Abstract
Experimental autoimmune thyroiditis (EAT) is inducible in mice by immunization with mouse thyroglobulin (mTg) together with adjuvant, either lipopolysaccharide (LPS) or complete Freund's adjuvant (CFA). The severity of the disease is dependent on the mouse strain and on the adjuvant used. We have previously shown that interleukin (IL)-12 deficient C57BL/6 mice immunized with mTg and CFA develop a significantly less severe thyroid infiltration in comparison to wild type C57 BL/6 mice. This result indicated a pivotal role for IL-12 in the development of thyroiditis induced with CFA and mTg. In the present study we demonstrate that IL-12 deficiency does not impair EAT induction when LPS is used as adjuvant. We also demonstrate that peritoneal exudate cells from IL-12-deficient mice stimulated in vitro either with LPS or IL-18 secrete high levels of tumour necrosis factor (TNF). Together the results emphasize the difference between the use of CFA and LPS in the induction of EAT, the importance of TNF-α for the pathogenesis of LPS-induced EAT, and also show the capacity of IL-12-deficient mice to develop a competent response to LPS.
Introduction
Experimental autoimmune thyroiditis (EAT) can be induced in mice by immunization with mouse thyroglobulin (mTg) plus adjuvants such as complete Freund's adjuvant (CFA) and lipopolysaccharide (LPS). The severity of the disease depends on the adjuvant and on the strain of mice used. EAT is considered a model of T helper 1 (Th1)-mediated disease because it has been shown that the in vivo administration of interferon-γ (IFN-γ) can exacerbate the severity of the pathology1 and the ablation of IFN-γ reduces the incidence and the severity of EAT.2
Interleukin (IL)-12 is mainly produced by monocytes and macrophages in response to bacterial products and is a pivotal cytokine in the development of Th1 lymphocytes. The pathogenic involvement of IL-12 in several animal models of Th1-mediated disease like type 1 diabetes (TD1),3 experimental autoimmune encephalomyelitis (EAE),4 autoimmune colitis,5 and experimental autoimmune uveitis has been extensively described.6 Our previous study showed that in vivo administration of non-physiological doses of IL-12 can either exacerbate or suppress the development of EAT depending on different factors such as the adjuvant used, timing of administration and dose.7 We found that a single dose of 300 ng of IL-12 administered with the first immunization with mTg and LPS increased the severity of the disease. In contrast a prolonged treatment of IL-12 in CFA induced EAT and mediated an immunosuppressive effect on the development of the disease.7 To investigate further the importance of IL-12 in the pathogenesis of EAT we immunized C57BL/6 IL-12 p40 knockout mice with mTg and CFA and found that these mice develop a less severe infiltration in the thyroid in comparison to the wild type. In either case these results demonstrate that IL-12 can play a role in the two different models of EAT.
In the present study we investigated the induction of EAT using the C57BL/6 IL-12 p40 knockout mice using LPS and mTg. In contrast to our previous result in the CFA induced EAT, we found that the IL-12 p40 knockout mice are susceptible to the development of EAT. To elucidate this result we also investigated the in vitro LPS response of peritoneal exudate cells (PEC) from IL-12-deficient mice. Once again we demonstrate variation between these different adjuvants in the induction of murine models of thyroiditis.
Materials and methods
Mice
EAT susceptible 6–8 week old CBA/J mice (H-2k) of both sexes were obtained from Harlan UK Ltd (Bicester, UK). C57BL/6 mice with the IL-12 p40 subunit knocked-out (obtained from Dr L. Adorini, Roche, Milan, Italy) and C57BL/6 (H-2b) mice were obtained from breeding colonies maintained in facilities of the Pathology Department, University of Cambridge. During the experiments all the mice were maintained in the same standard conditions at the Department of Pathology with free access to food and water.
Induction of EAT using adjuvant
Mouse thyroglobulin (mTg) was prepared by extraction from the pooled homogenized thyroids of normal outbred mice as previously described.8 Mice were given 50 µg mTg followed 3 hr later by 20 µg LPS (Salmonella enteritides; Sigma Chemical Co., St Louis, MO) both injected i.v. This was repeated on day 7, and the mice were bled and the thyroids taken on day 28.
