Abstract
Previous studies have shown that the antigen-specific T helper 2 (Th2) response induced by alum adjuvants is interleukin (IL)-4 independent. As a role for IL-18 in Th2 induction has recently been described, in addition to its role in enhancing Th1 responses, we have studied the Th2 response induced by ovalbumin (OVA) adsorbed to alum in wild-type and IL-18-deficient mice. Our results indicate that while endogenous IL-18 facilitates alum-induced IL-4 production, OVA-specific immunoglobulin G1 (IgG1) and IgE production remain unaffected. Furthermore, antigen-specific Th1 responses induced with alum/IL-12-adsorbed OVA were demonstrated to be highly IL-18 dependent. Despite these observations, injection of BALB/c mice with exogenous IL-18 adsorbed to alum/OVA did not alter IL-4 or interferon-γ production by T cells and had little effect on the relative production of IgG1/IgG2a antibody subclasses compared with alum/OVA inoculated mice. However, the previously described synergism between IL-12 and IL-18 in Th1 induction was evident as the Th1-promoting activity of alum/IL-12 against adsorbed OVA was greatly augmented by the coadministration of IL-18. These results indicate that while alum-induced IL-18 can facilitate Th2 induction, the addition of exogenous IL-18 cannot further enhance the alum-induced Th2 response.
Introduction
The induction of a T helper 2 (Th2)-dominated immune response remains a major limitation to the application of alum to modern vaccines. However, the mechanism(s) which allow alum to initiate Th2 responses against adsorbed antigens remain unclear, although they have been shown to be independent of the key Th2 associated cytokines, interleukin (IL)-4 or IL-13 and signalling via signal transducer and activator of transcription-6 (STAT-6).1 Nevertheless, previous studies have shown that coadsorption of IL-12 to alum gel elicits a switch in the alum-induced response from Th2 to Th1.2,3 These observations illustrate that adjuvants such as alum that are currently in clinical use have the potential to be improved. Consequently, an appreciation of the mechanisms of action of alum and alum/cytokine formulations would greatly facilitate the rational design of new safe adjuvants.
IL-18, a member of the IL-1 family was originally described as interferon-γ-inducing factor and has potent pro-inflammatory properties in conjunction with IL-12.4,5 Studies of the biological effects of IL-18 have highlighted its role in infectious disease and autoimmune conditions particularly through its ability to facilitate the induction of Th1 responses.6,7 However, recent reports indicate that IL-18 can also stimulate IL-4 production from basophils and CD4+ T cells and IL-13 production by mast cells.8,9 In addition, IL-18 has also been shown to indirectly induce B-cell isotype switching to IgE and together with its effects on Th2 cytokine production, has been demonstrated to play a role in allergic inflammation.10 In the absence of a role for IL-4 signalling1 the inductive mechanism for the production of Th2 responses by alum is uncertain. However, IL-18 clearly has the potential to fulfil this function. In order to characterize the role that endogenously synthesized IL-18 could play in the alum-induced Th2 response, we have analysed the alum-induced immune response against adsorbed ovalbumin (OVA) in IL-18-deficient mice. Furthermore, we have also examined the ability of exogenous IL-18 coadsorbed to alum/OVA to induce a similar switch in Th cell phenotype as that demonstrated previously with coadsorbed IL-12·2
Materials and methods
Adjuvant preparation
Alhydrogel (alum; purchased from Superfos BioSector a/s, Vedbaek, Denmark) was mixed with a predetermined quantity of highly purified ovalbumin (OVA; Worthington Biochemical Corporation, Lakewood, NJ) and incubated at room temperature for 30 min Recombinant IL-12 and IL-18 were purchased from R & D Systems (Oxford, UK).
