Abstract
MUC1 is a transmembrane mucin that is expressed on ductal epithelial cells and epithelial malignancies and has been proposed as a target antigen for immunotherapy. The expression of MUC1 has recently been reported on T and B cells. In this study we demonstrate that following activation in vivo or activation by different stimuli in vitro, human T cells expressed MUC1 at the cell surface. However, the level of expression in activated human T cells was significantly lower than that seen on normal epithelial cells or on breast cancer cells. In contrast, resting T cells did not bind MUC1-specific monoclonal antibodies (mAbs), nor was MUC1 mRNA detectable by reverse transcription–polymerase chain reaction (RT–PCR) or Northern blot analysis in these cells. The profile of activated T-cell reactivity with different MUC1-specific antibodies suggested that the glycoform of MUC1 expressed by the activated T cells carried core 2-based O-glycans, as opposed to the core 1 structures that dominate in the cancer-associated mucin. Confocal microscopy revealed that MUC1 was uniformly distributed on the surface of activated T cells. However, when the cells were polarized in response to a migratory chemokine, MUC1 was found on the leading edge rather than on the uropod, where other large mucin-like molecules on T cells are trafficked. The concentration of MUC1 at the leading edge of polarized activated human T cells suggests that MUC1 could be involved in early interactions between T cells and endothelial cells at inflammatory sites.
Introduction
The human epithelial mucin, MUC1, is a heavily O-glycosylated type I transmembrane glycoprotein expressed at the luminal surface of most glandular epithelial tissues. Expression of MUC1 is increased in many epithelial malignancies, notably breast, pancreatic and ovarian cancers, as well as in a proportion of colonic and lung cancers (reviewed in ref. 1). The extracellular domain of MUC1 consists largely of tandemly repeated sequences of 20 amino acids with ≈ 100 amino acids 5′ to this region and 180 amino acids 3′, followed by a transmembrane domain and a cytoplasmic tail.2 The number of tandem repeats (TR) in the MUC1 allele can vary between 25 and 100. Each of the TR contains five potential O-glycosylation sites, and the glycoforms produced by cancer cells can differ from those expressed by normal tissues.3
There have been reports of humoral and cellular immune responses to MUC1 in multiparous women and in patients with cancer.4–9 These data, together with the high level of expression in tumour cells, have led to a focus on MUC1 as a potential target for tumour immunotherapy. Several MUC1-derived cytotoxic T lymphocyte (CTL) epitopes have been identified10–13 and immunization with vectors expressing the full-length molecule or with peptides, have shown protection against MUC1-expressing tumours in mouse models.13,14 However, in transgenic mouse models, where MUC1 is expressed as a self-antigen, it is more difficult to demonstrate immune responses against MUC1,15–17 suggesting a degree of immunological tolerance. The degree of tolerance to a self-antigen is expected to be dependent on the level and location of expression of the self-antigen. Although the expression of MUC1 was originally thought to be restricted to epithelial tissues, recent work has suggested that MUC1 is also expressed by T and B cells.18–20 The expression of MUC1 on such cells has implications for both immune tolerance and autoimmunity. We therefore sought to investigate in detail the expression of MUC1 in human T cells, documenting both the level and duration of expression, the distribution of MUC1 on the T-cell surface and the specific form of the glycoprotein expressed.
Our data demonstrate that MUC1 is expressed by T cells activated both in vivo and in vitro, but that the level of expression is low. MUC1 on chronically stimulated T cells is co-expressed with the memory phenotype marker, CD45RO. The profile of reactivity of the T-cell glycoprotein with MUC1-specific antibodies indicates that the O-glycans added to the core protein are extended core 2-based structures, as seen in many normal tissues, rather than the truncated, predominantly core 1-based structures added to mucin produced by tumour cells. Moreover, confocal microscopy revealed that MUC1 expression on T cells is dispersed over the entire cell surface until polarization, at which point MUC1 becomes confined to the leading edge of the T cell.
Materials and methods
Cells and tissues
All samples were obtained after acquiring informed consent from the study participants and according to Ethics Committee Guidelines. Peripheral blood was obtained from healthy volunteers, patients with breast cancer and a patient with rheumatoid arthritis, or as a leukapheresis research product from the Central Blood Bank (Pittsburgh, PA). Peripheral blood mononuclear cells (PBMC) were isolated from whole blood by Ficoll–Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) density-gradient centrifugation. The synovial fluid was obtained by sterile needle aspiration from the acutely inflamed knee joint of a patient with active rheumatoid arthritis. The human breast cancer tissue was obtained from a patient at the time of primary surgery.
Antibodies
The monoclonal antibody (mAb) mouse anti-human CD3 (UCHT1), and mouse anti-human MUC1 mAbs HMFG1, HMFG2 and SM3 were obtained from the Cancer Research UK Hybridoma and Monoclonal Antibody Facility. Other MUC1-specific mAbs were: 232A1 (a gift from Dr J. Hilkens, the Netherlands Cancer Institute, Amsterdam, the Netherlands); mAb 12C10 (obtained from Dr R. B. Acres, Transgene, Strasbourg, France); and mAbs HMPV21 and MF0622 (obtained from ISOBM TD4 International Workshop on Monoclonal Antibodies against MUC1). In some experiments, biotinylated HMFG1 and 12C10 were used. Phycoerythrin (PE)-labelled mouse anti-human CD69, fluorescein isothiocyanate (FITC)-labelled mouse anti-human CD25, unlabelled isotype-control mouse immunoglobulin G1 (IgG1), PE-labelled anti-human CD45RO and PE-labelled isotype-control mouse immunoglobulin G2a (IgG2a) antibodies were purchased from Becton-Dickinson Pharmingen (San Jose, CA). Polyclonal rabbit anti-chicken spectrin antibody was a gift from Dr Elizabeth Repasky (Roswell Park Cancer Institute, Buffalo, NY). Alexa secondary antibodies and rhodamine phalloidin were purchased from Molecular Probes (Eugene, OR).
