Abstract
Mycobacteria are potent adjuvants, can survive intracellularly and have been safely used for many years as vaccines against tuberculosis and leprosy. They are thus important potential vectors for recombinant vaccines. Many of their adjuvant properties are mediated following phagocytosis by dendritic cells (DC), which are in turn critical for priming naïve T cells. Although the maturation of DC in response to mycobacteria, such as Mycobacterium bovis bacillus Calmette–Guérin (BCG), is well described the subsequent responses of autologous T cells to mycobacterium-infected DC remains uncharacterized. In our experiments DC infected with BCG expressed more co-stimulatory molecules than tumour-necrosis factor-α (TNF-α) -treated DC and stimulated more potent mixed leucocyte reactions. When autologous T cells were co-cultured with BCG-exposed DC they became highly activated, as determined by display of CD25, CD54 and CD71 on both CD4+ and CD8+ cells. In contrast, the response of T cells to TNF-α-matured DC was significantly less. Cytokine production from T cells cultured with BCG-exposed DC was enhanced with elevated secretion of interleukin-2 (IL-2), IL-10 and interferon-γ (IFN-γ) and was produced by both CD4+ and CD8+ lymphocytes as determined by intracellular staining. In particular, IFN-γ secretion was increased from 50 pg/ml to 25 000 pg/ml and IL-10 secretion increased from 20 pg/ml to 300 pg/ml in BCG-exposed DC co-cultures. Blocking antibodies to B7.1 and B7.2 or IL-12 significantly reduced the secretion of IFN-γ and reductions were also seen in the expression of CD25 and CD71 by CD4+ cells. These data demonstrate that mycobacterially infected DC are particularly potent activators of autologous T cells compared to TNF-α-exposed DC and that the resultant T cells are functionally superior.
Introduction
Mycobacterium bovis bacillus Calmette–Guérin (BCG) has been used since the 1920s as an attenuated vaccine against tuberculosis1 and since 1976 as immunotherapy against intravesicular bladder cancer.2 The history of safe use of the BCG vaccine and its immunostimulatory properties in bladder cancer treatment have led to renewed interest in genetically re-engineering this bacterium for use as a local immunotherapy or a specific vaccine against infectious disease and cancer.
The most potent professional antigen-presenting cell (APC), the dendritic cell (DC), has been the focus of much interest in recent years as it is the main initiator of naïve T-cell responses to immunological danger. On leaving the bone marrow, DC migrate to areas of the body where antigen entry is likely, i.e. mucosa. Here, they continually sample the environment by phagocytosis, receptor-mediated endocytosis, and pinocytosis. Upon contact with foreign organisms [characterized by lipopolysaccharide (LPS), bacterial DNA or double stranded RNA] or host inflammatory mediators [e.g. heat-shock proteins and tumour necrosis factor-α (TNF-α)] DC undergo a process of marked maturation, which increases the expression of various co-stimulatory molecules such as CD40, B7.1, and B7.2.3 Expression of such co-stimulatory molecules by DC plays a key role in generating efficient T-cell responses.4
It has recently been shown in vivo that the DC is the main host cell reservoir for live BCG organisms. Recombinant BCG expressing the MalE reporter gene from Escherichia coli was used to show that both macrophages and DC were infected with BCG to similar levels. However, major histocompatibility complex (MHC) class II antigen-presenting activity was only associated with the DC population.5 Several different investigators have demonstrated that mycobacteria such as BCG are potent activators of human DC.6–9 Along with an increase in co-stimulatory molecules enhanced secretion of interleukin-12 (IL-12) p70, TNF-α, IL-1, IL-8 and regulated on activated and normal T cell expressed and secreted (RANTES) is seen with BCG infection.10,11 However, there is conflicting evidence concerning the role of TNF-α in the maturation of infected DC. Thurnher et al. showed that maturation of BCG-infected DC was significantly inhibited by neutralizing TNF-α6 whereas Kim et al. suggested that TNF-α only played a role in down-regulation of BCG-induced IL-12 p70 production and not surface phenotype.7
Since the demonstration in 1991 that foreign epitopes inserted into BCG can be delivered to an MHC class I loading compartment, the potential of BCG as an anti-cancer vaccine has been realized.12,13 The co-stimulatory capacity of BCG-infected DC, along with their secretion of T helper type 1 (Th1) inducing cytokines, such as IL-12, indicates that using BCG as a vaccine vehicle may provide a non-specific immunostimulatory effect that is beneficial for the generation of naïve or previously tolerised antigen-specific T-cell responses to tumour antigens. The aim of this study was to determine whether BCG-infected DC stimulated autologous T cells over and above cytokine-exposed DC. We observed that autologous T cells cultured with BCG-infected DC demonstrated increased proliferation, activation and cytokine secretion compared to T cells cultured with TNF-α-exposed DC. Our results presented below suggest that BCG may have beneficial adjuvancy effects that may make it a better tumour vaccine candidate than other current strategies.
