Abstract
The production of nitric oxide (NO) by intraperitoneal macrophages of mice during secondary infection with Echinococcus multilocularis mediates immunosuppression at early and late stages of infection. We addressed the role of NO in host resistance against this extracellular metazoan parasite by infecting inducible nitric oxide synthase knockout ((iNOS-KO) mice (of the C57BL/6 background) with 100 metacestode vesicles. The parasite weight was significantly lower in iNOS-KO mice when compared with wild-type (WT) mice at 4 months postinfection (late stage), thus demonstrating that iNOS deficiency confers a certain degree of resistance against persistent chronic infection. However, histological analysis of periparasitic tissue showed no differences between WT and iNOS-KO mice, as both exhibited granuloma formation and the presence of giant cells. Together with histology, the production of a high level of interferon-γ (IFN-γ) in infected iNOS-KO mice upon stimulation with concanavalin A (Con A) and VF-antigen indicated normal T-cell signalling in these animals. As expected, peritoneal exudate cells (PEC) from infected iNOS-KO mice produced no detectable NO, while the PEC from infected WT mice produced high levels of NO after stimulation with lipopolysaccharide (LPS) and parasite protein or carbohydrate antigen, or even without in vitro stimulation. Consequently, the high level of NO production observed during chronic infection in WT mice appears to contribute more to immunosuppression than to limitation of parasite growth. This is also reflected by the fact that splenocyte proliferation was significantly higher and parasite masses lower in iNOS-KO mice (at 1 and 4 months postinfection) than in WT mice.
Introduction
Alveolar echinococcosis (AE) is a chronic parasitic disease predominantly of the liver. AE is characterized by tumour-like growth of the larval cestode (metacestode) of Echinococcus multilocularis. This exclusively extracellular metazoan parasite is able to induce immunosuppressive mechanisms similar to those of intracellular pathogens dealing with macrophage activation.1 Recently, we have described two immunological characteristics involved in the modulation of the host immune response, both taking part in the survival strategy of the parasite. One relates to the expression of a major carbohydrate antigen, Em2(G11), within the laminated layer of the metacestode. The laminated layer functions as a protective shield against host effector mechanisms. The Em2(G11) antigen induces an immunoglobulin G (IgG) response independent of T-cell receptor (TCR)-αβ+ and CD4+ T cells.2 Therefore, it is a T-cell independent antigen and may be responsible for the restricted T-cell activation and for an IgG response lacking avidity maturation after infection with E. multilocularis. The other key factor modulating the host–parasite interplay during chronic infection with E. multilocularis is immunosuppression induced by a high level of nitric oxide (NO) production by peritoneal exudate cells (PEC).1
A number of studies have shown that NO has important modulatory functions in a variety of pathological processes associated with infectious organisms or with protection against some microbial infections. NO-based restriction of the growth of pathogens, but also restriction/suppression of cell-mediated defences against intracellular pathogens, have been shown with the predominantly T helper 1 (Th1)-controlled intracellular infections with Mycobacterium tuberculosis,3,4Leishmania major5,6 and Toxoplasma gondii.7 The antimicrobial activity of reactive nitrogen intermediates (RNIs) has been convincingly demonstrated by the use of transgenic mice deficient for one or several antimicrobial effector pathways.8 Nevertheless, evidence from inducible nitric oxide synthase (iNOS)-deficient mice also demonstrates a marked variety in the role of RNIs. Depending on the infection, the contribution of iNOS to host protection is critical, ancillary, deleterious or imperceptible.9 However, NO has also a variety of other bioregulatory activities and iNOS is expressed in many cell types upon stimulation. Accordingly, the role of NO appears to vary considerably in pathological processes, depending on the tissues affected, as shown in a murine Schistosoma japonicum model.10 NO production was even shown to be detrimental to the host infected with M. avium,11,12 as inducible NO appeared to be more important for immunopathology than for host resistance.13
To date, the roles of NO in E. multilocularis infection have been relatively poorly defined. NO intermediates appeared to be instrumental in the in vitro killing of protoscolices of the parasite.14 However, as shown in a previous study, NO production by macrophages mediated immunosuppression at an early stage, as well as at a late stage of infection.1 Therefore, the question was raised as to whether NO production – in response to infection with E. multilocularis– was protective by limiting the parasite growth, or detrimental (exacerbative) by mediating immunosuppression or immune-mediated lesion formation in secondarily infected mice. In this study, we further addressed the role of NO in host resistance against this extracellular metazoan parasite by infecting iNOS-knockout (KO) mice (of the C57BL/6 background) and corresponding wild-type (WT) mice, and by assessing the parasite proliferative potential in these mice, the association of parasite growth with NO levels expressed by PEC, and the splenocyte proliferative response to various stimulators.
