Abstract
Invariant human natural killer T cells (NKT) express a restricted T-cell receptor (TCR) Vα24Vβ11 repertoire. These cells share both phenotypic and functional similarities between NK and T cells. Given the emerging role of NKT cells as critical cells in bridging the gap between innate and adaptive immunity, we examined their susceptibility to productive human immunodeficiency virus (HIV) infection by T-tropic, M-tropic, and primary isolates of HIV. We generated three human NKT cell clones (CA5, CA29, and CA31). Phenotypic characterization of these Vα24+ Vβ11+ clones indicated that they were predominately positive for CD4, CD161, HLA-DR, CD38, CD45RO, and CD95 expression. The NKT cell clones expressed significantly more surface CCR5 molecules/cell and lower CXCR4 molecules/cell than phytohaemagglutinin-stimulated peripheral blood mononuclear cells (PBMC). Consistent with the surface expression of CCR5 and CXCR4, the NKT clones were also selectively susceptible to HIV M-tropic, T-tropic, and primary isolate infection, as evaluated by both HIV p24 enzyme-linked immunosorbent assay and intracellular staining of HIV proteins. The amount of p24 production was dependent on the NKT clone studied and the HIV strain used. Clones CA29 and CA31 were also susceptible to HIV IIIB infection. The virions produced by these clones were able to productively infect PHA-stimulated PBMCs with the same kinetics as for primary infection of CD4+ blast. Collectively, this data demonstrates that NKT cells can be a target for productive HIV infection but with a lag in the time to peak p24 production.
Introduction
Natural killer T (NKT) cells, as their name implies, share functional and phenotypic homology with both natural killer cells and T cells. NKT cells express NK markers and a restricted (invariant) T-cell recpetor (TCR) repertoire.1 In humans, invariant NKT cells express Vα24JαQ chain that preferentially pairs with a Vβ11 chain.2–4 The human invariant TCRαβ pair is highly homologous toVα14 and Vβ8 found in mice.5 NKT cells are CD3+ but they may either express CD4 or CD8 or neither of these two coreceptors. Invariant NKT cells, recognize glycolipid antigens presented by a non-polymorphic major histocompatibility complex (MHC) class 1b protein, the CD1d molecule, which is expressed on the surface of antigen-presenting cells.6–9
CD1d-restricted T cells are thought to regulate a wide variety of immune responses.1,10,11 Subsequent to TCR ligation, NKT cells can produce both type 1 (interleukin (IL)-2, tumour necrosis factor (TNF)-α, interferon (INF)-γ) and type 2 (IL-4, IL-10, IL-13) cytokines suggesting that they may play a critical role in immune regulation. NKT cells have been reported to play a role in tumour surveillance by direct cytotoxicity and by activating NK cells that in turn leads to lysis of tumours and the target cells.12–16 Recently, NKT cells were linked to the prevention of type 1 diabetes in non-obese diabetic (NOD) mouse model of type 1 diabetes.17–19 In humans, NKT cells were present at high frequency in non-diabetic siblings in comparison to monozygotic twins and triplets.20 CD1d-restricted T cells were shown to be important for generating appropriate immune responses against a variety of pathogens including viral21 bacterial (Mycobacterium tuberculosis,22–24 Salmonella choleraesuis,25 Listeria monocytogenes,26 Toxoplasma gondii,27,28 and Leishmania major29), and fungal (Cryptococcus neoformans30,31) infections. Thus, CD1d-restricted T cells are important for tumour surveillance, autoimmunity, and in immune responses to a wide variety of human pathogens.
To date, there has been one study that evaluated the frequency of NKT cells in human immunodeficiency virus (HIV) disease and concluded that the frequency of Vα24Vβ11 NKT cells was lower in HIV+ individuals than HIV-donors.32 This finding suggests that NKT cells may be negatively impacted in HIV disease. To investigate the effect of HIV infection on CD1d-restricted cells, human CD1d-restricted clones were generated and evaluated for susceptibility to infection by T-tropic, M-tropic, and primary isolates of HIV.
