jurkat cells, and that the activation of JNK apparently occurs before activation of Akt in T cells. This suggests that the IGF-1R could act as a mediator or enhancer of PI-3 kinase and facilitate JNK activation in response to CD28 co-stimulation of T cells.
The PI-3 kinase inhibitor LY294002 blocks IGF-1-induced protection from Fas-mediated cell death in T cells, but not in Jurkat/IGF-1R cells
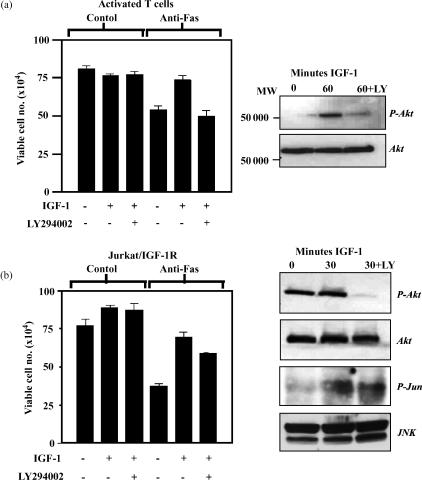
As shown above, both Akt and JNK are activated in response to IGF-1 stimulation of T cells. In order to determine whether the PI-3 kinase pathway is required for IGF-1-mediated protection from Fas-induced cell death, we used LY294002, a PI-3 kinase inhibitor that blocks Akt phosphorylation and activation in different cell types.44,45 As can be seen in Fig. 4(a) the protective effect of IGF-1 from Fas killing in activated T cells was blocked completely by LY294002 (20 μm), and this was accompanied by inhibition of IGF-1-induced phosphorylation of Akt (shown in right panel). This suggests that PI-3 kinase is essential for IGF-1-mediated survival signalling in T cells. The p38 specific inhibitor SB203580 (15 μm) had no affect on the viability of activated T cells or on IGF-1 protection, even though it suppressed basal levels of p38 phosphorylation (not shown). This suggests that p38 activity does not contribute to the survival of these cells and that IGF-1-induced dephosphorylation [visible in Fig. 3(a)] is not associated with protection from Fas killing.
Figure 4.
IGF-1-induced Akt phosphorylation protects T cells from Fas killing and inhibits Fas killing in Jurkat/IGF-1R cells. (a) T cells were activated for 4 days with ConA (5 μg/ml) before being washed extensively and cultured in reduced serum conditions. Cells were then either preincubated with LY294002 (20 μm) or not for 20 min before addition of IGF-1 and anti-Fas antibody. Viability was assessed by trypan blue exclusion after 6 hr. Data are presented as mean viable cell numbers from several independent experiments and vertical bars represent standard deviations from the mean. Akt and phospho-Akt were analysed 0 and 60 min following IGF-1 stimulation by Western blot analysis. (b) Jurkat/IGF-1R cells were washed and cultured in reduced serum medium, and the assay was carried out as with T cells in (a); the data are presented in an identical manner. Western blotting with anti-phospho-Akt and anti-Akt antibodies were used to assess Akt activation and loading, respectively. Anti-phospho-Jun and anti-JNK antibodies were used to detect JNK activity and loading.
In Jurkat/IGF-1R cells, LY294002 only partially inhibited IGF-1-mediated protection from Fas killing although it completely suppressed Akt phosphorylation in these cells (Fig. 4b). This suggests that another IGF-1-activated signalling pathway in addition to the PI-3 kinase pathway is required to promote the survival of these cells. The Erk inhibitor PD89059 had no affect on cell viability or IGF-1 function (not shown). This ruled out the MAP kinase pathway, which has been found to be involved in IGF-1-mediated protection from TNF killing in epithelial tumour cells.46
Interestingly, LY294002 had no affect on IGF-1-induced JNK activation, as demonstrated by phosphorylation of its substrate c-Jun on Serine 73 (Fig. 4b). This is in agreement with our previous observations in FL5.12 cells where IGF-1-induced JNK activity occurs in a PI-3-kinase-independent manner and suggests that the JNK pathway may be involved in the survival of Jurkat cells.
