Abstract
Interleukin-12 (IL-12) is essential to resistance to Trypanosoma cruzi infection because it stimulates the synthesis of interferon-γ (IFN-γ) that activates macrophages to a parasiticidal effect. Investigation of mice deprived of IL-12 genes (IL-12 knockout mice) has confirmed the important role of IL-12 and IFN-γ in controlling parasitism in T. cruzi infection. However, it has not yet been addressed whether a shift towards a T helper type 2 (Th2) pattern of cytokine response occurred in these mice that might have contributed to the aggravation of the infection caused by IL-12 deprivation. We examined the course of T. cruzi (Y strain) infection and the regulation of cytokine responses and nitric oxide production in C57BL/6 IL-12 p40-knockout mice. The mutant mice were extremely susceptible to the infection as evidenced by increased parasitaemia, tissue parasitism and mortality in comparison with the control C57BL/6 mouse strain (wild-type) that is resistant to T. cruzi. A severe depletion of parasite-antigen-specific IFN-γ response, without an increase in IL-4 or IL-10 production, accompanied by reduced levels of nitric oxide production was observed in IL-12 knockout mice. We found no evidence of a shift towards a Th2-type cytokine response. In IL-12 knockout mice, the residual IFN-γ production is down-regulated by IL-10 but not by IL-4 and nitric oxide production is stimulated by tumour necrosis factor-α. Parasite-specific immunoglobulin G1 antibody levels were similar in IL-12 knockout and wild-type mice, whereas IL-12 knockout mice had much higher levels of immunoglobulin G2b.
Introduction
Trypanosoma cruzi is the causative agent of Chagas' disease in man. This digenetic protozoon determines a systemic infection in man and in other mammals that is controlled, although not completely eliminated, by T-cell-dependent immune responses. Control of parasitism in the acute phase of infection is critically dependent on intracellular killing by cytokine-activated macrophages.
The cytokine interleukin-12 (IL-12) stimulates interferon-γ (IFN-γ) production by natural killer cells that is an important mechanism of innate immunity and is also essential to the development of type 1 cytokine-producing T-lymphocyte populations that will further supply IFN-γ as adaptive immunity is activated. IL-12 participates in the resistance to several intracellular pathogens including T. cruzi.1,2 It is also a growth factor for natural killer and T lymphocytes and we have recently shown that endogenous IL-12 is stimulatory of parasite-antigen-specific cell proliferation in the first week of murine T. cruzi infection.3
Treatment of mice with anti-IFN-γ, anti-tumour necrosis factor (TNF) or anti-IL-12 neutralizing monoclonal antibodies (mAbs) leads to aggravation of T. cruzi infection reinforcing the importance of these cytokines in the in vivo resistance to this parasite.4–7 Mice treated with anti-IL-12 neutralizing mAb had reduced IFN-γ synthesis by spleen cells indicating that IFN-γ production during infection depends on IL-12.5 Moreover, lower IFN-γ production correlated with lower nitric oxide (NO) production and aggravation of parasitism.5 Treatment with anti-IL-12 mAb of mice that have disrupted recombinase-activating genes (RAG knockout), thus lacking B and T cells, aggravated T. cruzi infection, clearly demonstrating a protective role for IL-12 in innate immunity.5 However, it is not clear from the in vivo neutralizing experiments in the intact host whether a shift towards a T helper type 2 (Th2) pattern of cytokine response occurred that might have contributed to the aggravation of the infection caused by IL-12 and IFN-γ reduction. Moreover, in this type of experiment, cytokine depletion is not complete and a putative compensatory effect by other Th1 stimulating cytokines is difficult to assess. Recently, the study of mice deprived of IL-12 genes (IL-12 knockout mice) has confirmed the important roles of IL-12 and IFN-γ in controlling parasitism in T. cruzi infection.8 In addition, treatment of T. cruzi-infected IL-12 knockout mice with anti-IL-18 mAbs further reduced serum IFN-γ levels and increased parasitaemia levels.9 However, so far, the question of whether a shift towards higher production of Th2 cytokines occurs in infected IL-12 knockout mice was not addressed.
In the present study, we examined the course of T. cruzi (strain Y) infection and cytokine responses in C57BL/6 mice deprived of IL-12 p40 genes (IL-12 knockout). We show that the mutant mice are extremely susceptible to the infection, as shown by increased parasitaemia, tissue parasitism and mortality in comparison with the control C57BL/6 mouse strain (wild-type) that is resistant to T. cruzi. A severe depletion of parasite-antigen-specific IFN-γ response, without increase in IL-4 or IL-10 production, accompanied by reduced levels of NO production was observed in the splenic compartment of IL-12 knockout mice.
Materials and methods
Animals and infection with T. cruzi
Female C57BL/6 IL-12 p40–/– (IL-12 knockout) and C57BL/6 IL-12 p40+/+ (wild-type) mice (6–8-week-old, specific pathogen-free), were bred in the specific pathogen-free mouse breeding facilities (Biotério de Camundongos Isogênicos) of the Department of Immunology, ICB/USP. The mice were housed five to a micro-isolator cage and offered food and water ad libitum. All animal procedures were performed in accordance with the principles of the Brazilian Code for the Use of Laboratory Animals and the project was approved by the Ethical Committee for Animal Research from ICB/USP. Groups of six or eight animals were infected intraperitoneally with T. cruzi Y strain blood trypomastigotes obtained as previously described.10 Wild-type and IL-12 knockout mice were infected with 5000 blood trypomastigotes. Parasitaemia counts were performed by counting the parasites in 5 μl of citrated blood obtained from the lateral tail veins. Mortality was evaluated by daily inspection of the cages.
