Abstract
γδ T cells are unique, and their localization at sites of infection is considered critical in immune defence. We demonstrate the accumulation of γδ T cells in rat regional popliteal lymph nodes (PLNi) starting 2 days after inoculation of cytomegalovirus (CMV) into the footpad. Early-appearance PLNi γδ T cells significantly inhibited plaque development and the spread of CMV infection. These γδ T cells were negative for CD4 and CD8beta receptors, proliferated in response to interleukin-2 (IL-2) and contained high levels of interferon-γ (IFN-γ), the appearance of which correlated with the curing of fibroblasts from virus infection. The addition of anti-IFN-γ abolished the ability of fibroblast monolayers to be cured from CMV infection. In contrast, this protection was not abolished by the addition of anti-rat IL-2 or anti-rat TNF-α, or by the depletion of NKR-P1-bearing cells within γδ T cells. In addition, the present study shows that while γδ T cells derived from naive and CMV-infected rats are able to kill both YAC-1 targets and CMV-infected syngeneic fibroblasts in vitro, only the latter are able to clear CMV-infected fibroblast monolayers. Finally, our data suggest that the expression of NKR-P1 by γδ T cells is critical for cytotoxicity, but its contribution to the curing from CMV infection was limited.
Introduction
The majority of T cells in the circulation and lymphoid organs express the αβ T-cell receptor (TCR), commonly in conjunction with the co-receptor of either CD4 (≈ 70%) or CD8 (≈ 20–30%).1,2 A less frequent population of T cells expresses the γδ TCR, usually in the absence of CD4 and CD8.2,3 There is also evidence that, particularly in rats, γδ T cells from lymphoid organs express the CD8-αβ heterodimer together with CD2 and CD5.4,5γδ T cells are fundamentally different from αβ T cells, and their presence at sites of infection is considered critical for their unique role in immune defence.1,6–8
Currently, there is clear evidence of the participation of both αβ and γδ T cells in clearing virus infections.9–11 CD8 αβ T cells are the major component in the adaptive immune system, providing specific pathogen recognition and long-term memory. Yet, during a primary immune response, there is a lag period of ≈ 3–4 days before antigen-specific responses are evident, and αβ T-cell responses do not peak until ≈ day 7. Therefore, it is essential that other strategies are employed by the immune system in order to mount an aggressive early immune response.12 Expansion of γδ T cells was observed in the early phases of human skin infection with Mycobacterium leprae and in the lungs of mice exposed to aerosol containing M. tuberculosis antigen.13 In another study, a significant increase was found in the number of γδ T cells in the spleen of normal C57BL/6 mice, starting 2 days after infection with vaccinia virus (VV).14 In addition, the maximal frequencies of γδ T cells at the earliest time-point (3 days) tested after challenge in mice with influenza A virus were determined.15,16
It is well accepted that the elimination of virus infection is achieved by the co-participation of direct lysis of infected cells and the action of cytokines.9,10,15,17
The potent cytotoxic responses of γδ T lymphocytes against cells infected with viruses may be essential for overall antiviral defence. However, not all γδ T cells reactive with virus-infected cells are cytotoxic. Therefore, further analysis of antiviral immunosurveillance by γδ T cells is crucial for understanding the unique biological role of this lymphocyte subset.
Like αβ T cells, γδ T cells differentiate into interferon-γ (IFN-γ) [T helper 1 (Th1-like)]- and interleukin (IL)-4 [T helper 2 (Th2-like)]-producing cells.18 Importantly, in γδ T-cell-mediated protection, IFN-γ is the main candidate for the effector molecule because IFN-γ is indispensable in innate immunity against viruses9,19 and γδ T cells predominantly produce IFN-γ upon activation.20
In the present study we analysed the phenotype, cytokine profile and functions of γδ T cells present in the popliteal lymph nodes (PLN) of rats after a single injection of cytomegalovirus (CMV) into the footpad. We also clarified the possible mechanisms of their action in order to reveal the role of IL-2, IFN-γ and tumour necrosis factor-α (TNF-α) in regulating their potential activity. These results are discussed in light of the potential function of γδ T cells as a first line of defence against CMV in lymphoid tissues.
Materials and methods
Viruses
The Smith's strain murine CMV (MCMV) was obtained from Dr B. Rager-Zisman (Ben-Gurion University, Beersheba, Israel). In order to adapt the MCMV to the rat system, the virus was passaged three or four times through the rat fibroblast monolayer (aMCMV). Then, the aMCMV was titrated by development of cytopathic plaques in mouse and rat fibroblast monolayers.17
Animals
Spraque-Dawley and inbred DA rats, weighing 240–330 g, were used in the experiments. Animal care and all experiments were performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals, and the protocol was approved by the Animal Care and Use Committee of the Technion.
Immunization
Rats were infected by injecting 10 000 plaque-forming units (PFU) of aMCMV in 0·1 ml phosphate-buffered saline (PBS) into the footpad. Control legs were either left uninfected or infected with mock-infected culture supernatant (Ben-Gurion University, Beersheba, Israel). Two and 6 days after virus inoculation, cell suspensions were prepared from both the draining PLN (PLNi) and the contralateral PLN (PLNc), as described previously.21 Each experiment was conducted on groups of four to six rats.
Fractionation of cells
PLN cells were fractionated using magnetic microbeads conjugated with anti-fluorescein isothiocyanate (FITC) and a magnetic field system (Miltenyi Biotec, Bergisch Gladbach, Germany), as described previously.21 The mouse monoclonal antibodies (mAbs) – anti-TCR γδ (clone V45) and anti-CD8beta (clone 341) (PharMingen, San Diego, CA) – were used. Fractions used in experiments were 90–95% pure, as assessed by flow cytometry.