Overall the data suggest that IGF-1-induced activation of PI-3 kinase and Akt phosphorylation are essential for IGF-1-mediated protection from Fas-induced cell death in activated T cells but are only partially required for IGF-1-mediated function in Jurkat cells.
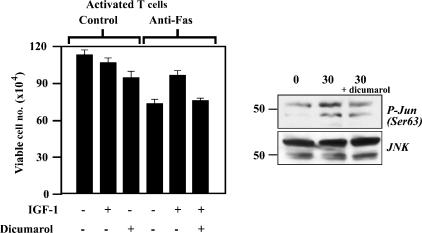
Dicumarol inhibits IGF-1-mediated protection from Fas killing in T cells
Although inhibition of PI-3 kinase activity by LY294002 was sufficient to block IGF-1-mediated protection from Fas killing in activated T cells, since IGF-1-mediated JNK phosphorylaion precedes Akt phosphorylation we were interested to determine if the JNK pathway also contributes to IGF-1R signalling in these cells. To address this we used the quinone reductase inhibitor dicumarol, which we had previously shown to inhibit JNK activation and IGF-1-mediated anti-apoptotic activity in FL5.12 cells.21 Cells were activated and incubated with the anti-Fas antibody under the same conditions as used for the PI-3 kinase inhibitor, but dicumarol (75 μm) was added to untreated cells and to anti-Fas-treated cells in the presence of IGF-1. The results, shown in Fig. 5, demonstrate that dicumarol by itself was very slightly toxic to T cells, but it reversed the protective effect of IGF-1. This suggests that the JNK pathway contributes to IGF-1-mediated protection from Fas killing in T cells. Thus both the PI-3 kinase and JNK pathways are active in mediating IGF-1R signalling in these cells, and inhibition of either pathway is sufficient to block IGF-1R activity.
Figure 5.
T cells were activated for 4 days with ConA (5 μg/ml) before being washed extensively and cultured in reduced serum conditions. Cells were then either preincubated with dicumarol (75 μm) or not for 20 min before addition of IGF-1 and anti-Fas antibody. Viability was assessed by trypan blue exclusion after 6 hr. Data are presented as mean viable cell numbers from several independent experiments and vertical bars represent standard deviations from the mean. Western blotting with anti-phospho-c-Jun and anti-JNK antibodies were used to assess c-Jun phosphorylation and loading, respectively.
Transient expression of dominant negative JNK inhibits IGF-1-mediated protection from Fas killing in Jurkat/IGF-1R cells
We were next interested to determine whether transient activation of JNK by IGF-1 contributes to protection from Fas-induced cell death in Jurkat/IGF-1R cells. The results with the PI-3 kinase inhibitor suggest that JNK activity is present in these cells when the PI-3 kinase pathway is inactive. Preliminary experiments with dicumarol suggested that it could abrogate IGF-1-mediated survival in these cells as it does in activated T cells and FL5.12 cells (not shown). However, we were also interested to test a specific inhibitor of JNK activation, therefore a dominant negative (DN) JNK1 protein, which could inhibit IGF-1-mediated protection from Fas killing, was investigated.
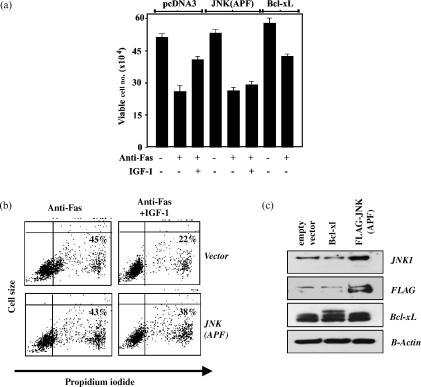
Jurkat/IGF-1R cells were transiently transfected with a DN JNK1 (APF) encoding plasmid or control plasmid, and following 24 hr in culture IGF-1-mediated protection from Fas killing was measured. A representative of several experiments with similar results is shown in Fig. 6. Cultures transfected with DN JNK1 or pcDNA3 contained similar numbers of viable cells in the absence of anti-Fas antibody or added IGF-1, which demonstrates that DN JNK1 is not toxic to Jurkat/IGF-1R cells. This was also evident in cultures that were maintained for more than 48 hr after transfection (not shown). In the presence of the anti-Fas cross-linking antibody the vector-transfected and DN JNK1-transfected cells displayed a similar decline of approximately 50% in viable cell numbers. However, upon addition of IGF-1 a substantial increase in cell viability was observed in the vector-transfected cells that was not observed in the DN-JNK1-transfected cells. As a control, cells were also transfected with Bcl-xL, which can protect T cells from Fas-mediated apoptosis,5 and as shown in Fig. 6(a) expression of Bcl-xL increased the numbers of viable cells in both the presence or absence of the anti-Fas antibody.
