Abstract
Peripheral blood monocytes extravasate and differentiate into tissue macrophages to mediate effective local defence, but how tissue-specific stimuli and environments may influence their functions remains unknown. Here, we found that peripheral blood monocytes gained the ability to produce granulocyte–macrophage colony-stimulating factor (GM-CSF) upon exposure to breast milk and differentiated into CD1+ dendritic cells (DCs) in the presence of exogenous interleukin-4 (IL-4) alone. This in vitro observation appeared physiologically relevant since macrophages that were freshly isolated from breast milk were also found to produce GM-CSF spontaneously. Furthermore, in contrast to peripheral blood monocytes that differentiated into DCs only in the presence of both exogenous GM-CSF and IL-4, differentiation of breast milk macrophages into DCs was induced by incubation with exogenous IL-4 alone. These IL-4-stimulated breast milk macrophages were efficient in stimulating T cells, suggesting their potential role in mediating T-cell-dependent immune responses in situ. On the other hand, unexpected expression of DC-SIGN, a DC-specific receptor for human immunodeficiency virus (HIV), even in unstimulated breast milk macrophages, may favour HIV infection, resulting in an increased risk of mother-to-infant vertical transmission of the virus via breast milk. Thus, tissue-specific development of macrophages is often linked to effective local immunity, but may potentially provide an opportunity for a pathogen to spread and transmit.
Introduction
Monocytes exit the bloodstream and differentiate into macrophages in the tissue. These tissue macrophages have developed outstanding bactericidal activity, and thus function as sentinels against invading pathogens.1 In addition, following phagocytosis of antigens encountered, some tissue macrophages differentiate into T-cell stimulatory dendritic cells (DCs) while migrating into the T-cell area of draining lymph nodes.2 Thus, tissue macrophages may potentially play a critical role not only in innate immunity but also in induction of antigen-specific T-cell responses of acquired immunity. However, the in vivo state of differentiation of monocyte-derived cells in unaffected naive tissues remains elusive.
To address this question, our attention has been directed to macrophages observed in breast milk. Breast milk is unique among body fluids in that it contains a large number of macrophages.3 These mononuclear phagocytes comprise up to 80% of the total cells present in the colostrum and early human milk, and appear to play an immunoprotective role in situ and even in the recipient infant.4 Like other tissue macrophages, breast milk macrophages (BrMMφ) are thought to derive from peripheral blood monocytes (PBMo), which extravasate and subsequently migrate into breast milk through the epithelium of the mammary gland.3 In arriving at the breast milk, BrMMφ show highly phagocytic activity and ingest a variety of milk components, resulting in the cytoplasmic accumulation of lipid-rich inclusions.3 Since recent in vitro and in vivo studies have underscored a profound effect of transmigration and phagocytosis on monocyte/macrophage differentiation,2,5 we hypothesized that BrMMφ might significantly modify their functions and could be substantially different from their precursor PBMo.
To test this hypothesis, we isolated BrMMφ from human breast milk and compared them with PBMo. We found that BrMMφ, but not PBMo, were capable of producing granulocyte–macrophage colony-stimulating factor (GM-CSF) spontaneously and differentiating into CD1+ DCs by stimulation with exogenous interleukin-4 (IL-4) alone. These unique functional features of BrMMφ were likely to be induced specifically by phagocytosis of breast milk components since PBMo that were allowed to phagocytose breast milk, but not Latex beads, exerted similar functions. Furthermore, unlike PBMo, BrMMφ expressed DC-SIGN, a DC-specific lectin that mediates interaction with human immunodeficiency virus (HIV),6 suggesting a critical role for BrMMφ in the vertical transmission of HIV via breast milk. These findings emphasize that BrMMφ are unique and potent immune cells that may function in both normal and pathological conditions, rather than terminally differentiated cells that are discarded into breast milk.