Materials and methods
Generation of NKT cell clones
CD1d-restricted T-cell clones were generated by single cell sorting as described.20,33 Briefly, NKT cells were sorted using the 6B11-fluorochrome conjugated antibody and single-cell sorts were grown with a mixture of irradiated (5000 rad) allogeneic peripheral blood mononuclear cells (PBMC) at 50 000 per well and irradiated 721.221 cells at 5000 per well. The cell mixture was supplemented with phytohaemagglutinin (PHA; 1 µg/ml), IL-2 (10 U/ml) and IL-7 (10 U/ml). NKT cell clones were verified by both flow cytometric analysis and CDR3 TCR sequencing, using reverse transcriptase–polymerase chain reaction (RT–PCR) as described.20,33 The 6B11 monoclonal antibody was previously identified to be specific for the CDR3 loop of Vα24+ NKT cells and recognizes CD1d-restricted human invariant NKT. The NKT clones were then frozen in liquid nitrogen until further use. When thawed, the clones were expanded using the protocol described below.
Expansion and culture of Vα24+ Vβ11+ NKT cells
NKT cell clones (CA5, CA29, and CA31) were expanded by culturing in RPMI-1640-supplemented with 10% autologous human serum, 25 × 106 mitomycin-treated PBMC, mitomycin-treated 5 × 106 B-lymphoblastoid JY cells (American Tissue Culture Collection, Manassas, VA), and 1 µg/ml anti-CD3. The PBMC used in the above mixture were isolated by Ficoll-Hypaque density gradient centrifugation from venous blood collected from healthy donors as previously described.34 Mitomycin treatment of cells consisted of incubating the cells with 50 µg/ml of mitomycin C at 37° for 30 min, washing three times with phosphate-buffered saline (PBS), then using these cells as feeders for the expansion of NKT cells. After coculturing the NKT cells with the feeder cells and anti-CD3, on day 1, recombinant IL-2 was added to a final concentration of 50 U/ml. On day 5, half of the medium was removed and fresh media was added along with IL-2. On days 10–12, NKT cells were analysed for expansion using light microscopy and split for further expansion. Purity of expanded cells was determined by flow cytometry using Vα24 and Vβ11 fluorochrome-conjugated antibodies.
Immunostaining and flow cytometric analysis
NKT cell clones were phenotypically characterized by using a panel of cell surface antibodies-conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), or allophycocyanin. Briefly, cells were incubated at room temperature for 20 min with the appropriate conjugated antibody panel. FITC-conjugated antibodies consisted of CD3, CD27, HLA-DR, CD28, CD45RA, Vα24, CD14, and immunoglobulin G (IgG) isotype. PE-conjugated antibodies consisted of Vβ11, CD95, CD62L, CD25, CD38, CXCR4, CCR5, and IgG2 isotype. PerCP-conjugated antibodies consisted of CD4, CD8, CD56, CD45RO, and IgG isotype. CD161 was conjugated to allophycocyanin and used in combination with IgG–allophycocyanin isotype. The cells were then washed with 1× PBS and fixed in 1% formaldehyde. Flow cytometric analyses were performed using a FACSCalibur flow cytometer utilizing CELLQuest software (Becton Dickinson, San Jose, CA). All antibodies were purchased from either Becton Dickinson or PharMingen (San Diego, CA), except for CXCR4-PE and CCR5-PE, which were obtained from Southern Biotechnology Associates (Birmingham, AL) and PharMingen Corporation, respectively. Vα24-FITC and Vβ11-PE were obtained from Coulter-Immunotech (Miami, FL). Recombinant human IL-2 was obtained from the Institutes of Health, AIDS Research and Reference Reagent Program (Bethesda, MD). IL-7 was purchased from PharMingen. 6B11, a human monoclonal antibody, specific for the invariant CDR3 loop of the AV24AJ18 rearrangement in NKT cells was also used to purify NKT cell clone, as it was determined to select for Vα24Vβ11 NKT cells.35
HIV-1 infection
NKT cell clones were infected with IIIB, Bal, or a primary isolate (302056) (National Institutes of Health, AIDS Research and Reference Reagent Program) of HIV at 5 ng HIV p24 per 1 × 106 cells for 2 hr at 37°. Subsequently. HIV-exposed cultures were washed three times to remove unbound virus and the cells cultured in the presence of IL-2. HIV isolate 302056 was determined to use CCR5 and not CXCR4 by ghost cell analysis.34,35 Briefly, ghost cells are HOS cells that express, in this case either CXCR4 or CCR5, and are stably transfected with a construct of HIV-2 leukotriene (LTR) linked to green fluorescence protein (GFP). Infection based on coreceptor utilization is detected by expression of GFP, which is monitored by flow cytometry.