Cell cultures and anti-cytokine mAbs
Spleen cell suspensions were prepared from wild-type and IL-12 knockout mice on days 7 and 14 after the infection. For each experiment, the spleen cells from three mice were pooled. The cells were cultured in 24-well culture plates at a density of 5 × 106/ml, in RPMI-1640 containing 5% fetal calf serum, 2 mm l-glutamine, 0·05 mm 2-mercaptoethanol, and penicillin and streptomycin (100 U/ml and 100 μg/ml, respectively) (Sigma Chemical Co., St Louis, MO). The cultures were stimulated with plate-bound anti-CD3 mAb (145-2C11, Pharmingen, San Diego, CA). Individual wells were coated with 0·5 ml of anti-CD3 mAb diluted to 10 μg/ml in 0·01 m phosphate-buffered saline (PBS), pH 7·0, for 1 hr at 37° and washed three times with PBS before the cells were added.11 Alternatively, spleen cell cultures were stimulated with 5 × 106 freeze–thawed tissue culture trypomastigotes [trypomastigotes' antigen (T-Ag)] prepared as described previously.12 Treatment of spleen cell cultures with anti-cytokine mAbs was performed by adding at the beginning of the cultures the following mAbs: 2A5 (anti-IL-10), 11B11 (anti-IL-4), XT22.11 (anti-TNF-α) or GL113 [immunoglobulin G1 (IgG1) isotype control] at 20 μg/ml; these mAbs were obtained in our laboratory by growing the rat anti-mouse hybridomas donated by Dr Robert Coffman and the DNAX Research Institute (Palo Alto, CA). The 51817.111 (anti-IL-18) mAb was bought from R & D Systems, Minneapolis, MN and used at 10 μg/ml. Supernatants were collected after 20 and 48 hr to measure the levels of cytokines and nitrite.
Detection of cytokines and nitrite production
The cytokine levels in the duplicate culture supernatants were detected by two-site sandwich enzyme-linked immunosorbent assay as described.11 IFN-γ, IL-10 and NO were determined in the 48-hr supernatants and IL-4 was determined in both the 20-hr and the 48-hr supernatants. The minimum concentration of each cytokine detectable in the conditions of our assays is indicated in parentheses: IFN-γ (0·78 ng/ml); IL-10 (0·312 U/ml) and IL-4 (38 pg/ml).
The nitrite content in the supernatants was measured by adding 50 μl of freshly prepared Griess reagent to 50 μl of the sample in 96-well plates and reading the optical density (OD) at 540 nm 10 min later by comparisons with the OD curves of serial dilutions of sodium nitrite in complete culture medium.13 The minimal detectable concentration was 1·56 μm.
The results of cytokine and nitrite determinations are shown as arithmetic means ± standard deviations from duplicate cultures.
Histopathology
The heart and brain from wild-type and IL-12 knockout mice on days 7 and 14 after infection were fixed in neutral 10% formalin, embedded in paraffin, sectioned, stained with haematoxylin & eosin and examined by light microscopy. Tissue parasitism was scored by counting the total number of amastigote nests using a 40× objective. Four semi-serial sections were counted for each animal. The results are shown as arithmetic means ± standard deviations of the total number of amastigote nests found in the sections obtained from five mice per group.
Parasite-specific IgG1, IgG2a and IgG2b
For this assay, microtitre plates (Falcon, Lincoln Park, NJ) were coated with trypomastigote antigens (15 μg/ml in carbonate buffer, 50 μl/well) and incubated overnight at 4°. After blocking with 1% bovine serum albumin in 10 mm PBS (PBS-BSA), pH 7·4, for 2 hr at room temperature, 50 μl of the appropriate sera diluted in PBS-BSA was added and incubated for 2 hr at room temperature. The plates were then washed five times with 0·05% PBS-Tween-20 and rabbit anti-mouse isotype-specific antibodies to IgG1, IgG2a and IgG2b (50 μl/well; Zymed, South San Francisco, CA) for 2 hr at room temperature. Following three washes the plates were incubated with peroxidase-labelled anti-mouse antibody (Sigma Chemical Co) for 1 hr. The plates were subsequently washed five times with PBS-Tween-20 and the reaction was developed by addition of H2O2 and orthophenylenediamine (OPD) and incubation for 10 min at room temperature. The reaction was stopped by the addition of 50 µl of 2·5 N H2SO4 and the OD of the resulting colour was read at 492 nm. The results are reported as the mean OD accompanied by the SD obtained from the sera from five mice. Non-specific reactions had OD < 0·05.
Data analysis
Means of control and experimental groups were compared using the Student's t-test. Comparisons of multiple groups were performed by analysis of variance (anova) and Bonferroni's test. Differences were considered significant when P < 0·05. Statistical analysis was performed using Graphpad Instat 3·0.