Materials and methods
Mice
Female C57BL/6 mice (8–10 weeks of age) were purchased from the Biotechnology & Animal Breeding Division (Füllinsdorf, Switzerland). The iNOS-KO mice (C57BL/6 background) were kindly provided by Dr H. Mossmann (Max-Planck-Institute for Immunobiology, Freiburg, Germany); the animals were originally established by MacMicking et al.15 In all experiments, animals were matched for age and weight. Each experimental group consisted of five animals (unless otherwise indicated in the Results or the figure legends). The number of groups and repetition of experiments are given in the Results section and in the legends to the figures. All mice were raised, housed and handled under standard aseptic animal laboratory conditions according to the rules of the Swiss regulations for animal experimentation.
Parasites, experimental infections and parasite antigen preparation
The parasite used in this study was a cloned E. multilocularis (KF5) isolate (as used in a previous study; see ref. 1), maintained by serial passages (vegetative transfer) in C57BL/6 mice. Animals were injected intraperitoneally (using a syringe and 1·2 × 40-mm needles) with 100 freshly prepared acephalic vesicular cysts suspended in RPMI-1640 (Gibco, Basel, Switzerland) to a final volume of 100 µl per mouse. Control mice received a corresponding volume of RPMI-1640.
A somatic E. multilocularis metacestode antigen generated in vitro (VF-antigen) was used for proliferation assays. It is identical to that employed previously.1 Briefly, the antigen isolation was based upon a host cell-free in vitro culture of E. multilocularis metacestode vesicles. These were washed with RPMI-1640 prior to aspiration of the vesicle cyst fluid. The fluid was directly used as antigen, storage was at −80°, and it was shown to be free of any bacterial endotoxins, as assessed using a standard turbidimetric kinetic-Limulus assay (Amoebocyte lysate assay; Haemachem Inc., St. Louis, MO).
At necropsy, metacestode tissue was carefully removed from the peritoneal cavity and from liver lobes by using microsurgery forceps and scalpel blades. The parasite tissue – thus free of host tissue – was immediately weighed on a Mettler AE160 scale (Mettler Toledo AG, Greifensee, Switzerland).
Cell preparations
Spleen-cell suspensions were prepared from infected or non-infected control mice, as previously described.1 PEC were obtained by performing peritoneal washings with 5 ml of RPMI-1640; the sediments of the washings were resuspended in RPMI-1640 complete medium and processed further, as described previously.1
Cell cultures and lymphocyte proliferation assays
Spleen cells were cultured in 96-well round-bottom plates at a concentration of 2 × 105 cells/well. The cell cultures were stimulated with concanavalin A (Con A) (2 µg/ml; Sigma Chemical Co., Basel, Switzerland), lipopolysaccharide (LPS) (2 µg/ml; Sigma Chemical Co.), parasite VF-antigen (Faub-isolate, 10 µg/ml), or affinity-purified carbohydrate Em2G11-antigen,2 or were left unstimulated as negative controls. All tests were performed in quadruplicate. Cells were stimulated with VF-antigen and all other parasite antigens for 96 hr, and with Con A or LPS, respectively, for 72 hr. Subsequently, the cells were pulsed with 1 µCi/well of [3H]thymidine (NEN, Boston, MA) and harvested 16–18 hr later. The results were calculated as the mean counts per minute (c.p.m.) of quadruplicate wells and expressed as geometric mean minus background (Δc.p.m.), where background = c.p.m. of wells containing pulsed but unstimulated cells. The background was accepted when it was < 10% of the values of the stimulated, positive wells. Proliferation assays and the experiments for collecting supernatants for quantification of NO and interferon-γ (IFN-γ) production were carried out in parallel. Supernatants were harvested from quadruplicate cultures after 24, 48 or 96 hr, and stored at −80°.
IFN-γ was measured by using sandwich enzyme-linked immunosorbent assays (ELISAs). Paired capture and biotinylated detection antibodies (Abs) were purchased from PharMingen GmbH (Heidelberg, Germany). Alkaline phosphatase covalently linked to streptavidin (PharMingen GmbH) was used to induce a colour reaction for the quantitative detection of secondary Abs. The absorbance was subsequently measured at 405 nm.