Preparation of PEC
To maximize macrophage recovery IL-12 p40 knockout mice, C57BL/6 and CBA/J mice were pretreated with thioglycollate (Difco, East Molesey, UK) by injection with 2 ml of a 3% solution into the peritoneal cavity. After 5, days PEC were collected using sterile ice-cold phosphate-buffered saline (PBS) containing streptomycin (100 mg/ml) and penicillin (100 U/ml). Erythrocytes were lysed and peritoneal exudate cells washed and resuspended in complete medium (RPMI-1640 plus supplements). Cells were stimulated by the addition of 5 µg/ml LPS and plated out in 96-well flat-bottomed plates at 2 × 105 cells/well. Plates were then incubated at 37° in 5% CO2 and supernatants removed for analysis of cytokines at various intervals over a 24–48-hr period.
Detection of cytokines in culture supernatants
Supernatants were collected from cell cultures at several time points and tested for IL-12, IL-18, IL-10 and TNF-α using an enzyme-linked immunosorbent assay (ELISA). Capture and secondary antibodies were all obtained from BioSource (Camarillo, CA) and the assays performed according to the manufacturer's instructions. IL-18 was assayed using an ELISA kit from Medical & Biological Laboratory Co., LTD (Nagoya, Japan). NO was also detected in the supernatants using the Griess reagent method. The sensitivity of detection for IL-18 was 4·5 pg/ml, for IL-10 62·5 pg/ml, for IL-12 15 pg/ml, for TNF-α 31 pg/ml and for ΝΟ 1·5 µm/ml.
Detection of antithyroglobulin autoantibodies
These were assayed using standard ELISA methods. Briefly, plates were coated overnight with mTg diluted to 10 µg/ml in a coating buffer pH 9·6 and left at 4°. After blocking with PBS/Tween/BSA, the sera were appropriately diluted and incubated on the plates for 1–2 hr at room temperature. Developing antibodies, conjugated to alkaline phosphatase, were added after washing; these were polyvalent anti-mouse immunoglobulin G (IgG) at 1/500 (Sigma Chemicals, Poole, UK) or anti-mouse IgG1, 2a or 2b at 1/1000 (Southern Biotechnology Associates Inc., Birmingham, AL) and the plates incubated for a further hour and then washed. The substrate, p-nitrophenyl phosphate (Sigma) 1 mg/ml, was added at 100 µl/well and the reaction stopped with 25 µl/well 4 m NaOH after which the optical density was read at 410 nm. In all assays a dilution curve was carried out for experimental and control serum samples. For clarity of presentation, specific serum dilutions were selected which are given in the figure legends.
Evaluation of thyroid infiltration
Thyroids were fixed in 10% phosphate-buffered formalin and 5 µm sections at six levels were stained with haematoxylin–eosin. The criteria used for scoring were those previously employed in our laboratory.8 For each mouse there are 12 possible scores since there are two lobes to the gland and six levels for each. Grade 0 = no infiltration, 1 = any definite infiltration up to 20%, 2 = between 20 and 50%, 3 = between 50 and 75%, 4 = gland totally infiltrated but follicles still discernible, 5 = no follicles detectable. The severity of the infiltration was assessed blind and the final score for each mouse is expressed as the median of the 12 possible scores.
Statistical analysis
Serum data were analysed by means of a one way anova. Cytokine data and histological data were analysed using a paired t-test and Wilcoxon signed rank test, respectively.
Results
Induction of EAT with mTg and LPS in C57BL/6 IL-12 p40 knockout mice
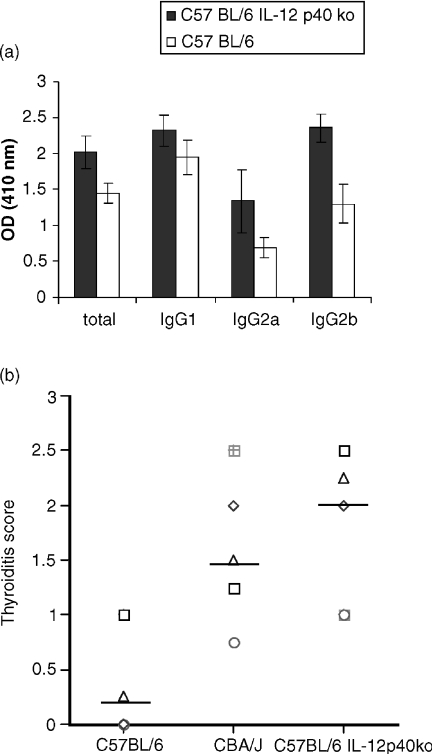
Following immunization with mTg and LPS IL-12 p40 knockout mice developed a more severe form of EAT if compared to the wild type mice. The levels of IgG1 anti thyroglobulin antibodies were similar to the control C57BL/6 mice while total IgG2a and IgG2b antithyroglobulin antibodies were increased (Fig. 1a). Figure 1(b) shows that the IL-12 p40 knockout mice also develop a more severe infiltration in the thyroid in comparison to the wild type (the data are representative of two different experiments). Figure 1(b) also shows that the IL-12 p40 knockout mice develop an infiltration that is comparable to a good responder mouse strain for EAT, the CBA/J mice.