Figure 6.
Transient expression of dominant negative JNK (APF) blocks IGF-1 protection from Fas killing in Jurkat/IGF-1R cells. Jurkat/GF-1R cells were transiently transfected with DN JNK (APF), Bcl-xL or empty vector as described in the Materials and Methods. Cells were allowed to recover for 20 hr and were washed in serum-free medium, and then incubated with or without the anti-Fas cross-linking antibody ± IGF-1 for 6 hr. Cell viability was then examined (a) by trypan blue exclusion or (b) by propidium iodide incorporation. 20 hr following transfection cell lysates were prepared and analysed for expression of transfected proteins by Western blotting. Anti-JNK and anti-Flag antibodies were used to detect Flag-tagged JNK (APF). Anti-Bcl-xL antibody was used to detect transfected Bcl-xL, which migrates as a slightly higher molecular weight isoform than endogenous Bcl-xL. The experiment shown is a representative of four independent transfections that gave similar results.
The effects of DN JNK1 on IGF-1-mediated protection from Fas were also measured by flow cytometric analysis of propidium iodide incorporation into the cells (Fig. 6b). IGF-1 decreased the number of cells that take up propidium iodide in the presence of a Fas cross-linking antibody from 45 to 22% in vector-transfected cells and in the DN JNK1-transfected cells IGF-1 decreased the number of cells taking up propidium iodide from 43 to 38%. This indicates that expression of DN-JNK1 suppresses IGF-1-mediated protection from Fas killing. The expression levels of transfected DN JNK1 in Jurkat/IGF-1R cell are shown by Western blotting with anti-JNK and anti-Flag antibodies, and transfected Bcl-xL protein, detected as a slightly higher molecular weight than endogenous Bcl-xL, is shown in Fig. 6(c). Altogether the data demonstrate that DN JNK1 blocks IGF-1-mediated protection from Fas-induced cell death and suggest that IGF-1-stimulated JNK activity is required for IGF-1 anti-apoptotic activity in Jurkat/IGF-1R cells.
Discussion
In this report we have shown that IGF-1 can protect T cells and Jurkat cells from Fas-induced death and this requires both the PI-3 kinase and JNK signalling pathways. Our data suggest that there is a difference between primary T cells and Jurkat cells in the relative contribution of the IGF-1-activated PI-3 kinase and JNK pathways to promote cell survival. Inhibition of the PI-3 kinase is sufficient to block survival in T cells, but not in Jurkat cells. Thus the JNK/c-Jun pathway may play an important role in the survival of transformed T cells and primary T cells.