Activation of human T cells in vitro
PBMC were cultured (1 × 106 cells/well in a 24-well plate) in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 2 mm l-glutamine, 50 µm β-mercaptoethanol (RPMI−10% FCS) and stimulated with phytohaemagglutinin (PHA; Abbot-Murex, Dartford, UK) at 1 µg/ml or with immobilized anti-CD3 mAb. Plates were incubated with 0·5 ml of purified anti-CD3 mAb [10 µg/ml in phosphate-buffered saline (PBS)] for 2 hr at 37°. The plates were then washed three times with PBS and blocked with RPMI−10% FCS before use. Alternatively, the PBMC in Fig. 4 were incubated with AIM-V medium (Gibco, Carlsbad, CA), supplemented with 10% human serum (Cellgro, Herndon, VA) containing 1% l-glutamine, in the presence of PHA (1 µg/ml; Sigma, St. Louis, MO) and 20 U/ml interleukin-2 (IL-2) (Dupont, Wilmington, DE). For antigen-specific activation in vitro, PBMC in RPMI−10% FCS were stimulated in a mixed lymphocyte reaction (MLR). Responder PBMC (1·5 × 106 cells/well of a 24-well plate) were co-cultured with irradiated (2000 rads) allogeneic stimulator cells (responder : stimulator ratio of 1 : 1) for at least 6 days. Cells from the MLR were purified by Ficoll–Paque centrifugation before staining. For chronic stimulation of T cells, PBMC were stimulated with irradiated allogeneic PBMC in AIM-V human serum medium containing 20 U/ml of IL-2 (Dupont). Three to five days after stimulation, half of the medium from each well was replaced with new medium containing fresh IL-2. The stimulation was repeated every 7 days.
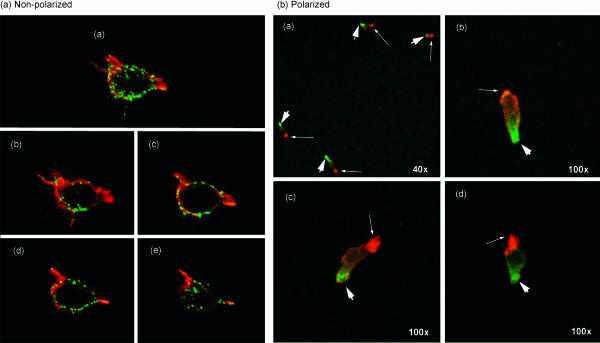
Figure 4.
Analysis by confocal microscopy of MUC1 on activated T cells. (a) Activated T cells were stained for MUC1 (green) and then counterstained for actin (red). The image shown in (a) represents a projection of eight images acquired as 0·5-µm-thick scanned sections, four of which are shown in b–e. Magnification is 100×. (b) Activated T cells adherent to fibronectin-treated slides were treated with regulated on activation, normal, T-cell expressed, and secreted (RANTES) chemokine prior to staining for MUC1 (green; thick arrows). Cells were then permeabilized and stained for spectrin (red; thin arrowheads), a marker for uropods. The magnification of image a is 40×. The magnification of images b–d is 100×. These confocal microscopy images are projections of 16 stacked sections through the cells.
Flow cytometric analysis
Cells were stained with mAb HMFG1, HMFG2, SM3, 12C10 or 232A1, followed by FITC-conjugated rabbit anti-mouse immunoglobulins (Dako, High Wycombe, UK). When biotinylated antibodies were used, binding was detected with streptavidin-PE (Southern Biotechnologies, Birmingham, AL). Cells were also stained with directly conjugated antibodies to CD3, CD25 and CD69. For staining of chronically stimulated T cells (Fig. 3b) cells were stained with mouse anti-human MUC1 mAb MF06 or isotype-control mouse IgG1 antibody, followed by a secondary antibody – goat anti-mouse Alexa488. Cells were then fixed for 10 min at room temperature in 1% paraformaldehyde and empty binding sites of the goat anti-mouse secondary antibody were blocked with unlabelled mouse IgG1. Cells were finally stained with PE-conjugated isotype control or PE-conjugated anti-CD45RO mAb. Samples were analysed using an XL Flow Cytometer (Beckman-Coulter, High Wycombe, UK) and WinMDA software (Scripps Research Institute, La Jolla, CA) or a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA) and FlowJo 3·2 software (Tree Star, Inc., San Carlos, CA). Dead cells were excluded on the basis of forward and side light scatter.
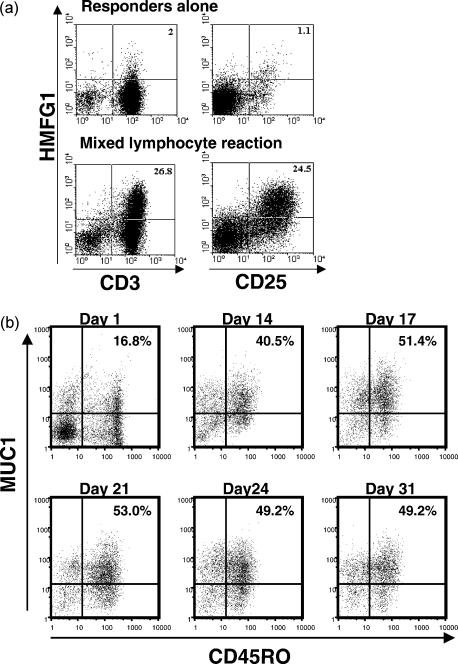
Figure 3.
Expression of MUC1 in activated T cells from a mixed lymphocyte reaction. (a) Responder peripheral blood mononuclear cells (PBMC), incubated for 6 days in the presence or absence of irradiated allogeneic stimulator cells, were stained with HMFG1–biotin, in association with CD3–fluorescein isothiocyanate (FITC) or CD25–FITC and analysed by flow cytometry. Numbers indicate the percentage of gated cells in the quadrant. (b) Cells activated with allogeneic PBMC every 7 days were stained for CD45RO and MUC1 (MF06 monoclonal antibody) and analysed by flow cytometry.
Confocal immunofluorescence microscopy
PHA-activated T cells were separated from dead cells by Ficoll–Paque gradient centrifugation and cultured overnight on fibronectin (Sigma)-coated four-well chamber slides (Nalge Nunc, Naperville, IL). To induce polarization we added regulated on activation, normal, T-cell expressed, and secreted (RANTES) chemokine (10 ng/ml; Sigma) to the cells 30 min prior to staining. Following chemokine treatment, the cells were fixed in 2% paraformaldehyde for 10 min at room temperature and then washed extensively in PBS containing 10% FCS. Indirect surface staining for MUC1 was performed using the mouse anti-human MUC1 mAb, HMPV, and Alexa488-labelled goat anti-mouse as a secondary antibody. Following the MUC1 staining, cells were fixed again and then permeabilized with 0·2% Triton-X-100 for 10 min at room temperature. Intracellular staining was performed with rhodamine to stain the actin filaments, or polyclonal rabbit anti-chicken spectrin, followed by a red fluorescent Alexa546 goat anti-rabbit secondary antibody. Following staining, cells were immediately analysed by confocal laser microscopy at the University of Pittsburgh Center for Biological Imaging Facility, using a Leica TCS NT confocal LSM microscope (Rockleigh, NJ). Images were collected as serial sections using (unless otherwise indicated) the ×100 objective. Images are shown as either individual sections or as projections of stacked images.