HIV-1 p24 assay
HIV viral core (p24) antigen was measured from the supernatant of infected cells using p24 enzyme-linked immunosorbent assay (ELISA) purchased from the AIDS Vaccine Program (Frederick, MD) and performed according to the manufacturer's instructions.
Intracellular p24 staining
Intracellular p24 staining was performed by initially fixing the NKT cell clones or lymphocytes with fluorescence activated cell sorting (FACS) lyse solution (Beckton Dickinson). The fixed cells were then permeabilized by incubation with FACS permeabilization buffer (Beckton Dickinson), followed by intracellular staining with KC57 PE antibody (detects both p17 and p24) and cell surface staining with Vα24-FITC or CD4-FITC. Flow cytometric analysis was performed using a FACS Calibur flow cytometer utilizing CELLQuest software (Becton Dickinson).
Results
Phenotypic analysis of NKT cell clones
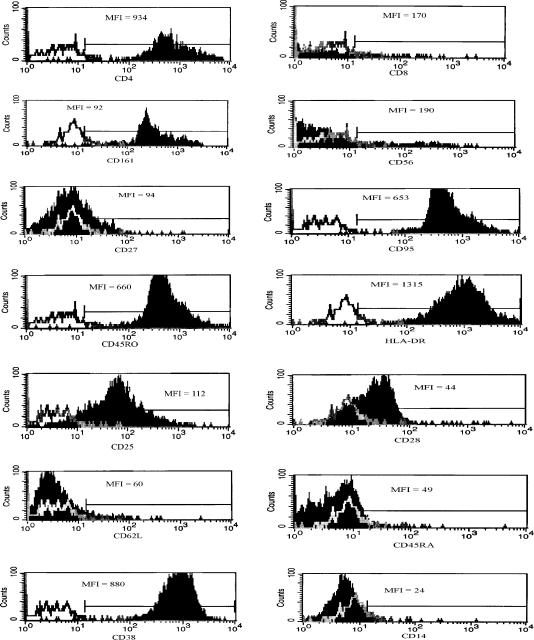
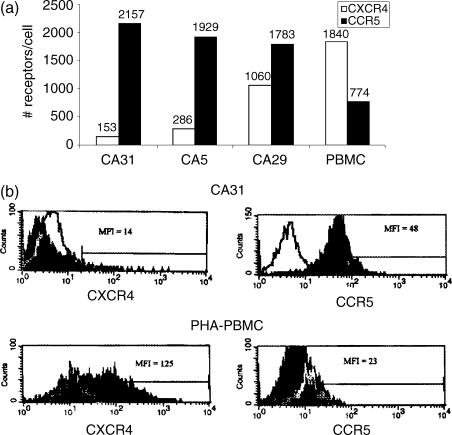
Three CD4+ human invariant NKT cell clones were generated from a normal donor (Fig. 1). CD1d restriction and TCR Vα chain, Vβ chain sequences were verified as previously described.20 To phenotypically characterize the invariant NKT cell clones, FACS analyses for functional (CD161, CD56, CD4, CD95, CD25, CD14, CD27, CCR5, and CXCR4) and activation (HLA-DR, CD38, CD45RO, CD45RA) markers were performed. The three CD1d-restricted NKT clones (CA5, CA29, and CA31) were uniformly positive for CD161, CD4, CD95, HLA-DR, CD38, and CD45RO (Table 1 and Fig. 2). Approximately 50% of the cells within each clone were positive for CD56 while all were negative for CD45RA and CD14, and only a fraction expressed low levels (10–28%) of CD27. Expression of CXCR4 was low (5–15%) when compared to CCR5 expression (73–80%) (Table 1). Using quantibright bead analysis, the median number of CCR5 molecules/cell on CA31 clone was approximately 2000 molecules/cell, on CA5 clone was 1900 molecules/cell, and on CA29 clone was approximately 1800 molecules/cell (Fig. 3). Median CXCR4 molecules/cell on CA31 was about 150, on CA5 was about 290 molecules/cell, and on CA29 was about 1060 molecules/cell (Fig. 3). However, PHA-stimulated PBMC from a normal donor expressed median of about 770 CCR5 molecules/cell and median of about 1840 CXCR4 molecules/cell.