Mice and inoculations
All mice were immunized subcutaneously into the footpad with 50 µl of OVA (100 µg) in phosphate-bufferd saline (PBS) or adsorbed to alum ± cytokines. rIL-12 or rIL-18 (1 µg/mouse) was coadsorbed to alum/OVA and inoculated into groups of four female, 8–10 week old, BALB/c mice (purchased from Harlan Olac, Oxford, UK). Boosting inoculations were performed in the same fashion 2 weeks later. IL-18−/− mice were constructed as described previously.7 IL-18−/− DBA/1 mice were back-crossed into the BALB/c mouse strain and greater than fifth generation mice were used for experiments. Homozygous (IL-18−/−) mice were obtained by intercrossing IL-18+/− BALB/c mice and progeny littermates were used for experiments. Groups of four male, 8–10 weeks old, IL-18−/− or wild type BALB/c mice were inoculated with alum/OVA or alum/OVA and IL-12 as described above. All animals were monitored in accordance with the guidelines of the Home Office, UK.
Determination of plasma antibody titres
Blood samples were taken from mice 4 weeks after primary inoculation with rIL-12 and rIL-18 adsorbed to alum. IL-18−/− and control mice also had blood samples taken at weeks 2 and 6 after the first immunization. Enzyme-linked immunosorbent assays (ELISA) were performed as described previously1 to detect OVA-specific IgG1, IgG2a and total IgE in plasma. Results are expressed as end-point dilutions where the end-point is determined as the final plasma dilution, which yields a higher absorbance than a negative control plasma sample, included in the assay. Levels of specific IgE were analysed using biotin labelled OVA as described previously11 and are expressed as optical densities. Comparisons between groups were performed using the Mann–Whitney U-test.
Lymph node culture
Cells isolated from the inguinal and popliteal lymph nodes (2 × 106/ml) were cultured with graded concentrations of OVA for up to 72 hr at 37°/5% CO2 in RPMI-1640 medium supplemented with 2 mm l-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin and 10% fetal calf serum (FCS; all Life Technologies/BRL, Paisley, UK). Proliferation assays were performed in triplicate in U-bottom 96-well plates (Corning Costar, NY). Supernatants from parallel triplicate cultures were stored at −70° until estimation of cytokine content by ELISA.
Cytokine assays
Murine interferon-γ (IFN-γ; R & D Systems), IL-4 and IL-2 (both Biosource, Nivelles, Belgium) were assayed by ELISA using paired antibodies according to the manufacturer's instructions. Lower limits of detection were as follows: IL-4 at 10 pg/ml, IL-2 at 20 pg/ml and IFN-γ at 20 pg/ml. Cytokine levels were compared using Student's t-test.
Results
Effect of exogenous cytokines on alum-induced Th1/Th2 responses
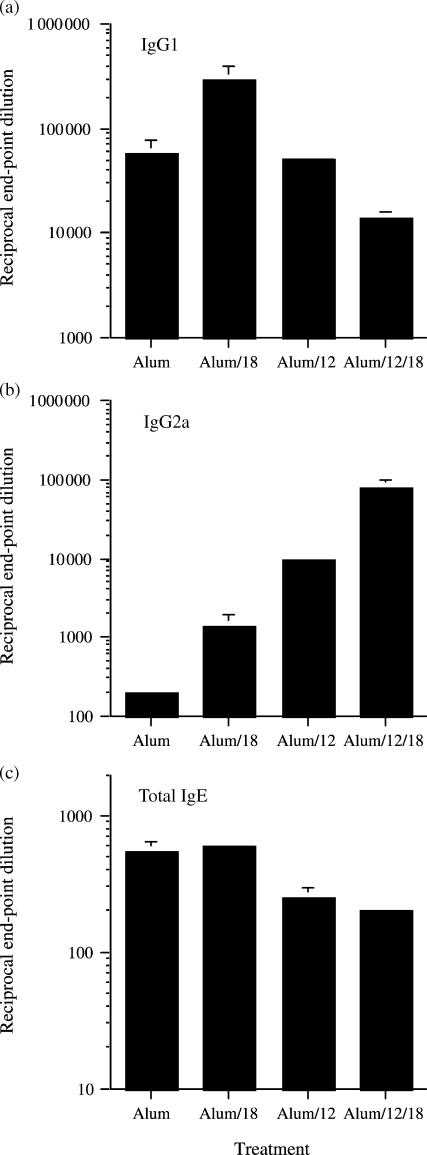
As described previously1 inoculation of BALB/c mice with OVA prepared in alum produced high titres of antigen-specific IgG1 (Fig. 1a) and negligible amounts of IgG2a (Fig. 1b), a phenotype typical of a polarized Th2 response. In the present study, IL-18 coadsorbed to alum/OVA (alum/IL-18/OVA) increased both OVA-specific IgG1 and IgG2a (P = 0·05) but had no effect on total IgE production (Fig. 1c) compared with alum/OVA-inoculated mice. Adsorption of IL-12 to alum/OVA (alum/IL-12/OVA) resulted in a bias towards a Th1 response with a significant increase (P = 0·001) in IgG2a and a significant decrease (P = 0·05) in IgE levels compared with alum/OVA-immunized mice. The extent of Th1 polarization obtained with alum/IL-12/OVA was greater than that seen with alum/IL-18/OVA because levels of IgG2a and IgE were significantly higher (P = 0·01) and lower (P = 0·05), respectively, in the alum/IL-12/OVA-immunized group. However, mice immunized with IL-12 and IL-18 coadsorbed to alum/OVA exhibited the most polarized Th1 response of all groups analysed with significantly more (P = 0·05) IgG2a being produced than that detected in the alum/IL-12/OVA treated mice.