Results
T. cruzi-infected IL-12 knockout mice develop higher parasitaemia and mortality than control wild-type C57BL/6 mice
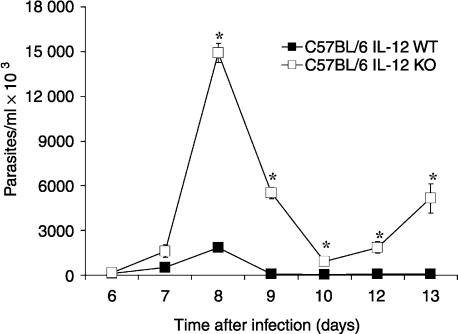
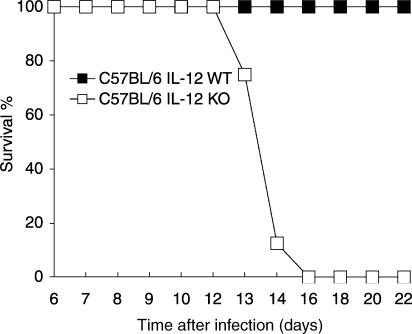
C57BL/6 mice are considered relatively resistant to infection by the Y strain of T. cruzi. When infected with an inoculum of 5000 parasites, these mice developed patent parasitaemia by day 6, reaching the highest counts by day 8 of infection; thereafter, parasite blood counts remained very low (Fig. 1). All the mice survived beyond the acute phase of infection (Fig. 2) and infection persisted as a chronic phase throughout the animals' lifetime. In contrast, mice of the same genetic background deprived of IL-12 p40 genes (IL-12 knockout) developed much higher levels of parasitaemia; although capable of controlling blood parasite counts, mortality by the 16th day of infection was 100%.
Figure 1.
Parasite counts in the blood from C57BL/6 IL-12 knockout mice and in wild-type C57BL/6 controls infected intraperitoneally with 5000 blood forms of Trypanosoma cruzi (Y strain). Arithmetic means from eight mice per group ± SD; *P < 0·05.
Figure 2.
Survival rates of C57BL/6 IL-12 knockout and wild-type C57BL/6 mice infected intraperitoneally with 5000 blood forms of T. cruzi (Y strain). Mean per cent survival rates of eight mice per group.
Failure of IL-12 knockout mice to control infection is associated with low production of IFN-γ and of NO but not with elevated IL-4 or IL-10 synthesis
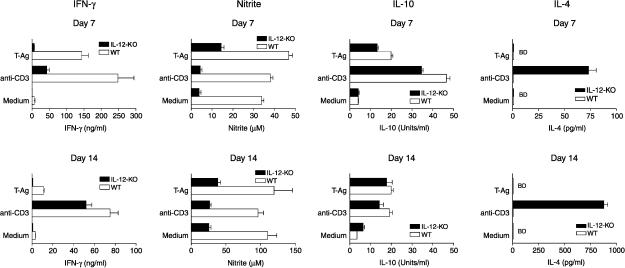
Synthesis of IFN-γ by spleen cells from infected IL-12 knockout mice to parasite antigen (T-Ag) stimulation was severely reduced (about 6 ng/ml on day 7 and 1·5 ng/ml on day 14 of infection) compared to that of similarly infected wild-type mice (Fig. 3). Production of IFN-γ by T-Ag-stimulated cultures from uninfected IL-12 knockout and wild-type mice was 0·78 ng/ml and 1·78 ng/ml, respectively, whereas in unstimulated cultures from either IL-12 knockout or wild-type mice the levels detected were 0·78 ng/ml. By day 14 of infection, T-Ag-stimulated IFN-γ synthesis by IL-12 knockout mice remained low, although on polyclonal T-cell stimulation by anti-CD3 it was not significantly different from wild-type mice, suggesting that the lack of IL-12 affected mostly antigen-induced responses.
Figure 3.
Production of the cytokines IFN-γ, IL-10 and IL-4 and of nitrite by spleen cells from C57BL/6 IL-12 knockout and wild-type C57BL/6 mice infected with T. cruzi. Cultures were performed on days 7 and 14 after infection and were kept only in culture medium or stimulated with parasite-antigen, T-Ag, or with plate-bound anti-CD3. Cytokines and nitrite were measured in the supernatants. Results are expressed as the arithmetic means from duplicate cultures ± SD. The results are representative of three experiments.
Levels of nitrite, indicative of NO production, were already elevated by day 7 of infection in spleen cell cultures from infected wild-type mice kept only in culture medium and an increase of about 50% in NO production was seen in T-Ag-stimulated cultures (Fig. 3). By day 14 of infection, NO production by cultures kept in culture medium or by T-Ag-stimulated cultures had increased to about three-fold the amount seen on day 7 of infection. In contrast to the high levels of NO production in wild-type mice, mice lacking IL-12 produced much lower levels of NO in T-Ag-stimulated cultures and in cultures that did not receive additional exogenous stimulation (7th and 14th day of infection); nevertheless, even in IL-12 knockout mice, NO production increased by day 14 compared to day 7 of infection. It should be pointed out that cultures that are maintained only in medium without additional in vitro stimulation are derived from T. cruzi-infected mice and therefore contain parasite antigens in infected macrophages or carried and/or presented by dendritic or B cells. The observation that variations in NO production levels do not always correlate with IFN-γ production levels is indicative that other cytokines or parasite products participate in the regulation of inducible NO synthesis.