For some experiments the negative-selected γδ T cells were prepared using a fluorescein isothiocanate (FITC)-labelled cocktail consisting of anti-αβ TCR, CD8beta, CD4 and NKR-P1 mAbs. As shown by fluorescence-activated cell sorter (FACS) analysis, this fraction was 90% pure for γδ T cells and negative for αβ T cells, and CD4 CD8 and NKR-P1-bearing cells.
Flow cytometric analysis
The cell-surface phenotype was determined using mouse mAbs that reacted with the following rat molecules: CD8beta (clone 341); CD4 (OX-35); αβ TCR (clone R73); γδ TCR (clone V45) (PharMingen); and CD161 (also known as NKR-PI, clone 10/78; Serotec, Oxford, UK). Determinations were made in a Becton-Dickinson (Lincoln, Park, NJ) flow cytometer (FACSCalibur model). In parallel, for each animal, an autofluorescence control (unstained cells) and an isotypic control were analysed. Isotype controls yielded results of < 1%, and have not been included in the graphs.
Staining for intracellular immunofluorescence
The levels of intracellular IFN-γ, IL-2, IL-4 and TNF-α were determined by using a Cytofix/Cytoperm Kit (PharMingen). All procedures were performed strictly in accordance with the manufacturer's instructions. Purified rabbit anti-rat IFN-γ (Immunogenetics, Zwijndrecht, Belgium), anti-rat TNF-α mAbs (Zymed Laboratories Inc., San Francisco, CA) and anti-rat IL-2 (PharMingen) were used at concentrations of 1 µg/106 cells. R-phycoerythrin-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma, Rechovot, Israel) was employed as the second antibody and used with an irrelevant isotype-matched Ab to control background staining.
Cytotoxicity assay
The cytotoxic activity of PLN-derived lymphocytes against CMV-infected and -uninfected fibroblasts was determined as described previously21 by a 51Cr-release assay. After 2 days, γ-radioactivity was counted separately in medium and in cell fractions. 51Cr release was calculated, in counts per minute (c.p.m.), in triplicate wells according to the standard formula:
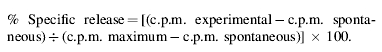 |
The spontaneous release was obtained from fibroblasts incubated with medium only. For maximal release, targets were incubated with 1% Triton-X-100 (≈ 97–98% release).
The cytolytic activity of PLN cells against natural killer (NK)-sensitive mouse YAC-1 cells was measured by using flow cytometry, as described previously.22,23 YAC-1 cells were grown in RPMI-1640 supplemented with 10% fetal calf serum (FCS) (Biological Industries, Kibbutz Beit HaEmek, Israel). Effector cells and washed YAC-1 cells were mixed at effector to target (E : T) ratios of 5 : 1, 10 : 1 and 20 : 1 in 200 µl of the culture medium, incubated for 1 hr at 37° in an atmosphere of 5% CO2, and analysed by flow cytometry. Live and dead target cells were gated and counted separately. The majority of effector cells could be gated out upon their scattering parameters, and the spillage of gates corresponding to target cells was subtracted. The percentage of dead YAC-1 cells was calculated using the following formula:
where DC = number of dead cells and LC = number of live cells. The cytotoxicity index (CTI) was determined by subtraction of the target cell spontaneous death values. The viability of targets was > 95%.
Three distinct cell populations were detected and gated on the plot of forward (FSC) and side (SSC) light scatters, corresponding to effector cells, live and dead targets. The population with relatively lower FSC and slightly higher SSC value was determined as dead cells.22,23
Cytokines
Recombinant human IL-2 (rhIL-2) and rat IL-2 (rIL-2) (Genzyme Diagnostic, Cambridge, MA) were used for the cell cultures. For neutralization of the effects of endogenous IL-2, 20 µg/ml of anti-rat IL-2 (PharMingen) was added to the fibroblast monolayers simultaneously with lymphocytes.
The polyclonal rabbit anti-rat IFN-γ (Immunogenetics) neutralized the antiviral effect of 10 U IFN-γ at a dose of 2·5 µg/ml (0·5 µg/well). Rabbit anti-rat TNF-α (Zymed Laboratories Inc.) was added at a dose of 20 µg/ml (4 µg/well).
Proliferation assay
Purified CD8beta+, CD4+ and γδ T+ cells (2–2·5 × 104/200-µl well) were cultured with 100 U/ml rhIL-2 for 48–72 hr in round-bottom 96-well plates. Cells were pulsed with 1 µCi [3H]thymidine ([3H]TdR) for 12–16 hr before harvesting on glass-fibre filters. [3H]TdR incorporation was measured by liquid scintillation counting and expressed as c.p.m.
Statistics
Data were expressed as mean ± SD. Kruskal–Wallis analysis of rank was used to determine if a variable changed significantly with respect to time. Differences between groups (dependent and independent variables) were evaluated using the Student's t-test of paired differences and the Wilcoxon rank-sum (Mann–Whitney U) test. Probabilities of ≤ 0·05 were considered significant.