Reverse transcription–polymerase chain reaction
Total RNA was prepared using Trizol reagent (Gibco BRL) according to the manufacturer's instructions. cDNA was generated from total RNA using the reverse transcription–polymerase chain reaction (RT–PCR) kit (Stratagene, La Jolla, CA), according to the manufacturer's instructions (in all experiments 10 µg of total RNA was used to generate cDNA). The cDNA was subsequently amplified using MUC1-specific primers 5′-GCCAGCCATAGCACCAAGACTG-3′ and 5′-AGCCCCAGACTGGGCAGAGAA-3′. These primers correspond to a sequence 3′ of the TR encoded by exons 2 and 5 and would result in the amplification of a 446-bp fragment from RNA and a 838-bp fragment from genomic DNA. For the semiquantitative RT–PCR, cDNA was diluted, as indicated, before the PCR amplification and the same primer set was used. In other experiments, the following primer sets were also used: 5′-TCTCAAGCAGCCAGCGCCTGCCTG-3′ and 5′-TCCCCAGGTGGCAGCTGAACC-3′ to yield a 331-bp product, and 5′-GCCAGCCATAGCACCAAGACTG-3′ with 5′-TGAAGAACCTGAGTGGAGTGG-3′ to yield an 816-bp product.
Northern blotting
Total RNA was extracted from cells using Trizol reagent (Gibco), according to the manufacturer's instructions. RNA (10 µg/lane) was run on a 1% agarose, 2·2 m formaldehyde gel and then transferred onto optimized nylon membrane and fixed. The membrane was prehybridized for 1 hr at 65° in hybridization buffer [1% bovine serum albumin (BSA), 0·25 m sodium dodecyl sulphate (SDS), 0·25 m disodium hydrogen orthophosphate, 0·25 m sodium dihydrogen orthophosphate-1-hydrate]. Probes (20 ng) were labelled using random primers and the MegaPrime kit (Amersham Pharmacia Biotech), following the manufacturer's instructions. Probes were hybridized to the blot overnight at 65°. Membranes were washed for 1 hr in buffer A (0·08 m sodium phosphate, 2 mm EDTA, 5% BSA, 10% SDS) at 65°, and then twice in buffer B (0·16 m sodium phosphate, 4 mm EDTA, 4% SDS) for 1 hr at 65° and then exposed to a phosphor screen (Amersham Pharmacia Biotech). Screens were exposed overnight at room temperature and visualized using a TYPHOON 8600 scanner system (Molecular Dynamics, Little Chalfont, UK). Blots were stripped in a solution of 0·06 × saline sodium citrate (SSC), 10 mm EDTA and 0·1% SDS at 100° for 10 min and then reprobed, as described above.
To verify the expression of MUC1 mRNA in normal human tissues, a multiple tissue-expression array (Clonetech, Basingstoke, UK) containing RNA from normal human tissues was hybridized with the MUC1 probe, as described above.
Probes
The following probes were used: MUC1, seven tandem repeats; C2GnT1, 950-bp PstI fragment; C2GnT2, 1234-bp EcoRI fragment; C2GnT3, 1361-bp EcoRI–BamHI fragment; 18S from Ambion (Austin, TX).
Results
Expression of MUC1 on T cells activated in vivo
MUC1 expression on human T cells was investigated using MUC1-specific mAbs and flow cytometry. The mAbs used were HMFG1, HMFG2, SM3, HMPV and MF06, all of which react with repetitive epitopes in the TR domain.22 mAbs 232A1 and 12C10, which bind to epitopes outside the TR region and therefore should react with all glycoforms, were also used.
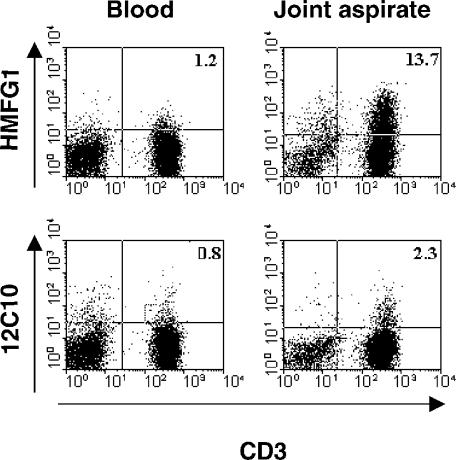
Previously published work on the expression of MUC1 on T cells has employed in vitro methods of activation. We sought to determine if this observation was true also for T cells activated in vivo. To examine this issue, an aspirate from an acutely inflamed joint of a patient with rheumatoid arthritis was obtained, and MUC1 expression was ascertained by HMFG1 binding and flow cytometric analysis. As shown in Fig. 1, more than 10% of the T cells from the aspirate were HMFG1 positive, while no staining was observed on T cells from the patient's blood. Similar results were obtained with a joint aspirate from a patient with osteoarthritis. This indicates that T cells taken from a site of an active immune response, i.e. activated in vivo, also express MUC1.
Figure 1.
Expression of MUC1 in T cells activated in vivo. Peripheral blood mononuclear cells (PBMC) from blood or a joint aspirate of a patient with rheumatoid arthritis were stained with the indicated antibodies and analysed by flow cytometry. Numbers indicate the percentage of gated cells in the quadrant.
Reactivity of MUC1-specific antibodies with human T cells activated in vitro
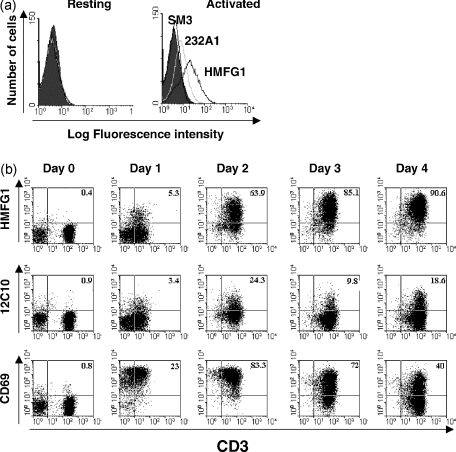
Flow cytometric analysis of human T cells with multiple anti-MUC1 mAbs showed that none bound to resting T cells and only mAbs HMFG1, HMPV and MF06 reproducibly bound to activated T cells (Fig. 2a and data not shown). Staining of activated T cells with mAb 232A1 was detectable, but the fraction of cells recognized by this antibody was low (Fig. 2a). To increase the intensity of signal, 12C10 and HMFG1 mAbs were biotinylated and streptavidin-PE was used for detection. Staining of T cells with HMFG1 was demonstrable 24 hr after in vitro activation with immobilized anti-CD3 mAb, and after 4 days > 90% of cells were stained by the antibody (Fig. 2b). Staining with biotinylated 12C10 mAb (also an IgG1, like HMFG1) was also seen, but at a much lower level than that for HMFG1 (Fig. 2b). Staining for the activation marker, CD69, indicated that MUC1 expression was a later event than CD69 expression during T-cell activation (Fig. 2b). Expression of MUC1 on activated T cells was similar on both CD4+ and CD8+ T cells (data not shown).
