Abstract
The airway epithelium is the first cellular component of the lung to be encountered by the particles and pathogens present in inhaled air. In addition to its role as a physical barrier, the immunological activity of the airway epithelium is an essential part of the pulmonary immune system. This means that the symptoms of lung diseases that involve immunological mechanisms are frequently exacerbated by infection of the airway epithelium with respiratory viruses. The virus-induced enhancement of immunological activity in infected epithelial cells is well characterized. However, the effects that contaminants of inhaled air have upon the infectivity and replication of respiratory viruses and the inflammation they cause, are comparatively unknown. In this study, we have shown that pre-exposure of airway epithelial cells to bacterial lipopolysaccharides or a proteolytically active house dust mite allergen, is able to, respectively, inhibit or enhance the level of cellular infection with respiratory syncytial virus and similarly alter virus-induced expression of the inflammatory chemokine interleukin-8. These results suggest that respiratory syncytial virus infection and the inflammation caused by respiratory syncytial virus may be modified by the biologically active contaminants of indoor air.
Introduction
Respiratory-virus-infected epithelial cells release a range of inflammatory mediators that co-ordinate local and systemic immunity and initiate repair at the site of infection. Whilst the activation of pulmonary immunity by respiratory viruses has been studied extensively, particularly in the context of exacerbations of disease (reviewed in ref. 1), the additional effects of biologically active contaminants of indoor air on the infectivity and replication of the viruses has not yet been considered in detail.
The impact of respiratory virus infections on human health is confounded by the unavoidable exposure of the lungs to highly variable levels and types of insult, such as those found in poor quality indoor air. Exposure to high levels of contaminants derived from pets, fungi, bacteria, arthropods and the combustion of fuels are associated with poor pulmonary health in their own right, but they can also influence the susceptibility to respiratory infection,2 and exacerbate disease in those with pre-existing conditions such as chronic obstructive pulmonary disease and asthma.3–13
In this study we have investigated how two biologically active contaminants of indoor air, bacterial lipopolysaccharides and a house dust mite allergen, influence the infectivity and replication of respiratory syncytial virus, a common cause of bronchiolitis in infants, and a significant cause of mortality and morbidity in the elderly.
Materials and methods
Respiratory syncytial virus (RSV) A2 strain was kindly provided by Prof. Peter Openshaw (The National Heart and Lung Institute, Imperial College of Science, Technology and Medicine at St Mary's Hospital, London, UK). Lysates of RSV-infected cells were prepared as follows: small 25 cm2 tissue culture flasks were plated with 1·5 × 106 HEp-2 cells and cultured for 16 hr. Cells were infected with RSV at 10 plaque-forming units (PFU) per cell in Eagle's modified minimum essential medium (MEM; Life Technologies, Paisley, UK) supplemented with 1% non-essential amino acids (NEAA; Life Technologies), and 2% fetal calf serum (FCS; Sigma, Poole, Dorset, UK.) for 24 hr. Infected cells were scraped from the culture flask, resuspended in serum-free medium, and snap frozen in liquid nitrogen. Because RSV retains a close association with the membranous components of the cells it is grown in and is very pleomorphic, ranging in size from 100 nm spherical particles to filaments greater than 400 nm in length; virus was not routinely purified. Control lysates of uninfected cells were prepared alongside the RSV-infected cells.
Antibodies
A monoclonal antibody (mAb), 021/1G specific for the RSV glycoprotein (G-protein), was kindly provided as ascitic fluid by Dr J. A. Melero (Centro Nacional be Biolgia Celular y Retrovirus, Instituto de Salud ‘Carlos III’, Madrid, Spain). The antibodies were titrated on RSV-infected cells and used between 1 : 5000 and 1 : 10 000 in immunoplaque assays.
Cell lines
HEp-2, a human laryngeal carcinoma-derived cell line, was obtained from the European Collection of Animal Cell Cultures (CAMR, Porton Down, Wiltshire UK). HEp-2 cells were cultured in Eagle's MEM supplemented with 5 mm Glutamax, 10% heat-inactivated FCS and 1% NEAA. All cells were cultured in a 100% humidified, 5% CO2 atmosphere at 37°. BEAS-2B, a human bronchial epithelial cell line transformed with Ad-12-SV40 was kindly provided by Dr Peter Lackie (Department of Immunopharmacology, University of Southampton, UK). BEAS-2B were cultured in 50% Eagle's MEM, 50% Ham's F12 nutrient medium, supplemented with 5 mm Glutamax, 1% NEAA and 2% Ultroser (Life Technologies).