Materials and methods
Isolation and culture of BrMMφ and PBMo
Breast milk was collected from healthy women within 3–6 days of delivery after informed consent under a protocol approved by the Institutional Review Board of the Nippon Medical School. Fresh BrMMφ were isolated from breast milk by Ficoll–Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation, followed by adherence to polystyrene tissue culture dishes for 1 hr at 37°. The adherent cells were then removed by incubation with 5 mm ethylenediaminetetraacetic acid for 30 min at 4°. Flow cytometric analysis of the cells thus obtained revealed that the cell preparation contained homogeneous CD14+ cells with no apparent contaminating cells (data not shown). In some experiments in which any in vitro stimulation needed to be avoided, this plastic adherence step was omitted. PBMo were isolated from human peripheral blood either by plastic adherence or by the previously described magnetic antibody cell sorting procedure.7 For treatment of BrMMφ and PBMo with cytokines, the cells were plated at 6 × 105/ml in RPMI-1640 medium (Sigma Chemical Co., St Louis, MO) supplemented with 10% fetal calf serum (Moregate, Bulimba, Australia), and cultured at 37° for 5 days either in the presence or absence of GM-CSF (50 ng/ml) and/or IL-4 (1000 U/ml) (both from Biosource International, Inc., Camarillo, CA).
GM-CSF secretion assays
Freshly isolated BrMMφ and PBMo were placed in the wells of 96-well tissue culture plates (2 × 105/well) containing 200 μl of RPMI-1640 medium supplemented with 10% fetal calf serum. After 1-day incubation at 37°, the culture supernatants were collected and GM-CSF concentration was determined, using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Biosource International). In some experiments, PBMo (1 × 106/ml) were incubated in 24-well culture plates for 4 hr with either breast milk supernatant at a concentration of 25% (v/v) or 0·1% latex beads (Polysciences, Inc., Warrington, PA). At the end of the incubation, the cells were harvested, and washed extensively. The cells were then cultured for an additional 30 hr in 96-well tissue culture plates, and GM-CSF concentration in the culture supernatants was determined as described above.
Antibodies and flow cytometry
Fluorescein isothiocyanate-conjugated antibodies to CD14 and CD40 as well as phycoerythrin-conjugated CD11b, CD83, and CD86 were purchased from PharMingen (San Diego, CA) and phycoerythrin-conjugated anti-human DC-SIGN monoclonal antibody (mAb) was from R & D Systems (Minneapolis, MN). The mAbs against CD1a, CD1b, and HLA-DR have been described previously.8,9 Fluorescein isothiocyanate-conjugated antibodies against mouse immunoglobulins were purchased from Immunotech (Marseille, France). Cells were preincubated at 4° for 30 min in RPMI-1640 media containing 10% human AB serum to block Fc receptors, and then labelled with indicated antibodies as described previously.10 Labelled cells were analysed using a FACScan (Becton Dickinson, Mountain View, CA) with propidium iodide gating for viable cells.
Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted from 3 × 105 cells of each cell preparation by using the commercial RNeasy kit (QIAGEN, Hilden, Germany), and the first-strand DNA was synthesized as described previously.7 Transcripts of CD83, GM-CSF, and DC-SIGN as well as the house-keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were amplified by PCR reactions. The primer sets used were: CD83 sense, 5′-ATT ATT GTA GGG TGG TGA AGA GAG G-3′; CD83 antisense, 5′-GTG AGG AGT CAC TAG CCC TAA ATG C-3′; GM-CSF sense, 5′-GCT GCT GAG ATG AAT GAA AC-3′; GM-CSF antisense, 5′-AGT CAA AGG GGA TGA CAA G-3′; DC-SIGN sense, 5′-GAG CTT AGC AGG GTG TCT TG-3′; DC-SIGN antisense, 5′-GCA GGC GGT GAT GGA GTC GT-3′; GAPDH sense, 5′-GCC TCA AGA TCA TCA GCA ATG C-3′; GAPDH antisense, 5′-ATG CCA GTG AGC TTC CCG TTC-3′. After 30 cycles of PCR reactions, the PCR products were resolved by electrophoresis in agarose gels and visualized by ethidium bromide staining using a UV light source.