Figure 2.
Expression of MUC1 in activated T cells. (a) Resting and phytohaemagglutinin (PHA)-activated T cells were stained using different MUC1-specific monoclonal antibodies (mAbs) followed by fluorescein isothiocyanate (FITC)-labelled rabbit anti-mouse immunoglobulin. (b) Unstimulated peripheral blood mononuclear cells (PBMC) (day 0) or PBMC stimulated with anti-CD3 mAb for the indicated periods of time were stained with anti-CD3–FITC, in combination with anti-CD69–PE, HMFG1–biotin or 12C10–biotin, and analysed by flow cytometry. Numbers indicate the percentage of gated cells in the quadrant.
The above experiments utilized potent polyclonal stimuli that might not be considered physiological, and in further studies we examined MUC1 expression by T cells stimulated by alloantigens in an MLR. After 6 days in culture with allogeneic stimulator cells, ≈ 25% of responding T cells stained with HMFG1 mAb (Fig. 3a). The pattern of HMFG1 staining mirrored the expression of the activation marker CD25. There was no significant staining of the non-stimulated T cells from the same donor. Cells repeatedly stimulated every 7 days over a 1-month period demonstrated persistent MUC1 expression. During this chronic stimulation, T cells acquired the memory phenotype, gaining expression of CD45RO, which was co-expressed with MUC1 on the majority of T cells (Fig. 3b).
Differential distribution of MUC1 on the surface of activated and polarized T cells
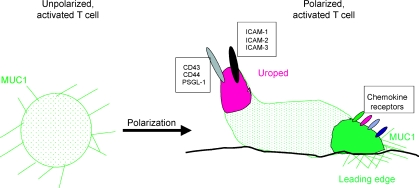
The distribution of MUC1 on the surface of T cells was studied by confocal microscopy using the MUC1-specific mAb, HMPV. Activated T cells displayed MUC1 evenly over the entire surface (Fig. 4a). When these cells were then exposed to the chemokine RANTES, they responded predictably by changing morphologically and assuming a polarized shape needed for migration, with a leading edge and a trailing edge (uropod). In these polarized cells, MUC1 was immediately sequestered to one of the poles (Fig. 4b). By staining for spectrin (in red), which is a known marker of the T-cell uropod, and for MUC1 (in green), we were able to determine that MUC1 is concentrated opposite the uropod and on the leading edge of the T cell (Fig. 4b).
Expression of MUC1 mRNA in activated T cells
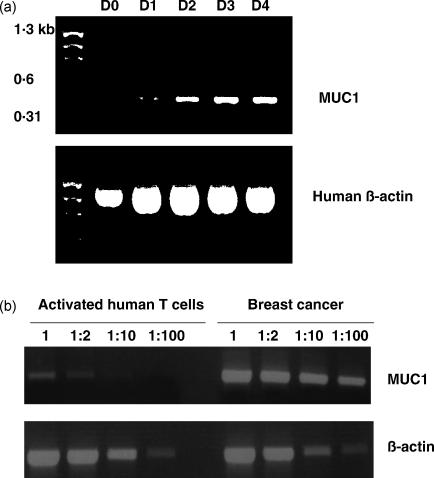
The results from the antibody staining suggested that MUC1 was expressed in activated, and not resting, human T cells. We confirmed this observation at the level of MUC1 RNA by RT–PCR from T cells at different time-points after activation. Figure 5a shows the presence of the predicted 446-bp RT–PCR fragment using primers 3′ to the TR domain of MUC1 on T cells activated with anti-CD3 antibody. No RNA was detectable on day 0, but the level of expression appears to increase with time after activation (Fig. 5a). Extraction of the bands and DNA sequencing confirmed these to be the expected fragment of MUC1 mRNA. Similar results were obtained using primers yielding a 331-bp fragment from the region 5′ to the TR domain (data not shown). Sequencing of this fragment also showed it to correspond to the expected MUC1 nucleotide sequence. Using semiquantitative RT–PCR, a comparison was made of levels of MUC1 mRNA in activated T cells and breast cancer cells from the same individual. Figure 5b shows that using cDNA from the breast cancer cells, strong bands were produced up to dilutions of 1 : 100, whereas a weak band was obtained from equivalent amounts of undiluted cDNA from activated T cells, which was lost rapidly on dilution. The data suggest that the level of expression of the glycoprotein in activated T cells was at least 50 times lower than in the breast cancer cells.
Figure 5.
Detection by reverse transcription–polymerase chain reaction (RT–PCR) of MUC1 transcript in activated T cells. (a) Human T cells were activated in vitro by anti-CD3 antibody. Total RNA was extracted and RT–PCR performed on the indicated days (D0, day 0; D1, day 1; D2, day 2; D3, day 3; D4, day 4). MUC1 transcript (446 bp) was identified after at least 24 hr. Human β-actin was included as a positive control, and a molecular weight marker is present in lane 1. (b) Semiquantitative RT–PCR for MUC1 in activated T cells and autologous breast cancer. Total RNA was extracted from autologous breast cancer cells and from purified human T cells following 4 days of in vitro activation using anti-CD3 antibody. cDNA was synthesized and then used as the template for RT–PCR at the dilutions shown. Human β-actin was included as a positive control.
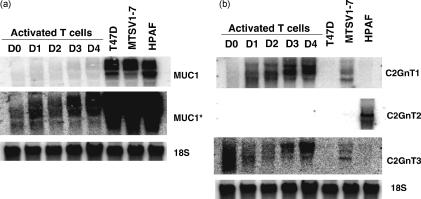
Northern blot analysis of MUC1 RNA expression after stimulation of T cells with PHA or CD3 antibody also showed expression, but at very low levels, beginning to appear after 1 day. Figure 6a shows that with a level of sensitivity sufficient to detect a strong signal for MUC1 RNA expressed by a breast cancer cell line, T47D, no transcript was detected in activated T cells. A much higher level of sensitivity was necessary to detect MUC1 RNA in the activated T cells. In the Northern blots the probe used was from the TR domain, and the size of the transcripts was as expected for full-length MUC1. We conclude that activation of T cells is accompanied by low-level expression of the full-length MUC1 RNA.
Figure 6.