Immunoplaque assay
RSV was titred by immunoplaque assay in HEp-2 cells. Cells were plated at 3 × 104 cells per well in 96-well flat-bottomed plates (Nunc, Rochester, NY). After 24 hr of culture, RSV-containing cell lysates, or control lysates (50 μl) were incubated for 2 hr with the cells in serum-free Eagle's MEM containing 5 mm Glutamax and 1% NEAA (Life Technologies). Cells were cultured for a further 24 hr in medium supplemented with 10% FCS then washed twice with phosphate-buffered saline containing 1% bovine serum albumin and 0·1% sodium azide (PBS BSA), followed by fixation with methanol containing 2% hydrogen peroxide for 20 min at room temperature. After washing twice in PBS BSA, cells were stained with the mouse mAb 021/1G specific for the RSV G protein, at a dilution of 1 : 10 000, for 30 min on ice. Cells were washed twice in PBS BSA and incubated with a 1 : 10 000 dilution of horseradish peroxidase (HRP)-conjugated rabbit anti-mouse polyclonal antibody specific for mouse immunoglobulins (Dako, Glostrup, Denmark). Following two washes with PBS BSA, cells were incubated with the HRP substrate 3-amino-9-ethyl-carbazol, in phosphate citrate buffer (Sigma) for 30 min at room temperature. The reaction was stopped by washing with PBS BSA containing 0·01% sodium azide. Stained cells were counted by light microscopy. In the absence of RSV infection, irrespective of whether the cells had been treated with Der p I or lipopolysaccharide (LPS), no cells were stained using this protocol (data not shown). Similarly, when an isotype-matched control antibody was used in place of the mAb 021/1G specific for the RSV G protein, cells that had been infected with RSV did not stain positive, irrespective of whether the cells had been treated with Der p I or LPS (data not shown).
Der p I and LPS
Der p 1 was purified from laboratory cultures of Dermatophagoides pteronyssinus by affinity chromatography, using a monoclonal anti-group I mite allergen antibody 4C1 (Indoor Biotechnologies, Cardiff, UK) coupled to cyanogen bromide-activated Sepharose 4B (Sigma). Whole mite culture (15 g) was stirred for 16 hr at 4° in 200 ml PBS pH 8·0. Debris and insoluble material was removed by centrifugation at 13 000 g and 0·2 μm filtration. The extract was applied to the 4C1 affinity column, which was washed extensively with PBS pH 8·0 and eluted with 50 mm glycine 50% ethylene glycol, pH 10·0. After dialysis against PBS pH 8·0, Der p I was concentrated to 1 mg/ml using a Centriprep 10 ultrafiltration unit (Amicon, Warford, UK) and stored at − 80°. Purity was assessed by silver-stained sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and zymogram.
LPS from Escherichia coli O26 : B6 and Pseudomonas aeruginosa were purchased from Sigma.
RSV infection of cells treated with Der p I or LPS
HEp-2 or BEAS-2B airway epithelial cells were plated at 3 × 104 cells per well in flat-bottomed 96-well plates and cultured for 16 hr. Cultures were infected with RSV that was diluted 1 : 1500 from a 4·5 × 106 PFU/ml stock to give a multiplicity of infection of 0·005 PFU per cell. Cells were treated with LPS or Der p I as follows: Der p I was diluted in Eagle's MEM supplemented with 1% NEAA and 5 mm l-cysteine to give a range of final concentrations of Der p I between 0 and 1 μg/ml. LPS was diluted in Eagle's MEM supplemented with 1% NEAA to give a range of final concentrations of LPS between 100 pg/ml and 10 μg/ml. After 2 hr incubation at 37° the cultures were supplemented with 10% FCS and were cultured for a further 24 hr. Cells were stained for the expression of RSV G protein using the RSV G protein immunoplaque assay. Results are expressed as the titre of RSV in PFU/ml.
RSV infection of cells treated with proteolytically inactive Der p I
To examine whether the proteolytic activity of the mite allergen influenced infection with RSV, Der p I was incubated with irreversible cysteine and serine protease inhibitors before addition to the cultures. Der p I was incubated for 30 min at 37° with 10 μg/ml (2·8 μm) of l-trans-epoxysuccinyl-leucylamide-[4-guanidino]-butane (E64) (Calbiochem, Nottingham, UK), and 10 μg/ml (4·6 μm) of 4-[amidinophenyl] methanesulphonyl fluoride (APMSF; Calbiochem) in Eagle's MEM supplemented with 1% NEAA and 5 mm l-cysteine. Supernatants were taken from the cells at 24 hr, and the RSV was titrated using the RSV G protein immunoplaque assay. The titration was performed on HEp-2 cells irrespective of whether the original infection/protease treatment was performed using HEp-2 or BEAS-2B cells.