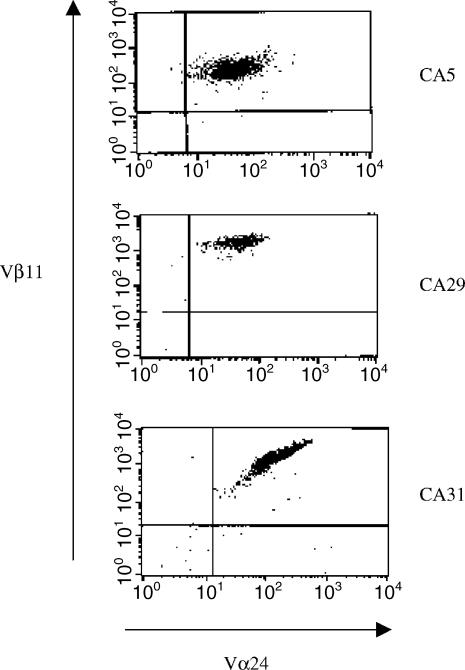
Figure 1.
Generation of NKT cell clones. NKT cell clones were generated by single cell sorting using the 6B11 antibody. These clones were then expanded as described in materials and methods. The flow scatter plot shown for clones CA5, CA29, and CA31 are 100% positive for Vα24Vβ11.
Table 1.
Phenotypic analysis of invariant NKT cell clones. Percent expression of each phenotypic marker on CA5, CA29, and CA31 was measured by flow cytometry. All clones were Vα24+Vβ11+
| Cell surface marker | CA 5 | CA 29 | CA 31 |
|---|---|---|---|
| CD4 | 97 | 99 | 99 |
| CD8 | 4 | 7 | 5 |
| CD161 | 99 | 95 | 99 |
| CD56 | 70 | 50 | 50 |
| CD27 | 10 | 28 | 12 |
| CD95 | 99 | 99 | 99 |
| CD45RO | 94 | 95 | 99 |
| HLA-DR | 89 | 90 | 100 |
| CD25 | 29 | 20 | 83 |
| CD28 | 43 | 36 | 61 |
| CD62L | 6 | 6 | 5.5 |
| CD45RA | 0 | 0 | 2 |
| CD38 | 99 | 99 | 99 |
| CD14 | 0 | 0 | 0.9 |
| CXCR4 | 5 | 15 | 5 |
| CCR5 | 74 | 73 | 80 |
Figure 2.
Expression of cell surface markers on invariant NKT cell clones. (a) A representative example of phenotypic cell surface marker expression on clone CA31 is shown. Vα24+ Vβ11+ T cells, determined by gating on these cells, were also stained for a number of functional (CD4, CD8, CD161, CD56, CD27, CD95, CD25, CD28, CD38, CD14) and activation (CD45RA, CD45RO, HLA-DR, CD38, CD62L) markers. White histogram represents isotype-matched control and dark histogram represents the phenotypic marker of interest. Mean fluorescence intensity (MFI) for each marker is shown within each figure. Results are representative of the three clones.
Figure 3.
Expression of CCR5 and CXCR4 on NKT cells. NKT cell clones (CA31, CA5, CA29) or PHA-stimulated PBMC were evaluated for CXCR4 and CCR5 cell surface expression using rainbow quantibright bead flow cytometric analyses. (a) The relative number of CXCR4 and CCR5 molecules/cell is shown for each clone and for PHA-stimulated PBMCs. (b) A representative flow histogram of CXCR4 and CCR5 expression on clone CA31 or on PHA-stimulated PBMCs are shown. White histogram represents isotype-matched control and dark histogram represents either CXCR4 or CCR5 expression, as indicated in the Y-Axis. The MFI is indicated within each histogram. Results are representative of the three clones.