Northern blot analysis of resting and activated human T cells. (a) Resting T cells (D0) or T cells activated by anti-CD3 antibody for the indicated periods of time (D0, day 0; D1, day 1; D2, day 2; D3, day 3; D4, day 4) were analysed by Northern blot analysis for expression of MUC1. MUC1* represents the same blot with increased sensitivity. (b) The O-glycosylation enzymes C2GnT1, C2GnT2 and C2GnT3 from resting T cells (D0) or T cells activated by anti-CD3 antibody for the indicated time-periods were analysed for expression by Northern blot analysis. The cell lines T47D, MTSV1-7 and HPAF were included as positive controls.
Expression of glycosyl transferases synthesizing core 2 structures in activated T cells
The lack of reactivity of the SM3 and HMFG2 antibodies with MUC1 expressed on activated T cells suggested that the O-glycans on the MUC1 expressed by these cells were core 2 based.23,24 Indeed, the first cDNA coding for a core 2-synthesizing enzyme, β6GlcNAc-transferase 1 (C2GnT1), was isolated by expression cloning from activated T cells.25 Two further β6GlcNAc transferase enzymes, C2GnT2 and C2GnT3, have now been isolated,26,27 and we examined the expression of transcripts coding for each of the three enzymes by Northern blot analysis in resting and in activated T cells (Fig. 6b). An increase in the expression of C2GnT1 mRNA was seen upon T-cell activation, C2GnT2 transcripts were not detected, whereas the level of expression of C2GnT3 appeared to fluctuate, both in size and level of expression. We conclude from this data that the increased activity responsible for synthesizing core 2 structures in activated T cells is probably the result of increased expression of the C2GnT1 enzyme, as had been previously assumed.28
Expression of MUC1 mRNA in normal adult tissues
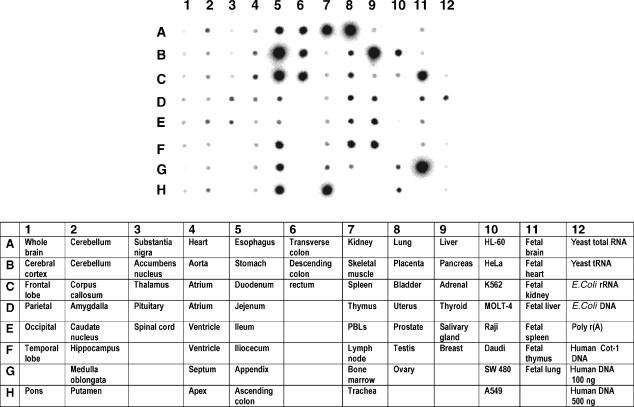
Expression of MUC1 in normal adult tissues has been documented by immunohistochemical staining using antibodies to epitopes in the TR domain. These studies have suggested that MUC1 expression is largely seen in epithelial cells, particularly those of the lung, stomach, pancreas, kidney, lactating mammary gland and salivary gland.29–31 While mRNA levels do not necessarily predict the levels of expressed glycoprotein, detection of the transcript avoids the problem of different glycoforms being differentially recognized by antibodies to the TR domain. To define the profile of expression of MUC1 mRNA in normal adult tissues, a dot-blot of polyA+ RNA was subjected to Northern analysis using a probe to the TR domain of MUC1. Figure 7 shows that, as expected, a high expression of MUC1 is seen in epithelial tissues. However the levels of transcript detected in the intestinal tract and trachea were much higher than those seen with antibody staining,32 presumably because of the extensive glycosylation that blocks access of antibody to TR epitopes. Fetal lung and kidney also showed high expression levels. Significantly, MUC1 transcripts were not detected in thymus, spleen and PBL, confirming our observation that MUC1 is not expressed in resting T cells and only at low levels in activated T cells.
Figure 7.
The presence of MUC1 mRNA transcript in fetal and adult human tissues. A commercial multiple tissue expression array was assayed for MUC1 transcript using a probe to the tandem repeat (TR) region.
More detailed analysis by flow cytometry of some other haemopoietic lineages, using the same antibodies, indicated that MUC1 is not expressed on monocytes or monocyte-derived dendritic cells, and only very low levels could be detected on B cells (data not shown). Finally, T cells in lymph nodes removed at surgery for breast cancer did not stain with the antibodies in immunohistochemistry, whereas metastatic breast cancer cells in the lymph nodes did stain strongly with all the antibodies.
Discussion
In this study, we have analysed in detail the expression of MUC1 in human T cells. Following activation in vivo and by different stimuli in vitro, human T cells expressed MUC1 at the cell surface, although the level of expression was much lower than that seen in breast cancer cells. Our findings demonstrated that resting T cells do not bind anti-MUC1 mAbs, nor is MUC1 mRNA detectable by RT–PCR or Northern blot analysis in these cells. As the activated T cells progress to the memory state, they maintain MUC1 on the cell surface. Confocal microscopy revealed that MUC1 was uniformly expressed at the cell surface until a migratory chemokine was present; following such a stimulus, the cells focused MUC1 to the leading edge (Fig. 8). The profile of reactivity with different antibodies suggests that the glycoform of MUC1 expressed by the activated T cells carries core 2-based O-glycans as opposed to the core 1 structures that dominate in the cancer-associated mucin.
Figure 8.
After polarization, MUC1 is concentrated at the leading edge of activated T cells. During polarization, many mucin-like molecules on T cells are directed to the uropod, but MUC1 is directed to the leading edge. In that site, MUC 1 could participate in, or modify, specific interactions with molecules on inflamed endothelial cells or other cells in sites of inflammation.
It is well established that the glycosylation pattern of MUC1 can vary with the cell type expressing the glycoprotein.33–35 The binding of antibodies to epitopes in the TR domain of MUC1 is strongly influenced by the composition and density of the O-glycans attached to serines and threonines in this domain. The preferential reactivity of the HMFG1 mAb with activated T cells probably reflected the fact that it can recognize MUC1 carrying core 2-based structures (as well as the cancer-associated glycoforms; see refs 23 and 24). The same must be true for mAbs HMPV and MF06. As activated T cells are known to synthesize core 2 O-glycans,25 MUC1 will probably carry core 2-based structures. In contrast, the mAbs SM3 and HMFG2, which react better with MUC1 glycoforms carrying core 1-dominated structures,24 bound activated T cells poorly. Northern analysis of the transcripts for the three enzymes able to synthesize core 2 O-glycans in the activated cells, suggested that the increase in C2GnT1 was responsible for the increased capacity to synthesize core 2 structures in activated T cells, although a role for C2GnT3 cannot be excluded. C2GnT1 expression in lymphocytes is also involved in the optimal expression of selectin ligands for adhesion and lymphocyte-homing properties, and it has been reported that its expression is regulated by the cytokine milieu subsequent to T-cell activation.28,36
The mAbs 232A1 and 12C10, reactive with single epitopes outside the TR region, and therefore unaffected by glycosylation patterns, did show positive staining of a fraction of activated T cells, but the percentage of cells staining was less than that seen with HMFG1. This is probably a result of the fact that lower levels of expression of MUC1 could be detected using an antibody recognizing an epitope repeated 25–100 times (depending on the allele), such as HMFG1, as compared to the level which can be detected by an antibody binding to a single epitope. The mAb B27·29, used in a previous study where expression of MUC1 in activated T cells was described,18 also recognizes an epitope in the TR region, overlapping with that recognized by HMFG1, HMPV and MF06 mAbs.22
Previous reports had examined MUC1 expression by human T cells. One of these studies used the mAb DF3-P and suggested that MUC1 was present in resting human T cells and the leukaemia cell line Jurkat.19 This antibody, like mAb SM3, has been reported to bind to cancer-associated glycoforms of MUC1, where core 1 structures predominate. However, in our study, the antibody SM3 did not bind to resting T cells, and MUC1 mRNA could not be detected. The HMFG1 antibody, which can recognize MUC1 carrying extended core 2-based O-glycans, as well as cancer-associated glycoforms, also did not bind to resting T cells. Our findings are substantiated by other studies that demonstrated minimal staining of Jurkat cells using DF3-P and no staining of resting T cells with MUC1-specific mAbs VU-4H5 and VU-3C6,20 and no staining of resting T cells with MUC1-specific mAb B27·29.18 Therefore, the majority of published reports support our observation that MUC1 is not expressed by resting human T cells.