Materials and methods
Reagents for cell culture, antibodies and cytokines
Cultures were maintained in RPMI-1640 medium (Gibco BRL, Invitrogen Life Technologies, Paisley, UK) supplemented with 10% fetal calf serum (FCS; HarlaSera Laboratories, Harlan-Sera Labs, Loughborough, UK), 2 mm l-glutamine and 1 mm sodium pyruvate (Gibco BRL). Recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) was purchased from Leucomax (Sandoz Pharmaceuticals, Camberley, UK) and recombinant IL-4 and TNF-α were obtained from R & D Systems (R&D Systems Abingoon, Oxfordshire, UK). Mycobacterium bovis BCG Pasteur strain (American Type Culture Collection, LGC Promochen, Teddington, UK) was grown in 7H9 media supplemented with OADc enrichment media (Middlebrooks, Difco, Becton Dickinson, Cowley, UK) and resuspended in RPMI-1640 for use. Monoclonal antibodies against the following human cell-surface markers were used, conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), or (Peridinin Chlorophy II Protein (PerCP)): CD1a-PE, CD4-FITC, CD8-PerCP, CD11c-PE, CD14-PE, CD25-PE, CD54-PE, B7·1-PE, and HLA-DR-PE (Becton Dickinson, BD Biosciences, Cowley, UK), CD40-PE (Serotech, Kidlington, Oxford, UK), CD71-PE, CD83-PE and B7·2-PE (BD-Pharmingen, BD Biosciences, Cowley, UK), mouse immunoglobulin G1 (IgG1) fluorescently conjugated to FITC, PE, or PercP. PE-conjugated antibodies to IL-2, IL-4, IL-10 and interferon-γ (IFN-γ; BD-Pharmingen) were used for the determination of intracellular cytokine production.
Generation of dendritic cells from peripheral blood
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats diluted 1 : 1 with Hanks' balanced salt solution (HBSS; GibcoBRL) by density centrifugation over Lymphoprep (Nycomed, Invitrogen Life Technologies, Paisley, UK), washed twice in HBSS and resuspended at 6·6 × 106 PBMCs/ml in RPMI-1640 supplemented with 1% FCS, 2 mm l-glutamine and 1 mm sodium pyruvate. Cells were allowed to adhere in six-well plates (Costar, Western Laboratory Services LTD, Aldershot, UK) at 20 × 106/well for 1·5 hr at 37°. The non-adherent cells were cryopreserved and the adherent cells were cultured for 5 days in RPMI-1640, 10% FCS supplemented with 800 U/ml GM-CSF and 500 U/ml IL-4 (DC media). Cells were supplemented with an additional 1 ml of cytokine-containing medium on day 3 and harvested on day 5–6 at which point they were deemed to be immature dendritic cells (imm DC).
Maturation of dendritic cells
Imm DC were plated in DC media at a density of 1 × 106/ml in 24-well plates (Costar) and either left untreated or matured by the addition of TNF-α at 1000 U/ml or Mycobacterium bovis BCG [1 × 107 colony-forming units (CFU)/ml] for 48 hr (multiplicity of infection = 10).
Tuberculin purified protein derivative (PPD) responsiveness of donors
PBMC from each donor were plated out in triplicate at a density of 1·5 × 105/well in 96-well round-bottomed plates and stimulated for 3 days with 2·5 μg/ml phytohaemagglutinin (Sigma, Sigma-Aldrich LTD, Poole, UK) for 6 days with media alone or PPD (Staten Serums Institute, Copenhagen, Denmark) at 5 μg/ml. Cells were pulsed for the final 18 hr of culture with 0·5 μCi of methyl-[3H]thymidine. Those donors having a stimulation index greater than 3 were deemed to be PPD responsive.
Flow cytometric analysis of DC maturation
Day 7 DC were analysed by flow cytometry to determine their cell surface phenotype. Cells were washed in phosphate-buffered saline (PBS) with 1% FCS and 0·1% sodium azide, incubated with antibody for 30 min in the dark at 4°, and then washed and analysed on a Becton Dickinson FACscan with Cell Quest software.
Determination of T-cell proliferation
Immature and mature DC were irradiated with a 137caesium source at a dose of 30 Gy and plated out in sextuplet in 96-well round-bottomed plates (Costar) at 10 consecutive 1 : 2 serial dilutions starting with 104 DC/well. The non-adherent responder T-cell populations (both autologous and allogeneic) were recovered from liquid nitrogen, and added at a density of 1 × 105/well. Cell proliferation during the final 18 hr of a 5-day culture was determined by uptake of 0·5 μCi methyl-[3H]thymidine (Amersham, Amersham Biosciences, Amersham, UK). Cells were harvested onto printed filter mats (Wallac) and radioactivity was determined in a 1450 Wallac Microbeta Jet Scintillation counter. Statistics were determined using the analysis of covariance on spss.
Determination of T-cell activation
Media-exposed DC, TNF-α-exposed DC, or BCG-exposed DC were irradiated (30 Gy) and cultured in 24-well plates with autologous peripheral blood lymphocytes at a ratio of 1 : 5 (105 DC: 5 × 105 PBLs). Following 15, 24, 48, 72 and 96 hr of culture cells were harvested, supernatants were collected and stored at −80° until required and 105 cells were analysed for T-cell activation by flow cytometry. Lymphocytes were gated according to their forward and side scatter (FSC/SSC) profile and CD4+ and CD8+ cells were analysed for their expression of CD25, CD54 and CD71. Statistics were determined using the students paired t-test.
Cytokine determination
Supernatants from 96-h co-cultures were assayed for IL-2, IL-4, IL-10 and IFN-γ by commercially available enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems) and levels were quantified using a Titretek Multiskan microplate reader (IL-10 and IFN-γ) or a 1450 Wallac Microbeta Jet luminometer. The lower levels of detection of the kits were 1·6 pg/ml (IL-2), 1·6 pg/ml (IL-4), 15·6 pg/ml (IL-10) and 15·6 pg/ml (IFN-γ).
Intracellular cytokine production was determined by repeating the DC : T-cell co-cultures and analysing the cells at 24, 48, 72 and 96 hr for the intracellular accumulation of IL-2, IL-4, IL-10 and IFN-γ. Briefly, 105 cells were stained with fluorescently conjugated CD4 or CD8, washed, fixed in 4% paraformaldehyde (Sigma) and permeablized in 1% saponin (Sigma). Intracellular staining was performed in 0·1% saponin for 30 min in the dark at 4°. Ten thousand events were collected on a Becton Dickinson FACScan and analysed for cytokine expression.