Interleukin-8 (IL-8) enzyme-linked immunosorbent assay (ELISA)
The wells of a 96-well ELISA plate were coated with 100 μl of mouse anti-human IL-8 monoclonal capture antibody (6217.111) at 4 μg/ml in PBS (R & D Systems, Minneapolis, MN) for 16 hr at room temperature. The plates were washed three times with PBS containing Tween-20 (0·05%) (PBST) and blocked for 1 hr at room temperature with 200 μl/well of PBS containing 5% glucose, 1% BSA and 0·05% sodium azide. Following two washes with PBST, recombinant IL-8 (R & D System) was used to generate a standard curve, and 100 μl of the standards or tissue culture supernatants were added to each well and incubated for 2 hr at room temperature. Plates were washed three times with PBST, and incubated for 2 hr with 100 μl/well of biotinylated polyclonal goat anti-human IL-8 at 20 ng/ml in PBS (R & D systems). After washing with PBST, 100 μl of avidin–HRP was added to each well and incubated for 20 min at room temperature. Bound HRP was detected after three washes with PBST using 50 μl of tetramethylbenzidine at 100 μg/ml in phosphate citrate perborate buffer (Sigma). The reaction was stopped after 5 min with 25 μl of 2 m H2SO4, and the absorbance was read at a wavelength of 450 nm (Anthos Reader 2001). The mean of triplicate determinations was used to establish from the standard curve, the level of IL-8 in each supernatant.
Statistical analysis
The Student's t-test function of the graph plotting and analysis software (Sigma Plot for Windows version 8·00, SPSS) and the anova function of Microsoft Excel 2000 were used to determine the significance of findings. Figures with error bars show the mean ± the SEM.
Results
Bacterial LPS inhibits the infection of airway epithelial cells by RSV
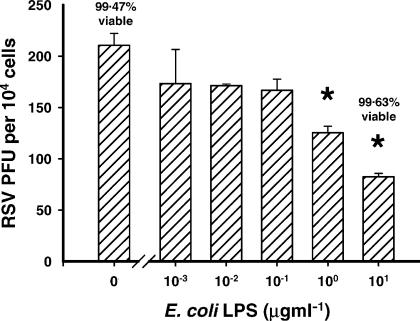
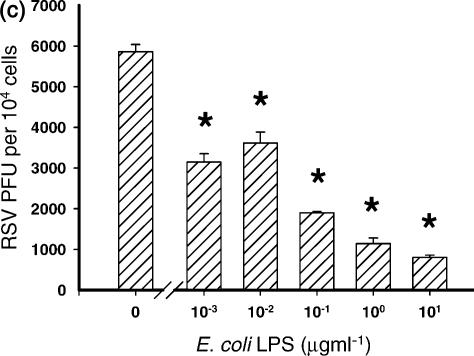
LPS from E. coli O26:B6 was titrated into the cultures at the same time as a constant titre of RSV was added. There was a significant decrease in the number of RSV-infected cells observed after 24 hr of culture (P ≤ 0·01) at, and above 1 μg ml of LPS (Fig. 1). Similar results were obtained using BEAS-2B human bronchial epithelial cells and with LPS isolated from Pseudomonas aeruginosa (data not shown). Irrespective of the dose of LPS used, the cells were always greater than 99% viable after 24 hr of culture with RSV (Fig. 1).
Figure 1.
LPS inhibits the infection of airway epithelial cells by RSV. LPS was titrated onto cells at the same time as they were infected with RSV. There was an LPS dose-dependent decrease in the number of RSV-infected cells observed (*P ≤ 0·01).
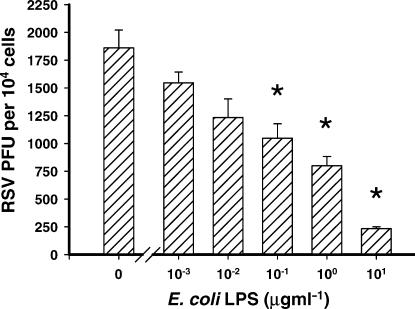
The LPS dose-dependent decrease in the number of cells infected with RSV was reflected by the amount of new progeny virus produced. Lysates of RSV-infected cells that had been treated with LPS were titrated onto fresh HEp-2 cells. As before, there was a significant decrease in the number of infected cells observed (P ≤ 0·02) when the cells producing the virus were treated with LPS at a concentration greater than 100 ng/ml of LPS (Fig. 2). This suggests that the LPS was not simply inhibiting the detection of RSV, and that the reduction in infectivity seen in Fig. 1, was reflected in the amount of new progeny virus produced.
Figure 2.
LPS inhibits the production of new progeny RSV by airway epithelial cells. Lysates from cells infected in the presence of LPS were titrated onto fresh cells. The LPS dose-dependent decrease in the number of cells infected with RSV was reflected by the amount of new progeny virus produced (*P ≤ 0·02).