Nitrite accumulation in the cell culture supernatants was measured by using the Griess assay. Briefly, 100 µl of sample and standard reagent sodium nitrite (NaNO2) were added to individual wells of a 96-well plate, followed by 100 µl of Griess reagent (1% sulphanilamide in 2·5% H3PO4, 0·1% naphthylethylenediamine dihydrochloride in 2·5% H3PO4). After 10 min of incubation, the absorbance was read at 543 nm.
Histology
After killing infected mice by CO2, livers containing macroscopically visible metacestode tissue were fixed in 4% buffered paraformaldehyde solution. Paraffin-embedded tissue sections were stained with haematoxylin & eosin (H & E).
Statistical analysis
Groups were compared using the Student's t-test (Microsoft Excel; MacOffice2001). A P-value of < 0·05 was considered statistically significant.
Results
Increased resistance to secondary chronic E. multilocularis infection in iNOS-deficient mice
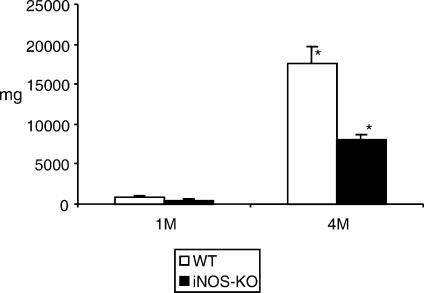
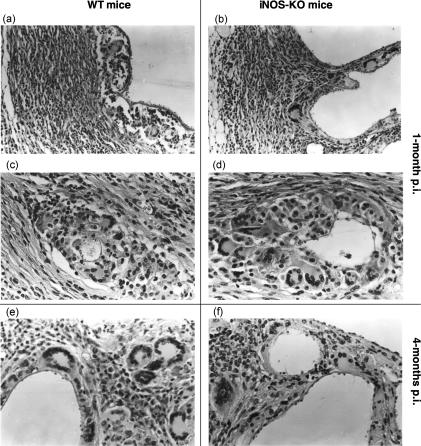
All infected mice (five animals per group, experiments repeated four times) exhibited the presence of metacestode at necropsy. The total metacestode weight recovered (including metastases) was assessed per animal, i.e. 20 iNOS-KO and 20 WT mice at 1 and 4 months postinfection (p.i.). As shown in Fig. 1, the parasite weight from iNOS-KO mice was significantly lower (P < 0·01) than that of WT mice at 4 months p.i. The metacestodes appeared viable upon histological examination in both types of mice, and no difference was detected in parasite morphology, or host reaction to the parasite, between WT mice and those of iNOS-KO origin (Fig. 2). These data demonstrated that the host immune response – independent of NO synthesis – failed principally to kill and eliminate the parasite, but NO contributed essentially to the promotion of the metacestode proliferative potential.
Figure 1.
Mean metacestode tissue masses (in mg) at 1 and 4 months postinfection (p.i.) (1 M and 4 M, respectively). White bars represent the values obtained from wild-type (WT) mice, black bars the values from inducible nitric oxide synthase-knockout (iNOS-KO) mice. Indicated are the mean values + standard errors per group of 20 mice (data represent four independent experiments with five animals per each group). *Statistically significant differences (P < 0.05).
Figure 2.
Comparative histopathological investigation of Echinococcus multilocularis-infected wild-type (WT) mice (panels a, c and e) and inducible nitric oxide synthase (iNOS)-deficient mice (panels b, d and f) showed no difference between lesions of WT and iNOS-knockout (KO) mice at 1 and 4 months postinfection (p.i.). Panels (a), (b), (c) and (d) show lesions of mice at 1 month p.i. at low and high magnification. There was a massive fibrosis (panels a and b) with numerous multinucleated giant cells and epithelioid cells (panels c and d) in both wild-type (2a, 2c) and iNOS-KO mice (2b, 2d). There was still no significant difference between lesions of WT (Fig. 2e) and KO (Fig. 2f) mice at 4 months p.i. The magnification was ×160 for (a) and (b), and ×320 for (c), (d) and (f).