Activated Akt has recently been shown to be sufficient to replace CD28 in activation of the IL-2 promoter.21 However, although Akt can mediate the same signalling responses that occur in response to CD28 co-stimulation it is still not clear how much direct recruitment of PI-3 kinase to CD28 contributes to Akt activation. It has even been suggested that PI-3 kinase recruitment to CD28 could be a negative regulator of IL-2 production.47 We have previously reported that up-regulation of IGF-1R on activated T cells is dependent on ligation of the CD28 co-stimulatory receptor.6 It has also been established that CD28 co-stimulation is a prerequisite for JNK activity as a result of T-cell activation, and interestingly, this activity is only demonstrable approximately 30 hr subsequent to T-cell activation.40 IGF-1R levels are significantly increased 24 hr after T-cell activation.6 Our data here suggest that CD28 could stimulate the activation of the PI-3 kinase and also the JNK pathways indirectly by increasing the levels of IGF-1R on T cells and thus enhancing IGF-1-mediated activation of these pathways. Such a role for the IGF-1R is also consistent with the observations from others and us on the contribution of the IGF system to cell survival, growth and differentiation in different cells.48–50
The survival function of IGF-1 signalling is largely associated with the activation of Akt in a PI-3 kinase-dependent manner, and the results here indicate that activation of Akt through PI-3 kinase contributes to the protective effect of the IGF-1R in T cells. Inhibition of Akt activation with the PI-3 kinase inhibitor, LY294002, completely abolished IGF-1 protection of primary activated T cells. However in Jurkat/IGF-1R cells, where Akt activity is constitutively high and is not significantly increased through IGF-1 stimulation, the JNK pathway could mediate an alternative PI-3-kinase independent survival signal. Transient activation of JNK by IGF-1 in these cells occurred following phosphorylation of MKK4/SEK1, an upstream activator of JNK that has previously been implicated in T-cell survival51 and DN JNK1 (APF) blocked IGF-1-mediated protection from Fas-induced cell death. This role for JNK in promoting cell survival in T lymphoblastic cells is similar to its activity in IGF-1-mediated protection from IL-3 withdrawal in the FL5.12 B lymphoblastic cell line.23 Similarly it has also been shown that stimulation of focal adhesion kinase (FAK) leads to anchorage-dependent survival of primary fibroblasts through the activation of JNK in a MKK4-specific manner.34
Much of the literature on stress-activated kinases has focused on the role of JNK signalling in the context of cell death. A wide range of apoptosis-inducing stimuli, ranging from ultraviolet radiation to death receptor signalling, all involve prolonged activation of JNK signalling.52 However, in the case of apoptosis induced by Fas ligation it has been established that prolonged activation of JNK occurs as a result of caspase activation, and that Fas-induced apoptosis can proceed in the absence of JNK activation.53 Other reports indicate that neither JNK nor a putative Fas interacting JNK activating protein DAXX are required for Fas-induced apoptosis.54,55 This indicates that prolonged JNK activation occurs as a result of stress associated with the onset of apoptosis.
In primary T cells JNK activation by IGF-1 preceded Akt activation. However, unlike our observations in Jurkat cells, inhibition of the PI-3 kinase pathway was sufficient to abolish IGF-1-mediated protection from apoptosis. Dicumarol also inhibited IGF-1-mediated survival in T cells. This suggests that the PI-3 kinase pathway is sufficient to promote T-cell survival, but activation of the JNK pathway by IGF-1 can also support survival. This does not rule out a potential additional contribution of other signalling pathways such as those controlled by NF-κB, which was observed in activated T cells within 20 min of IGF-1 stimulation (data not shown). Many cytokines, including TNF, IGF-1, and epidermal growth factor (EGF), can transiently activate JNKs and this is associated with a survival response rather than a pro-apoptotic response.56 JNK and c-Jun activity is also associated with cellular transformation by EGF57 and the c-Met oncogene.58 In Jurkat cells IGF-1R expression is enhanced to levels that promote JNK activation and survival by CD28 stimulation. This raises the question whether CD28 acts in T-cell leukaemias/lymphomas to augment IGF-1R expression and thus promote cell survival and maintain the transformed phenotype through subsequent activation of JNKs. Indeed CD28 expression has recently been reported on B-cell myelomas where it augmented IL-8 production.59 IGF-1R function has also been implicated in the survival of myeloma cells.12 This, together with our results in Jurkat cells, suggests that there is a connection between CD28 and IGF-1R expression leading to JNK activity in the maintenance and progression of lymphoid tumours.
In conclusion we have shown that IGF-1 activates both the JNK and PI-3-kinase-mediated signalling pathways in primary activated T cells and Jurkat cells. Inhibition of JNK in both cell types is sufficient to block IGF-1-mediated cell survival, but while inhibition of PI-3 kinase can totally suppress IGF-1-mediated survival in T cells it only partially suppresses IGF-1R function in Jurkat cells. This suggests that CD28 mediated enhancement of IGF-1R on T cells and may play an important role in the survival of T-cell leukemias, as well as in the survival of normal T cells.