Results
Response of γδ T cells to CMV infection
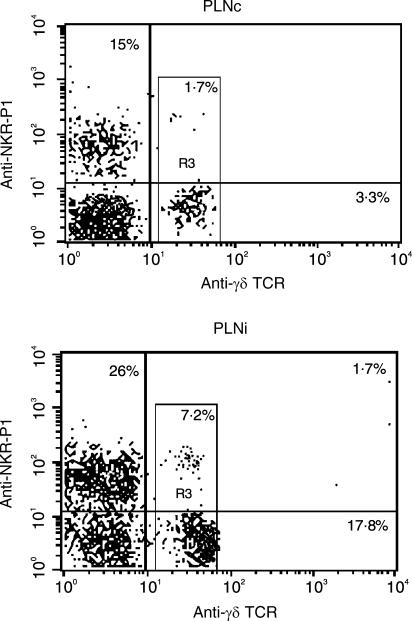
On day 6 after inoculation of aMCMV into the footpad, the draining PLN became swollen and contained 4·3 ± 1·9 × 107 cells, as compared with 2·3 ± 0·7 × 106 cells in the draining PLN of rats on day 2 postinfection (P < 0·05) and with 1·3 ± 0·3 × 106 cells in contralateral PLN (P < 0·01). The number of cells in contralateral PLN was similar to those observed in the PLN of uninfected rats (data not shown). The γδ T-cell percentage rose dramatically (P < 0·01) in the draining PLN starting 2 days after infection with aMCMV (18·7 ± 2·1%) as compared with contralateral PLN (3·3 ± 1·5%) (Figs 1 and 2). Thus, although the percentages of γδ T cells detected on days 2 and 6 postinfection (15·8 ± 3·7%) were similar, at least 10-fold more γδ T cells were present in PLN from rats on day 6 postinfection.
Figure 1.
Co-expression of NKR-P1 (vertical line) with γδ T-cell receptor (TCR) (horizontal line) on preparations of contralateral popliteal lymph nodes (PLNc) and draining PLN (PLNi) from rats 2 days postinfection. Cells were prepared from freshly isolated PLN, stained with monoclonal antibodies (mAbs) and analysed as described in the Materials and methods. Numbers in quadrants of the dot-plots represent the percentage of lymphocytes. Numbers within R3 area correspond to the percentage of double-staining NKR-P1+ γδ TCR+ cells among γδ T cells (R3). Results are typical of six replicate experiments.
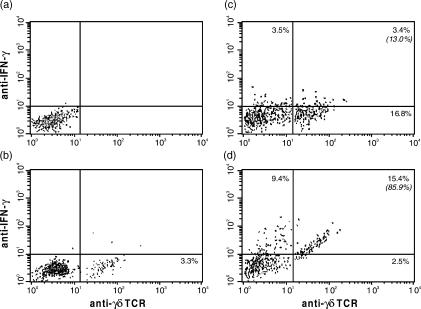
Figure 2.
The detection of cell-associated interferon-γ (IFN-γ) content in rat popliteal lymph node (PLN)-derived γδ T cells using two-colour flow cytometry. Dot-plots of a representative result among five tests are shown. (a) Background staining with isotypic immunoglobulin G–fluorescein isothiocyanate (IgG–FITC) and phycoerythrin (PE)-conjugated secondary antibodies. (b) Contralateral PLN (PLNc) from rats 6 days postinfection. (c) Draining PLN (PLCi) from rats 2 days postinfection. (d) PLNi from rats 6 days postinfection. Cytokine-positive cells were identified according to background staining (< 1% of the analysed subset). Numbers in quadrants of the dot-plots represent the percentage of lymphocytes. Numbers in parenthesis correspond to the percentage of interferon-γ+ (IFN-γ+) cells among γδ T cells. TCR, T-cell receptor.
Freshly isolated cells from draining PLN and contralateral PLN were used for separation. Fifty thousand cells of γδ T+ and γδ T− cell fractions were plated on fibroblast monolayers preinfected with 50 PFU of aMCMV/well. For comparison, 50 000 cells of the CD8beta+ fraction [previously described as a cytotoxic T lymphocyte (CTL) with specific antiviral activity]17,21 were also plated. The results are summarized in Table 1.
Table 1.
Inhibition of plaque development in syngeneic monolayer by different types of rat lymph node-derived cell populations
| No of plaques (Mean ± SD) | PFU/ml in culture fluid | |||||
|---|---|---|---|---|---|---|
| Type of cells* | Days after infection† | Day 4 | Day 7 | Day 8 | Day 10 | Day 12 |
| 1. Infected monolayer only | 47·0 ± 3·7 | > 200 | Confluent | Confluent | 64000 | |
| 2. PLNc, γδ T+ | 2 | 7·0 ± 2·6 | 19·7 ± 2·5 | > 20d | Confluent | 68000 |
| 3. PLNi, γδ T+ | 2 | 2·5 ± 1·3 | 9·3 ± 1·5 | 9·3 ± 1·5 | 9·3 ± 1·5‡ | 3000 |
| 4. PLNi, γδ T− | 2 | 8·3 ± 0·96 | 51·0 ± 6·6 | Confluent | Confluent | 52000 |
| 5. PLNi, CD8+ | 6 | 1·3 ± 0·96 | 0·3 ± 0·5 | 0·3 ± 0·5 | 0 | 0 |
| 6. PLNi, γδ T− | 6 | 4·5 ± 1·3 | 4·0 ± 0·8 | 3·3 ± 1·0 | 3·3 ± 1·0§ | 0 |
| 7. PLNi, γδ T+ | 6 | 0 | 2·3 ± 1·9 | 2·3 ± 1·9 | 2·3 ± 1·9§ | 0 |
| 8. PLNi, γδ T+ (NKR-PI depleted) | 6 | 4·8 ± 1·7 | 4·3 ± 1·0 | 4·3 ± 0·96 | 1·8 ± 0·9 | 0 |
| 9. PLNi, γδ T+(NKR-PI depleted)anti-IFN-γ | 6 | 29·3 ± 4·6 | > 200 | Confluent | Confluent | 64000 |
A total of 50 000 γδ T+ or γδ T− cell fractions, as well as CD8+ cells, were plated on syngeneic fibroblast monolayers (10 000 fibroblasts/well) preinfected 2 hr previously with 50 plaque-forming units (PFU) of aMCMV [Smith's strain murine cytomegalovirus (MCMV), passaged three or four times through the rat fibroblast monolayer] in 0·1 ml of Dulbecco's modified Eagle's minimal essential medium (DMEM); in rows 8 and 9 the negative selected γδ T cells depleted from NKR-P1+ were used. PLNc, contralateral popliteal lymph nodes; PLNi, draining popliteal lymph nodes.