As IL-12 knockout mice, besides lacking IL-12, have much lower IFN-γ production compared to wild-type mice, it was pertinent to ask whether the former, when infected with T. cruzi, would overproduce IL-10 and/or IL-4 indicative of a shift towards a Th2-type response. As shown in Fig. 3, IL-10 production levels in spleen cell cultures from infected IL-12 knockout mice were about the same as those observed in spleen cell cultures from infected wild-type mice, regardless of the stimulus used in the cultures. IL-4 production in cultures derived from IL-12 knockout or wild-type mice, whether kept in medium or stimulated with parasite-antigen, was below the detection levels of our assay (38 pg/ml). Only in cultures from infected IL-12 knockout spleen cells stimulated with anti-CD3 was IL-4 detected and no IL-4 production was measurable in similarly stimulated cultures from infected wild-type mice. Thus, spleen cells from infected IL-12 knockout mice, obtained ex vivo and cultured either only in medium or further stimulated with parasite antigen in vitro did not produce more IL-10 or IL-4 than similarly processed cells derived from wild-type mice.
Regulation of IFN-γ production levels by IL-10 and TNF-α in wild-type and in IL-12 knockout infected mice
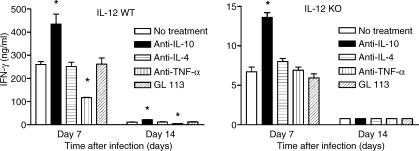
IFN-γ production in intact mice is primarily dependent on stimulation by IL-12. Treatment of spleen cell cultures with high (100 μg) concentrations of the anti-IL-12 neutralizing C17.8 mAb reduced IFN-γ production by 80–90% (data not shown). IL-12 knockout mice had even more intense reduction of IFN-γ production as shown in Figs 3 and 4. It was of interest to verify how IFN-γ production levels would be regulated in wild-type and in IL-12 knockout mice. In addition to IL-12, TNF-α also stimulated IFN-γ synthesis in wild-type T. cruzi-infected mice because neutralization by anti-TNF-α mAb reduced IFN-γ levels by 50% (Fig. 4). However, in the absence of IL-12 (IL-12 knockout mice), neutralization of TNF-α did not modify IFN-γ production. Regarding the participation of IL-18 as stimulator of IFN-γ production, we used the neutralizing anti-IL-18 mAb 51817.111 at 10 μg/ml (a dose that inhibits 100% of the biological activities of 15 ng/ml of IL-18) and did not observe an effect on IFN-γ production levels in spleen cell cultures from T. cruzi-infected wild-type or IL-12 knockout mice (data not shown). In relation to cytokines that may act as negative regulators of IFN-γ synthesis, IL-10 neutralization in culture significantly increased IFN-γ levels in T-Ag-stimulated cultures from infected wild-type and IL-12 knockout mice, while anti-IL-4 treatment had no effect in either situation (Fig. 4). Thus, although IL-10 production was not higher in IL-12 knockout mice versus wild-type mice (cf. Fig. 3), yet IL-10 exerted a suppressive effect on IFN-γ synthesis in IL-12 knockout mice.
Figure 4.
Regulation by cytokines of splenic cells IFN-γ production in C57BL/6 IL-12 knockout and wild-type C57BL/6 mice infected with T. cruzi. Cultures were performed on days 7 and 14 after infection and stimulated with parasite-antigen, T-Ag. At the beginning of the cultures the neutralizing anti-cytokine mAbs, anti-IL-10, anti-IL-4 and anti-TNF-α, were added; GL.113 was used as isotype control mAb. The supernatants were harvested after 72 hr and IFN-γ was measured by enzyme-linked immunosorbent assay. Results are expressed as the arithmetic means from duplicate cultures ± SD. *P < 0·05. The results are representative of three experiments.
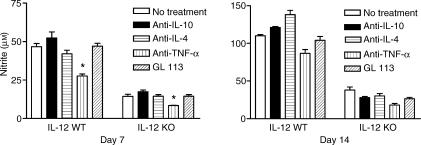
TNF-α stimulates NO production in wild-type and in IL-12 knockout infected mice
It has been previously shown that NO production by macrophages from T. cruzi-infected mice is dependent on activation by IFN-γ and TNF-α.14,15 Although IL-12 knockout mice had a severe reduction of IFN-γ production levels, reduction of NO production was not as severe (Figs 3 and 5). TNF-α stimulated NO production in both wild-type and IL-12 knockout mice on day 7 after infection, as shown by the reduction of nitrite levels in the cultures treated with neutralizing anti-TNF mAb (Fig. 5). Thus, TNF-α may partly supply the stimulus for NO production in IL-12 knockout mice. The negative regulatory action of IL-10 or IL-4 on NO synthesis by activated macrophages has been described before.16,17 However, neutralization of IL-10 or IL-4 did not significantly modify NO production levels in T-Ag-stimulated spleen cell cultures from either wild-type or IL-12 knockout mice infected with T. cruzi (Fig. 5).
Figure 5.
Regulation by cytokines of splenic cell nitrite production in C57BL/6 IL-12 knockout and wild-type C57BL/6 mice infected with T. cruzi. Cultures were performed on days 7 and 14 after infection and stimulated with parasite-antigen, T-Ag. At the beginning of the cultures the neutralizing anti-cytokine mAbs, anti-IL-10, anti-IL-4 and anti-TNF-α, were added; GL.113 was used as isotype control mAb. The supernatants were harvested after 72 hr and nitrite was measured by the Griess reaction. Results are expressed as the arithmetic means from duplicates ± SD. *P < 0·05. The results are representative of three experiments.