Figure 1.
Plasma levels of OVA-specific IgG1 (a), IgG2a (b) and total IgE (c) from BALB/c mice were determined 2 weeks post boost with OVA adsorbed to alum ± cytokines. Antibody titres were determined by ELISA and are expressed as mean end-point dilution ± SEM (n = 4). Data is representative of two independent experiments and comparisons between groups were made using a Mann–Whitney U-test.
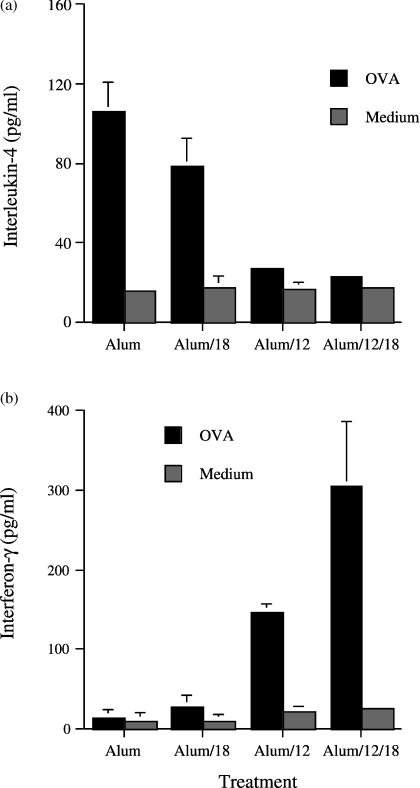
When cells from the draining lymph nodes of alum/OVA or alum/cytokine/OVA-treated mice were re-stimulated with antigen in vitro, no significant differences in the proliferative responses were detected between the three groups (data not shown). Cytokine analysis of supernatants from these cultures revealed that while alum/OVA and alum/IL-18/OVA-immunized mice produced significant levels of IL-4 compared with control (unstimulated) cells (P = 0·02), this was not the case with lymph nodes isolated from alum/IL-12/OVA-immunized mice (Fig. 2a). As previously demonstrated, lymph node cells from alum/OVA-inoculated BALB/c mice did not produce significant levels of IFN-γ and coadsorption of IL-18 to alum did not modify this response (Fig. 2b). In contrast, in alum/IL-12/OVA-treated mice, a significant amount of IFN-γ was produced on re-stimulation with antigen compared with both alum/OVA- and alum/IL-18/OVA-immunized mice (P = 0·001; Fig. 2b). However, production of IFN-γ by antigen-stimulated lymph node cells could be further increased (P = 0·05) by the addition of IL-18 to the alum/IL-12/OVA combination during immunization (Fig. 2b).
Figure 2.