Susceptibility of NKT clones to HIV infection
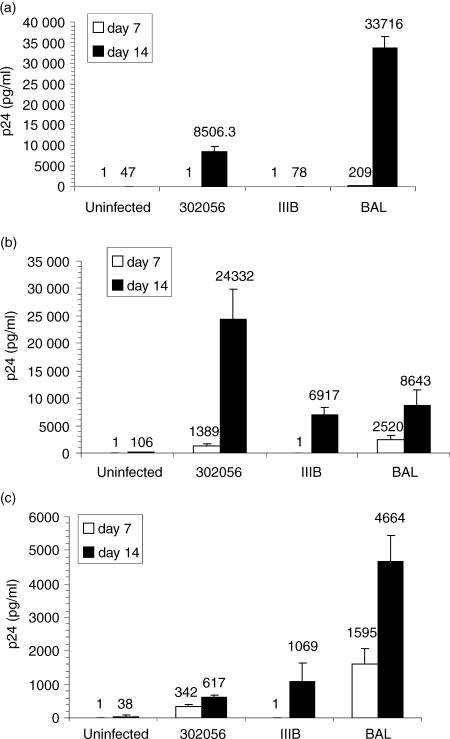
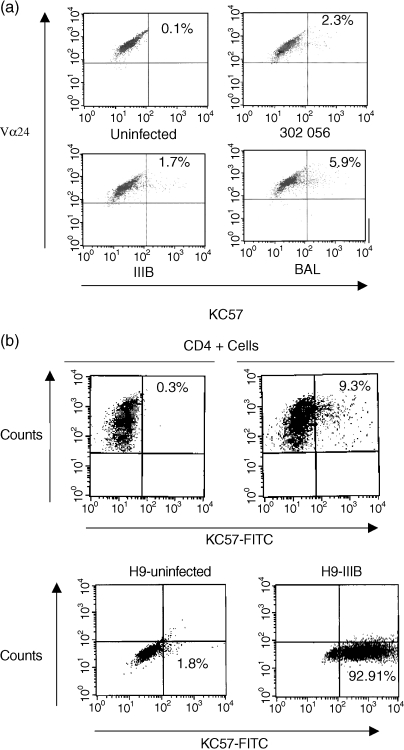
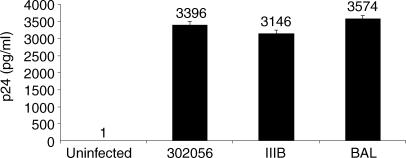
To evaluate whether the surface phenotype (CD4+ CCR5high CXCR4low) was predictive of susceptibility to various HIV subtypes, the clones were infected with T-tropic (IIIB), M-tropic (Bal), and primary isolate of HIV (302056, NIH AIDS Research and Reference Reagent Program). Strain 302056 was determined to preferentially utilize CCR5 through ghost cell analysis. All three clones, CA5, CA29, and CA31, were productively infected by strain 302056 and Bal as was evident by p24 ELISA (Fig. 4). Both clones CA29 and CA31 were susceptible to HIV IIIB infection, but CA5 was not (Fig. 4). To further confirm HIV infection of these NKT clones, intracellular staining for HIV proteins using KC57 antibody that specifically detects intracellular p24 and p17 proteins were analyzed by FACS. NKT cell clones were infected with strain IIIB, 302056, or Bal and 14 days postinfection, the cells were surface stained for Vα24Vβ11 and intracellular staining for p24 and p17 were determined (Fig. 5). Gating on Vα24+ Vβ11+ populations, intracellular background expression of KC57+ cells was approximately 0·1% for clone CA5 (Fig. 5; uninfected panel). Intracellular expression of IIIB infected T cells, however, was 1·7%, 302056 infected cells was 2·3%, and Bal infected cultures was 5·9%. As additional controls for the staining of HIV+ NKT cells, levels of KC57+ infected cells were measured from both infected PBMC and chronically infected H9 cells (H9IIIB). Total PBMC infected with IIIB and stained for CD4+ and KC57+ cells demonstrated approximately 9·0% HIV+ positive cells. Using H9IIIB we show that the level of KC57+ cells at 92·9%. Thus, NKT cells are susceptible to HIV infection, but production of progeny virions were delayed in comparison with PHA blasts.
Figure 4.