T-cell proliferation assay
Cord blood mononuclear cells were isolated by Ficoll–Hypaque density gradient centrifugation and non-T cells were removed, using the Lympho-kwik (One Lambda, Canoga Park, CA) according to the manufacturer's instruction. The remaining cells were then passed through nylon wool column. The T-cell population thus obtained comprised over 98% CD45RA+, CD3+ naive T cells, and were used as responder T cells. BrMMφ were pretreated with GM-CSF and/or IL-4 as mentioned above, and the cells were then washed, irradiated (5000 rads) and used as antigen-presenting cells. Using 96-well, U-bottomed tissue culture plates, purified native T cells (5 × 104/well) were cultured with indicated numbers of antigen-presenting cells at 37° for 5 days, and the [3H]thymidine (1 μCi/well) incorporation during the last 18 hr of culture was determined as described previously.7
Results and discussion
Distinct phenotypes of BrMMφ and PBMo
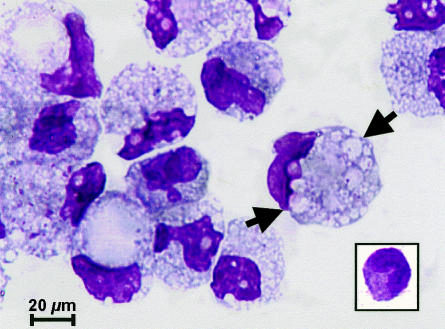
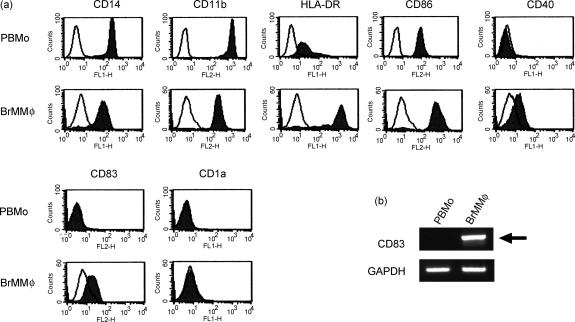
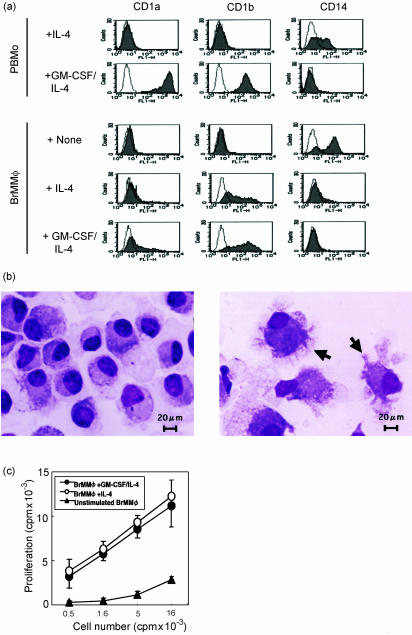
In order to detect differences between BrMMφ and their possible precursor PBMo, BrMMφ were purified from the colostrum and compared with PBMo. As shown in Fig. 1, freshly isolated BrMMφ were larger than PBMo (inset), and contained numerous inclusions in the cytoplasm (indicated with arrows). Both cell types expressed monocyte/macrophage lineage markers, such as CD14 and CD11b, but notably higher levels of surface expression for these marker proteins were observed on PBMo than on BrMMφ. In contrast, some of the molecules, such as HLA-DR, CD86 and CD40, that were involved in T-cell stimulation, were expressed more prominently on BrMMφ than on PBMo. Strikingly, cell surface expression of CD83, a glycoprotein expressed in mature DCs and in activated cells of some lineages,11,12 was detected only on BrMMφ, but not on PBMo (Fig. 2a). Biosynthesis of CD83 in BrMMφ and the lack of its expression in PBMo were further confirmed at the transcriptional level by RT-PCR (Fig. 2b). These phenotypic studies suggested that BrMMφ and PBMo might be at different stages of cell activation and/or differentiation. Although the possibility that BrMMφ were fully differentiated DCs was ruled out since these cells lacked the expression of CD1a (Fig. 2a) and CD1b (data not shown), down-regulation of monocyte/macrophage markers and up-regulation of major histocompatibilty complex class II and T-cell co-stimulatory molecules as well as induction of CD83 expression in BrMMφ led to the hypothesis that these cells might be committed to differentiating into DCs.
Figure 1.
BMMφ were morphologically distinct from their possible precursor PBMo. BMMφ and PBMo (inset) were subjected to May–Grünwald staining, and viewed under a light microscope. Note that BMMφ contained numerous inclusions (arrows) in their large cytoplasm.
Figure 2.