Parasitism in organs is higher in IL-12 knockout T. cruzi-infected mice but acute-phase inflammation is much reduced
Parasitism in the heart of wild-type mice was much more scarce than in IL-12 knockout mice, in which intensely parasitized cardiac fibres (parasite nests) were frequent. The number of ‘nests’ was counted in heart sections of infected IL-12 knockout and wild-type mice. Organs obtained on day 7 of infection showed no difference in mean nest counts between wild-type and IL-12 knockout: 0·6 ± 0·4 versus 1·6 ± 0·8, respectively. In contrast, by day 14 of infection, heart parasitism was 9·2 ± 3·3 in wild-type versus 997·6 ± 262·6 in IL-12 knockout mice, that is about 100-fold more intense in IL-12-deficient mice. An obvious difference was also seen in the degree of inflammatory infiltrate in the tissues. An intense, predominantly mononuclear cell infiltrate was observed in the hearts from wild-type mice, whereas the infiltrate was almost absent in the myocardium of IL-12 knockout mice, save for occasional areas of polymorphonuclear neutrophil accumulation near a ruptured parasite nest (data not shown). The brains obtained from infected wild-type and knockout mice were also examined. We did not find parasites or inflammatory infiltrate in the brains from infected IL-12 knockout or wild-type mice on day 14 after infection, when parasitism or inflammation of the heart was very intense (data not shown).
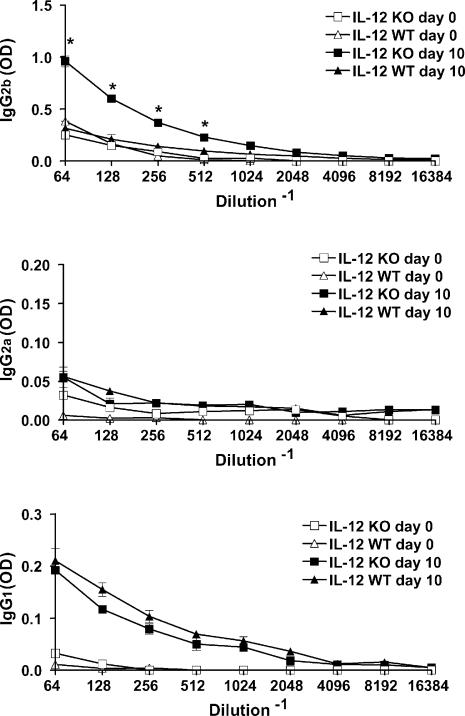
IL-12 knockout mice develop a higher IgG2b parasite-specific antibody response in the acute phase of infection
We tested the sera from infected IL-12 knockout and wild-type mice for anti-T. cruzi antibody levels during the first 2 weeks of infection. Results obtained on day 10 of infection are shown in Fig. 6. IgG1 antibody titres increased to similar levels in infected wild-type and IL-12 knockout mice in comparison to uninfected mice, whereas no increase was seen in specific IgG2a in the sera from infected wild-type or IL-12 knockout mice. However, IgG2b-specific antibody was clearly augmented only in sera from infected IL-12 knockout mice; no increase in IgG2b antibody levels was seen in the sera from infected wild-type mice.
Figure 6.
Parasite-specific antibodies determination in the sera from day 10 infected C57BL/6 IL-12 knockout and wild-type C57BL/6 mice. Day 0 refers to uninfected mice. IgG1, IgG2a and IgG2b antibody levels are expressed by the mean OD accompanied by the SD, obtained in the sera from three mice at the reciprocal of the tested serum dilutions. *P < 0·05.
Discussion
Our data showing increased parasitaemia levels and mortality of T. cruzi (Y strain)-infected IL-12 p40–/– mice compared to wild-type C57BL/6 mice are in agreement with the aggravation of infection reported in either IL-12 p40–/– mice infected with the Colombian strain of T. cruzi8 and in IL-12 p35–/– mice infected with the Tulahúen strain.9 Interestingly, we found that while both male and female IL-12 p40–/– C57BL/6 mice succumb to the infection equally and have very intense tissue parasitism, females, but not males (data not shown), control parasitaemia levels. The resistance to T. cruzi (Y strain) infection is higher in females of several mouse strains and of the wild rodent Calomys callosus and is related to female gonadal hormones.18,19 Parasite-specific antibodies participate in the removal of parasites from circulation and thus affect parasitaemia levels; IL-12 knockout mice had, on day 10 of infection, increased serum anti-T. cruzi IgG1 and IgG2b that are effective in clearance of blood trypomastigotes.20
In our model of infection, parasite-anitgen-stimulated IFN-γ production was markedly low in IL-12 knockout mice as compared with wild-type mice at both infection days 7 (6 versus 150 ng/ml) and 14 (1·5 versus 12 ng/ml). We did not find any evidence of increased production of IL-10 or IL-4 by spleen cells from infected IL-12 knockout mice in cultures kept only in medium (subject to endogenous stimulation by parasites or their products) or stimulated by added T-Ag as a recall response. Thus, there was no evidence that the lack of IL-12 would shift the cytokine pattern of response towards a Th2 type. The only circumstance in which we observed increased IL-4 production in IL-12 knockout spleen cell cultures, as compared to the very low levels in wild-type mice, was when the cultures were stimulated with plate-bound anti-CD3, that acts as a polyclonal T-cell stimulator. Levels of IL-10, however, were not different between IL-12 knockout and wild-type spleen cell cultures stimulated with anti-CD3 mAb. This is in accordance with a previous observation that anti-CD3 is a stronger stimulator of IL-4 production and that neutralization of IFN-γ in these cultures increases IL-4 levels.11 Moreover, we could not find significant differences in the levels of IgG1 anti-T. cruzi antibodies between IL-12 knockout and wild-type mice in the early phase of infection. Given the activity of IL-4 in isotype switching to IgG1, if IL-4 levels were increased in IL-12 knockout mice, higher IgG1 levels could be expected in IL-12 knockout in comparison with wild-type mice but this did not happen. However, we found higher IgG2b-specific antibody levels in IL-12 knockout mice. Switching to IgG2b secretion in murine B cells is stimulated by transforming growth factor-β (TGF-β) and is inhibited by IL-4 (reviewed in ref. 21). Although we did not measure TGF-β levels, macrophages from IL-12 knockout mice produce high levels of this cytokine.22 Therefore, the enhanced IgG2b response in infected IL-12 knockout versus wild-type mice could reflect the increased TGF-β synthesis by the former animals and reinforce the absence of a shift in cytokine synthesis towards IL-4.