HIV-1 infection of invariant NKT cell clones. CA5 (a), CA29 (b), and CA 31 (c) NKT cells were infected using HIV IIIB, Bal, or 302056 isolate. HIV p24 levels were measured at both day 7 and day 14. Data is representative of at least six experiments for each clone.
Figure 5.
Intracellular HIV protein detection. (a) NKT cell clone (CA29) was infected with IIIB, Bal, or 302056 and HIV intracellular proteins (p24 and p17) were detected. The cells were surface stained for Vα24 expression and intracellularly stained using KC57 antibody. (b) HIV intracellular protein expression from IIIB infected PHA-stimulated PBMCs (gating on CD4+ T cells) and H9 cells chronically infected with HIV IIIB were also evaluated.
Given that there was a delay in the kinetics of HIV infection of NKT cells, we examined whether a low level of virus released from NKT cell clones on day 7 is still infectious. NKT cells were initially infected with IIIB, 302056, or Bal for 2 hr, as previously described, then washed extensively. The infected cells were allowed to be cultured for 7 days then the supernatant from those cells were added to PHA-stimulated PBMC and p24 was measured 7 days later. The supernatant from infected NKT clones contained infectious virions that were able to infect PHA-stimulated PBMC to levels that were approximately 3 ng/ml of p24 (Fig. 6) This data indicate that even at day 7, where a low level of HIV is measured from the infected clones, the supernatant still contained infectious virions that are able to transmit the virus to susceptible cells, without the marked delay seen in the CD1d-restricted clones.
Figure 6.
Infected NKT cells produce infectious virions. NKT cells (CA29) were infected with IIIB, Bal, or 302056 and seven day postinfection, the supernatant from the infected NKT cells were used to infect PHA-stimulated PBMCs. HIV p24 was subsequently measured 7 days postinfection of PHA-stimulated PBMCs. Results are representative of at least three experiments
Discussion
Although the main targets of HIV infection are CD4+ primed/memory T cells and monocytes/macrophages, considerable data point to other cells types as being an active source of HIV replication. Specifically, we36 and others37,38 have demonstrated that CD4 expression on CD8+ T cells leads to productive HIV replication. More recently, we have also demonstrated that the environmental milieu impacts HIV productive infection of naïve T cells.39
NKT cells are emerging as key players in the immune regulatory response. Since CD1d-restricted cells were recently reported to be depleted in HIV+ patients, we examined the susceptibility of NKT cells to HIV infection. A set of CD4+ clones with invariant Vα24 Vβ11 TCR was chosen for these studies. The clones were sequenced and their CD1d-restriction was confirmed (data not shown). These clones also expressed surface proteins characteristic of CD1d-restricted T cells. Additional phenotypic characterization revealed that CCR5 chemokine coreceptor expression was also higher on these cells than what would be expected from PHA-stimulated PBMC, while CXCR4 expression was much lower (5–15%). This finding suggested that these cells might be more susceptible to infection by CCR5-utilizing viruses than CXCR4 utilizing HIV strains.
To determine NKT cell susceptibility to HIV infection, the clones were infected with T-tropic (IIIB), M-tropic (Bal), or primary (302056) strain of HIV. All three CD1d-restricted NKT cell clones were susceptible to Bal and 302056 infection, but with delayed kinetics. Specifically, virions from infected NKT cells were released by day 14 rather than day 7, as usually observed from CD4+ blasts. Clones CA31 and CA29 were also susceptible to infection by HIV IIIB, which in the case of clone CA31 can be explained by the higher level of CXCR4 molecules present on these cells. HIV infection of these cells was also confirmed using intracellular staining of HIV p24. Finally, transmission of infectious virion to PHA-stimulated PBMC was also confirmed by demonstrating that supernatant from infected NKT cells can efficiently infect PHA-stimulated PBMC. Therefore, CD1d-restricted T cells can be a productive target for HIV infection of predominately CCR5 utilizing strains but CXCR4 utilizing strain infection can also occur, depending on the level of expression of this chemokine receptor. Recently, Motsinger and colleagues reported that human NKT cells are susceptible to HIV infection40 at a higher rate than CD4+ T cells. These NKT cells, however, were stimulated with α-galaceramide-pulsed dendritic cells, which may have led to their hyperactivation and subsequently the reported higher rate of HIV infection.40 NKT cell clones used in these studies were not activated with α-galaceramide. It is also important to note that based on our experience and that of others, HIV infection of CD4+ T-cell clones, such as H9, SUPT-1, CEM (ss), and HPB-ALL, as measured by p24 ELISA is always potently detected by day 6–7 postinfection, which is at a faster kinetic rate than that reported here for NKT cell infections.