Blocking antibody experiments
DC were matured with BCG for 48 hr in the presence or absence of a blocking antibody to IL-12 (20 μg/ml, clone 24910.1; R & D Systems). DC were then co-cultured with autologous T cells at a ratio of 5 : 1 in the presence or absence of mouse monoclonal azide-free blocking antibodies to B7.1 (5 μg/ml, clone 5B5, Autogen Bioclear LTD, Calne, UK), B7.2 (10 μg/ml, clone 1G10, Autogen Bioclear), B7.1 and B7.2 or IL-12. T cells were pretreated with the blocking antibodies for 30 min before addition to the co-cultures. After 72 hr and 96 hr supernatants were harvested, centrifuged at 10 000 g, stored at − 80° and then assayed for IFN-γ levels by solid-phase ELISA (R & D Systems). Cells were also stained for CD4, CD8, CD25, CD54 and CD71 as described above.
Results
BCG infection of DC induces a more mature phenotype than TNF-α
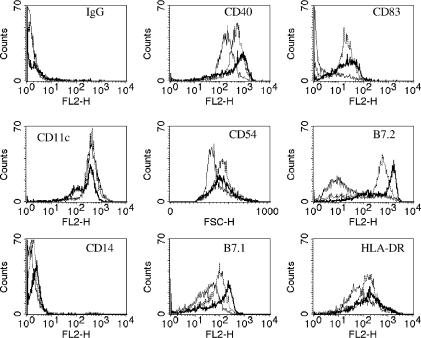
Maturation of DC is associated with alterations in the expression of lineage and cell-surface markers. In agreement with previous studies BCG infection increased the levels of these markers substantially more than TNF-α treatment (Fig. 1).6,7,10,11 Upon exposure to BCG or TNF-α only 50–80% of DC were recovered, compared to around 80% of media exposed DC. Between 70% and 80% of DC were infected with BCG after 48 hr as determined by uptake of Green Fluorescent Protein (BCG)-GFP (data not shown).
Figure 1.
Functional analysis of monocyte-derived DC. Day 5 monocyte-derived DC were incubated for a further 48 hr with DC media (thin-line) (medium-exposed DC), 1000 U/ml TNF-α (broken line) (TNF-α-exposed DC) or M. bovis BCG at a multiplicity of infection of 10 (bold line) (BCG-exposed DC) prior to staining with a panel of PE-conjugated antibodies for cell surface markers. Representative FACs plots of one donor are shown.
Response of allogeneic and autologous T cells to BCG-exposed DC
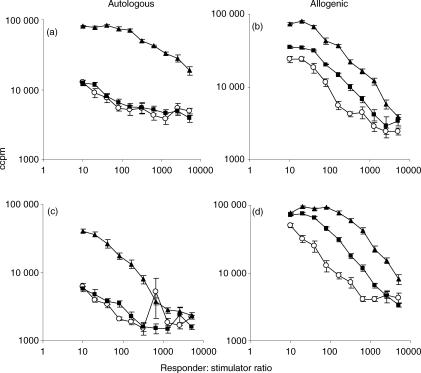
The ability of mature DC to stimulate allogeneic T-cell proliferation is a key indicator of their state of maturity. Therefore the ability of BCG-infected DC, TNF-α-treated DC and media-exposed DC (M-DC) to stimulate mixed leucocyte reactions was examined in nine donors. In all experiments T-cell proliferation was greatest when BCG-exposed DC were used as stimulators (Fig. 2). In autologous systems a seven- to eight-fold increase in T-cell proliferation was seen with BCG-exposed DC as stimulators compared to TNF-α-exposed DC (at a ratio of 10 : 1). In allogeneic experiments, where the baseline was higher there was a one- to three-fold increase in proliferation. TNF-α-DC were better stimulators than M-DC of allogeneic proliferation but not of autologous T-cell proliferation.
Figure 2.
Autologous (a,c) and allogeneic (b,d) mixed leucocyte reactions between media-exposed DC (○), TNF-α-exposed DC (▪); BCG-exposed DC (▴) and peripheral blood lymphocytes. Irradiated DC (stimulators) were cultured with autologous or allogenic PBL (responders) for 5 days. [3H]thymidine incorporation was determined during the last 18 hr of culture to measure T-cell proliferation. (a,b) show a representative PPD-responsive donor and (c,d) a PPD-non-responsive donor.
To determine whether the enhanced proliferation was a result of recall responses to BCG antigens panels of both PPD-positive and PPD-negative donors were studied (five and four donors, respectively). BCG-exposed DC from PPD-positive donors stimulated autologous T-cell proliferation more than the other DC types (Fig. 2a). When PPD-negative donors were used the responder : stimulator ratio was much more influential, with only lower ratios having an enhanced effect (Fig. 2c). However, in allogeneic DC : T-cell experiments the PPD status of the donor was not statistically significant (Fig. 2b,d). Table 1 summarizes the statistical significance of the data from 10 donors, of which two representative donors are shown in Fig. 2. Compared to the random error there are statistically significant differences (P < 0·05) in T-cell proliferation depending on maturation stimulus and PPD status of the donor in the autologous setting whereas in the allogeneic setting the PPD status of the donor is not significant.
Table 1.
Statistical significance of enhanced T-cell proliferation with BCG-matured DC as shown in Fig. 2 was determined using the analysis of co-variance on spss for Windows
| Variable | P-value (autologous) | P-value (allogeneic) |
|---|---|---|
| PPD status | <0·001 | 0·739 |
| Donor | <0·001 | <0·001 |
| Maturation stimulus | <0·001 | <0·001 |
| PPD status and maturation | <0·001 | <0·001 |
| Log dilution | <0·001 | <0·001 |
| PPD status and dilution | <0·001 | 0·967 |
| Maturation and dilution | 0·168 | 0·556 |
| PPD * Maturation * dilution | <0·001 | 0·928 |
PPD, protein purified derivative. Calculated P-values are shown for the different variables and autologous or allogeneic co-cultures. A P-value < 0·05 was taken to be a statistically significant.