The results we obtained pointing to a lack of Th2 stimulation in T. cruzi-infected IL-12 knockout mice are in contrast with what is observed in Leishmania major infection, as IL-12 p35–/– or p40–/– mice on a 129/Sv/Ev background were found to have high IL-4 production accompanying their intense susceptibility to L. major infection.23 On the other hand, NF-κB2–/– mice have marked IL-12 reduction because of impaired CD40 signalling in macrophages, are more susceptible to L. major infection in comparison with the resistant wild-type counterparts, but do not show enhanced IL-4 synthesis.24
However, in agreement with our observations in T. cruzi infection, T cells from IL-12 p40–/– mice infected with Toxoplasma gondii also fail to produce IL-4 even after repeated stimulation with the parasites.25 In addition, IL-12 p40–/– mice are extremely susceptible to Mycobacterium tuberculosis, presenting only a very low IFN-γ response but without the induction of IL-4.26
Further evidence that IL-4 was not involved in regulating IFN-γ production levels in T. cruzi-infected IL-12 knockout mice came from the in vitro neutralization experiments shown in Fig. 4. Treatment with neutralizing anti-IL-4 mAb 11B11 at high concentration did not modify IFN-γ production in parasite-antigen-stimulated cultures from IL-12 knockout mice. It should be stressed that the same treatment did not affect IFN-γ production by cells from wild-type mice either. This last observation confirms previous results by our group that IFN-γ production is not regulated by IL-4 in mice infected with the Y strain of T. cruzi.27
In contrast with the lack of effect of IL-4 neutralization in culture, mAb neutralization of IL-10 significantly increased IFN-γ production in wild-type and also in IL-12 knockout mice. Thus, however, little IFN-γ is being produced by spleen cells from infected IL-12 knockout mice, it is under negative regulation by IL-10. Neutralization of TGF-β in culture did not have a significant effect in wild-type mice (data not shown).
Residual IFN-γ production was still observed in spleen cell cultures from IL-12 knockout T. cruzi-infected mice although this amounted to less than 10% of the levels observed in similar cultures from infected wild-type mice. About the same ratio of reduction was reported by other authors.9 IL-18 is a cytokine that is stimulatory for IFN-γ synthesis. In our study, treatment with anti-IL-18 neutralizing mAb of spleen cell cultures from T. cruzi-infected mice had no reducing effect on IFN-γ production by wild-type mice or on the residual IFN-γ production seen in IL-12 p40 knockout mice. However, treatment with anti-IL-18 mAb of IL-12 p35–/– mice infected with the Tulahúen strain of T. cruzi results in decreased serum IFN-γ levels and increased parasitaemia.9 We did not analyse the blood compartment and the observed differences may reflect distinct methodology and/or strains of T. cruzi used in the study. Another cytokine that stimulates IFN-γ production is IL-23. However, this molecule cannot be considered a potential stimulator of IFN-γ in our study because the IL-12 p40 knockout mice also lack IL-23.28
Regarding the requirements for the generation of Th1 IFN-γ-producing T CD4+ cells, their frequency in T. gondii-infected IL-12 p40–/– mice is similar to that found in wild-type mice suggesting that IL-12 is not essential to Th1 generation but enhances IFN-γ-production by already committed precursors.25 In addition, parasite-antigen-specific lymphocyte proliferation is of similar intensity in IL-12 knockout and in wild-type immunized mice indicating similar levels of CD4+ T-cell priming.25 Our results showing that IL-12 knockout mice did not have, in comparison to wild-type mice, reduction of T. cruzi-specific IgG antibody levels suggest that T helper cell priming occurred in IL-12 knockout mice.