In vitro production of IL-4 (a) and IFN-γ (b) by cultured lymph nodes removed from mice immunized with alum/OVA alone or coadsorbed with IL-18 or IL-12 or both IL-12 and IL-18. Cytokine analysis was performed on supernatants taken from cell cultures stimulated with graded concentrations of OVA after 72 hr. Values represent mean cytokine concentration as determined by ELISA ± SEM (n = 4). Data is representative of two independent experiments and comparisons between groups were performed by Student's t-test.
Analysis of the role of endogenous IL-18 on the Th2/Th1 response in alum- or alum/IL-12-inoculated mice
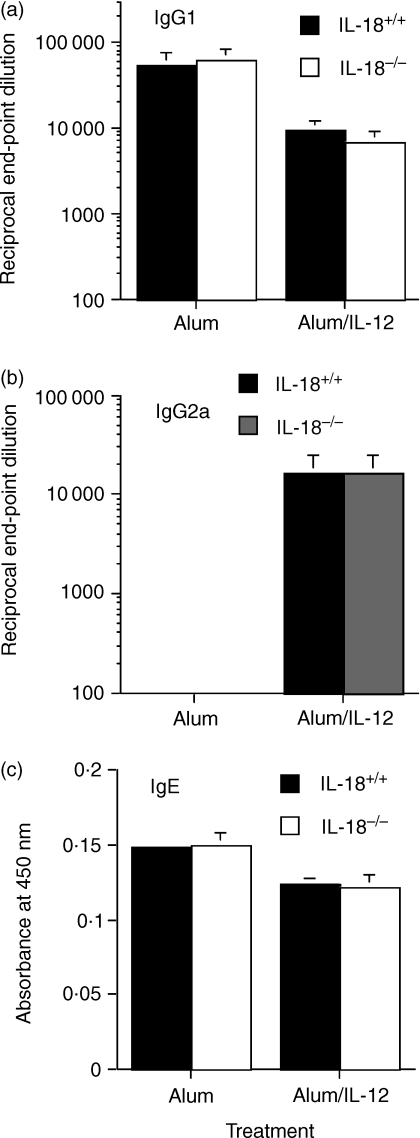
As previously shown, immunization of BALB/c mice with alum/OVA produced high levels of IgG1 and negligible amounts of IgG2a, a profile suggestive of a Th2 response (Figs 3a,b) whereas inoculation of mice with alum/IL-12/OVA produced antibody isotypes consistent with a Th1 response. Mice lacking IL-18 did not display any significant differences in IgG1 or IgG2a titres when immunized with either alum/OVA or alum/IL-12/OVA compared with wild-type mice (Figs 3a,b). Endogenous IL-18 production was also shown to be unimportant in alum-induced IgE production (Fig. 3c).
Figure 3.
Plasma levels of OVA-specific IgG1 (a), IgG2a (b) and IgE (c) from wild-type and IL-18-deficient BALB/c mice were determined 2 weeks post boost with OVA adsorbed to alum or both OVA and IL-12 adsorbed to alum. Values represent mean end-point dilution ± SEM (n = 4).