Both CD4 and CD8 T lymphocytes react to BCG-exposed DC by up-regulating CD25, CD54 and CD71: a comparison with TNF-α-exposed DC
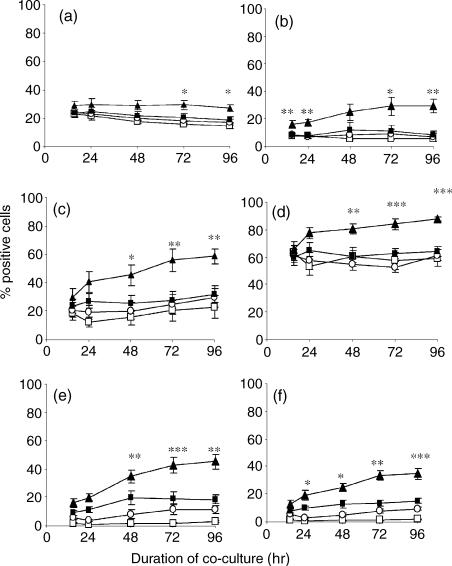
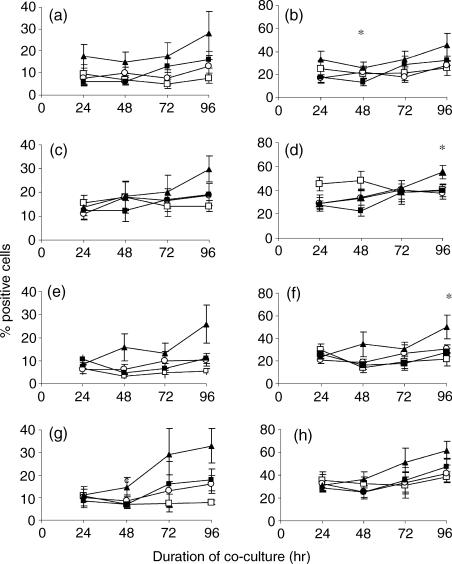
As BCG-matured DC enhance T-cell proliferation the activation status of these T cells was investigated. BCG-exposed DC significantly increased the levels of the IL-2 receptor α chain CD25 (P < 0·05), the adhesion receptor CD54 (P < 0·01) and the transferrin receptor CD71 (P < 0·01) on autologous CD4+ and CD8+ lymphocytes (Fig. 3) following 96 hr co-culture. This effect was significantly greater than that observed with TNF-α-exposed DC. Despite the marked change in the phenotype of DC following the addition of TNF-α, these DC were relatively poorly able to activate T cells. To determine the importance of mycobacterial recall responses in T-cell activation the data were evaluated separately on the six PPD-positive and four PPD-negative donors that were used to calculate the mean percentage of positive cells shown in Fig. 3. Statistical analysis showed that PPD-negative donors also showed significantly increased T-cell activation with BCG-exposed DC compared to TNF-α-exposed DC (Table 2). Statistical significance was often observed at other time-points if not at 96 hr (data not shown).
Figure 3.
Autologous peripheral blood lymphocytes were co-cultured with media (□), media-exposed DC (○), TNF-α-exposed DC (▪) or BCG-exposed DC (▴) at a ratio of 5 : 1 and the percentage of CD4+ (a,c,e) and CD8+ lymphocytes (b,d,f) expressing CD25 (a,b), CD54 (c,d), and CD71 (e,f) was determined by flow cytometry over a 96-hr period. The mean percentage of 10 donors is shown here. Any statistically significant increase of CD25, CD54, or CD71 on the surface of CD4 or CD8 T lymphocytes is indicated by *P < 0·05, **P < 0·01, ***P < 0·0001 (Student's t-test).
Table 2.
The paired Student's t-test assuming unequal variance was used to determine the statistical significance of enhanced T-cell activation as shown in Fig. 4
| T-cell population | P-value (overall) | P-value (PPD +ve) | P-value (PPD –ve) |
|---|---|---|---|
| CD4+ CD25+ | 0·05 | >0·05 | 0·05 |
| CD8+ CD25+ | 0·01 | >0·05 | 0·01 |
| CD4+ CD54+ | 0·01 | 0·05 | 0·05 |
| CD8+ CD54+ | 0·0001 | 0·01 | 0·05 |
| CD4+ CD71+ | 0·01 | 0·05 | >0·05 |
| CD8+ CD71+ | 0·001 | 0·05 | 0·01 |
BCG-exposed DC co-cultures were compared to TNF-α-exposed DC co-cultures at 96 hours. A P-value < 0·05 was taken to show a statistically significant increase in T-cell activation.