BrMMφ were phenotypically distinct from PBMo. (a) Purified PBMo (upper panels) and BrMMφ (lower panels) were analysed for surface expression of indicated proteins by flow cytometry. Specific staining (filled area) and negative control staining (open area) were shown in each panel. (b) PBMo and BrMMφ were analysed for mRNA expression of CD83 (upper panel) and GAPDH (lower panel) by RT-PCR. Note that a specific band representing CD83 mRNA expression (arrow) was detected only for BrMMφ, but not for PBMo, whereas both cell types expressed the house-keeping gene, GAPDH.
Spontaneous production of GM-CSF by BrMMφ
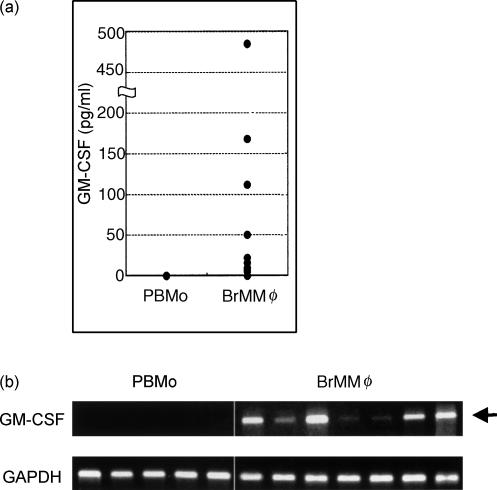
It has been established that GM-CSF is the most potent cytokine that induces differentiation of monocytes into DCs.13 However, it seems unlikely that BrMMφ are stimulated in situ by exogenous GM-CSF derived from other cell types since the level of GM-CSF in breast milk, if detectable by sensitive assays, is comparable to that in the serum.14 Alternatively, we considered the possibility that BrMMφ might spontaneously produce GM-CSF and stimulate themselves in an autocrine fashion. Indeed, when freshly isolated BrMMφ were left unstimulated in culture for 2 days, significant levels of GM-CSF were detected in the culture supernatants (Fig. 3a). For comparison, culture supernatants of PBMo incubated under identical conditions did not contain detectable levels of the cytokine (Fig. 3a). To rule out the possibility that BrMMφ might be stimulated non-specifically with bovine serum components to produce GM-CSF, freshly isolated BrMMφ were analysed immediately for the expression of GM-CSF mRNA by RT-PCR. Transcription of the GM-CSF gene was observed in all the BrMMφ preparations from seven different donors, but not in any of the PBMo preparations so far analysed (Fig. 3b). Thus, the unique ability of BrMMφ to produce GM-CSF spontaneously was demonstrated both at the transcriptional and at the translational levels.
Figure 3.
BrMMφ, but not PBMo, spontaneously produced GM-CSF. (a) Freshly isolated PBMo from six subjects and BrMMφ from 13 subjects were cultured separately for 1 day, and the amount of GM-CSF released into the medium was determined by ELISA and plotted. Note that, whereas no PBMo preparations secreted detectable levels of the cytokine, BrMMφ isolated from most donors produced significant levels of GM-CSF (P < 0·0035; Mann–Whitney U-test). (b) GM-CSF expression in PBMo and BrMMφ was also examined at the transcriptional level by RT-PCR. All the BrMMφ preparations from seven different donors were found to express the GM-CSF gene (arrow) whereas none of the five PBMo preparations expressed the gene.
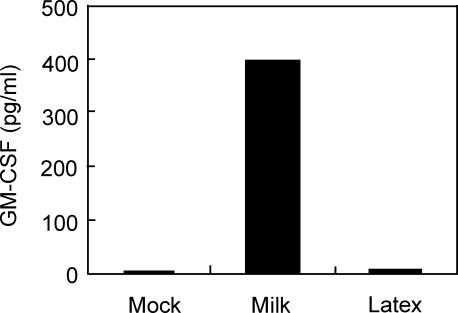
It remained to be determined how BrMMφ acquired the ability to produce GM-CSF. However, one of the critical differences between BrMMφ and their possible precursor PBMo is that BrMMφ prominently phagocytose breast milk components, which PBMo is never exposed to. Thus, we considered the possibility that PBMo might gain the ability to produce GM-CSF after an exposure to breast milk. To test this possibility, freshly isolated PBMo were allowed to phagocytose either breast milk or control latex beads for 4 hr, and then the cells were washed and cultured for an additional 30 hr. Production of GM-CSF by these cells was assessed by measuring its concentration in the culture medium. As shown in Fig. 4, breast-milk-treated PBMo gained the ability to produce a significant amount of GM-CSF whereas latex-bead-treated cells, as well as mock-treated cells, did not secrete detectable levels of GM-CSF. These results demonstrated that phagocytosis of breast milk was likely to be a potent stimulus for GM-CSF production, which may account for spontaneous production of the cytokine by BrMMφ.