DA rats were injected with 10 000 PFU of aMCMV into the footpad; PLN cells were derived either day 2 or day 6 after inoculation with aMCMV.
Plaques were dramatically enlarged in size.
Plaques were arrested.
IFN-γ, interferon-γ.
It was shown that while confluent cytopathic plaques developed in control cultures of fibroblasts alone (Table 1, row 1), different patterns of plaque development were found in cultures of different cell fractions. Observations on a daily basis identified curing of the fibroblast monolayer from aMCMV infection (the infected fibroblasts disappeared and the number of fibroblasts in the plaque area expanded so that plaques could no longer be distinguished) by CD8beta+ (Table 1, row 5) and γδ T+ cells from rats on day 6 postinfection (Table 1, row 7). We also found that γδ T-cell depletion had no influence on development of the immune response induced by CD8beta+ CTL (Table 1, row 6).
The curing of fibroblast monolayers from virus infection was not abolished by the depletion of NKR-P1+ cells within γδ T cells (Table 1, row 8).
The protective effects of γδ T cells were mainly caused by the presence of IFN-γ, as the addition of anti-IFN-γ abolished the curing of fibroblast monolayers from aMCMV infection (Table 1, row 9). However, the protection was not abolished by the addition of anti-rat IL-2 or anti-rat TNF-α (data not shown).
In contrast to lymphocytes obtained from rats on day 6 postinfection, when lymphocytes were obtained from rats on day 2 postinfection, only draining PLN-derived γδ T cells (Table 1, row 3) induced the inhibition of cytomegalic plaque development. However, this protection was not absolute, and after 10 days plaques dramatically increased in size and aMCMV was detected in the culture medium.
Phenotype and cytokine profile of γδ T cells
The phenotypes of γδ T cells determined by double labelling showed that only 1·5 ± 0·2% of contralateral PLN γδ T cells, and 1·67 ± 0·4 of draining PLN γδ T cells in rats on day 6 postinfection were CD8beta+. Moreover, γδ T− fractions from all rat groups contained the highest proportion (from 17·7 to 21·6%) of CD8beta+ lymphocytes. The γδ TCR and CD8beta (marker of CTL) are thus expressed on different cell populations in this virus-induced process. No cells were categorized as γδ T+ CD4+ cells.
Recently, it was found that a large proportion of γδ T cells express different types of NK receptors. Based on this observation we also studied the co-expression of NKR-PI receptors on γδ T cells. NKR.P1+ γδ T+ cells constituted 1·7 ± 0·3% of the total number of γδ T cells in contralateral PLN, as well as in PLN of uninfected rats (data not shown), and their percentage increased to 6·8 ± 1·4% on day 2 and to 7·0 ± 4·3% on day 6 after inoculation with aMCMV. A representative result of six independent experiments is shown in Fig. 1.
The intracellular content of IL-2, IL-4, IFN-γ and TNF-α in γδ T cells of PLNs from rats on days 2 and 6 postinfection is presented in Table 2. It was found that the percentage of γδ T cells containing IL-2 significantly increased in the draining PLN of rats on day 6 postinfection as compared with contralateral PLN or draining PLN of rats on day 2 postinfection (P < 0·001). Only a relatively small number of γδ T cells contained IL-4 and TNF-α, and there was no difference in the number of γδ T cells containing IL-4 and TNF-α in the PLN between infected and uninfected rats. We also examined whether γδ T cells were activated by aMCMV to produce IFN-γ. On day 2 postinfection, 13·0 ± 1·8% of the γδ T cells from draining PLN contained IFN-γ (Table 2, Fig. 2c). A considerable increase in the percentage of γδ T cells containing IFN-γ was observed in draining PLN on day 6 postinfection (81·2 ± 4·9%; P < 0·0001, Table 2; Fig. 2d).
Table 2.
Intracellular content of cytokines in rat lymph node-derived γδ T lymphocytes
| γδ T cells containing cytokines (%) | |||
|---|---|---|---|
| Cytokines | PLNc, 2 days after inoculation with aMCMV | PLNi, 2 days after inoculation with aMCMV | PLNi, 6 days after inoculation with aMCMV |
| IL-2 | 0 | 0 | 14·3 ± 1·6*† |
| IL4 | 0·9 ± 0·5 | 1·6 ± 0·5 | 1·6 ± 0·5 |
| TNF-α | 4·2 ± 0·5 | 4·8 ± 1·5 | 4·2 ± 0·5 |
| IFN-γ | 1·5 ± 0·9 | 13·0 ± 1·8* | 81·2 ± 4·9*† |
P < 0·05 compared with contralateral popliteal lymph nodes (PLNc).
P < 0·05 compared with draining popliteal lymph nodes (PLNi), 2 days after inoculation with aMCMV [Smith's strain murine cytomegalovirus (MCMV), passaged three or four times through the rat fibroblast monolayer].
IFN-γ, interferon-γ; TNF-α, tumour necrosis factor-α.