BCG-matured DC enhance production of cytokines by T cells
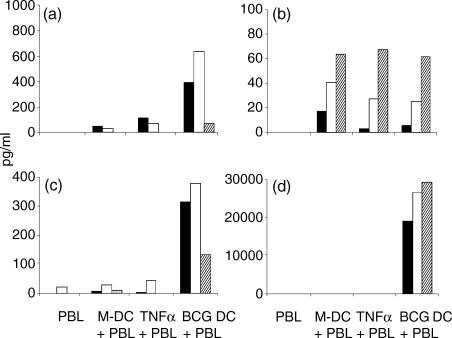
Upon activation T cells secrete a variety of cytokines including IL-2, IFN-γ, IL-4 and also IL-10. Analysis of T-cell culture supernatants after 96 hr showed that in cultures containing BCG-exposed DC there were significantly elevated levels of secreted IL-2 (6/10 donors), IL-10 (9/10 donors) and IFN-γ (10/10 donors) (Fig. 4). In contrast, co-culture of T cells with BCG-exposed DC did not increase IL-4 secretion and in some donors IL-4 levels were highest in cultures stimulated by media-exposed DC (3/10 donors). As the IL-4 receptor is up-regulated within 24 hr of activation measurement of cell-free IL-4 may not accurately reflect IL-4 levels.14 To examine further cytokine production in the co-culture system, intracellular cytokine determination was employed. Staining revealed that production of these cytokines was not maximal before 96 hr (Fig. 5). Production of IL-2, IL-4, IL-10 and IFN-γ was elevated in both CD4+ and CD8+ lymphocytes by 96 hr in BCG-exposed DC co-cultures. A statistically significant increase (P < 0·05) in CD8+ IL-2+, IL-4+ and IL-10+ cells was seen over TNF-α-exposed DC at 48, 96 and 96 hr, respectively. Some donor variability was seen but this did not bear any significant relation to their measured response to PPD and the mean of six donors is shown to illustrate the overall trend of cytokine production, which shows a mixed phenotype.
Figure 4.
Peripheral blood lymphocytes were co-cultured with autologous DC for 96 hr at a ratio of 5 : 1 and the supernatants were analysed for the presence of IL-2 (a), IL-4 (b), IL-10 (c) and IFN-γ (d) by solid-phase ELISAs. Three representative donors of 10 are shown (black, white and hatched bars). The lower levels of detection of the kits were 1·6, 1·6, 15·6 and 15·6 pg/ml for IL-2, IL-4, IL-10 and IFN-γ, respectively.
Figure 5.
Autologous peripheral blood lymphocytes were co-cultured with media (□), media-exposed DC (○), TNF-α-exposed DC (▪), or BCG-exposed DC (▴) for 24–96 hr. A panel of fluorescently conjugated antibodies was used to determine intracellular cytokine production on CD4+ (a,c,e,g) and CD8+ (b,d,f,h) lymphocytes and the mean percentage of cells producing IL-2 (a,b), IL-4 (c,d), IL-10 (e,f) and IFN-γ (g,h) from six donors is shown here. Any statistically significant increase of IL-2, IL-4, IL-10, or IFN-γ production in CD4 or CD8 T lymphocytes is indicated by *P < 0·05 (Student's t-test).
Decreased release of IFN-γ by autologous PBL co-cultured with BCG-exposed DC in the presence of B7 or IL-12-blocking antibodies, a process uncoupled from the level of their activation marker expression
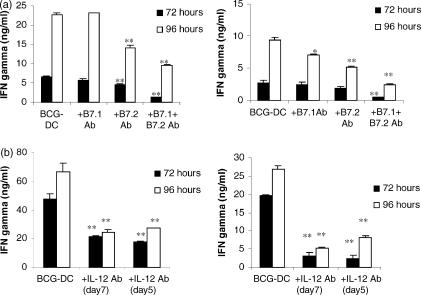
To investigate the mechanism of BCG-exposed DC being notably potent activators of autologous T cells, blocking antibodies were employed against B7.1, B7.2 and IL-12. When B7.1 was blocked during co-culture little effect on IFN-γ secretion was seen. Blockade of B7.2 led to decreased levels of IFN-γ after 72 and 96 hr of co-culture that was further enhanced by the inclusion of anti-B7.1 antibody (Fig. 6a).
Figure 6.
IFN-γ secretion as determined by solid-phase ELISA in co-culture supernatants. (a) Autologous peripheral blood lymphocytes were co-cultured with BCG-exposed DC at a ratio of 5 : 1 for 72 and 96 hr in the presence or absence of blocking antibodies to B7.1, B7.2, or B7.1 and B7.2 at concentrations of 5 μg/ml and 10 μg/ml, respectively. Two representative donors of three are shown. (b) DC were matured with BCG in the presence or absence of an anti-IL-12 antibody (20 μg/ml) and then co-cultured with autologous T cells in the presence or absence of an anti-IL-12 antibody. IFN-γ was measured in supernatants at 72 and 96 hr from co-cultures of BCG-exposed DC and T cells, co-cultures of BCG-exposed DC, T cells and an anti-IL-12 antibody and co-cultures of BCG-exposed DC that had been treated with an anti-IL-12 antibody, T cells and an anti-IL-12 antibody. Two representative donors are shown. Any statistically significant decrease in IFN-γ by inclusion of blocking antibodies compared to levels in unblocked BCG-exposed DC: T-cell co-cultures is indicated by *P < 0·05, **P < 0·01, ***P < 0·0001 (Student's t-test).
Neutralization of the biological effect of IL-12 by inclusion of an anti-IL-12 antibody also dramatically decreased IFN-γ levels (Fig. 6b). Little difference in reduction was seen between neutralization of IL-12 during co-culture alone and during co-culture and maturation of DC, suggesting that IL-12 is still being produced during co-culture and has a direct correlation with IFN-γ production rather than on the function of the DC.