CD1d-restricted T cells are found at a low percentage in the periphery (0·1–1%).1 However, tissues in the liver, spleen, gut and other mucosal surfaces are thought to be enriched for these cells. Thus their presence at antigen-rich sites suggests a potential role in the transmission of HIV. Moreover, the importance of CD1d-restricted T cells in immunologic responses against tumours and infections suggest that dysfunction of these cells in HIV would be of clinical importance.
Acknowledgments
Richardson Fleuridor is a Mark Weiss Memorial Fellow. We thank this organization for their continued support of HIV research. Brian Wilson is supported by NIH RO1 A YS051.
Abbreviations
- NKT
natural killer T cells
- PBMC
peripheral blood mononuclear cells
- FITC
fluorescein isothioc yanate
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein
- NOD
non-obese diabetic
- INF-γ
interferon-γ
- TNF-α
tumour necrosis factor-α
- IL-2
interleukin-2
References
- 1.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 2.Nicol A, Nieda M, Koezuka Y, Porcelli S, Suzuki K, Tadokoro K, Durrant S, Juji T. Human invariant valpha24+ natural killer T cells activated by alpha-galactosylceramide (KRN7000) have cytotoxic anti-tumour activity through mechanisms distinct from T cells and natural killer cells. Immunology. 2000;99:229. doi: 10.1046/j.1365-2567.2000.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Nieda M, Koezuka Y, et al. Analysis of human V alpha 24+ CD4+ NKT cells activated by alpha-glycosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2000;164:4458. doi: 10.4049/jimmunol.164.9.4458. [DOI] [PubMed] [Google Scholar]
- 4.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumperz JE, Roy C, Makowska A, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211. doi: 10.1016/s1074-7613(00)80174-0. February. [DOI] [PubMed] [Google Scholar]
- 6.Wilson SB, Kent SC, Horton HF, Hill AA, Bollyky PL, Hafler DA, Strominger JL, Byrne MC. Multiple differences in gene expression in regulatory Valpha 24Jalpha Q T cells from identical twins discordant for type I diabetes. Proc Natl Acad Sci USA. 2000;97:7411. doi: 10.1073/pnas.120161297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostolou I, Cumano A, Gachelin G, Kourilsky P. Evidence for two subgroups of CD4− CD8− NKT cells with distinct TCR alpha beta repertoires and differential distribution in lymphoid tissues. J Immunol. 2000;165:2481. doi: 10.4049/jimmunol.165.5.2481. [DOI] [PubMed] [Google Scholar]
- 8.Baur N, Nerz G, Nil A, Eichmann K. Expression and selection of productively rearranged TCR beta VDJ genes are sequentially regulated by CD3 signaling in the development of NK1.1 (+) alpha beta T cells. Int Immunol. 2001;13:1031. doi: 10.1093/intimm/13.8.1031. [DOI] [PubMed] [Google Scholar]
- 9.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 10.Hammond KJ, Pelikan SB, Crowe NY, et al. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999;29:3768. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995;7:367. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 12.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 13.Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, Kronenberg M, Seeger RC. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa K, Shibasaki C, Hiratsuka M, Arakawa M, Takahashi K, Takeuchi T. Antitumor spectrum of deoxyspergualin and its lack of cross-resistance to other antitumor agents. J Antibiot (Tokyo) 1991;44:1101. doi: 10.7164/antibiotics.44.1101. [DOI] [PubMed] [Google Scholar]
- 15.Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8 (+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97:2923. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 16.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 17.Hong S, Wilson MT, Serizawa I, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 18.Naumov YN, Bahjat KS, Gausling R, et al. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc Natl Acad Sci USA. 2001;98:13838. doi: 10.1073/pnas.251531798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune type 1 diabetes. Nat Med. 2001;7:1057. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 1998;391:177. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 21.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 22.