γδ T cells as cytotoxic effector cells
Given the importance of cytotoxicity in the immune response to CMV, we next determined the cytotoxic effector functions of γδ T+ and γδ T− fractions obtained from the same donor in a Cr51-release assay. Fifty thousand cells of γδ T+ and γδ T− fractions, obtained from rats 2 and 6 days after virus inoculation, were plated on 51Cr-labelled fibroblast monolayers preinfected with 1000 PFU of aMCMV/well. Lysis of infected fibroblasts by effector cells was determined only at 2 days postinfection, because the infected cells do not release 51Cr during the 2–3 days after infection. After 5–6 days, the infected fibroblasts progress into confluent cytopathic plaques and die.
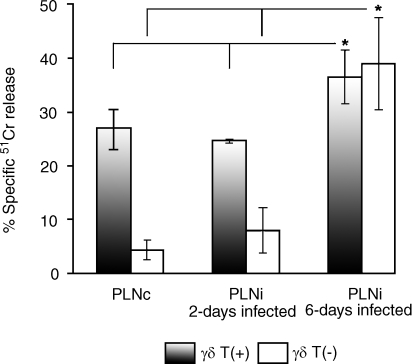
It was found that at an E : T ratio of 5 : 1, both contralateral PLN and draining PLN γδ T+ cells from rats on day 2 postinfection showed a similar ablility to kill aMCMV-infected fibroblasts (27 ± 3·5% and 24·6 ± 0·4%, respectively, P > 0·05) (Fig. 3). However, the γδ T+-cell cytotoxicity against infected fibroblasts was significantly elevated to 36·5 ± 3·7% by day 6 after infection with aMCMV (P = 0·03 versus contralateral PLN, and P = 0·004 versus draining PLN from rats on day 2 postinfection).
Figure 3.
Lysis of aMCMV [Smith's strain murine cytomegalovirus (MCMV), passaged three or four times through the rat fibroblast monolayer]-infected fibroblast monolayers (10 000 fibroblasts/well) by popliteal lymph node (PLN)-derived γδ T+ and γδ T− cell fractions from rats 2 and 6 days after inoculation with 10 000 plaque-forming units (PFU) of aMCMV into the footpad. Freshly isolated PLN cells were fractionated using a magnetic field system with monoclonal antibodies (mAbs) to rat γδ T-cell receptor (TCR). Fifty thousand purified cells were plated on 51Cr-labelled syngeneic monolayers preinfected 2 hr in advance with 1000 PFU of aMCMV/10 000 fibroblasts. Analysis was performed 2 days after addition of γδ T cells. The infected fibroblast monolayer maintained without lymphocytes did not release 51Cr. Results represent the mean value ± SD from four independent experiments. *Statistically significant difference (P < 0·05). PLNc, contralateral popliteal lymph node; PLNi, draining popliteal lymph node.
In contrast, γδ T− fractions of contralateral PLN from the same rats failed to lyse the infected targets (< 5%). Moreover, γδ T− fractions from draining PLN derived from rats on day 2 postinfection revealed very low cytolytic activity against aMCMV-infected fibroblasts compared with γδ T− fractions from draining PLN derived from rats on day 6 postinfection (8·0 ± 3·5% versus 37·7 ± 6·4%, P = 0·0007). In the latter, strong lysis is attributed to the occurrence of CD8+ CTL.
During the first 3 days of culture there was no significant lysis of uninfected fibroblasts as compared with infected monolayers in γδ T+ cultures (4·9 ± 1·2% for contralateral PLN; 4·3 ± 0·4% for draining PLN, P > 0·05) or in cultures of γδ T− fractions (0%).
In order to determine whether the lysis of infected fibroblasts is dependent upon the presence of NKR-P1 cells, in further experiments we used negatively selected γδ T cells depleted of NKR-P1+ cells (see the Materials and methods). It was found that depletion of NKR-P1+ cells from the γδ T cells resulted in a reduction in the death of cells in the infected monolayer, from 26 ± 5·9% to 13·9 ± 3·7% in contralateral PLN (P = 0·01) and from 30 ± 8·4% to 9·8 ± 4·5% (P = 0·004) in draining PLN from rats on day 6 postinfection.
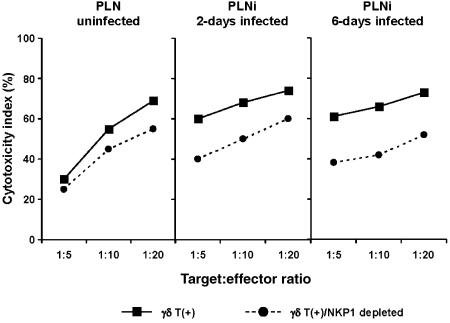
γδ T cells were also tested for their ability to lyse YAC-1, a known target for natural killer cells. It was found that at the E : T ratios of 10 : 1 and 20 : 1, γδ T cells from both uninfected and infected PLN were equally cytotoxic for YAC-1 targets. Moreover, the γδ T+ sets from draining PLN of rats 6 days postinfection mediated the same lysis against the YAC-1 target as compared with lysis mediated by γδ T+ cells from PLN of rats 2 days postinfection. As in the case of aMCMV-infected targets, the removal of the NKR-P1+ cells resulted in a decreased death of the YAC-1 target (Fig. 4).
Figure 4.
YAC-1 lysis by draining popliteal lymph node (PLNi)-derived γδ T+ cells from rats 2 and 6 days after inoculation of 10 000 plaque-forming units (PFU) of cytomegalovirus (CMV) into the footpad, as compared to PLN from uninfected rats.
Cultured γδ T cells
To further characterize the γδ T cells developed following infection with CMV, we examined the response of the purified, positively selected γδ T cells from rats on day 6 postinfection, cultured in the presence of IL-2.