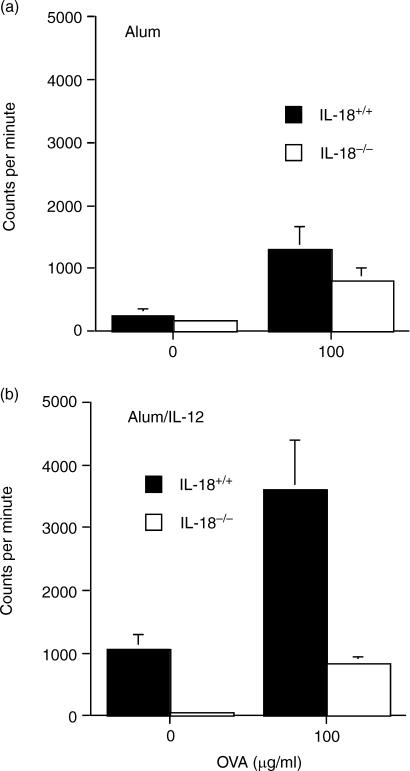
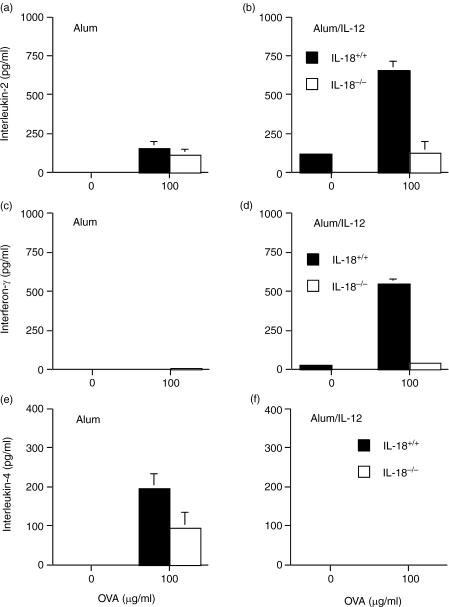
Lymph node cells isolated from alum/OVA-immunized IL-18−/− and control mice proliferated equally strongly in response to OVA (Fig. 4a). In marked contrast, when mice were inoculated with alum/IL-12/OVA, IL-18−/− mice had markedly reduced (P = 0·01) OVA-specific proliferation at the dose of OVA tested compared with control mice (Fig. 4b). When lymph node cells were stimulated with mitogen (concanavalin A), significantly reduced levels of proliferation were also observed in unimmunized IL-18-deficient compared with wild-type mice (data not shown), suggesting that IL-18−/− cells may have a reduced ability to proliferate in response to certain stimuli. The OVA-specific production of the Th1 cytokines IFN-γ and IL-2 by draining lymph node cells was significantly increased (both P < 0·001) by the addition of IL-12 to alum/OVA (Fig. 5a–d). While the absence of endogenous IL-18 had no effect on lymph node cytokine production in animals inoculated with alum/OVA alone (Fig. 5a and c), the production of IFN-γ and IL-2 in animals inoculated with alum/IL-12/OVA was in large part dependent on endogenous IL-18 (Fig. 5b,d). Thus, IFN-γ and IL-2 production was significantly reduced (P < 0·001 and P < 0·01, respectively) in IL-18−/− animals inoculated with alum/IL-12/OVA than wild-type mice. Furthermore, lymph nodes from alum/OVA inoculated mice (Fig. 5e), unlike those inoculated with alum/IL-12/OVA (Fig. 5f) produced the Th2 cytokine IL-4 and this was partially dependent on endogenous IL-18 (P = 0·025). The lower levels of IFN-γ and IL-2 cytokine production seen in alum/IL-12/OVA immunized IL-18−/− versus wild-type mice may reflect a general reduction in responsiveness of Th1-type cells in these mice, rather than a switch to Th2, as proliferation was also reduced and IL-4 production was not increased under these conditions.
Figure 4.
Comparison of proliferative responses to OVA in vitro in wild type and IL-18 knockout mice immunized with OVA adsorbed to alum (a) or OVA and IL-12 adsorbed to alum (b). Lymph node cells were incubated with graded concentrations of OVA or medium (unstimulated) and measured as 3H-thymidine incorporation at 96 hr. Values are the mean of three independent cultures set up from individual mice (n = 4).
Figure 5.
In vitro production of IL-2 (a, b), IFN-γ (c, d) and IL-4 (e, f) by cultured lymph node cells removed from wild-type and IL-18-deficient mice immunized with OVA adsorbed to alum (a, c and e) or OVA and IL-12 adsorbed to alum (b, d and f).