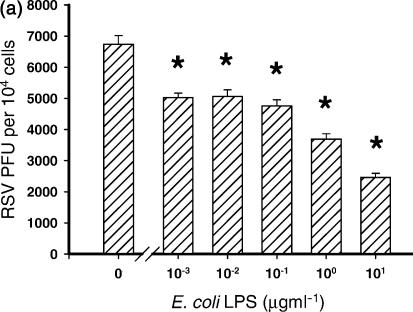
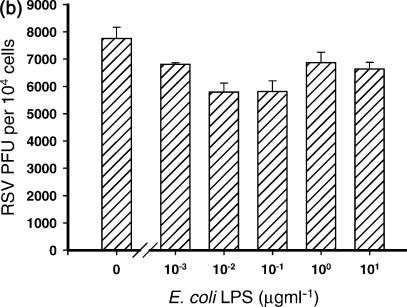
The level of inhibition of RSV infection by LPS was dependent upon the order in which the cells were treated with LPS and RSV. Three conditions were examined in which cells were (a) treated with LPS 4 hr before infection with RSV; (b) treated with LPS 4 hr after infection with RSV; or (c) treated with LPS at the same time as infection with RSV.
When cells were pretreated for 4 hr with LPS, significant inhibition of RSV infection was observed at the lowest concentration of LPS used (10 ng/ml) and at all concentrations above this (P ≤ 0·03) (Fig. 3a). In contrast, the level of RSV infection when cells were infected first, and then 4 hr later treated with LPS was not significantly inhibited by any concentration of LPS (P ≥ 0·05) (Fig. 3b).
Figure 3.
Inhibition of RSV infection by LPS was dependent upon the order in which the cells were treated with LPS and RSV. Three conditions were examined in which cells were (a) treated with LPS 4 hr before infection with RSV *P ≤ 0·03); (b) treated with LPS 4 hr after infection with RSV (*P ≤ 0·05); and (c) treated with LPS at the same time as infection with RSV (significance range from P = 0·007 with 10 ng/ml LPS to 0·00004 with 10 μg/ml LPS). Cells treated with LPS at the same time as infection with RSV inhibited infection of the cells most efficiently.
Cells treated with premixed RSV and LPS showed the most dramatic decrease in the level of RSV infection. All concentrations of LPS used, significantly inhibited infection of the cells with RSV (significance range from P = 0·007 with 10 ng/ml LPS to 0·00004 with 10 μg/ml LPS) (Fig. 3c).
Bacterial LPS inhibits the expression of IL-8 by RSV-infected airway epithelial cells
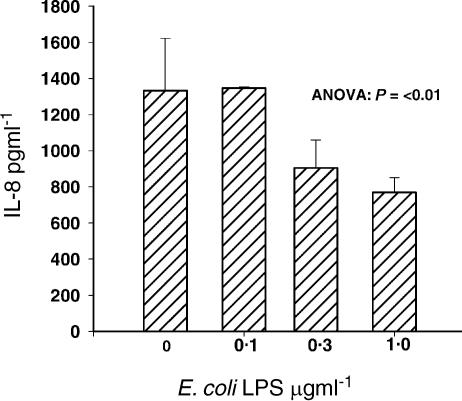
Because non-replicating RSV is able to induce transient chemokine gene expression in epithelial cells,14 we were interested to determine whether LPS-treated virus retained the potential to cause inflammation by inducing the release of IL-8. We therefore examined whether the reduction of RSV infectivity by LPS is reflected by a decrease in the expression of an early inflammatory chemokine, IL-8. In this series of experiments, LPS was added to the cells at the same time as the virus.
In the absence of infection by RSV, at any concentration of LPS tested, IL-8 expression never exceeded 291 pg/ml SD = 46·5 pg/ml. However, in line with the reduction in infection and the production of new progeny virus, the RSV-induced release of IL-8 into the supernatant was inhibited as LPS was titrated into the culture system (P ≤ 0·01). (Fig. 4).
Figure 4.
The reduction of RSV infectivity by LPS is reflected by a decrease in the expression of an early inflammatory chemokine, IL-8. In line with the reduction in infection and the production of new progeny virus, the RSV-induced IL-8 measured by ELISA in culture supernatants was inhibited as LPS was titrated into the culture system.