As shown in Table 3, the proliferation of γδ T+ cells in response to 48 hr of stimulation by IL-2 in vitro was higher compared with the proliferation of purified CD4+ (P = 0·03) or CD8beta+ (P = 0·0002) lymphocytes. However, during the next 5 days of culture in the presence of rhIL-2 or rIL-2 (data not shown), a high level of blastogenesis was observed in all types of cultures. It was found that curative and cytolytic activities were particularly effective when blasts from these established cultures were transferred to syngeneic aMCMV-preinfected fibroblasts.
Table 3.
Effect of recombinant human interleukin-2 (rhIL-2) on the proliferative capacity of CD8beta+, CD4+ and γδ T+ cell subsets derived from rat draining popliteal lymph nodes (PLNi)
| Type of culture¶ | |||||
|---|---|---|---|---|---|
| Type of cells* | Addition of IL-2† | c.p.m.‡ after 48 hr | P-value§ | 2 days | 5 days |
| CD4+ | + | 4123 ± 495 | 0·03 | 30–40% blasts | 80–90% blasts |
| CD4+ | − | 374 ± 90 | Lymphocytes | Lymphocytes | |
| CD8+ | + | 813 ± 44 | 0·0002 | 10–15% blasts | 50–70% blasts |
| CD8+ | − | 235 ± 2 | Lymphocytes | Lymphocytes | |
| γδ T+ | + | 5900 ± 524 | 50–70% blasts | 80–90% blasts | |
| γδ T+ | – | 421 ± 156 | Lymphocytes | Lymphocytes | |
PLN were used from rats 6 days postinfection. Positively selected γδ T+, CD8beta+ and CD4+ cells were used at 10 000/well. Approximately 90–95% of positive fractions expressed the corresponding markers when analysed using flow cytometry.
Cells were cultured with or without 100 U/ml hrIL2 in culture plates with round-bottom wells.
[3H]Thymidine (1 µCi) was present in each well for the last 16 hr of culture before determining the incorporated radioactivity using a Wallac liquid scintillation counter. Representative data out of four experiments are shown as counts per minute (c.p.m.), which are means of triplicate wells.
Compared with IL-2-activated γδ T+ lymphocytes.
Number of blasts/100 cells.
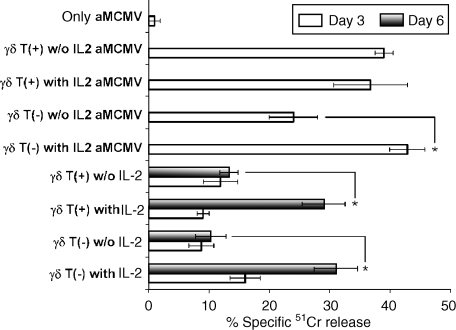
In contrast to γδ T+ cells, in which lytic activity does not depend on the presence of IL-2, the blasts from γδ T− fractions induced stronger lysis of infected fibroblasts in the presence of IL-2 (Fig. 5). In addition, when blasts developed from both γδ T+ and γδ T− fractions were transferred to fresh, uninfected syngeneic monolayers, strong IL-2-dependent lysis was observed 6 days after passage (Fig. 5).
Figure 5.
Lysis of aMCMV [Smith's strain murine cytomegalovirus (MCMV), passaged three or four times through the rat fibroblast monolayer]-infected and -uninfected fibroblast monolayers by interleukin-2 (IL-2)-induced blasts developed from γδ T+ and γδ T− draining popliteal lymph node (PLNi)-derived lymphocytes. Fifty thousand cells of γδ T+ and γδ T− cell fractions were cultured on syngeneic fibroblast monolayers with 100 U/ml human recombinant IL-2 (hrIL-2). Five days later, when clear blasts developed, the cells were harvested, washed twice, and 50 000 cells/well were plated on a 51Cr-labelled monolayer, untreated or infected 2 hr previously with 1000 plaque-forming units (PFU) of aMCMV with or without 100 U/ml hrIL-2. The percentage of specific 51Cr release was counted 3 and 6 days after passage. Results represent the mean value ± SD from three independent experiments. *Statistically significant difference (P < 0.01).
Discussion
γδ T cells are apparently activated in response to CMV infection,19,24 but precise mechanisms of their protective/curative effects are still poorly understood. In some experimental disease models, γδ T cells appear to reduce damage, whereas in others, results imply that they exacerbate it. Even within a single disease model, the effects depend upon the time-point at which the γδ T cells are examined. Moreover, it was shown that the various γδ T-cell subsets carry out contrasting roles in host resistance and that these subsets normally balance one another out.25 Therefore, further information on the activational stages of the γδ T cells during their development is needed to address this issue.
Our fundamental finding is that we identified and characterized the rat γδ T lymphocytes that appeared in draining PLN after a single injection of aMCMV into the footpad, as compared with contralateral PLN-derived γδ T cells. The protective effect of draining PLN γδ T cells can be detected 2 days postinfection. The absolute number of γδ T cells showed a relative increase of at least 10-fold by day 6 postinfection. These γδ T cells were found to exhibit high cytolytic activity, extensive proliferation in response to IL-2 activation in vitro, and the majority (81%) also produced IFN-γ. Similarly to the classical CD8 CTL, they can completely protect fibroblast monolayers from infection with aMCMV in vitro. The phenotypic analysis showed that the γδ TCR and CD8beta markers are expressed on different cell populations in this virus-induced process. Therefore, antiviral activity of γδ T cells is not dependent upon the presence of CD8beta+ CTL.