Discussion
Previous studies have clearly shown that subcutaneous administration of antigen prepared in alum induces a Th2-polarized response.1 While a number of in vitro studies have demonstrated a fundamental role for IL-4 in the generation and maintenance of Th2 responses, in vivo analysis has demonstrated that the Th2 response induced by alum is independent not only of IL-41,12 but of other Th2-associated cytokines such as IL-613 and IL-13.1 Indeed, these previous studies have indicated that the major role of alum induced IL-4 production is to down-regulate the Th1 response. Therefore the question remains, how do alum adjuvants induce Th2 responses? Recently, it has been reported that IL-18 can substantially enhance IL-4 production from both NK1 1+ T cells14 and induce IgE expression by B cells.9 Furthermore, in models of Th2 induction, such as the ragweed mouse model of allergic asthma, IL-18 has been shown to have potent Th2-stimulating capabilities.10 In our current studies, the production of signature Th2 antibody isotypes was similar in both wild-type and IL-18-deficient mice following inoculation with OVA prepared in alum. However, IL-18-deficient mice had reduced IL-4 production by lymph node cells following inoculation with OVA prepared in alum compared with wild-type mice. The failure of the decrease in IL-4 to be reflected in decreased serum IgG1, may be a result of the poor correlation between this antibody subclass and IL-4 production. In fact, previous studies have demonstrated that alum can induce IgG1 production independently of IL-4.1,12 Therefore, our data suggest that IL-18-mediated signalling may facilitate some aspects of the alum-induced Th2 response. This result appears to corroborate previous studies demonstrating that application of exogenous IL-18 may allow polarization of the response to Th2, although this is the first study in IL-18-deficient mice that supports a role for endogenous production of this cytokine in Th2 induction. Interestingly, a significant role was also found for endogenous IL-18 in a Th1-polarized response, using IL-12 coadsorbed with antigen to alum gels. Under these immunization conditions, there was both a reduction in Th1 cytokine production and proliferation of T cells. Although the proliferative responses of IL-18-deficient T cells were also reduced compared with wild-type cells when incubated with mitogen, antigen specific responses were not reduced when alum/OVA was the immunising stimulus. Therefore, there was not a global reduction in the ability of IL-18-deficient T cells to proliferate, but more likely, a specific inhibition of Th1 proliferation and cytokine production. These results correlate well with a recent study showing that IL-18 knockout mice had reduced incidence and severity of collagen-induced arthritis, which was associated with a deficiency in collagen-specific proliferation and IL-2 and IFN-γ production.7
Previous studies have demonstrated that adsorption of cytokines onto alum gels together with antigen, can influence the Th1/Th2 profile of the induced response. This suggests that the Th2 profile of alum, which has been a major obstacle to its widespread use in modern vaccines, can be modified by the rational application of immunomodulators, in particular IL-12. Indeed, studies in mice2 and monkeys3 have demonstrated that this approach works with putative vaccine antigens, although the reactogenicity of IL-12 may be an obstacle to the use of this adjuvant in humans.15,16 As our studies in cytokine-deficient mice demonstrated that IL-18 plays a significant role in the Th1 response induced by IL-12 adsorbed to alum, we decided to examine whether IL-18 could be applied to alum in a similar manner. However, the lack of a clear change in cytokine profile following this regime indicates that IL-18 cannot act in isolation to alter the Th1 or Th2 profile of the developing alum-induced immune response. Although levels of the Th2-associated antibody subclass, IgG1, could be increased on addition of IL-18 to alum, levels of the Th1-associated IgG2a subclass were similarly increased, indicating that IL-18 may act to increase antibody titres in general rather than inducing a switch in the relative Th1/Th2 response. In contrast, the addition of an equivalent amount of IL-12 to alum clearly produced a switch in the OVA-specific immune response from Th2 towards a Th1 type, as has been described previously.2 Our data also indicate that with two doses of cytokine adsorbed to alum/OVA, IL-12 inhibits total plasma IgE levels while IL-18 alone has no effect. Other studies have recently shown that treatment of mice with exogenous IL-18 can induce Th2 responses, in particular IgE production.8 Although the doses of IL-18 required to induce these responses were often higher than those used in the present study and were injected on several occasions, the adsorption of cytokines to alum gel enhances their in vivo effectiveness,2 presumably by delivering the cytokine more effectively: for example, in association with antigen or by creating a slow release depot of cytokine. Furthermore, the IL-18 adsorbed to alum was clearly available at biologically active levels as coadministration of similar quantities of IL-18 in combination with IL-12 was able to further enhance the previously reported alum/IL-12-induced Th1 response.17,18 Collectively, our results suggest that addition of IL-18 to alum does not act to polarize the alum induced response towards either a Th1 or a Th2 response. However, while alum/IL-12 can induce Th1 responses, IL-18 can synergize with this combination to induce enhanced Th1 responses.