Der p I enhances the infection of airway epithelial cells by RSV
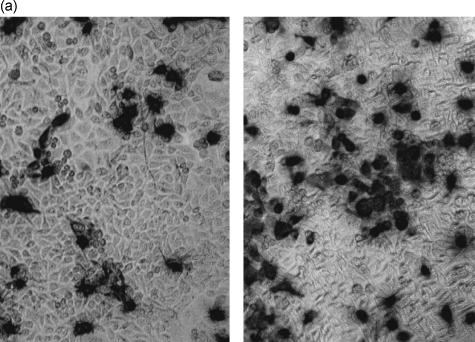
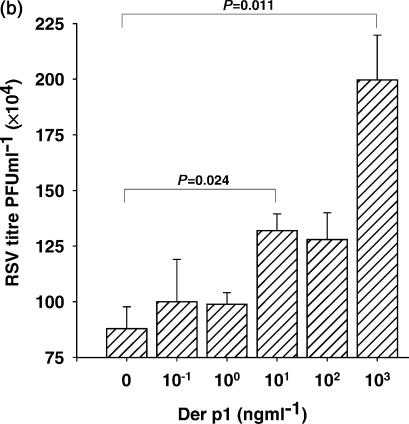
The number of cells infected after culture with RSV for 24 hr was enumerated by immunohistochemistry using an anti-RSV G protein-specific antibody. In cultures infected with RSV in the absence of Der p I, the baseline level of infection with RSV under these conditions was confirmed. As Der p I was titrated into the system at the same time as the virus, there was a dose-dependent increase in the number of infected cells observed (Fig. 5a,b). Similar results were obtained using BEAS-2B a human bronchial epithelial cell line. These results indicate that in contrast to LPS, Der p I increases the susceptibility of airway epithelial cells to infection with RSV.
Figure 5.
(a) Der p I enhances the infection of airway epithelial cells by RSV. The number of cells infected after culture with RSV in the absence (left panel) and presence of Der p I (right panel) for 24 hr was enumerated by immunohistochemistry using an anti-RSV G protein-specific antibody. (b) As Der p I was titrated into the system, there was a dose-dependent increase in the number of infected cells observed.
Der p I enhances the replication of RSV in airway epithelial cells
Fresh epithelial cells infected with titrated supernatants from 24-hr cultures of RSV-infected cells, were used to estimate the degree of RSV replication and the release of new progeny virus from epithelial cells. When compared with supernatants from cells infected with RSV in the absence of Der p I, the supernatants from cells infected with RSV in the presence of Der p I were found to contain a four-fold higher amount of infectious virus (Fig. 6). This indicates that the increased susceptibility of cells to infection with RSV after treatment with Der p I is reflected in the ability of the cells to support the replication and release of infectious virus from the cells.
Figure 6.
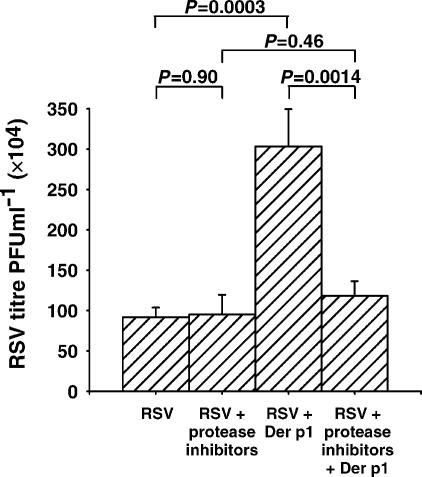
The proteolytic activity of Der p I is responsible for enhanced replication of RSV in airway epithelial cells. Supernatants from cells infected with RSV in the presence of Der p I contained a four-fold higher titre of infectious virus than those infected in the absence of Der p I. Irreversible class-specific inhibitors of proteases inhibited the Der p I-induced increase in the replication and release of RSV.
The proteolytic activity of Der p I is responsible for the observed increase in the infection and replication of RSV
Several groups have found that the proteolytic activity of Der p I may influence immunoregulation15–17 and the integrity of the epithelium in the airway.18 To identify whether proteolytic activity was involved in the enhanced infection and replication of RSV in the presence of Der p I, mechanistic class-specific inhibitors of proteases were used to inhibit irreversibly the proteolytic activity of the allergen. Cells were infected in the presence and absence of Der p I that was proteolytically active, or inactivated with E64 and APMSF. After 24 hr, the supernatant was titrated onto fresh cells.
Cells exposed to protease inhibitors alone (RSV + protease inhibitors) supported the replication and release of RSV as efficiently as untreated cells (RSV). As already described, supernatants from cells exposed to both Der p I and RSV contained considerably more infectious virus than cells exposed to RSV alone (RSV + Der p I). Inhibition of the proteolytic activity of Der p I (RSV + protease inhibitors + Der p I, however, reduced the amount of RSV in the supernatant to a level similar to that produced by cells exposed to RSV alone, or by cells treated with RSV and inhibitors (Fig. 6). These results suggest that the proteolytic activity of Der p I is probably responsible for the increased infection and support of RSV in airway epithelial cells.