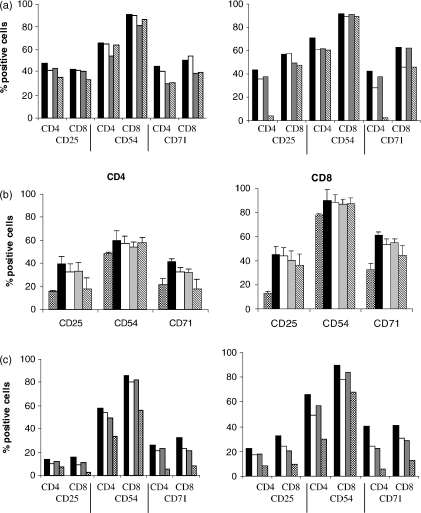
When the above co-cultures were examined for levels of CD25, CD54 and CD71 on CD4+ and CD8+ T cells only small decreases in expression of these markers was seen with B7 blocking antibodies (Fig. 7a). Expression of CD25 and CD71 on CD4+ T cells in the presence of blocking antibodies to B7.1 and B7.2 was reduced to levels comparable with those seen in TNF-α-exposed DC co-cultures (Fig. 7b). Treatment with IL-12 blocking antibody also partly reduced levels of CD25, CD54 and CD71 but not to levels seen with TNF-α-exposed DC co-cultures (Fig. 7c).
Figure 7.
T-cell activation was assessed after 96 hr of co-culture by flow cytometric analysis. Cells were stained for CD4, CD8 and CD25, CD54 or CD71. (a) Autologous peripheral blood lymphocytes were co-cultured with BCG-exposed DC at a ratio of 5 : 1 for 96 hr (black bars) and in the presence of blocking antibodies to B7.1 (white bars), B7.2 (grey bars) or B7.1 and B7.2 (hatched bars) at concentrations of 5 μg/ml and 10 μg/ml. Two representative donors of three are shown. (b). The mean percentage of CD4+ and CD8+ T cells that are positive for CD25, CD54 and CD71 from three donors is shown. Bars represent the same conditions as shown in (a) with the additional condition of TNF-α-exposed DC co-cultures (checked bars). (c) DC from a further two donors were matured with BCG in the presence or absence of an anti-IL-12 antibody (10 μg/ml) and then co-cultured with autologous T cells. Levels of CD25, CD54 and CD71 on CD4+ and CD8+ lymphocytes are shown for co-cultures of BCG-exposed DC and T cells (black bars), co-cultures of BCG-exposed DC, T cells and an anti-IL-12 antibody (white bars) and co-cultures of BCG-exposed DC that had been treated with an anti-IL-12 antibody, T cells and an anti-IL-12 antibody (grey bars). TNF-α-exposed DC co-cultures are shown by checked bars.
Discussion
The unique T-cell co-stimulatory capacity of DC is not shared by other APC, such as macrophages, and may reflect the ability of DC to sense microbial products via innate immune receptors. Clinical trials with DC-based vaccine strategies use a variety of different maturation stimuli. However, the effect of these on T lymphocytes has not been satisfactorily addressed. This study examines the differential responses of autologous T cells to cytokine-matured or bacterially matured human monocyte-derived DC.
In this study Mycobacterium bovis BCG-infected DC were found to be particularly potent activators of T cells, producing levels of activation far exceeding those obtained with conventionally TNF-α-exposed DC. Although there have been several reports on the consequences of BCG infection of DC its impact on T-cell activation and function has been little investigated.6–9 In agreement with the studies of Hope et al. in cattle we found that BCG-infected DC stimulated marked increases in the proliferation of autologous T cells.15 The above authors found that T cells from unvaccinated cattle also proliferated to BCG-exposed DC, although to a lesser extent than BCG-vaccinated cattle. This is similar to our findings in PPD responder and PPD non-responder humans. However, Kim et al. found that BCG-infected DC did not stimulate proliferation of autologous lymphocytes from BCG-vaccinated healthy donors. One possible explanation for this is their use of a 10-fold lower dose of BCG to infect DC than that employed in our study. The above results show that BCG-infected DC are able to stimulate naïve T cells to proliferate more strongly than TNF-α-exposed DC. This could be because of presentation of antigens from BCG onto MHC class I and MHC class II,16–18 or increased levels of co-stimulatory molecules or cytokine secretion.19 It is beyond the scope of this paper to address whether the T-cell activation and proliferation is a result of presentation of BCG antigens on MHC class I and II or to increased T-cell responses to self antigens that are presented on these more ‘mature’ DC. Increased T-cell responses in donors that have no recall response to PPD antigens suggests that it is a naïve T-cell response. Generation of a naïve T-cell response to antigens from BCG suggests that this may be of interest as a recombinant vaccine vehicle as well as a DC maturation agent.