In conclusion, our data clearly demonstrate that the induction of a Th1 response by IL-12 coadsorbed to alum/OVA is facilitated by endogenous IL-18 and this response can be further enhanced by the addition of exogenous IL-18. However, while endogenous IL-18 also facilitates the production of IL-4 during the alum induced Th2 response, other aspects of the Th2 response such as IgE production were not increased and the addition of exogenous IL-18 could not enhance these responses. Therefore, further study will be necessary to characterize the STAT-6-independent signalling events that result in the Th2-promoting activity of alum adjuvant.
Acknowledgments
J.M.B. is in receipt of a Wellcome Trust Career Development Fellowship. J.A. was in receipt of Wellcome Trust sponsored Research Leave.
References
- 1.Brewer JM, Conacher M, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen specific Th2 responses in the absence of IL-4 or IL-13 mediated signalling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 2.Jankovic D, Caspar P, Zweig M, Garcia-Moll M, Showalter SD, Vogel FR, Sher A. Adsorption to aluminum hydroxide promotes the activity of IL-12 as an adjuvant for antibody as well as type 1 cytokine responses to HIV-1 gp120. J Immunol. 1997;159:2409–17. [PubMed] [Google Scholar]
- 3.Kenney RT, Sacks DL, Sypek JP, Vilela L, Gam AA, Evans-Davis K. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J Immunol. 1999;163:4481–8. [PubMed] [Google Scholar]
- 4.Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 5.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–64. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 6.Ohkusu K, Yoshimoto T, Takeda K, et al. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect Immun. 2000;68:2449–56. doi: 10.1128/iai.68.5.2449-2456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei XQ, Leung BP, Arthur HM, McInnes IB, Liew FY. Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J Immunol. 2001;166:517–21. doi: 10.4049/jimmunol.166.1.517. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimoto T, Tsutsui H, Tominaga K, Hoshino K, Okamura H, Akira S, Paul WE, Nakanishi K. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci USA. 1999;96:13962–6. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimoto T, Mizutani H, Tsutsui H, et al. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000;1:132–7. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 10.Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam R, Kurimoto M, Sur S. IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164:2701–10. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 11.Burstein HJ, Shea CM, Abbas AK. Aqueous antigens induce in vivo tolerance selectively in IL-2- and IFN-gamma- producing (Th1) cells. J Immunol. 1992;148:3687–91. [PubMed] [Google Scholar]
- 12.Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4 deficient mice, Alum not only generates T helper-1 responses equivalent to Freund's complete adjuvant, but continues to induce T helper-2 cytokine production. Eur J Immunol. 1996;26:2062–6. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 13.Brewer JM, Conacher M, Gaffney M, Douglas M, Bluethmann H, Alexander J. Neither interleukin-6 nor signalling via tumour necrosis factor receptor-1 contribute to the adjuvant activity of alum and Freund's adjuvant. Immunol. 1998;93:41–8. doi: 10.1046/j.1365-2567.1998.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leite-d-e-Moraes MC, Hameg A, Pacilio M, et al. IL-18 enhances IL-4 production by ligand-activated NKT lymphocytes: a pro-Th2 effect of IL-18 exerted through NKT cells. J Immunol. 2001;166:945–51. doi: 10.4049/jimmunol.166.2.945. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 16.Atkins MB, Robertson MJ, Gordon M, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3:409–17. [PubMed] [Google Scholar]
- 17.Leung BP, McInnes IB, Esfandiari E, Wei XQ, Liew FY. Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. J Immunol. 2000;164:6495–502. doi: 10.4049/jimmunol.164.12.6495. [DOI] [PubMed] [Google Scholar]
- 18.Eberl M, Beck E, Coulson PS, Okamura H, Wilson RA, Mountford AP. IL-18 potentiates the adjuvant properties of IL-12 in the induction of a strong Th1 type immune response against a recombinant antigen. Vaccine. 2000;18:2002–8. doi: 10.1016/s0264-410x(99)00532-0. [DOI] [PubMed] [Google Scholar]