Der p I enhances the expression of IL-8 by RSV-infected airway epithelial cells
Our data suggest that Der p I increases the severity of RSV infection in airway epithelial cells. To determine whether the enhanced infection is reflected by an increase in the inflammatory potential of RSV and Der p I, the expression of IL-8 protein by the cells was investigated after challenge.
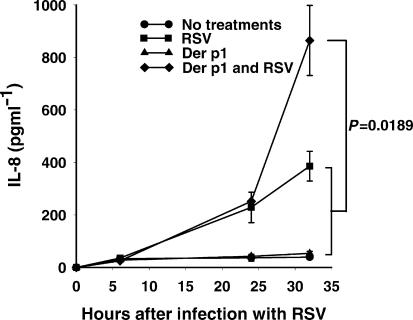
Supernatants taken 24 hr after the application of treatments showed a low level of constitutive IL-8 expression and in the presence of Der p I alone, the level of IL-8 expression was only slightly increased. When the cells were infected with RSV alone, there was a substantial increase in expression; confirming the well-established finding that RSV is a potent inducer of IL-8 expression. When cells were infected with RSV and treated with Der p I together, the stimuli synergised to induce substantial IL-8 mRNA expression (Fig. 7).
Figure 7.
Der p I enhances the expression of IL-8 by RSV-infected airway epithelial cells. IL-8 protein expression, measured by ELISA, followed a similar trend to the findings with IL-8 mRNA; the sum of the levels of IL-8 released by treatment of cells with RSV and Der p I separately induced a significantly lower level of IL-8 protein than when cells were treated with virus and protease simultaneously. P-values refer to comparisons at the 32-hr time-point.
The sum of the levels of IL-8 released by treatment of cells with RSV and Der p I separately induced a significantly lower level of IL-8 protein than when cells were treated with virus and protease simultaneously (Fig. 7). No IL-8 was detected at 0 hr after infection, indicating that IL-8 was not carried over into the experiment from the 1 : 1500 diluted stock virus preparation used to achieve an multiplicity of infection of 0·005 PFU per cell.
Discussion
We have shown that LPS inhibits the infection of human epithelial cells with RSV, decreases the production of new progeny virus and inhibits RSV-induced expression of IL-8. In contrast, Der p I enhances the infection of human epithelial cells with RSV, increases the production of new progeny virus and enhances RSV-induced expression of IL-8.
The finding that RSV infection was most efficiently inhibited when LPS and virus were mixed immediately before inoculation strongly suggests that LPS may interfere with the attachment or fusion of the virus to the cells. Furthermore, because cells pretreated with LPS were able to support RSV infection and the production of new progeny virus, it is unlikely that the inhibitory effect of LPS involved the induction of antiviral cytokines.
Recently, the cellular receptors for RSV have been shown to involve cell-surface-associated, sulphated glycoasaminoglycans (GAG).19–21 Accordingly, co-incubation of RSV with sulphated GAGs, such as heparin, inhibits the infection of cells with RSV.19 In a series of experiments using chemically modified heparin chains that lacked their N-, C6-O, or C2-O-sulphate groups, it was concluded that of these three sulphates, only the N-sulphate was essential and that at least 10 saccharide subunits were required to inhibit RSV infection.22 Although LPS from E. coli O26:B6 is not sulphated, it is not inconceivable that the O side chain of saccharides in LPS could perhaps interfere with the interaction between the oligomeric complex of RSV G, F and SH proteins that have been found to interact with heparin,23 and cellular GAGs during infection.
There are now several reports showing that proteolytic allergens cleave immunoregulatory receptors, for example CD23 and CD25.15,16 These and other studies speculate that disturbance of immune regulation caused by receptor cleavage may be an important factor in the maintenance of allergic immunity. However, the concept that an extracellular protease, whether an allergen or not, has influence upon the progression of an intracellular infection, is unexpected.
Recently however, it has become recognized that many of the activities ascribed to proteases arise from their ability to activate cells directly via protease-activated receptors (PAR) (reviewed in refs 24 and 25), and we suspect that PAR may provide a link between proteases and the enhancement of infection with RSV.
There are alternative explanations for our data, however. In a study of murine influenza A infection, inhalation of aerosolized mite protease augmented the replication of influenza A virus via direct cleavage of viral haemagglutinin.26 Although in RSV the F (fusion) protein is usually cleaved by a ubiquitous intracellular protease, furin, we could find no evidence to support the notion that RSV F protein was cleaved by Der p I (data not shown).
It is important to place this work in the context of the pathogenesis of disease. Of particular interest is the influence of infection upon predisposition to or protection from the development of atopy and airway inflammation. There is evidence that exposure to Der p I and LPS, respectively, promotes or inhibits the cytokine milieu causally involved in atopy;27–34 and RSV is (controversially) linked with the development of asthma35,36 and effector mechanisms typical of type 2 cytokines,37–41 however, it is not yet known whether LPS or Der p I influences RSV infection or the sequelae of infection in vivo.