Figure 1.
C57BL/6 IL-12 p40-deficient mice develop a more severe mTg and LPS induced thyroiditis in comparison to the wild type C57BL/6. 5 C57BL/6 IL-12 p40 deficient mice and 5 C57BL/6 mice were immunized with mTg and LPS, 28 days after the first immunization sera and thyroids were collected for analysis. (a) Serum (diluted 1/50) antibody response against mTg, total immunoglobulins (P < 0·0006), IgG2a (P < 0·0074) and IgG2b (P < 0·0003) are significantly increased in the C57BL/6 IL-12 p40-deficient mice, there is no significant difference in the anti mTg IgG1 isotype (P < 0·0259). (b) Histological score of thyroid lesion. Thyroids from C57BL/6 IL-12 p40 deficient mice are more infiltrated than C57BL/6 mice thyroids.
In vitro response of peritoneal exudates from CBA/J and C57BL/6 to LPS
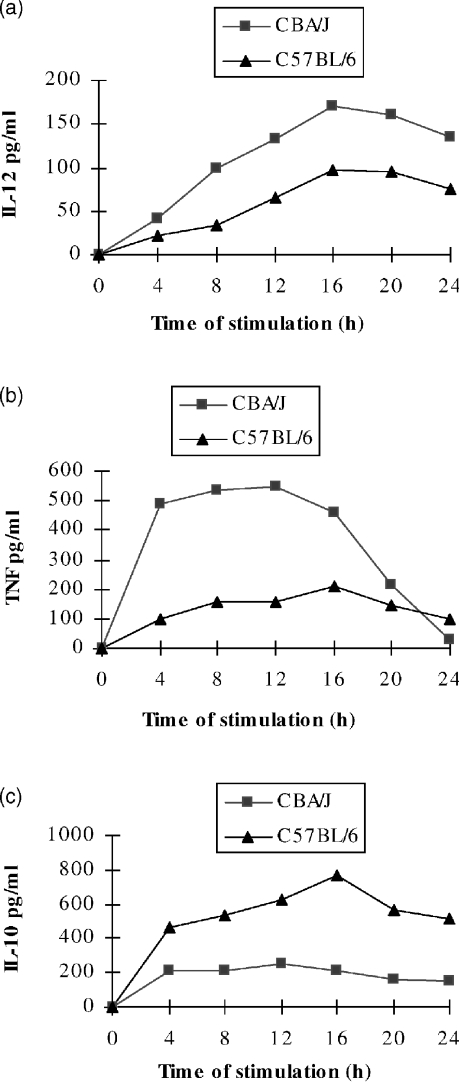
To establish if the susceptibility to EAT correlated with the capacity to respond to the adjuvant, PEC from CBA/J and C57BL/6 mice were stimulated in vitro with LPS (5 µg/ml). Figure 2 shows that macrophages from CBA/J mice secrete more IL-12, TNF-α and less IL-10 in comparison to C57BL/6 mice.
Figure 2.
CBA/J PEC secrete a more Th1-like panel of cytokines. PEC from thioglycollate-treated mice were stimulated in vitro with LPS (5 µg/ml), supernatants were sampled every 4 hr for measurement of cytokine concentrations. Unstimulated cells did not produce detectable amounts of cytokines (not shown). (a) PEC from CBA/J mice produce more IL-12 (P < 0·005) (b) more TNF-α (P < 0·05), and (c) less IL-10 (P < 0·0002) than PEC from C57BL/6 mice.