The importance of CD4+ T cells in immunity to M. tuberculosis has been well documented,20 but it has only become apparent in more recent years that CD8+ T cells also play a role in tuberculosis infection with the study of Beta-2 Microglobulin (β2-M) knockout mice.21 In the absence of functional MHC class I molecules immune responses to pathogenic mycobacteria are severely attenuated or ablated with 70% of β2-M knockout mice dying within 6 weeks after challenge with M. tuberculosis compared with survival of the wild-type mice of > 20 weeks. Since then several groups have shown that activated cytolytic CD8+ T cells can be generated against BCG antigens by stimulating isolated PBMCs with live BCG.17,22 When we infected monocyte-derived DC with BCG we found that both CD8+ and CD4+ T cells displayed enhanced expression of activation markers compared to TNF-α-exposed DC. As both PPD responder and non-responder donors had increased T-cell activation it seems unlikely that this is purely a recall response to BCG. Freshly isolated peripheral blood DC, when pretreated for 48 hr with GM-CSF, efficiently stimulate an autologous mixed leucocyte reaction that is dependent on B7.1 and B7.2.23 In an attempt to determine the mechanism of this enhanced activation with BCG-infected DC blocking antibodies to B7.1 and B7.2 were employed during co-culture, however, no significant reduction in activation was seen. When we compared levels of IFN-γ in co-culture supernatants we found that blocking B7.2 or B7.2 and B7.1 significantly reduced levels of IFN-γ. This is similar to the findings of Lespagnard et al. where proliferation and IL-2 secretion in response to injection of keyhole limpet haemocyanin-pulsed DC into mice was inhibited by blocking the interaction of B7.1 and B7.2 with their cognate ligands.24 As BCG-exposed DC secrete IL-12 p70 whereas TNF-α-exposed DC do not we investigated the effect of neutralizing IL-12 during DC maturation and during co-culture, however, no effect on T-cell activation marker expression was seen.10
BCG-exposed DC prime T cells to secrete significantly larger quantities of IFN-γ, IL-2 and IL-10 than TNF-α-exposed DC. Intracellular cytokine production confirms the existence of a mixed phenotype, however, surprisingly the kinetics of cytokine production were similar for all cytokines. The production of IL-12 by the DC may be responsible for polarizing a type 1 response, typical of mycobacterial infections. When we neutralized IL-12 in our DC–T-cell co-cultures we found that levels of IFN-γ, a classical Th1 cytokine produced in response to mycobacterial infection, were significantly reduced. Neutralization of IL-12 during DC maturation had no additional effect on IFN-γ secretion. Although the effects on DC maturation were not studied it can be concluded that either no effect on DC phenotype would have been observed or that DC phenotype is not responsible for enhanced IFN-γ secretion in BCG-DC–T-cell co-cultures. This is supported by the findings of Giacomini et al. who showed that IFN-γ secretion from T cells cultured with supernatants from DC infected with M. tuberculosis could be inhibited by inclusion of a neutralizing antibody to IL-12.25 It has been shown that Th1 polarization by IL-12-producing DC is only transient with a Th2 or Th0 response being primed at later time-points,26 which may explain the high levels of IL-10 in culture supernatants as we only examined one time-point. Levels of the cytokines measured were greatest after 96 hr suggesting that if there is some down-regulation of a Th1 response and up-regulation of a Th2/Th0 response it is not complete by 96 hr of co-culture. The co-stimulatory molecules are also influential for T-cell polarization with B7.1 controlling Th1 development and B7.2 a Th2 response.27,28 As both B7.1 and B7.2 are up-regulated on BCG-exposed DC this may explain how both type 1 and type 2 cytokines are produced by T cells co-cultured with BCG-exposed DC. Blocking B7.2 led to reductions in IFN-γ, a Th1 cytokine, whereas blockade of B7.1 did not. However, blockade of both B7.2 and B7.1 further reduced levels of IFN-γ, suggesting that the roles that B7.1 and B7.2 play in Th1/Th2 balance are complex. The data presented suggest the existence of multiple mechanisms for the activation of human T cells by BCG and that the involvement of different APCs may influence which mechanisms are involved. Attempts to block these processes may have a limited effect with only certain aspects of T-cell responses being affected. It has recently been shown that bacterially infected murine DC produce IL-2 in response to bacterial infection.29 IL-2 production is specific to DC and is not produced by bacterially infected macrophages, for example. Secretion of IL-2 is transient and occurs at early time-points of infection (4–8 hr and 10 hr later). As IL-2 is responsible for up-regulating the IL-2R α-subunit it may be that BCG-infected DC are able to up-regulate activation markers such as CD25 by IL-2 secretion above TNF-α-exposed DC which do not secrete IL-2. However, it must be remembered that in our system DC were matured with BCG for 48 hr and then washed before co-culture with T cells. This does not fit in with the described kinetics of IL-2 production by DC observed by Granucci et al.29
Infection of human monocyte-derived DC with BCG leads to a much more potent autologous T-cell response than using immature DC or TNF-α-exposed DC. This has implications both for the use of BCG as a recombinant antigen-specific tumour vaccine and in the use of ex vivo melanoma antigen peptide pulsed DC as tumour therapies. Peptide-pulsed DC or TNF-α-exposed DC are currently in clinical trials in patients with advanced melanoma. The use of peptide-pulsed DC led to tumour regression or stabilization in up to 20% of patients and the use of TNF-α-matured DC led to similar results in other Phase I trials.30,31 In this study CD34+ progenitor-derived DC were pulsed with gp100 and tyrosinase. A small-scale study with HER-2/neu or MUC-a pulsed TNF-α-matured monocyte-derived DC in patients with breast and ovarian cancer showed specific cytotoxic T lymphocytes in five of 10 patients.32 In summary, the data presented suggest that BCG-exposed DC are superior at activating autologous T cells than conventionally matured TNF-α-treated DC. From the results presented here it is possible that the use of an immunostimulatory adjuvant such as BCG to mature DC would result in enhanced co-stimulation and activation of patient tumour-specific T-cell responses.
Acknowledgments
This work was funded by Cancer Research UK (registered charity number 1089464) and the University of Leeds. The authors wish to thank Dr Dearbhaile O'Donnell for critical comments during the preparation of this manuscript, Dr S. Young and J. Porte for technical assistance and support and Dr Colin Johnston for help with statistical analysis.