Importantly, while γδ T cells derived from naive and aMCMV-infected rats are able to kill both aMCMV-infected fibroblasts and YAC-1 targets, only draining PLN, which produce large amounts of IFN-γ, are able to clear CMV infection in vitro. Our data demonstrated that the appearance of IFN-γ is correlated with the curing of fibroblasts from virus infection, and that the neutralization of endogeneous IFN-γ abolished the protective antiviral effects of γδ T cells from draining PLN. The activation of IFN-γ-secreting γδ T cells at 2 days postinfection with VV, was observed by Selin et al.14 The IFN-γ-producing γδ T cells that appeared at an early stage of infection in the peritoneal cavity and the liver after intraperitoneal (i.p.) infection with MCMV were also analysed in mice by Ninomiya et al.19 The kinetics of these mouse γδ T cells resemble those of γδ T cells in the draining PLN of rat, as described in our experiments.
The addition of anti-rat TNF-α or anti-rat IL-2 had no effect on the curing of fibroblasts from aMCMV infection. Nevertheless, an accumulation of IL-2-positive γδ T cells in draining PLN occurred 6 days after infection. As IL-2 stimulation of lymphocytes resulted in extensive lysis of both infected and uninfected syngeneic fibroblasts, we proposed that γδ T cells from draining PLN could activate other T-cell subsets via cytokine release. In this context, the observed IL-2-induced cytotoxicity against uninfected syngeneic fibroblasts is worthy of mention in light of developing autoimmunity, which may occur post-CMV infection.
Recently, it was found that the γδ T-lymphocyte subset has the potential to kill targets by using different mechanisms, including Granzymes A and B, Fas ligand, perforin, lymphotoxin β and a gene implicated in generating the lytic response of NK cells.26 Human and murine γδ T cells express inhibitory NK receptors27,28 as well as NKG2D, an NK type of activating receptor that is also expressed by cytolytic CD8+αβ T cells and NK cells.29
NKR-P1 is a marker of interest on γδ T cells. Rat NKR-P1 is a type-II integral membrane protein with an extracellular C-type lectin domain, which is an NK cell-activating receptor. NKR-P1 knockout and NKR-P1-inhibition studies suggest a role of this receptor in target-cell recognition. Eichlberger & Doherty described a population of NK1.1+ γδ T+ cells that developed in cultures of influenza-infected mice with cytotoxic activity.15 However, these cells were unable to lyse the virus-specific target.
Based on this observation, the expression of NKR-P1 was assessed on PLN-derived rat γδ T cells. The total number of NKR.P1+ γδ T+ cells increased four-fold by day 2 after inoculation with aMCMV. The depletion of NKR-P1+ cells within γδ T cells significantly decreased, but did not abolish the cytotoxicity against either infected fibroblasts or YAC-1 targets. This implies that the NKR-P1 receptor is involved in the killing induced by γδ T cells. In contrast, as we can see from Table 1, the expression of NKR-P1 by γδ T cells has little functional relevance in curing fibroblasts from CMV infection. As IFN-γ is an essential molecule in the innate immunity against CMV infection, we assume that in our rat model the NKR.P1+ γδ T+ cells are weak producers of IFN-γ. This suggestion is consistent with the observation that depletion of NK cells expressing the DX-5 antigen (murine pan-NK marker) from γδ T-cell-enriched spleen cells did not affect IFN-γ production in response to Listeria monocytogenes infection.9 Nor can we exclude the fact that other types of NK receptors, such as Ly-49 or the NKG2 family of C-type lectins, including NKG2A, NKG2C, NKG2D and NKG2E, may be involved in the antiviral effects of γδ T cells.
In conclusion, our results show that γδ T cells can manifest their effects as early as 2 days postinfection, before the conventional primary CD8- and CD4-αβ T-cell populations develop. Thus, γδ T cells can provide an important line of defence against CMV, linking innate and adaptive immune responses. The virus-induced enormous increase in the absolute number of γδ T cells, their high cytotoxicity and active production of IFN-γ, making the surrounding cells refractory to infection, may be a mechanism of early protection during CMV infection.
Acknowledgments
This study was supported by a joint grant from Center for Absorption in Science of the Ministry of Immigration Absorption and the Committee for Planning and Budgeting of the Council for Higher Education under the framework of the KAMEA programme.