Acknowledgments
We thank Dr Barbara J. Hart of the Royal Agricultural College, Cirencester, UK, for cultured mites. This work was generously supported by a grant from The NHS Executive R & D Trent Regional Research Scheme. C.R.A.H. is grateful for additional support from The Wellcome Trust and the Midlands Asthma and Allergy Research Association. K.J.B. was supported by a Medical Research Council M.Res. studentship. M.E.L.G. was supported by a University of Leicester Faculty of Medicine and Biological Sciences Ph.D. studentship.
reference
- 1.Message SD, Johnston SL. The immunology of virus infection in asthma. Eur Resp J. 2001;18:1013–25. doi: 10.1183/09031936.01.00228701. [DOI] [PubMed] [Google Scholar]
- 2.Kilpelainen M, Terho EO, Helenius H, Koskenvuo M. Home dampness, current allergic diseases, and respiratory infections among young adults. Thorax. 2001;56:462–7. doi: 10.1136/thorax.56.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strachan DP. The role of environmental factors in asthma. Br Med Bulletin. 2000;56:865–82. doi: 10.1258/0007142001903562. [DOI] [PubMed] [Google Scholar]
- 4.Burr ML, Anderson HR, Austin JB, Harkins LS, Kaur B, Strachan DP, Warner JO. Respiratory symptoms and home environment in children: a national survey. Thorax. 1999;54:27–32. doi: 10.1136/thx.54.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrett MH, Rayment PR, Hooper MA, Abramson MJ, Hooper BM. Indoor airborne fungal spores, house dampness and associations with environmental factors and respiratory health in children. Clin Exp Allergy. 1998;28:459–67. doi: 10.1046/j.1365-2222.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 6.Gehring U, Heinrich J, Jacob B, Richter K, Fahlbusch B, Schlenvoigt G, Bischof W, Wichmann HE. Respiratory symptoms in relation to indoor exposure to mite and cat allergens and endotoxins. Eur Resp J. 2001;18:555–63. doi: 10.1183/09031936.01.00096801. [DOI] [PubMed] [Google Scholar]
- 7.Koskinen OM, Husman TM, Meklin TM, Nevalainen AI. The relationship between moisture or mould observations in houses and the state of health of their occupants. Eur Resp J. 1999;14:1363–7. [PubMed] [Google Scholar]
- 8.Pirhonen I, Nevalainen A, Husman T, Pekkanen J. Home dampness, moulds and their influence on respiratory infections and symptoms in adults in Finland. Eur Resp J. 1996;9:2618–22. doi: 10.1183/09031936.96.09122618. [DOI] [PubMed] [Google Scholar]
- 9.Rylander R, Lin RH. (1 → 3)-beta-d-glucan – relationship to indoor air-related symptoms, allergy and asthma. Toxicology. 2000;152:47–52. doi: 10.1016/s0300-483x(00)00291-2. [DOI] [PubMed] [Google Scholar]
- 10.Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, Pauwels R, Sergysels R. Severity of asthma is related to endotoxin in house dust. Am J Resp Crit Care Med. 1996;154:1641–6. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 11.Peden DB. Air pollution in asthma: effect of pollutants on airway inflammation. Ann Allergy Asthma Immunol. 2001;87:12–7. doi: 10.1016/s1081-1206(10)62334-4. [DOI] [PubMed] [Google Scholar]
- 12.Andrae S, Axelson O, Bjorksten B, Fredriksson M, Kjellman NIM. Symptoms of bronchial hyperreactivity and asthma in relation to environmental factors. Arch Dis Childhood. 1988;63:473–8. doi: 10.1136/adc.63.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sporik R, Holgate ST, Plattsmills TAE, Cogswell JJ. Exposure to house-dust mite allergen (Der-P-I) and the development of asthma in childhood – a prospective-study. N Engl J Med. 1990;323:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 14.Fiedler MA, WernkeDollries K, Stark JM. Respiratory syncytial virus increases IL-8 gene expression and protein release in A549 cells. Am J Physiol-Lung Cellular Mol Physiol. 1995;13:L865–72. doi: 10.1152/ajplung.1995.269.6.L865. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt CRA, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: Innate protection by antiproteases. J Exp Med. 1995;182:1537–44. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz O, Sewell HF, Shakib F. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J Exp Med. 1998;187:271–5. doi: 10.1084/jem.187.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161:3645–51. [PubMed] [Google Scholar]
- 18.Wan H, Winton HL, Soeller C, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–33. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krusat T, Streckert HJ. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch Virol. 1997;142:1247–54. doi: 10.1007/s007050050156. [DOI] [PubMed] [Google Scholar]