Histopathology and splenocytic IFN-γ-production in E. multilocularis-infected WT and iNOS-deficient mice
When metacestode-infected liver samples were analysed histopathologically, no difference was detected between lesions of WT and iNOS-KO mice at 1 and 4 months after infection (Fig. 2a–2e). A periparasitic granulomatous inflammation with massive fibrosis and numerous multinucleated giant cells and epithelioid cells was consistently observed in both WT and iNOS-KO mice.
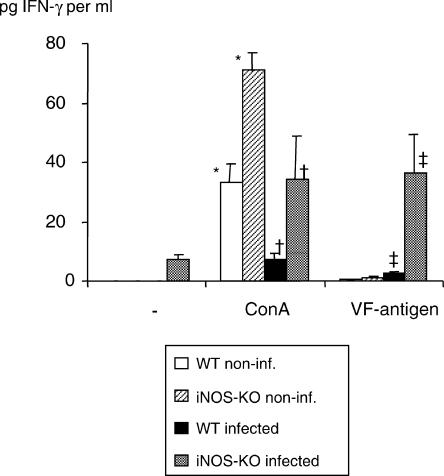
The splenocytes from infected iNOS-KO mice exhibited a high level of IFN-γ at 1 (data not shown) and 4 months (Fig. 3) after infection upon stimulation with Con A and VF-antigen, whereas the corresponding responses were almost abrogated in infected WT mice. IFN-γ expression was also higher in Con A-stimulated splenocytes from non-infected iNOS-KO mice than in WT mice, while VF-antigen stimulation did not result in any significant IFN-γ production in these two non-infected mouse groups, as expected.
Figure 3.
Interferon-γ (IFN-γ) production in infected wild-type (WT) and inducible nitric oxide synthase-knockout (iNOS-KO) mice upon stimulation with concanavalin A (Con A) and VF-antigen in vitro. Splenocytes were collected at 4 months postinfection (p.i.). Identical results were obtained from animals at 1 month p.i. (data not shown). The figure shows results from one representative out of two independent experiments. *, † and ‡ indicate statistically significant differences, assessed upon five different measurement points per group (P < 0.05).
These data together principally indicate normal T-cell signalling in iNOS-KO mice.
High NO production by PECs in BL/6 WT mice after infection with E. multilocularis
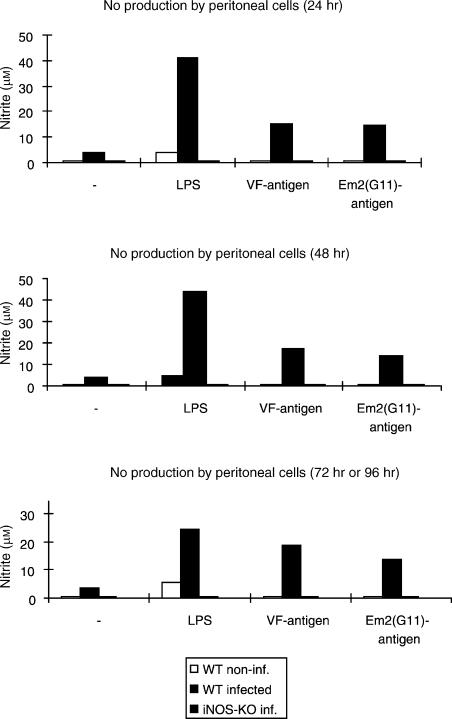
As a consequence of finding different parasite masses in WT and iNOS-KO mice at 4 months p.i., we assessed NO production specifically at this time-point of infection. As expected, PECs from infected iNOS-KO mice generated no detectable NO, while PECs from infected WT mice produced high levels of NO upon stimulation with LPS, and also with the different parasite antigens used, and – to a minor extent – even in the absence of stimulation (Fig. 4). NO production was also seen in the supernatants of stimulated splenocytes from uninfected WT mice, but not from corresponding iNOS-KO mice (data not shown).
Figure 4.
Determination of nitric oxide (NO) production in culture supernatants. Peritoneal exudate cells (PEC) from 1-month-infected mice were left unstimulated (–) or were stimulated in vitro with lipopolysaccharide (LPS) or parasite VF- and Em2(G11)-antigen. White bars refer to non-infected control mice, black bars to wild-type (WT) mice and striped bars to inducible nitric oxide synthase-knockout (iNOS-KO) mice. Each point was measured in quadruplicate. Supernatants were harvested after 24, 48 and 72 hr (LPS) or 96 hr (VF-antigen and Em2(G11)-antigen), respectively; nitrite (NO2−) accumulation was assessed using the Griess method. This is one representative out of four independent experiments with similar patterns of NO production.