References
- 1.Hayday AC. γδ Cells. A right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Lawetzky A, Tiefenthaler G, Kubo R, Hunig T. TcR α/β and γ/δ expression in the rat. Eur J Immunol. 1990;20:343–9. doi: 10.1002/eji.1830200217. [DOI] [PubMed] [Google Scholar]
- 3.Itohara S, Nakanishi N, Kanagawa O, Kubo K, Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor γ/δ: analysis of γ/δ T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci USA. 1989;86:5094–8. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhnlein P, Vicente A, Varas A, Hunig T, Zapata A. Gamma/delta T cells in fetal, neonatal, and adult rat lymphoid organs. Dev Immunol. 1995;4:181–8. doi: 10.1155/1995/73127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhnlein P, Park JH, Herrmann T, Elbe A, Hunig T. Identification and characterization of rat gamma/delta T lymphocytes in peripheral lymphoid organs, small intestine, and skin with monoclonal antibody to a constant determinant of the gamma/delta T cell receptor. J Immunol. 1994;153:979–86. [PubMed] [Google Scholar]
- 6.Carding SR, Egan PJ. The importance of γδ T cells in the resolution of pathogen-induced inflammatory immune responses. Immunol Rev. 2000;173:98–108. doi: 10.1034/j.1600-065x.2000.917302.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Kamath A, Das H, Li L, Bukowski JF. Antibacterial effect of human V gamma 2V delta 2 T cells in vivo. J Clin Invest. 2000;108:1349–57. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki G, Yamada H, Kishihara K, Yoshikai Y, Nomoto K. Mechanism of murine V gamma1(+) T cell-mediated innate immune response against Listeria monocytogenes infection. Eur J Immunol. 2002;32:928–35. doi: 10.1002/1521-4141(200204)32:4<928::AID-IMMU928>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Wallace M, Malkovsky M, Carding SR. Gamma/delta T lymphocytes in viral infections. J Leukoc Biol. 1995;58:277–83. doi: 10.1002/jlb.58.3.277. [DOI] [PubMed] [Google Scholar]
- 11.Welsh RM, Lin MY, Lahman BL, Varga SM, Zarozinski CC, Selin LK. αβ and γδ T-cell networks and their roles in natural resistance to viral infections. Immunol Rev. 1997;159:79–93. doi: 10.1111/j.1600-065x.1997.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 12.Bluestone JA, Khattri R, Sciammas R, Sperling AI. TCR gamma delta cells: a specialized T cell subset in the immune system. Annu Rev Cell Dev Biol. 1995;11:307–53. doi: 10.1146/annurev.cb.11.110195.001515. [DOI] [PubMed] [Google Scholar]
- 13.Sim GK. Intraepithelial lymphocytes and the immune system. Adv Immunol. 1995;58:297–343. doi: 10.1016/s0065-2776(08)60622-7. [DOI] [PubMed] [Google Scholar]
- 14.Selin LK, Santolucino PA, Pinto AK, Szomolanyi-Tsuda E, Welsh M. Innate immunity to viruses: control of vaccinia virus infection by γδ T cells. J Immunol. 2001;166:6784–94. doi: 10.4049/jimmunol.166.11.6784. [DOI] [PubMed] [Google Scholar]
- 15.Eichelberger M, Doherty PC. γδ T cells from influenza-infected mice developed a natural killer cell phenotype following culture. Cell Immunol. 1994;159:94–102. doi: 10.1006/cimm.1994.1298. [DOI] [PubMed] [Google Scholar]
- 16.Carding SR, Alan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by gamma/delta+ T cells. J Exp Med. 1990;172:1225–31. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyugovskaya L, Ginsburg H. Cure of fibroblast monolayer from murine cytomegalovirus infection: phenotypic assessment of rat lymphoid cell population developed on fibroblast monolayers. Cell Immunol. 1996;169:30–9. doi: 10.1006/cimm.1996.0087. [DOI] [PubMed] [Google Scholar]
- 18.Wesch D, Glatzel A, Kabelitz D. Differentiation of resting human peripheral blood gamma delta cells toward Th1- or Th2 phenotype. Cell Immunol. 2001;15:110–7. doi: 10.1006/cimm.2001.1850. [DOI] [PubMed] [Google Scholar]
- 19.Ninomiya T, Takimoto H, Matsuzali G, Hamano S, Yoshihida H, Yoshikai Y, Kimura G, Nomoto K. Vγ1+γδ T cells play protective roles at an early phase of murine cytomegalovirus infection through production of interferon-γ. Immunology. 2000;99:187–94. doi: 10.1046/j.1365-2567.2000.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin Z, Chen C, Szabo SJ, Glimcher LH, Ray A, Craft J. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-gamma by gammadelta T cells. J Immunol. 2002;15:1566–71. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 21.Dyugovskaya L, Bashenko Y, Hirsh M, Krausz M. Immune potential of lymph node-derived lymphocytes in uncontrolled hemorrhagic shock. Shock. 2001;15:118–23. doi: 10.1097/00024382-200115020-00007. [DOI] [PubMed] [Google Scholar]
- 22.Vitale M, Neri LM, Comani S, Falcieri E, Rizzoli R, Rana R, Papa S. Natural killer function in flow cytometry. II. Evaluation of NK lytic activity by means of target cell morphological changes detected by right angle light scatter. J Immunol Methods. 1989;121:115–20. doi: 10.1016/0022-1759(89)90426-2. [DOI] [PubMed] [Google Scholar]
- 23.Zamai L, Mariani AR, Zauli G, Rodella L, Rezzani R, Manzoli F, Vitale M. Kinetics of in vitro natural killer activity against K562 cells as detected by flow cytometry. Cytometry. 1998;32:280–5. doi: 10.1002/(sici)1097-0320(19980801)32:4<280::aid-cyto4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Dechanet J, Merville P, Lim A, et al. Implication of γδ T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–49. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a γδ T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–9. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 26.Fahrer AM, Konigshofer Y, Kerr EM, Ghandour G, Mack DH, Davis MM, Chien Y-H. Attributes of γδ intraepithelial lymphocytes as suggested by their transcriptional profile. Proc Natl Acad Sci USA. 2001;98:10261–6. doi: 10.1073/pnas.171320798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura H, Washizu J, Naiki Y, Hara T, Fukui Y, Sasazuki T, Yoskikai Y. MHC Class II-dependent NK1.1+γδ T cells are induced in mice by Salmonella infection. J Immunol. 1999;162:1573–81. [PubMed] [Google Scholar]
- 28.Boullier S, Poque Y, Halary F, Bonneville M, Fournie JJ, Gougeon ML. Phosphoantigen activation induces surface translocation of intracellular CD94/NKG2A class I receptor on CD94-peripheral V gamma 9, V delta 2 T cells but not on CD94-thymic or mature gamma delta T cell clones. Eur J Immunol. 1998;28:3399–410. doi: 10.1002/(SICI)1521-4141(199811)28:11<3399::AID-IMMU3399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 29.Bauer S, Groh V, Steinle Wu J, Phillips JH, Lanier L, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]