- 20.Feldman SA, Hendry RM, Beeler JA. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virol. 1999;73:6610–7. doi: 10.1128/jvi.73.8.6610-6617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman SA, Audet S, Beeler JA. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J Virol. 2000;74:6442–7. doi: 10.1128/jvi.74.14.6442-6447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol. 2000;74:10508–13. doi: 10.1128/jvi.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman SA, Crim RL, Audet SA, Beeler JA. Human respiratory syncytial virus surface glycoproteins F, G and SH form an oligomeric complex. Arch Virol. 2001;146:2369–83. doi: 10.1007/s007050170009. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 25.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–82. [PubMed] [Google Scholar]
- 26.Akaike T, Maeda H, Maruo K, Sakata Y, Sato K. Potentiation of infectivity and pathogenesis of influenza-a virus by a house-dust mite protease. J Infect Dis. 1994;170:1023–6. doi: 10.1093/infdis/170.4.1023. [DOI] [PubMed] [Google Scholar]
- 27.Vedanthan PK, Mahesh PA, Holla AD, Vedanthan R, Liu AH. Lower prevalence of asthma, rhinitis and atopy in rural India is associated with higher house-dust endotoxin levels. J Allergy Clin Immunol. 2002;109:104. [Google Scholar]
- 28.Douwes J, Pearce N, Heederik D. Does environmental endotoxin exposure prevent asthma? Thorax. 2002;57:86–90. doi: 10.1136/thorax.57.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Mutius E. Infection: friend or foe in the development of atopy and asthma? The epidemiological evidence. Eur Resp J. 2001;18:872–81. doi: 10.1183/09031936.01.00268401. [DOI] [PubMed] [Google Scholar]
- 30.von Mutius E, Braun-Fahrlander C, Eder W, et al. Indoor exposure to endotoxin is inversely related to asthma, hay fever and atopy. Epidemiology. 2001;12:177. [Google Scholar]
- 31.von Mutius E, Braun-Fahrlander C, Schierl R, Riedler J, Ehlermann S, Maisch S, Waser M, Nowak D. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000;30:1230–4. doi: 10.1046/j.1365-2222.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 32.Gereda JE, Leung DYM, Thatayatikom A, Streib JE, Price MR, Klinnert MD, Liu AH. Relation between house-dust endotoxin exposure, type 1 T cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355:1680–3. doi: 10.1016/s0140-6736(00)02239-x. [DOI] [PubMed] [Google Scholar]
- 33.Wan GH, Li CS, Lin RH. Airborne endotoxin exposure and the development of airway antigen-specific allergic responses. Clin Exp Allergy. 2000;30:426–32. doi: 10.1046/j.1365-2222.2000.00730.x. [DOI] [PubMed] [Google Scholar]
- 34.Martinez FD, Holt PG. Role of microbial burden in aetiology of allergy and asthma. Lancet. 1999;354:S12–5. doi: 10.1016/s0140-6736(99)90437-3. [DOI] [PubMed] [Google Scholar]
- 35.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin-E antibodies after respiratory syncytial virus bronchiolitis – a prospective cohort study with matched controls. Pediatrics. 1995;95:500–5. [PubMed] [Google Scholar]
- 36.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respiratory Crit Care Med. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 37.Openshaw PJM. Immunity and immunopathology to respiratory syncytial virus – the mouse model. Am J Resp Crit Care Med. 1995;152:S59–62. doi: 10.1164/ajrccm/152.4_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- 38.Renzi PM, Turgeon JP, Yang JP, Drblik SP, Marcotte JE, Pedneault L, Spier S. Cellular immunity is activated and a TH-2 response is associated with early wheezing in infants after bronchiolitis. J Pediatr. 1997;130:584–93. doi: 10.1016/s0022-3476(97)70243-9. [DOI] [PubMed] [Google Scholar]
- 39.Yao JX, Wu JM. Respiratory syncytial virus bronchiolitis in infants is associated with predominant Th2-like response. Pediatr Res. 1999;45:92. [Google Scholar]
- 40.Roman M, Calhoun WJ, Hinton KL, Avendano LF, Simon V, Escobar AM, Gaggero A, Diaz PV. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Resp Crit Care Med. 1997;156:190–5. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 41.Rabatic S, Gagro A, LokarKolbas R, KrsulovicHresic V, Vrtar Z, PopowKraupp T, Drazenovic V, MlinaricGalinovic G. Increase in CD23 (+) B cells in infants with bronchiolitis is accompanied by appearance of IgE and IgG4 antibodies specific for respiratory syncytial virus. J Infect Dis. 1997;175:32–7. doi: 10.1093/infdis/175.1.32. [DOI] [PubMed] [Google Scholar]