Abbreviations
- BCG
bacillus Calmette–Guérin
- DC
dendritic cell
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IFN-γ
interferon-γ
- IL
interleukin
- PE
phycoerythrin
- PPD
purified protein derivative
- TNF-α
tumour necrosis factor-α
References
- 1.Huebner RE. BCG vaccination in the control of tuberculosis. Curr Top Microbiol Immunol. 1996;215:263–82. doi: 10.1007/978-3-642-80166-2_12. [DOI] [PubMed] [Google Scholar]
- 2.Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–94. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell co-stimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 5.Jiao X, Lo-Man R, Guermonprez P, et al. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J Immunol. 2002;168:1294–301. doi: 10.4049/jimmunol.168.3.1294. [DOI] [PubMed] [Google Scholar]
- 6.Thurnher M, Ramoner R, Gastl G, Radmayr C, Bock G, Herold M, Klocker H, Bartsch G. Bacillus Calmette-Guerin mycobacteria stimulate human blood dendritic cells. Int J Cancer. 1997;70:128–34. doi: 10.1002/(sici)1097-0215(19970106)70:1<128::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Kim KD, Lee HG, Kim JK, et al. Enhanced antigen-presenting activity and tumour necrosis factor-alpha-independent activation of dendritic cells following treatment with Mycobacterium bovis Bacillus Calmette-Guerin. Immunology. 1999;97:626–33. doi: 10.1046/j.1365-2567.1999.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demangel C, Bean AG, Martin E, Feng CG, Kamath AT, Britton WJ. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis Bacillus Calmette Guerin-infected dendritic cells. Eur J Immunol. 1999;29:1972–9. doi: 10.1002/(SICI)1521-4141(199906)29:06<1972::AID-IMMU1972>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Henderson RA, Watkins SC, Flynn JL. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–43. [PubMed] [Google Scholar]
- 10.Smith SG, Patel PM, Selby PJ, Jackson AM. The response of human dendritic cells to recombinant adenovirus, recombinant Mycobacterium bovis Bacillus Calmette Guerin and biolistic methods of antigen delivery: different induction of contact-dependant and soluble signals. Immunol Lett. 2001;76:79–88. doi: 10.1016/s0165-2478(00)00324-2. [DOI] [PubMed] [Google Scholar]
- 11.Ramoner R, Rieseries C, Herold M, Klocker H, Bartsch G, Stenzl A, Thurnher M. Activation of human dendritic cells by Bacillus Calmette-Guerin. J Urol. 1998;159:1488–92. doi: 10.1097/00005392-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Aldovini A, Young RA. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature. 1991;351:479–82. doi: 10.1038/351479a0. [DOI] [PubMed] [Google Scholar]
- 13.Stover CK, De La Cruz V, Fuerst TR, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–60. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 14.Bullens DM, Kasran A, Peng X, Lorre K, Ceuppens JL. Effects of anti-IL-4 receptor monoclonal antibody on in vitro T cell cytokine levels: IL-4 production by T cells from non-atopic donors. Clin Exp Immunol. 1998;113:320–6. doi: 10.1046/j.1365-2249.1998.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hope JC, Kwong LS, Sopp P, Collins RA, Howard CJ. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in Bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand J Immunol. 2000;52:285–91. doi: 10.1046/j.1365-3083.2000.00780.x. [DOI] [PubMed] [Google Scholar]
- 16.Feng CG, Demangel C, Kamath AT, Macdonald M, Britton WJ. Dendritic cells infected with Mycobacterium bovis Bacillus Calmette Guerin activate CD8 (+) T cells with specificity for a novel mycobacterial epitope. Int Immunol. 2001;13:451–8. doi: 10.1093/intimm/13.4.451. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM, Malin AS, Pauline T, Lukey Atkinson SE, Content J, Huygen K, Dockrell HM. Characterization of human Mycobacterium bovis Bacille Calmette-Guerin-reactive CD8+ T cells. Infect Immun. 1999;67:5223–30. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mustafa AS, Shaban FA, Abal AT, Al Attiyah R, Wiker HG, Lundin KE, Oftung F, Huygen K. Identification and HLA restriction of naturally derived Th1-cell epitopes from the secreted Mycobacterium tuberculosis antigen 85B recognized by antigen-specific human CD4 (+) T-cell lines. Infect Immun. 2000;68:3933–40. doi: 10.1128/iai.68.7.3933-3940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shortt J, Hart DN, Watson JD, Baird MA. Blockade of B7-2, not B7-1, inhibits purified protein derivative-primed T-lymphocyte responses but fails to influence the proportion of Th1 versus Th2 subsets. Scand J Immunol. 1998;47:355–62. doi: 10.1046/j.1365-3083.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- 20.Raupach B, Kaufmann SH. Immune responses to intracellular bacteria. Curr Opin Immunol. 2001;13:417–28. doi: 10.1016/s0952-7915(00)00236-3. [DOI] [PubMed] [Google Scholar]
- 21.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–17. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner J, Dockrell HM. Stimulation of human peripheral blood mononuclear cells with live Mycobacterium bovis BCG activates cytolytic CD8+ T cells in vitro. Immunology. 1996;87:339–42. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheinecker C, Machold KP, Majdic O, Hocker P, Knapp W, Smolen JS. Initiation of the autologous mixed lymphocyte reaction requires the expression of costimulatory molecules B7-1 and B7-2 on human peripheral blood dendritic cells. J Immunol. 1998;161:3966–73. [PubMed] [Google Scholar]
- 24.Lespagnard L, Mettens P, De Smedt T, Bazin H, Urbain J, Leo O, Moser M. The immune response induced in vivo by dendritic cells is dependent on B7-1 or B7-2, but the inhibition of both signals does not lead to tolerance. Int Immunol. 1998;10:295–304. doi: 10.1093/intimm/10.3.295. [DOI] [PubMed] [Google Scholar]
- 25.Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L, Orefici G, Julkunen I, Coccia EM. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001;166:7033–41. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]
- 26.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–16. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 27.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 28.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 29.Granucci F, Vizzardelli C, Pavelka N, et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–888. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- 30.Lau R, Wang F, Jeffery G, Marty V, Kuniyoshi J, Bade E, Ryback ME, Weber J. Phase I trial of intravenous peptide-pulsed dendritic cells in patients with metastatic melanoma. J Immunother. 2001;24:66–78. doi: 10.1097/00002371-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Mackensen A, Herbst B, Chen JL, et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34 (+) hematopoietic progenitor cells. Int J Cancer. 2000;86:385–92. doi: 10.1002/(sici)1097-0215(20000501)86:3<385::aid-ijc13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed denritic cells. Blood. 2000;96:3102–8. [PubMed] [Google Scholar]