Suppressive splenic proliferative responses to mitogen and VF-antigen stimulation during chronic AE
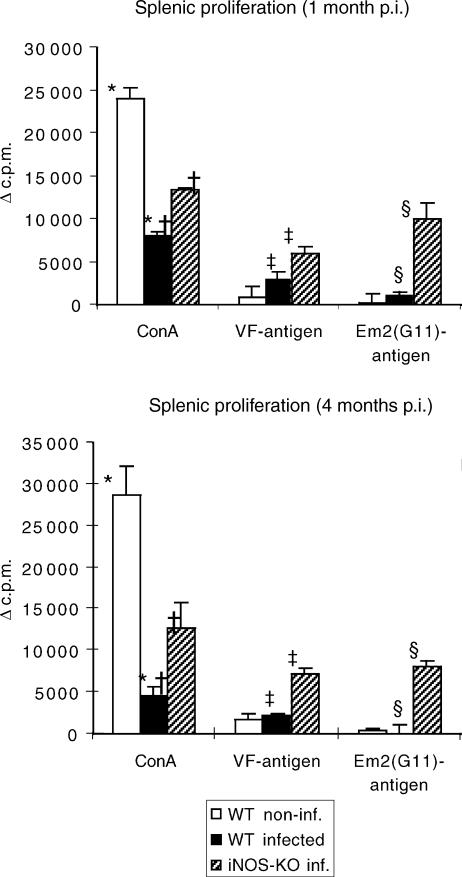
To address lymphocyte functions after experimental chronic infection of C57BL/6 WT and iNOS-KO mice with E. multilocularis, the proliferative responses to the T-cell mitogen Con A and to parasite VF- and Em2-antigen2 were investigated in vitro. Spleen cells were isolated from control mice and from E. multilocularis-infected WT and iNOS-KO mice at 1 and 4 month p.i., respectively. As shown in Fig. 5, the splenic proliferative responses to Con A stimulation in infected WT mice was significantly decreased at 1 month after infection, reaching only ≈ 30% of the values obtained from non-infected control mice. The respective decrease in iNOS-KO mice was less pronounced at the same time-point, the levels were ≈ 50% of the control values. The degree of suppression was determined in four independent experiments, respective results showing that the values for WT mice ranged between 20 and 40% and those for iNOS-KO mice between 50 and 70% of the control values. At 4 months p.i., spleen cells from infected WT mice expressed an even lower level of responsiveness to Con A (a decrease to ≈ 15% of control animals), conversely to iNOS-KO mice, which maintained approximately the suppression level exhibited at 1 month p.i.
Figure 5.
The proliferative responses to concanavalin A (Con A)- and parasite VF- and Em2-antigen stimulation of spleen cells from non-infected (white bars), infected wild type (WT) (black bars) and infected inducible nitric oxide synthase-knockout (iNOS-KO) mice (striped bars) were determined at different time-points [1 and 4 months postinfection (p.i.)]. The figure shows results from one representative out of four independent experiments exhibiting similar results. The suppression of concanavalin A (Con A)-derived proliferation at an early stage was repeated in four independent experiments, with comparable results obtained. *, †, ‡ and § indicate statistically significant differences. The results were calculated as the mean counts per minute (c.p.m.) of quadruplicate wells and expressed as geometric mean minus background (Δc.p.m.), where background = c.p.m. of wells containing pulsed but unstimulated cells.
In WT mice, the lymphoproliferative responses to (predominantly proteinic) VF- and carbohydrate Em2-antigen were very low at 1 month p.i. and practically abrogated at 4 months p.i. Conversely, a distinct and quantitatively even response to both types of parasite antigens was detectable in iNOS-KO mice at both time-points (1 and 4 months p.i., respectively).