The different reactivities of antibodies, which are affected by the glycosylation pattern of the cell producing the glycoprotein, emphasizes the importance of documenting the expression of MUC1 mRNA transcripts. The expression of full-length MUC1 was confirmed by sequencing the products of RT–PCR and by Northern blot analysis. Very low levels of transcripts were detected by Northern blot analysis as compared to levels in breast cancer cells, and in semiquantitative RT–PCR, the level of MUC1 transcript in activated human T cells was found to be at least 50-fold lower than that seen in human breast cancer. It is also important to note that using immunohistochemistry, there was no significant staining of T cells within activated lymph nodes. In contrast, micrometastases from breast cancer were readily identifiable within these same lymph nodes using HMFG1 or the other MUC1-specific mAbs used in the study. Therefore, the data presented here demonstrate that although MUC1 is expressed by activated human T cells, the level of expression is very low and certainly much lower than seen in breast cancer and normal epithelial tissues. The relative levels of target antigens are important in determining whether a cellular immune response is activated or is effective. The low level of expression in T cells probably precludes induction of autoimmunity as a result of MUC1 immunization strategies.
This information is of interest as MUC1-based immunotherapy is under investigation in the clinic, and the induction of autoimmunity or the lack of response, due to immunological tolerance, must be considered. In the trials carried out with radiolabelled HMFG1 mAb, no side-effects suggestive of toxicity to lymphocytes have been noted.37 On the other hand, the expression of MUC1 by cells of the immune system could result in higher-than-expected levels of immunological tolerance. Also, the high expression of MUC1 mRNA in the gastrointestinal tract (Fig. 7), if translated into protein, could also lead to high levels of immunological tolerance, as described for ovalbumin when expressed by intestinal cells.38
The function of MUC1 in activated T cells is uncertain. It has been proposed that MUC1 has a role in immune-response regulation,18 but the evidence is controversial. Inhibition of T-cell proliferation by synthetic MUC1 peptides (covering the TR sequence),39 or MUC1 from tumour-cell supernatants,40 has been reported. In another report, the T-cell inhibitory factor in tumour-cell supernatants could be separated from MUC1.41 We have found no effect of MUC1 TR peptides on the activation or function of T cells, and the level of MUC1 in the supernatants from activated T cells is barely detectable (T. Plunkett, J. Taylor-Papadimitriou, unpublished). It seems conceivable that it is the expression of MUC1 on the surface of T cells that plays some, as-yet undefined, role in T-cell function. Previous reports using tumour cells over-expressing MUC1 have indicated a role for MUC1 molecules in the inhibition of intercellular adhesion.42–44 However, a certain density of surface expression may be required for blocking such cell–cell interactions.
The confocal microscopic analysis of activated T cells showed two distinct patterns of MUC1 expression. The molecule is uniformly expressed over the entire cell surface in non-polarized T lymphocytes but, interestingly, it forms polar aggregates in T cells undergoing cytoskeletal rearrangements in response to a chemokine. It is known that to initiate migration, T lymphocytes switch from a spherical to a polarized shape.45 We have correlated the spatial distribution of MUC1 with that of other molecules associated with cytoskeletal rearrangement. Following RANTES-induced cell polarization we found MUC1 at the leading edge of the polarized T cell. This suggests that activated T cells may use MUC1 on their leading edge to affect interactions with endothelial cells as they travel to inflammatory sites and/or MUC1 plays a role in events that occur after the lymphocytes have passed through the endothelium into the underlying tissues (e.g. migration through tissue or interactions with target cells). Which molecules might be responsible for the interaction with MUC1, and the nature of this interaction, are yet to be defined. Although MUC1 may carry specific O-glycans (e.g. sialylated Lewis X) that are recognized by selectins, it is unlikely that MUC1 is interacting with selectins on endothelial cells because the functionality of selectin ligands appears to depend on modifications of the core protein as well as the specific O-glycans.46 It needs to be said that by virtue of the extended structure of the MUC1 molecules, they can inhibit cell interactions such as those mediated by integrins and E-cadherin47,48 as well as participate in cell adhesion through interaction with lectins such as sialoadhesin.49 Which of these effects predominate in the polarized T cell could depend on the immediate environment.
Rheumatoid arthritic joints are sites of chronic inflammatory disease, and activated effector/memory T cells are the dominant cell type present in synovial tissue.50 CD45RO+ T cells from these inflammatory infiltrates display a polarized morphology.51 In addition, there is increased expression of adhesion molecules on inflamed endothelium in arthritic joints.52 As MUC1 is expressed on the surface of activated memory T cells, it has the potential to play a role in T-cell migration into inflamed arthritic joints and/or in events inside the joints. Finding MUC1-expressing T cells in synovial fluid from a person with rheumatoid arthritis supports this idea.
Acknowledgments
We wish to thank Dr Constantino Pitzalis (Rheumatology Unit, Guy's, King's and St. Thomas', School of Medicine, London) for providing the synovial sample, and Dr Simon Watkins and Sean Alber, at the University of Pittsburgh Imaging Facility, for assistance in acquiring the confocal microscopy images. Our work was supported by NIH grant 5RO1 CD56103 and by a grant from the Arthritis Foundation, Western Pennsylvania Chapter. A.M. is supported by a fellowship from the Association for International Cancer Research (AICR 98–254).