Figure 4.
GM-CSF was produced from PBMo after phagocytosis of breast milk. Purified PBMo were cultured with either media alone, 25% breast milk supernatant, or 0·1% latex beads. After 4 hr, the cells were washed, and then cultured for an additional 30 hr. At the end of the culture, the culture supernatants were collected and GM-CSF concentration was determined by enzyme-linked immunosorbent assay. Light microscopic observation of latex-bead-treated cells revealed that over 90% of cells contained phagocytosed beads intracellularly (not shown).
Differentiation of BrMMφ into DCs by stimulation with exogenous IL-4
Given the impact of GM-CSF upon differentiation of myeloid cells into DCs in general,13 we reasoned that spontaneous production of GM-CSF by BrMMφ might influence their capability for differentiation into DCs. Since it is known that IL-4 supports GM-CSF-dependent DC differentiation,13 we examined if exogenously added IL-4 might act synergistically with endogenously produced GM-CSF and induce differentiation of BrMMφ into DCs. As reported previously,13 PBMo did not differentiate into CD1+ DCs and retained the expression of CD14 in the presence of exogenous IL-4 alone (Fig. 5a, top panels) whereas the cells fully differentiated into DCs after stimulation with the combination of GM-CSF and IL-4, evidenced by the induction of CD1a and CD1b expression and the loss of CD14 expression (second panels from top). In sharp contrast, significant levels of CD1b expression, as well as marginal induction of CD1a, were observed on BrMMφ stimulated with IL-4 alone (fourth panels from top). Consistent with their being DCs, these IL4-stimulated BrMMφ developed plasma membrane projections (indicated with arrows in right panel of Fig. 5b), which were not apparent in BrMMφ cultured without IL-4 (left panel of Fig. 5b). Furthermore, unlike mock-treated BrMMφ that retained the expression of CD14, virtually no expression of the monocyte/macrophage marker protein was detected on IL-4-stimulated BrMMφ. A similar phenotype was observed for IL-4-stimulated PBMo that had been preincubated with breast milk (data not shown), indicating that phagocytosis of breast milk could influence differentiation of myeloid cells into DCs. These findings underscored a remarkable ability of BrMMφ to initiate DC differentiation by stimulation with IL-4 alone even in the absence of exogenous GM-CSF. It is most likely that endogenous GM-CSF produced by BrMMφ acted synergistically with exogenous IL-4.
Figure 5.
BrMMφ differentiated into CD1+ dendritic cells with high T-cell stimulatory activity after incubation with IL-4 alone. (a)Surface expression of CD1a, CD1b and CD14 molecules on cytokine-activated PBMo and BrMMφ and mock-treated BrMMφ was determined by flow cytometry. Broken lines represent negative staining. Mock-treated PBMo were not analysed due to their poor viability. (b) IL-4-stimulated BrMMφ developed plasma membrane projections (arrows in right panel), which were not apparent in BrMMφ cultured without IL-4 (left panel). Cells were subjected to May–Grünwald staining, and viewed under a light microscope. (c)To assess the ability of cytokine-stimulated BrMMφ to activate naïve T cells, allogeneic cord blood T cells (1 × 104/well) were cultured for 5 days with the indicated numbers of irradiated BrMMφ that were preincubated with either GM-CSF plus IL-4, IL-4 alone, or medium alone, and the [3H]thymidine uptake during the last 12 hr of culture was measured. Results were expressed as counts per min ± SEM.
T-cell activation by IL-4-stimulated BrMMφ
DC differentiation is generally associated with increased T-cell stimulatory activity.15 Therefore, we examined if IL-4-stimulated BrMMφ might exhibit better T-cell stimulatory activity than unstimulated BrMMφ. As shown in Fig. 5(c), unstimulated BrMMφ did not efficiently induce proliferation by allogeneic cord blood-derived T cells. In contrast, IL-4-stimulated BrMMφ were significantly more potent in inducing T-cell activation than unstimulated BrMMφ and were almost as efficient as GM-CSF/IL-4-stimulated BrMMφ that had undergone full differentiation into DCs.