In vitro response of PEC from C57BL/6 IL-12 p40 knockout mice and C57BL/6 to LPS and IL-18
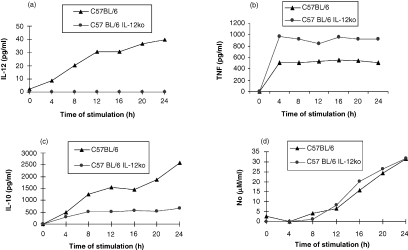
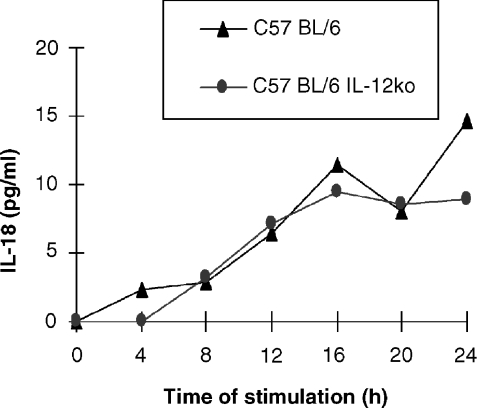
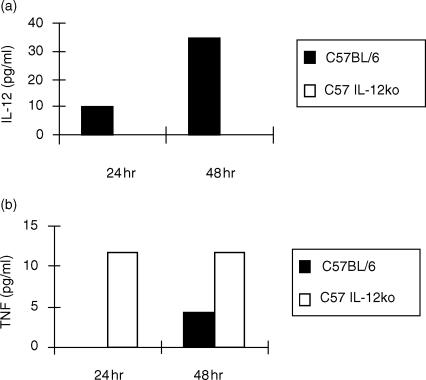
To address why the IL-12 p40 knockout mice develop a more severe form of EAT in comparison to the wild type mice despite the lack of functional IL-12 expression (Fig. 3a), we decided to compare the capacity of PEC to respond to LPS and IL-18. PEC from the knockout mice stimulated in vitro with LPS produce significant amounts of TNF-α (see Fig. 3b) and diminished levels of IL-10 (Fig. 3c) in comparison to the wild type. NO levels from LPS-stimulated macrophages are comparable between the two groups (see Fig. 3d). Figure 4 shows that LPS-stimulated macrophages from IL-12 p40 knockout mice produce similar levels of IL-18 to the control groups suggesting that IL-12 gene disruption was not counterbalanced by an overproduction of IL-18 in the C57BL/6 mice. We therefore wished to examine whether in the IL-12 knockout mice IL-18 alone could support TNF-α production. In vitro stimulation of PEC with IL-18 (10 ng/ml) induced secretion of significantly enhanced levels of TNF-α and with a faster kinetics in the IL-12 knockout mice (Fig. 5b) compared to the wild-type. Increasing the amount of IL-18 10-fold to 100 ng/ml, did not alter the pattern of TNF-α secretion by the two strains (data not shown). PEC from the knockout mice did not produce any IL-12 (Fig. 5a).
Figure 3.
Differences in the cytokine secretion profiles of wild-type and IL-12 p40 deficient mice. PEC from thioglycollate-treated mice were stimulated in vitro with LPS (5 µg/ml), supernatants were sampled every 4 hr for cytokine concentrations. Unstimulated cells did not produce detectable amounts of cytokines (not shown). (a) PEC from C57BL/6 IL-12 p40-deficient mice did not secrete IL-12; (b) PEC from C57BL/6 IL-12 p40-deficient mice secrete more TNF-α (P < 0·0005); (c) less IL-10 (P < 0·001) than C57BL/6 mice; and (d) similar levels of NO.
Figure 4.
PEC from C57BL/6 IL-12 p40-deficient mice secrete similar levels of IL-18 to the wildtype. Peritoneal exudate cells (PEC) from thioglycollate-treated mice were stimulated in vitro with LPS (5 µg/ml), supernatants were sampled every 4 hr for measurement of cytokine concentrations. Unstimulated cells did not produce detectable amounts of cytokines (not shown).
Figure 5.
PEC from IL-12 p40 knockout and wild type mice respond differently to stimulation by IL-18. Peritoneal exudate cells (PEC) from thioglycollate-treated mice were stimulated in vitro with IL-18 (10 ng/ml), supernatants were sampled at 24 and 48 hr for cytokine concentrations. Unstimulated cells did not produce detectable amounts of cytokines (not shown). (a) PEC from C57BL/6 IL-12 p40-deficient mice do not secrete IL-12. (b) PEC from C57BL/6 IL-12 p40-deficient mice secrete more TNF-α than C57BL/6 mice.