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999:1891973. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apostolou I, Takahama Y, Belmant C, et al. Murine natural killer T (NKT) cells [correction of natural killer cells] contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc Natl Acad Sci USA. 1999;96:5141. doi: 10.1073/pnas.96.9.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emoto M, Emoto Y, Buchwalow IB, Kaufmann SH. Induction of IFN-gamma-producing CD4+ natural killer T cells by Mycobacterium bovis bacillus Calmette Guerin. Eur J Immunol. 1999;29:650. doi: 10.1002/(SICI)1521-4141(199902)29:02<650::AID-IMMU650>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugawara I. Interleukin-18 (IL-18) and infectious diseases, with special emphasis on diseases induced by intracellular pathogens. Microbes Infect. 2000;2:1257. doi: 10.1016/s1286-4579(00)01279-x. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, et al. Alpha-galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano Y, Hisaeda H, Sakai T, Zhang M, Maekawa Y, Zhang T, Himeno K. Role of innate immune cells in protection against Toxoplasma gondii at inflamed site. J Med Invest. 2001;48:73. [PubMed] [Google Scholar]
- 29.Ishikawa H, Hisaeda H, Taniguchi M, et al. CD4 (+) v (alpha) 14 NKT cells play a crucial role in an early stage of protective immunity against infection with Leishmania major. Int Immunol. 2000;12:1267. doi: 10.1093/intimm/12.9.1267. [DOI] [PubMed] [Google Scholar]
- 30.Lim TS, Murphy JW. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980;30:5. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawakami K, Kinjo Y, Uezu K, et al. Monocyte chemoattractant protein-1-dependent increase of V alpha 14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J Immunol. 2001;167:6525. doi: 10.4049/jimmunol.167.11.6525. [DOI] [PubMed] [Google Scholar]
- 32.van der Vliet HJ, von Blomberg BM, Hazenberg MD, et al. Selective decrease in circulating V alpha 24+V beta 11+ NKT cells during HIV type 1 infection. Immunol. 2002;168:1490. doi: 10.4049/jimmunol.168.3.1490. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Bao M, Yoon JW. Intrinsic defects in the T-cell lineage results in natural killer T-cell deficiency and the development of diabetes in the nonobese diabetic mouse. Diabetes. 2001;50:2691. doi: 10.2337/diabetes.50.12.2691. [DOI] [PubMed] [Google Scholar]
- 34.Bastiani Lallos L, Cecilia D, Fenyo EM, Laal S, Zolla-Pazner S. HIV phenotype correlates with the relative amounts of lymphocyte function-related molecule 1 (LFA-1) and major histocompatibility complex (MHC) class II in the virion envelope. AIDS. 2000;14:1523. doi: 10.1097/00002030-200007280-00008. July 28. [DOI] [PubMed] [Google Scholar]
- 35.Cecilia D, Kewa I, Ramani VN, et al. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J Virol. 1998;72:6988. doi: 10.1128/jvi.72.9.6988-6996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan YB, Landay AL, Zack JA, Kitchen SG, Al-Harthi L. Upregulation of CD4 on CD8+ T cells: CD4dimCD8bright T cells constitute an activated phenotype of CD8+ T cells. Immunology. 2001;103:270. doi: 10.1046/j.1365-2567.2001.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitchen SG, Korin YD, Roth MD, Landay A, Zack JA. Costimulation of naive CD8 (+) lymphocytes induces CD4 expression and allows human immunodeficiency virus type 1 infection. J Virol. 1998;72:9054. doi: 10.1128/jvi.72.11.9054-9060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flamand L, Crowley RW, Lusso P, Colombini-Hatch S, Margolis DM, Gallo RC. Activation of CD8+ T lymphocytes through the T cell receptor turns on CD4 gene expression: implications for HIV pathogenesis. Proc Natl Acad Sci USA. 1998;95:3111. doi: 10.1073/pnas.95.6.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steffens CM, Smith KY, Landay A, Shott S, Truckenbrod A, Russert M, Al-Harthi L. T cell receptor excision circle (TREC) content following maximum HIV suppression is equivalent in HIV-infected and HIV-uninfected individuals. AIDS. 2001;15:1757. doi: 10.1097/00002030-200109280-00003. [DOI] [PubMed] [Google Scholar]
- 40.Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002;195:869. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]