Taken together, these results demonstrated that, after migration into breast milk, BrMMφ were no longer identical to their precursor monocytes. GM-CSF produced by BrMMφ could be a critical factor for local defence in both innate and T-cell dependent immunological settings. It has been recently reported that GM-CSF can augment phagocytosis and pathogen killing by macrophages in the PU.1 transcription factor-dependent manner.1 Thus, one can speculate that BrMMφ, activated by GM-CSF in an autocrine fashion, may be potentiated to mediate initial defence against invading pathogens. Indeed, a role for GM-CSF in preventing Staphylococcus aureus infections in the mammary gland has been noted.16 Furthermore, the ability of BrMMφ to differentiate into functional DCs when stimulated with exogenous IL-4 makes it likely that BrMMφ may undergo such differentiation at a site of mastitis and play a role in mediating specific T-cell responses locally since a small fraction of T cells in breast milk are known to secrete IL-4 upon inflammatory stimulation.17
Expression of DC-SIGN in BrMMφ
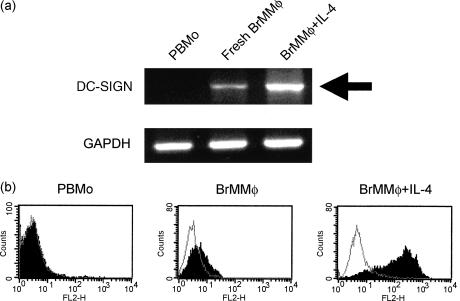
Although DCs are critical immune-competent cells that orchestrate effective immune responses, some pathogens, such as HIV, have been evolved to utilize DCs as their target for infection.18,19 Given that vertical transmission of HIV via breast milk requires living cells in breast milk,20 we were particularly interested in the expression of DC-SIGN, a DC-specific lectin that binds HIV envelope glycoproteins, in BrMMφ.6,21,22 Using RT-PCR, we found that freshly isolated BrMMφ, but not PBMo, expressed DC-SIGN even before their differentiation into DCs (Fig. 6a). The expression of DC-SIGN in BrMMφ was further up-regulated when the cells were stimulated by exogenous IL-4 alone to initiate DC differentiation (Fig. 6a). Based on these observations, we used flow cytometry to examine whether DC-SIGN is present on freshly isolated BrMMφ. As shown in Fig. 6(b), we could detect marginal but significant expression of DC-SIGN on BrMMφ, although we could not see any measurable expression on PBMo. As expected, strong enhancement of the DC-SIGN expression was observed on IL-4-treated BrMMφ (Fig. 6b). Thus, being committed to differentiating into DCs, BrMMφ could potentially provide a useful tool for HIV spread, resulting in efficient virus transmission to the infant via breast milk. Local production of IL-4 in mastitis may up-regulate the expression of DC-SIGN in BrMMφ, which may explain why mastitis is linked with higher HIV load in breast milk and higher risk of mother-to-infant vertical transmission of the virus, as noted recently.23
Figure 6.
Freshly isolated PBMo and BrMMφ as well as IL-4-stimulated BrMMφ were analysed (a)for mRNA expression of DC-SIGN (upper panel) and GAPDH (lower panel) by RT-PCR and (b) for protein expression of DC-SIGN by FACScan. In FACScan analysis, specific staining (filled area) and negative control staining (open area) were shown in each panel. Note that fresh BrMMφ, but not PBMo, expresses the DC-SIGN gene and protein and its expression was up-regulated after IL-4 stimulation.
The salient feature of BrMMφ demonstrated in the present study underscores an ability of monocyte-derived tissue macrophages to undergo morphological, phenotypic and functional modifications after extravasation. Such modifications are often associated with efficient local defence, but may provide an opportunity for a pathogen to spread and transmit.
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Science, Sport, and Culture (#10180102), from the Ministry of Health and Labor and Welfare, Japan, and from the Japanese Health Science Foundation.