Discussion
Susceptibility to EAT has been linked to the major histocompatibility locus H-2, with H-2s and H-2k mice being excellent responders to actively induced thyroiditis, whereas H-2b and H-2d were considered to be low responders.9 While C57BL/6 (H-2b) mice have been classified as ‘poor responders’ to the induction of thyroiditis with mTg and adjuvants in comparison to other strains like CBA/J mice (H-2k), they nevertheless develop a significant autoantibody response to thyroglobulin and an infiltration of mononuclear cells into the thyroid gland. Following immunization with mTg and LPS, C57BL/6 IL-12 p40 knockout mice also developed antithyroglobulin autoantibodies and a thyroid infiltrate. The knockout mice developed an infiltration that is equal to the good responder CBA/J strain. As our previous studies using CFA as an adjuvant had shown that both thyroid infiltration and production of autoantibodies were markedly impaired in IL-12 p40 knockout mice this result appeared to be paradoxical7 It is possible that different cytokines or differences in the timing of cytokine secretion are involved in the development of EAT when the different adjuvants are employed. The fact that IL-12-deficient mice can develop EAT suggests that other cytokines might be important in the pathogenesis of thyroiditis when LPS is used to induce disease. TNF-α for instance is also produced by macrophages after LPS stimulation and, together with IFN-γ, IL-1, IL-6, and NO contributes to the generation of Th1 cells. The importance of TNF-α in the development of LPS plus mTg-induced EAT is shown by the ability of soluble TNF-α receptor type I to significantly impair EAT development induced with this adjuvant.10 However, the soluble TNF-α receptor I does not influence EAT induced by mTg and CFA. The capacity of different strains of mice to resist infections or develop autoimmunity is related to their capacity to produce different patterns of cytokines in response to comparable stimuli. CBA/J mice are generally considered ‘Th1-type’ mice because of the capacity of their immune system to produce proinflammatory cytokines in response to pathogens. We have shown that PEC from CBA/J mice stimulated in vitro with LPS secrete different cytokine profiles: in particular more IL-12 and TNF-α and less IL-10 in comparison to C57BL/6 mice. IL-12 and TNF-α are pivotal cytokines in the polarization of T lymphocytes to the Th1 subset. On the contrary IL-10 is considered an anti-inflammatory factor because of its ability to inhibit cytokine synthesis from T cells, natural killer cells, monocytes, macrophages and dendritic cells. This result adds more information to the original observation that CBA/J mice are considered good responders to adjuvant induced EAT in comparison to poor responder strains such as C57BL/6. The high levels of TNF-α secreted by CBA/J macrophages in response to LPS may help to explain the ability of this strain to develop good Th1 responses. Moreover the low production of IL-10 may explain why this strain has a lower capacity to limit inflammatory responses when compared to C57BL/6 mice. In contrast, the low TNF-α and high IL-10 levels secreted by C57BL/6 macrophages in response to LPS make this strain capable of containing the inflammation. Because IL-12-deficient C57BL/6 mice can develop a good response to mTg and LPS, despite the lack of functional IL-12 expression and since their PEC respond to LPS by secreting significant amounts of TNF-α (see Fig. 3b) and diminished levels of IL-10 (Fig. 3c) in comparison to the wild type, we hypothesized that other cytokines could compensate for the lack of IL-12. IL-18 was originally identified as a factor capable of inducing IFN-γ in mice with endotoxic shock.11 This effect was shown to be independent of IL-12. In the mouse, IL-18 is mainly produced by activated macrophages and dendritic cells. Together with IL-12, IL-18 stimulates a strong Th1 immune response.11 Figure 4 demonstrates that LPS-stimulated macrophages from IL-12-deficient mice produce similar levels of IL-18 to the control groups, suggesting IL-12 gene disruption was not counterbalanced by an overproduction of IL-18 in the C57BL/6 mice. IL-18 stimulated PEC secrete significantly enhanced levels of TNF-α with a faster kinetics in C57BL/6 IL-12 p40 knockout mice (Fig. 5b) compared to the wild type. It is possible that C57BL/6 mice may compensate for IL-12 gene disruption by being more responsive to IL-18 with increased TNF-α and diminished IL-10 production. This may explain the different cytokine profiles induced by LPS in IL-12 p40-deficient and wild type C57BL/6 mice. This compensatory mechanism could help explain why IL-12 p40 knockout C57BL/6 mice develop a more pronounced thyroiditis than the wild type mice, if we consider TNF-α a key cytokine in the pathogenesis of LPS induced EAT and IL-10 a ‘macrophage deactivating factor’. From previous and present studies we conclude that IL-12 can exacerbate LPS-induced EAT but its absence can be balanced by other cytokines such as IL-18 and TNF-α. It is, however, not possible here to completely rule out the involvement of other inflammatory cytokines stimulated by LPS, particularly IL-1 and IL-6. Recent work has also implicated CD28 and CD40L in the breakage of mTg-induced tolerance;12 therefore examination of both costimulatory molecules and cytokines will facilitate progress to a fuller understanding of the mechanism of tolerance breakdown.