The residual IFN-γ production, in contrast to IFN-γ production in wild-type mice, is not being stimulated by TNF-α because adding neutralizing anti-TNF-α mAb to the cultures did not reduce IFN-γ levels in spleen cell cultures from IL-12 knockout mice (cf. Fig. 4). Many studies indicate that TNF-α acts with IL-12 to co-stimulate the synthesis of IFN-γ;1,29 however, mice deficient in TNF-α receptor p55 infected with T. cruzi have IFN-γ mRNA expression levels similar to wild-type infected mice,30 suggesting that although co-stimulatory, TNF-α is not essential to IFN-γ synthesis. On the other hand, IL-12 p35–/–T. cruzi (strain Tulahuen)-infected mice have, in comparison with wild-type infected mice, reduced blood TNF-α levels in the first 10 days of infection.9 Thus, the severe reduction of IFN-γ levels in IL-12 knockout mice described in this and in previous work8,9 may result from the compounded absence of IL-12 plus reduced TNF-α production. Although TNF-α did not have a stimulatory role on IFN-γ synthesis in IL-12 knockout, neutralization of TNF-α in spleen cell cultures from these mice (and also from wild-type mice) significantly reduced NO production, suggesting that TNF-α is activating inducible NO synthase in IL-12 knockout mice. NO synthesis in T. cruzi-infected macrophages is activated by both IFN-γ and TNF-α resulting in a parasiticidal effect by these cells.14,15,31 NO is involved in the control of T. cruzi parasitism in infected mice as shown by the extreme susceptibility of mice treated with inhibitors of NO synthesis32 or lacking inducible NO synthase genes;8,33 moreover, NO seems to be specially important in controlling parasitism in the acute phase of infection.34
Because parasitaemia levels were so much higher in IL-12 knockout mice, reflecting the lack of control of the infection by a severely impaired immune response, it was not surprising to find a much heavier parasitism in the organs exemplified by the intense parasitism of the heart. However, we did not find the central nervous system affected with parasites and inflammation as reported in the reactivation phase of infection (day 45) of benznidazole-treated IL-12 knockout mice infected with the Colombian strain of T. cruzi.8 Again, the use of a distinct T. cruzi strain or that brain parasitism is a late event may explain the results. What is striking in the heart pathology of infected IL-12 knockout mice is the almost total absence of inflammation in the presence of very heavy parasitism, as described also by other authors;8,9 a similar aspect is seen in the hearts from infected STAT-4 knockout mice,35 IFN-γ knockout mice8 and in IFN-γ-receptor knockout mice.33 In this regard, the CC chemokine RANTES, and the CXC chemokines IP-10 and MIG mRNAs, were those that were most conspicuously detected in the hearts of mice in the acute phase of T. cruzi infection (Colombian strain).36 These chemokines are induced by IFN-γ and are involved in lymphocyte recruitment; therefore, it is plausible that the intense reduction of IFN-γ production, seen in IL-12 knockout mice, would be accompanied by reduction in their synthesis and in mononuclear cell migration to the sites of infection. Nevertheless, it must be kept in mind that the parasite itself can also induce chemokine production by macrophages.36
In conclusion, the results obtained by studying T. cruzi infection in IL-12-deprived mice indicate that IL-12 is essential to the generation of parasite-antigen-specific lymphocytes that will produce IFN-γ which in turn activates parasiticidal effector mechanisms and is also involved in lymphocyte recruitment to sites of infection. The low production of IFN-γ in IL-12 knockout mice appears to be directly related to the lack of IL-12, that is not substituted by any other cytokine; furthermore, no evidence of a shift towards Th2-type cytokine production was found in IL-12-deprived mice infected with T. cruzi.
Acknowledgments
The authors are grateful to Dr Robert L. Coffman and to DNAX Research Institute for the gift of the anti-cytokine hybridomas. We thank Dr Gislaine A. Martins for help with the antibody assays. Mr Ademir Veras da Silva and Mr Bernardo Paulo Albe provided expert technical assistance. The authors thank Dr Mahasti S. de Macedo for reading the manuscript. A. P. Galvão da Silva was a recipient of a fellowship from FAPESP and this work is part of her thesis. This work was supported by FAPESP and CNPq.
References
- 1.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira MA, Santiago HC, Lisboa CR, Ceravollo IP, Trinchieri G, Gazzinelli RT, Vieira LQ. Leishmania sp: comparative study with Toxoplasma gondii and Trypanosoma cruzi in their ability to initialize IL-12 and IFN-gamma synthesis. Exp Parasitol. 2000;95:96–105. doi: 10.1006/expr.2000.4523. [DOI] [PubMed] [Google Scholar]
- 3.Galvao da Silva AP, Abrahamsohn IA. Interleukin-12 stimulation of lymphoproliferative responses in Trypanosoma cruzi infection. Immunology. 2001;104:349–54. doi: 10.1046/j.1365-2567.2001.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torrico F, Heremans H, Rivera MT, Van Marck E, Billiau A, Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626–32. [PubMed] [Google Scholar]