Abbreviations
- BrMMφ
breast milk macrophages
- DC
dendritic cell
- PBMo
peripheral blood monocytes
References
- 1.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU1. Immunity. 2001;15:557–67. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 2.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–61. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 3.Pitt J. The milk mononuclear phagocyte. Pediatrics. 1979;64:745–9. [PubMed] [Google Scholar]
- 4.Hughes A, Brock JH, Parrott DM, Cockburn F. The interaction of infant formula with macrophages: effect on phagocytic activity, relationship to expression of class II MHC antigen and survival of orally administered macrophages in the neonatal gut. Immunology. 1988;64:213–18. [PMC free article] [PubMed] [Google Scholar]
- 5.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–3. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 6.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 7.Spada FM, Borriello F, Sugita M, Watts GF, Koezuka Y, Porcelli SA. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur J Immunol. 2000;30:3468–77. doi: 10.1002/1521-4141(2000012)30:12<3468::AID-IMMU3468>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Olive D, Dubreuil P, Mawas C. Two distinct TL-like molecular subsets defined by monoclonal antibodies on the surface of human thymocytes with different expression on leukemia lines. Immunogenetics. 1984;20:253–64. doi: 10.1007/BF00364207. [DOI] [PubMed] [Google Scholar]
- 9.Lampson LA, Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980;125:293–9. [PubMed] [Google Scholar]
- 10.Pena-Cruz V, Ito S, Oukka M, Yoneda K, Dascher CC, Von Lichtenberg F, Sugita M. Extraction of human Langerhans cells: a method for isolation of epidermis-resident dendritic cells. J Immunol Methods. 2001;255:83–91. doi: 10.1016/s0022-1759(01)00432-x. [DOI] [PubMed] [Google Scholar]
- 11.Scholler N, Hayden-Ledbetter M, Hellstrom KE, Hellstrom I, Ledbetter JA. CD83 is a sialic acid-binding Ig-like lectin (Siglec) adhesion receptor that binds monocytes and a subset of activated CD8+ T cells. J Immunol. 2001;166:3865–72. doi: 10.4049/jimmunol.166.6.3865. [DOI] [PubMed] [Google Scholar]
- 12.Yamashiro S, Wang JM, Yang D, Gong WH, Kamohara H, Yoshimura T. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958–63. [PubMed] [Google Scholar]
- 13.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasparoni A, Chirico G, De Amici M, Ravagni-Probizer M, Ciardelli L, Marchesi ME, Rondini G. Granulocyte-marophage colony-stimulating factor in human milk. Eur J Pediatr. 1996;155:69. doi: 10.1007/BF02115636. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 16.Daley M, Williams T, Coyle P, Furda G, Dougherty R, Hayes P. Prevention and treatment of Staphylococcus aureus infections with recombinant cytokines. Cytokine. 1993;5:276–84. doi: 10.1016/1043-4666(93)90015-w. [DOI] [PubMed] [Google Scholar]
- 17.Skansen-Saphir U, Lindfors A, Andersson U. Cytokine production in mononuclear cells of human milk studied at the single-cell level. Pediatr Res. 1993;34:213–6. doi: 10.1203/00006450-199308000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Cameron PU, Lowe MG, Crowe SM, O'Doherty U, Pope M, Gezelter S, Steinman RM. Susceptibility of dendritic cells to HIV-1 infection in vitro. J Leukoc Biol. 1994;56:257–65. doi: 10.1002/jlb.56.3.257. [DOI] [PubMed] [Google Scholar]
- 19.Granelli-Piperno A, MoSeries B, Pope M, et al. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–8. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Southern SO. Milk-borne transmission of HIV. Characterization of productively infected cells in breast milk and interactions between milk and saliva. J Hum Virol. 1998;1:328–37. [PubMed] [Google Scholar]
- 21.Steinman RM. DC-SIGN: a guide to some mysteries of dendritic cells. Cell. 2000;100:491–4. doi: 10.1016/s0092-8674(00)80684-4. [DOI] [PubMed] [Google Scholar]
- 22.Sol-Foulon N, Moris A, Nobile C, et al. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity. 2002;16:145–55. doi: 10.1016/s1074-7613(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 23.Semba RD. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci. 2000;918:156–62. doi: 10.1111/j.1749-6632.2000.tb05484.x. [DOI] [PubMed] [Google Scholar]