Acknowledgments
We are grateful to the Wellcome Trust for their support. We would also like to acknowledge Mr B Potter for his technical support with histology.
Abbreviations
- EAT
experimental autoimmune thyroiditis
- mTg
mouse thyroglobulin
- PEC
peritoneal exudate cells
References
- 1.Remy JJ, Salamero J, Bechet MM, Charreire J. Experiment autoimmune thyroiditis induced by recombinant interferon-gamma. Immunol Today. 1987;8:73. doi: 10.1016/0167-5699(87)90845-0. [DOI] [PubMed] [Google Scholar]
- 2.Tang H, Mignon Godefroy K, Meroni PL, Garotta G, Charreire J, Nicoletti F. The effects of a monoclonal antibody to interferon-gamma on experimental autoimmune thyroiditis (EAT): prevention of disease and decrease of EAT-specific T cells. Eur J Immunol. 1993;23:275–8. doi: 10.1002/eji.1830230143. [DOI] [PubMed] [Google Scholar]
- 3.Tremblau S, Penna G, Bosi E, Mortara A, Gateley M, Adorini L. Interleukin 12 administration induces T helper type I cells and accelerates autoimmune diabetes in NOD mice. J Exp Med. 1995;181:817–21. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard JP, Kaldburger K, Goldman SJ. Prevention of autoimmune encephalomyelitis by antibodies against interleukin-12. J Exp Med. 1995;181:381–6. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neurath MF, Fuss I, Kelsall BL, Stueber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoi H, Kato K, Kezuka T, Sakai J, Usui M, Yagita H, Okamura K. Prevention of experimental autoimmune uveitis by monoclonal antibody to interleukin-12. Eur J Immunol. 1997;27:641–6. doi: 10.1002/eji.1830270310. [DOI] [PubMed] [Google Scholar]
- 7.Zaccone P, Hutchings P, Nicoletti F, Penna G, Adorini L, Cooke A. The involvement of IL-12 in murine experimentally induced autoimmune thyroid disease. Eur J Immunol. 1999;29:1933–42. doi: 10.1002/(SICI)1521-4141(199906)29:06<1933::AID-IMMU1933>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Hutchings PR, Parish NM, Cooke A. Experimental models of autoimmune thyroiditis. In: Lefkovits I, Adorini L, editors. The Immunology Methods Manual. London: Academic Press Ltd; 1997. pp. 1775–85. [Google Scholar]
- 9.Vladiutiu AO, Rose NR. Autoimmune murine thyroiditis: relation to histocompatibility (H-2) type. Science. 1971;174:1137–8. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- 10.Zaccone P, Fehervari Z, Blanchard L, Nicoletti F, Edwards CK, III, Cooke A. Autoimmune thyroid disease induced by thyroglobulin and lipolysaccharide is inhibited by soluble TNF receptor type I. Eur J Immunol. 2002;32:1021–8. doi: 10.1002/1521-4141(200204)32:4<1021::AID-IMMU1021>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Flynn JC, Kong Y-CM. IL-12 prevents tolerance induction with mouse thyroglobulin by priming pathogenic T cells in experimental autoimmune thyroiditis: Role of IFN-γ and the costimulatory molecules CD40L and CD28. Cell Immunol. 2001;208:52–61. doi: 10.1006/cimm.2001.1767. [DOI] [PubMed] [Google Scholar]