- 5.Abrahamsohn IA, Coffman RL. Trypanosoma cruzi: IL-10, TNF, IFN-γ and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol. 1996;84:231–44. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 6.Aliberti JC, Cardoso MA, Martins GA, Gazzinelli RT, Vieira LQ, Silva JS. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–7. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter CA, Slifer T, Araujo F. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infection Immunity. 1996;64:2381–6. doi: 10.1128/iai.64.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michailowsky V, Silva NM, Rocha CD, Vieira LQ, Lannes-Vieira J, Gazzinelli RT. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am J Pathol. 2001;159:1723–33. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller U, Kohler G, Mossmann H, Schaub GA, Alber G, Di Santo JP, Brombacher F, Holscher C. IL-12-independent IFN-gamma production by T cells in experimental Chagas' disease is mediated by IL-18. J Immunol. 2001;167:3346–53. doi: 10.4049/jimmunol.167.6.3346. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa de Oliveira LC, de Curotto Lafaille MA, Collet de Araujo Lima GM, Abrahamsohn IA. Antigen-specific IL-4- and IL-10-secreting CD4+ lymphocytes increase in vivo susceptibility to Trypanosoma cruzi infection. Cell Immunol. 1996;170:41–53. doi: 10.1006/cimm.1996.0132. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsohn IA, Coffman RL. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J Immunol. 1995;155:3955–63. [PubMed] [Google Scholar]
- 12.Curotto-de-Lafaille MA, Barbosa-de-Oliveira LC, Lima GC, Abrahamsohn IA. Trypanosoma cruzi: maintenance of parasite-specific T cell responses in lymph nodes during the acute phase of the infection. Exp Parasitol. 1990;70:164–74. doi: 10.1016/0014-4894(90)90097-v. [DOI] [PubMed] [Google Scholar]
- 13.Pinge-Filho P, Tadokoro CE, Abrahamsohn IA. Prostaglandins mediate suppression of lymphocyte proliferation and cytokine synthesis in acute Trypanosoma cruzi infection. Cell Immunol. 1999;193:90–8. doi: 10.1006/cimm.1999.1463. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz-Fernandez MA, Fernandez MA, Fresno M. Synergism between tumor necrosis factor-α and interferon-γ on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992;22:301–7. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 15.Gazzinelli RT, Oswald P, Hieny S, James SL, Sher A. The microbicidal activity of interferon-γ treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–6. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 16.Cunha FQ, Moncada S, Liew FY. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun. 1992;182:1155–9. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- 17.Liew FY. Regulation of nitric oxide synthesis in infectious and autoimmune diseases. Immunol Lett. 1994;43:95–8. doi: 10.1016/0165-2478(94)00157-x. [DOI] [PubMed] [Google Scholar]
- 18.de Souza EM, Rivera MT, Araujo-Jorge TC, de Castro SL. Modulation induced by estradiol in the acute phase of Trypanosoma cruzi infection in mice. Parasitol Res. 2001;87:513–20. doi: 10.1007/s004360100376. [DOI] [PubMed] [Google Scholar]
- 19.do Prado Junior JC, Leal M, Anselmo-Franci JA, de Andrade Junior H, Kloetzel JK. Influence of female gonadal hormones on the parasitemia of female Calomys callosus infected with the ‘Y’ strain of Trypanosoma cruzi. Parasitol Res. 1998;84:100–5. doi: 10.1007/s004360050364. [DOI] [PubMed] [Google Scholar]
- 20.Brodskyn CI, Silva AM, Takehara HÁ, Mota I. IgG subclasses responsible for immune clearance in mice infected with Trypanosoma cruzi. Immunol Cell Biol. 1989;67:343–8. doi: 10.1038/icb.1989.50. [DOI] [PubMed] [Google Scholar]
- 21.Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–70. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 22.Bastos KR, Alvarez JM, Marinho CR, Rizzo LV, Lima MR. Macrophages from IL-12p40-deficient mice have a bias toward the M2 activation profile. J Leukoc Biol. 2002;71:271–8. [PubMed] [Google Scholar]
- 23.Mattner F, Magram J, Ferrante J, et al. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–9. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 24.Speirs K, Caamano J, Goldschmidt MH, Hunter CA, Scott P. NF-kappa B2 is required for optimal CD40-induced IL-12 production but dispensable for Th1 cell Differentiation. J Immunol. 2002;168:4406–13. doi: 10.4049/jimmunol.168.9.4406. [DOI] [PubMed] [Google Scholar]
- 25.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 2002;16:429–39. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 26.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrahamsohn IA, da Silva AP, Coffman RL. Effects of interleukin-4 deprivation and treatment on resistance to Trypanosoma cruzi. Infect Immun. 2000;68:1975–9. doi: 10.1128/iai.68.4.1975-1979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 29.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–9. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castanos-Velez E, Maerlan S, Osorio LM, Aberg F, Biberfeld P, Orn A, Rottenberg ME. Trypanosoma cruzi infection in tumor necrosis factor receptor p55-deficient mice. Infect Immun. 1998;66:2960–8. doi: 10.1128/iai.66.6.2960-2968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva JS, Vespa GNR, Cardoso MAG, Aliberti JCS, Cunha FQ. Tumor necrosis factor alpha mediates resistance to Trypanososma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–7. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vespa GN, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–82. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holscher C, Kohler G, Muller U, Mossmann H, Schaub GA, Brombacher F. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–15. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saeftel M, Fleischer B, Hoerauf A. Stage-dependent role of nitric oxide in control of Trypanosoma cruzi infection. Infect Immun. 2001;69:2252–9. doi: 10.1128/IAI.69.4.2252-2259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarleton RL, Grusby MJ, Zhang L. Increased susceptibility of Stat4-deficient and enhanced resistance in Stat6-deficient mice to infection with Trypanosoma cruzi. J Immunol. 2000;165:1520–5. doi: 10.4049/jimmunol.165.3.1520. [DOI] [PubMed] [Google Scholar]
- 36.Talvani A, Ribeiro CS, Aliberti JC, et al. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect. 2000;2:851–66. doi: 10.1016/s1286-4579(00)00388-9. [DOI] [PubMed] [Google Scholar]