Acknowledgments
This work was funded by the Health Research Board of Ireland and the Higher Education Authority.
References
- 1.Scaffidi C, Kirchhoff S, Krammer PH, Peter ME. Apoptosis signaling in lymphocytes. Curr Opin Immunol. 1999;11:277–85. doi: 10.1016/s0952-7915(99)80045-4. [DOI] [PubMed] [Google Scholar]
- 2.Klas C, Debatin KM, Jonker RR, Krammer PH. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993;5:625–30. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 3.Hennino A, Berard M, Casamayor-Palleja M, Krammer PH, Defrance T. Regulation of the Fas death pathway by FLICE-inhibitory protein in primary human B cells. J Immunol. 2000;165:3023–30. doi: 10.4049/jimmunol.165.6.3023. [DOI] [PubMed] [Google Scholar]
- 4.Ward SG. CD28: a signalling perspective. Biochem J. 1996;318:361–77. doi: 10.1042/bj3180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 6.Walsh PT, O'Connor R. The insulin-like growth factor-I receptor is regulated by CD28 and protects activated T cells from apoptosis. Eur J Immunol. 2000;30:1010–18. doi: 10.1002/(SICI)1521-4141(200004)30:4<1010::AID-IMMU1010>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Kooijman R, Rijkers GT, Zegers BJ. IGF-I potentiates interleukin-2 production in human peripheral T cells. J Endocrinol. 1996;149:351–6. doi: 10.1677/joe.0.1490351. [DOI] [PubMed] [Google Scholar]
- 8.Kooijman R, Scholtens LE, Rijkers GT, Zegers BJ. Type I insulin-like growth factor receptor expression in different developmental stages of human thymocytes. J Endocrinol. 1995;147:203–9. doi: 10.1677/joe.0.1470203. [DOI] [PubMed] [Google Scholar]
- 9.Kozak RW, Haskell JF, Greenstein LA, Rechler MM, Waldmann TA, Nissley SP. Type I and II insulin-like growth factor receptors on human phytohemagglutinin-activated T lymphocytes. Cell Immunol. 1987;109:318–31. doi: 10.1016/0008-8749(87)90315-7. [DOI] [PubMed] [Google Scholar]
- 10.Krishnaraj R, Zaks A, Unterman T. Relationship between plasma IGF-I levels, in vitro correlates of immunity, and human senescence. Clin Immunol Immunopathol. 1998;88:264–70. doi: 10.1006/clin.1998.4578. [DOI] [PubMed] [Google Scholar]
- 11.Tu W, Cheung PT, Lau YL. IGF-I increases interferon-gamma and IL-6 mRNA expression and protein production in neonatal mononuclear cells. Pediatr Res. 1999;46:748–54. doi: 10.1203/00006450-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Ge NL, Rudikoff S. Insulin-like growth factor I is a dual effector of multiple myeloma cell growth. Blood. 2000;96:2856–61. [PubMed] [Google Scholar]
- 13.Rodriguez-Tarduchy G, Collins MK, Garcia I, Lopez-Rivas A. Insulin-like growth factor-I inhibits apoptosis in IL-3-dependent hemopoietic cells. J Immunol. 1992;149:535–40. [PubMed] [Google Scholar]
- 14.O'Connor R. Survival factors and apoptosis. Adv Biochem Eng Biotechnol. 1998;62:137–66. doi: 10.1007/BFb0102309. [DOI] [PubMed] [Google Scholar]
- 15.Adams TE, Epa VC, Garrett TP, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cell Mol Life Sci. 2000;57:1050–93. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–5. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 17.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 18.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 19.Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohn JL, Hueber AO, McCarthy NJ, Lyon D, Navarro P, Burgering BM, Evan G. The opposing roles of the Akt and c-Myc signalling pathways in survival from CD95-mediated apoptosis. Oncogene. 1998;17:2811–8. doi: 10.1038/sj.onc.1202393. [DOI] [PubMed] [Google Scholar]
- 21.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 22.Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 23.Krause D, Lyons A, Fennelly C, O'Connor R. Transient activation of Jun N terminal kinases (JNKs) and protection from apoptosis by the IGF-IR can be suppressed by dicoumarol. J Biol Chem. 2001;276:19244–52. doi: 10.1074/jbc.M008186200. [DOI] [PubMed] [Google Scholar]