Abbreviations
- C2GnT
β6GlcNAc transferase
- CTL
cytotoxic T lymphocyte
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- IL-2
interleukin-2
- mAb
monoclonal antibody
- MLR
mixed lymphocyte reaction
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- PHA
phytohaemaglutinin
- PBMC
peripheral blood mononuclear cells
- RANTES
regulated on activation, normal, T-cell expressed, and secreted
- TR
tandem repeat
References
- 1.Finn OJ, Jerome KR, Henderson RA, Pecher G, Domenech N, Magarian-Blander J, Barrat-Boyes S. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol Rev. 1995;145:61–89. doi: 10.1111/j.1600-065x.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 2.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, et al. Molecular cloning and expression of human tumour-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–93. [PubMed] [Google Scholar]
- 3.Lloyd KO, Burchell J, Kudryashov V, Yin BWT, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem. 1996;271:33325–34. doi: 10.1074/jbc.271.52.33325. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal B, Reddish MA, Krantz MJ, Longenecker BM. Does pregnancy immunize against breast cancer? Cancer Res. 1995;55:2257–61. [PubMed] [Google Scholar]
- 5.Jerome KR, Barnd DL, Bendt KM, Boyer CM, Taylor-Papadimitriou J, McKenzie IFC, Bast RC, Finn OJ. Cytotoxic T lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991;51:2908–16. [PubMed] [Google Scholar]
- 6.Feuerer M, Beckhove P, Bai L, et al. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–8. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 7.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–8. [PubMed] [Google Scholar]
- 8.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic and colon cancer patients. Cancer Res. 1994;54:2856–60. [PubMed] [Google Scholar]
- 9.von Mensdorff-Pouilly S, Verstraeten AA, Kenemans P, et al. Survival in early breast cancer patients is favorably influenced by a natural humoral response to polymorphic epithelial mucin. J Clin Oncol. 2000;18:574–83. doi: 10.1200/JCO.2000.18.3.574. [DOI] [PubMed] [Google Scholar]
- 10.Domenech N, Henderson RA, Finn OJ. Identification of an HLA-A11-restricted epitope from the tandem repeat domain of the epithelial tumor antigen mucin. J Immunol. 1995;155:4766–74. [PubMed] [Google Scholar]
- 11.Brossart P, Heinrich KS, Stuhler G, et al. Identification of HLA-A2-restricted T-cell epitopes derived from the MUC-1 tumour antigen for broadly applicable vaccine therapies. Blood. 1999;93:4309–17. [PubMed] [Google Scholar]
- 12.Carmon L, El-Shami KM, Paz A, et al. Novel breast-tumour-associated MUC1-derived peptides: characterization in Db−/−x β2 microglobulin single chain. Int J Cancer. 2000;85:391–7. [PubMed] [Google Scholar]
- 13.Heukamp LC, van der Burg SH, Drijfhout JW, Melief CJM, Taylor-Papadimitriou J, Offringa R. Identification of three non-VNTR MUC1-derived HLA-A*0201-restricted T cell epitopes that induce protective anti-tumour immunity in HLA-A2/Kb-transgenic mice. Int J Cancer. 2001;91:385–92. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1051>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Graham RA, Burchell JM, Beverly P, Taylor-Papadimitriou J. Intramuscular immunisation with MUC1 cDNA can protect C57 mice challenged with MUC1-expressing syngeneic mouse tumour cells. Int J Cancer. 1996;65:664–70. doi: 10.1002/(SICI)1097-0215(19960301)65:5<664::AID-IJC17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Tempero RM, van Lith ML, Morikane K, Rowse GJ, Gendler SJ, Hollingsworth MA. CD4+ lymphocytes provide MUC1-specific tumor immunity in vivo that is undetectable in vitro and is absent in MUC1 transgenic mice. J Immunol. 1998;161:5500–6. [PubMed] [Google Scholar]
- 16.Acres B, Apostolopoulos V, Balloul JM, et al. MUC1-specific immune responses in human MUC1 transgenic mice immunized with various human MUC1 vaccines. Cancer Immunol Immunother. 2000;48:588–94. doi: 10.1007/PL00006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumour-specific epitopes elicit distinct immune effector mechanism in wild-type versus MUC1-transgenic mice with different potential for tumour rejection. J Immunol. 2001;166:6555–63. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal B, Krantz MJ, Parker J, Longenecker BM. Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer Res. 1998;58:4079–81. [PubMed] [Google Scholar]
- 19.Chang F, Zhao HL, Phillips J, Greenburg G. The epithelial mucin MUC1 is expressed on resting T lymphocytes and can function as a negative regulator of T cell activation. Cell Immunol. 2000;201:83–8. doi: 10.1006/cimm.2000.1643. [DOI] [PubMed] [Google Scholar]
- 20.Treon SP, Maimonis P, Bua D, et al. Elevated soluble MUC1 levels and decreased anti-MUC1 antibody levels in patients with multiple myeloma. Blood. 2000;96:3147–53. [PubMed] [Google Scholar]
- 21.Xing PX, Prezonska J, McKenzie I. Epitope mapping of anti-breast and anti-ovarian mucin monoclonal antibodies. Mol Immunol. 1992;29:641–50. doi: 10.1016/0161-5890(92)90201-8. [DOI] [PubMed] [Google Scholar]
- 22.Blockzjil A, Nilsson K, Nilsson O. Epitope characterization of MUC1 antibodies. Tumor Biol. 1998;19(Suppl. 1):46–56. doi: 10.1159/000056504. [DOI] [PubMed] [Google Scholar]
- 23.Hilkens J, Boer M. Monoclonal antibodies against the nonmucin domain of MUC1/episialin. Tumour Biol. 1998;19(Suppl. 1):67–70. doi: 10.1159/000056506. [DOI] [PubMed] [Google Scholar]
- 24.Burchell J, Taylor-Papadimitriou J. Effect of modification of carbohydrate side chains on the reactivity of antibodies with core protein epitopes of the MUC1 gene product. Epithelial Cell Biol. 1993;2:155–62. [PubMed] [Google Scholar]
- 25.Bierhuizen MFA, Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc: Galβ1–3-GalNAc-R (GlcNAc to GalNAc) β1–6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumour antigen. Proc Natl Acad Sci USA. 1992;89:9326–30. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bierhuizen MFA, Mattei M-G, Fukoda M. Expression of the developmental I antigen by cloned human cDNA encoding a member of a β6, 6-N-acetylglucosaminyltransferase gene family. Genes Dev. 1993;7:468–78. doi: 10.1101/gad.7.3.468. [DOI] [PubMed] [Google Scholar]