- 24.Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–54. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 26.Rincon M, Flavell RA, Davis RA. The JNK and P38 MAP kinase signaling pathways in T cell-mediated immune responses. Free Radic Biol Med. 2000;28:1328–37. doi: 10.1016/s0891-5849(00)00219-7. [DOI] [PubMed] [Google Scholar]
- 27.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–36. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 28.Ghaffari-Tabrizi N, Bauer B, Villunger A, Baier-Bitterlich G, Altman A, Utermann G, Uberall F, Baier G. Protein kinase Ctheta, a selective upstream regulator of JNK/SAPK and IL-2 promoter activation in Jurkat T cells. Eur J Immunol. 1999;29:132–42. doi: 10.1002/(SICI)1521-4141(199901)29:01<132::AID-IMMU132>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. Embo J. 1998;17:3101–11. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong C, Yang DD, Tournier C, Whitmarsh AJ, Xu J, Davis RJ, Flavell RA. JNK is required for effector T-cell function but not for T-cell activation. Nature. 2000;405:91–4. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 31.Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–5. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 32.Roulston A, Reinhard C, Amiri P, Williams LT. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. J Biol Chem. 1998;273:10232–9. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Hutter D, Liu Y, Wang X, Sheikh MSM, Chan A, Holbrook NJ. Transforming growth factor-beta 1 suppresses serum deprivation-induced death of A549 cells through differential effects on c-Jun and JNK activities. J Biol Chem. 2000;275:18234–42. doi: 10.1074/jbc.M909431199. [DOI] [PubMed] [Google Scholar]
- 34.Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH (2) -terminal kinase. J Cell Biol. 2000;149:741–54. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng X, Xiao L, Lang W, Gao F, Ruvolo P, May WS., Jr Novel role for JNK as a stress-activated Bcl2 kinase. J Biol Chem. 2001;25:25. doi: 10.1074/jbc.M100279200. [DOI] [PubMed] [Google Scholar]
- 36.Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–26. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bozyczko-Coyne D, O'Kane TM, Wu ZL, Dobrzanski P, Murthy S, Vaught JL, Scott RW. CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Abeta-induced cortical neuron apoptosis. J Neurochem. 2001;77:849–63. doi: 10.1046/j.1471-4159.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- 38.Bruckner SR, Tammariello SP, Kuan CY, Flavell RA, Rakic P, Estus S. JNK3 contributes to c-Jun activation and apoptosis but not oxidative stress in nerve growth factor-deprived sympathetic neurons. J Neurochem. 2001;78:298–303. doi: 10.1046/j.1471-4159.2001.00400.x. [DOI] [PubMed] [Google Scholar]
- 39.Robertson MJ, Manley TJ, Pichert G, Cameron C, Cochran KJ, Levine H, Ritz J. Functional consequences of APO-1/Fas (CD95) antigen expression by normal and neoplastic hematopoietic cells. Leuk Lymphoma. 1995;17:51–61. doi: 10.3109/10428199509051703. [DOI] [PubMed] [Google Scholar]
- 40.Weiss L, Whitmarsh AJ, Yang DD, Rincon M, Davis RJ, Flavell RA. Regulation of c-Jun NH (2) -terminal kinase (Jnk) gene expression during T cell activation. J Exp Med. 2000;191:139–46. doi: 10.1084/jem.191.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawlor MA, Feng X, Everding DR, Sieger K, Stewart CE, Rotwein P. Dual control of muscle cell survival by distinct growth factor-regulated signaling pathways. Mol Cell Biol. 2000;20:3256–65. doi: 10.1128/mcb.20.9.3256-3265.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Gao JX, Salojin K, et al. Regulation of fas ligand expression during activation-induced cell death in T cells by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase. J Exp Med. 2000;191:1017–30. doi: 10.1084/jem.191.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shan X, Czar MJ, Bunnell SC, Liu PP, Liu Y, Schwartzberg PL, Wange RL. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol. 2000;20:6945–57. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madge LA, Pober JS. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFkappaB in human endothelial cells. J Biol Chem. 2000;275:15458–65. doi: 10.1074/jbc.M001237200. [DOI] [PubMed] [Google Scholar]
- 45.Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, Miyake S, Tohyama M. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem. 1999;73:2037–46. [PubMed] [Google Scholar]
- 46.Remacle-Bonnet MM, Garrouste FL, Heller S, Andre F, Marvaldi JL, Pommier JG. Insulin-like growth factor-I protects colon cancer cells from death factor-induced apoptosis by potentiating tumor necrosis factor alpha-induced mitogen-activated protein kinase and nuclear factor kappaB signaling pathways. Cancer Res. 2000;60:2007–17. [PubMed] [Google Scholar]
- 47.Harada Y, Tanabe E, Watanabe R, et al. Novel role of phosphatidylinositol 3-kinase in CD28-mediated costimulation. J Biol Chem. 2001;276:9003–8. doi: 10.1074/jbc.M005051200. [DOI] [PubMed] [Google Scholar]
- 48.Baserga R, Resnicoff M, D'Ambrosio C, Valentinis B. The role of the IGF-I receptor in apoptosis. Vitam Horm. 1997;53:65–98. doi: 10.1016/s0083-6729(08)60704-9. [DOI] [PubMed] [Google Scholar]
- 49.Niesler CU, Urso B, Prins JB, Siddle K. IGF-I inhibits apoptosis induced by serum withdrawal, but potentiates TNF-alpha-induced apoptosis, in 3T3-L1 preadipocytes. J Endocrinol. 2000;167:165–74. doi: 10.1677/joe.0.1670165. [DOI] [PubMed] [Google Scholar]
- 50.Niikura T, Hashimoto Y, Okamoto T, et al. Insulin-like growth factor I (IGF-I) protects cells from apoptosis by Alzheimer's V642I mutant amyloid precursor protein through IGF-I receptor in an IGF-binding protein-sensitive manner. J Neurosci. 2001;21:1902–10. doi: 10.1523/JNEUROSCI.21-06-01902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishina HK, Fischer D, Radvanyi L, et al. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–3. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 52.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–36. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 53.Abreu-Martin MT, Palladino AA, Faris M, Carramanzana NM, Nel AE, Targan SR. Fas activates the JNK pathway in human colonic epithelial cells: lack of a direct role in apoptosis. Am J Physiol. 1999;27:599–605. doi: 10.1152/ajpgi.1999.276.3.G599. [DOI] [PubMed] [Google Scholar]
- 54.Hofmann TG, Moller A, Hehner SP, Welsch D, Droge W, Schmitz ML. CD95-induced JNK activation signals are transmitted by the death-inducing signaling complex (DISC), but not by Daxx. Int J Cancer. 2001;93:185–91. doi: 10.1002/ijc.1316. [DOI] [PubMed] [Google Scholar]
- 55.Villunger AD, Huang C, Holler N, Tschopp J, StrasSeries A. Fas ligand-induced c-Jun kinase activation in lymphoid cells requires extensive receptor aggregation but is independent of DAXX, and Fas-mediated cell death does not involve DAXX, RIP, or RAIDD. J Immunol. 2000;165:1337–43. doi: 10.4049/jimmunol.165.3.1337. [DOI] [PubMed] [Google Scholar]
- 56.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–76. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 57.Bost F, McKay R, Bost M, Potapova O, Dean NM, Mercola D. The Jun kinase 2 isoform is preferentially required for epidermal growth factor-induced transformation of human A549 lung carcinoma cells. Mol Cell; Biol. 1999;19:1938–49. doi: 10.1128/mcb.19.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodrigues GA, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. Embo J. 1997;16:2634–45. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shapiro VS, Mollenauer MN, Weiss A. Endogenous CD28 expressed on myeloma cells up-regulates interleukin-8 production: implications for multiple myeloma progression. Blood. 2001;98:187–93. doi: 10.1182/blood.v98.1.187. [DOI] [PubMed] [Google Scholar]