- 27.Schwientek T, Yeh J-C, Levery SB, Keck B, Merkx G, van Kessel AG, Fukuda M, Clausen H. Control of O-glycan branch formation. J Biol Chem. 2000;275:11106–13. doi: 10.1074/jbc.275.15.11106. [DOI] [PubMed] [Google Scholar]
- 28.Carlow DA, Corbel SY, Williams MJ, Ziltener HJ. IL-2, -4 and -15 differentially regulate O-glycan branching and P-selectin ligand formation in activated CD8 T cells. J Immunol. 2001;167:6841–8. doi: 10.4049/jimmunol.167.12.6841. [DOI] [PubMed] [Google Scholar]
- 29.Zotter S, Hageman PC, Lossnitzer A, Mooi WJ, Hilgers J. Tissue and tumour distribution of human polymorphic epithelial mucin. Cancer Rev. 1988;11–12:55–101. [Google Scholar]
- 30.Girling A, Bartkova J, Burchell J, Gendler SJ, Gillet C, Taylor-Papadimitriou J. A core protein epitope of the PEM mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int J Cancer. 1989;43:1072–6. doi: 10.1002/ijc.2910430620. [DOI] [PubMed] [Google Scholar]
- 31.Peat N, Gendler SJ, Lalani E-N, Duhig T, Taylor-Papadimitriou J. Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Res. 1992;52:1954–60. [PubMed] [Google Scholar]
- 32.Sikut R, Sikut A, Zhang K, Baeckstrom D, Hansson GC. Reactivity of antibodies with highly glycosylated MUC1 mucins from colon carcinoma cells and bile. Tumour Blood. 1998;19(Suppl. 1):122–6. doi: 10.1159/000056513. [DOI] [PubMed] [Google Scholar]
- 33.Hanisch F-G, Uhlenbruck G, Peter-Katalinic J, Egge H, Dabrowski J, Dabrowski U. Structures of neutral O-linked polylactosaminoglycans on human skim milk mucins. A novel type of linearly extended poly-N-acetyllactosamine. J Biol Chem. 1989;264:872–83. [PubMed] [Google Scholar]
- 34.Hull SR, Bright A, Carraway KL, Abe M, Haye DF, Kufe DW. Oligosaccharide differences in the DF3 sialomucin antigen from normal human milk and the BT-20 human breast carcinoma cell line. Cancer Comms. 1989;1:261–7. [PubMed] [Google Scholar]
- 35.Lloyd KO, Burchell J, Kudryashov V, Yin BWT, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumour cells. J Biol Chem. 1996;271:33325–34. doi: 10.1074/jbc.271.52.33325. [DOI] [PubMed] [Google Scholar]
- 36.Lim Y-C, Xie H, Come CE, Alexander SI, Grusby MJ, Lichtman AH, Luscinskas FW. IL-12, STAT4-dependent up-regulation of CD4+T cell core 2 β-1,6-N-acetylglucosaminyltransferase, an enzyme essential for biosynthesis of P-selectin ligands. J Immunol. 2001;167:4476–84. doi: 10.4049/jimmunol.167.8.4476. [DOI] [PubMed] [Google Scholar]
- 37.Epenetos AA, Hird V, Lambert H, Mason P, Coulter C. Long term survival of patients with advanced ovarian cancer treated with intraperitoneal radioimmunotherapy. Int J Gynecol Cancer. 2000;10(Suppl. 1):44–6. doi: 10.1046/j.1525-1438.2000.99510.x. [DOI] [PubMed] [Google Scholar]
- 38.Vezys V, Olson S, Lefrancois L. Expression of intestinal-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–14. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal B, Krantz MJ, Reddish MA, Longenecker BM. Cancer-associated MUC1 mucin inhibits human T-cell proliferation, which is reversible by IL-2. Nat Med. 1998;4:43–9. doi: 10.1038/nm0198-043. [DOI] [PubMed] [Google Scholar]
- 40.Chan AK, Lockhart DC, von Bernstorff W, Spanjaard RA, Joo H-G, Eberlein TJ, Goedegebuure PS. Soluble MUC1 secreted by human epithelial cancer cells mediates immune suppression by blocking T-cell activation. Int J Cancer. 1999;82:721–6. doi: 10.1002/(sici)1097-0215(19990827)82:5<721::aid-ijc16>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 41.Paul S, Bizouarne N, Paul A, Price M, Hansson G, Kieny MP, Acres B. Lack of evidence for an immunosuppressive role for MUC1. Cancer Immunol Immunother. 1999;48:22–8. doi: 10.1007/s002620050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondo K, Kohno N, Yokoyama A, Hiwada K. Decreased MUC1 expression induces E-cadherin-mediated cell adhesion of breast cancer cell lines. Cancer Res. 1998;58:2014–9. [PubMed] [Google Scholar]
- 43.van de Wiel-van Kemenade E, Ligtenberg MJ, de Boer AJ, Zimmermann G, Hugh JC. Episialin (MUC1) inhibits cytotoxic lymphocyte–target cell interaction. J Immunol. 1993;151:767–76. [PubMed] [Google Scholar]
- 44.McDermott KM, Crocker PR, Harris A, Burdick MD, Hinoda Y, Hayashi T, Imai K, Hollingsworth MA. Overexpression of MUC1 reconfigures the binding properties of tumour cells. Int J Cancer. 2001;94:783–91. doi: 10.1002/ijc.1554. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–11. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramachandran V, Nollert MU, Qui H, Liu WJ, Cummings RD, Zhu C, McEver RP. Tyrosine replacement in P-selectin glycoprotein ligand-1 affects distinct kinetic and mechanical properties of bonds with P- and L-selectin. Proc Natl Acad Sci USA. 1999;96:13771–6. doi: 10.1073/pnas.96.24.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–65. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell–cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–77. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nath D, Hartnell A, Happerfield L, Miles DW, Burchell J, Taylor-Papadimitriou J. Macrophage–tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology. 1999;98:213–9. doi: 10.1046/j.1365-2567.1999.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohem CL, Brezinschek RI, Wisbey H, Tortorella C, Lipsky PE, Oppenheimer-Marks N. Enrichment of differentiated CD45dimCD27− memory T cells in the peripheral blood, synovial fluid, and synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:844–54. doi: 10.1002/art.1780390518. [DOI] [PubMed] [Google Scholar]
- 51.del Pozo MA, Cabanas C, Montoya MC, Ager A, Sanchez-Mateos P, Sanchez-Madrid F. ICAM redistributed by chemokines to cellular uropods as a mechanism for recruitment of T lymphocytes. J Cell Biol. 1997;137:493–508. doi: 10.1083/jcb.137.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.To SS, Newman PM, Hyland VJ, Robinson BG, Schrieber L. Regulation of adhesion molecule expression by human synovial microvascular endothelial cells in vitro. Arthritis Rheum. 1996;39:467–77. doi: 10.1002/art.1780390315. [DOI] [PubMed] [Google Scholar]