Discussion
In the present study, we infected iNOS-deficient and C57BL/6 WT mice to investigate the role of NO in the development of murine AE. The production of NO during the course of infection, flanking a specific immune response, may have a pivotal function with both protective and/or potentially pathogenic consequences. To date, NO has been shown to be instrumental in the in vitro killing of E. multilocularis protoscolices.14 However, this effect may be of little relevance in the effective control of the parasite growth, as protoscolices are only found at a late stage of infection and do not contribute to the pathology developed during infection. In one of our previous studies we showed that peritoneal macrophages from E. multilocularis-infected mice completely suppressed Con A- and VF-antigen-induced splenic lymphocyte proliferation, and that the peritoneal and splenic macrophage-mediated immunosuppression was caused by NO production in response to the E. multilocularis infection. By infecting iNOS-KO mice, we were able to show that production of high levels of NO during chronic infection appeared to contribute to, rather than suppress, the control of parasite growth in WT mice. Several factors supported this. First and most importantly, the parasite weight was significantly lower in iNOS-KO mice than in WT mice at a late stage of infection (i.e. 4 months p.i.). Second, granuloma formation, with marked fibrosis and multinucleated giant cell formation, also occurred in iNOS-KO mice. Third, splenocytes from iNOS-KO mice were able to produce very high levels of IFN-γ upon stimulation with Con A and VF-antigen, and this occurred at both 1 and 4 months p.i., respectively. Together with histological analyses, the production of high levels of IFN-γ in infected iNOS-KO mice indicated normal T-cell signalling and stimulation in E. multilocularis-infected animals. In particular, the production of high levels of IFN-γ in response to stimulation with VF-antigen appeared consistently with proliferation of high levels of T cells, and might contribute to the relative resistance found in iNOS-KO mice.
Therefore, we now confirm and extend our previous findings1 that, in contrast to several other murine infection models, NO seems not to have any antiparasitic activity in vivo to limit the metacestode growth. Thus, NO synthesis results rather in the impairment of an effective immune response, which might downregulate – at least partially – the proliferative potential of the metacestode, as shown by the significantly reduced parasite mass acquired during chronic infection in iNOS-deficient mice. Alternatively, the effect of high proliferation of parasites in WT mice (compared to iNOS-KO mice) may be linked to immunopathological events creating an environment that favours parasite growth. Lack of this pathophysiological condition may suppress parasite proliferation. We are presently addressing this latter hypothesis in vitro.
In E. multilocularis infection, cell-mediated immunity plays a crucial role in the control of E. multilocularis infections. Thus, regressive and progressive forms of disease – both in humans and rodents – correlated with a course-specific periparasitic granuloma cell composition and the induction of an antigen-specific T-cell response.16–18 In our present model, however, the relative resistance found in iNOS-KO mice does not correlate with a specific periparasitic cell composition, as judged by conventional histopathological comparison to infected WT animals. A massive fibrosis was visible in both groups of mice, comprising numerous multinucleated giant cells and epitheloid cells. The metacestodes themselves also exhibited no morphological differences between the two mouse groups with regard to microscopical aspects of the laminated and germinal layers. However, the comparatively lower parasite burden in infected iNOS-KO mice was associated with a partial recovery of the T-cell function during infection, when compared with infected WT mice upon their production of IFN-γ and T-cell proliferative response. This recovery of T-cell function is not complete in the absence of NO, suggesting that other mechanisms, such as suppressive CD8 T cells19 or increased concentrations of immune complexes,20 may also be involved in the immunosuppressive phenomenon presently studied, as discussed extensively in a preceding publication.1 Our data nevertheless clearly demonstrate that NO plays an important role in impairing T-cell function in E. multilocularis infection.
In the iNOS-KO mouse model, we now need to address several issues to elucidate parasite growth-suppression mechanisms in further detail. These issues include: potential differences in the periparasitic immune- and effector-cell composition between iNOS-KO and WT mice; comparative differences in the splenic architecture; the association of NO expression with the regulation of other inflammatory cytokines not yet tested; and the mechanisms underlying the apparent immunosuppressive effect of NO, especially the potential influence of expression of high levels of NO on the expression of Bcl genes or other apoptotic genes in T cells.
In summary, the present study revealed that the lack of iNOS expression by peritoneal, as well as splenic, macrophages was – in contrast to WT mice – not associated with a suppression of splenocyte proliferative responses, but rather with a marked repression of intraperitoneal metacestode proliferation. This suggests that NO indirectly promotes metacestode growth.
Acknowledgments
This work was supported by the Swiss National Science Foundation (grants no. 31-63615.00 and 32-54041.98), the Interreg II-project no. BWA 30.027 and the EU EchinoRisk-project QLK2-CT-2001-01995 (BBW no. 00.0586-1). We thank Dr H. Mossmann (Max-Planck-Institute for Immunobiology, Freiburg, Germany) for supplying iNOS-KO mice, and we are indebted to Dr H.-U. Ochs (Institute of Parasitology, University of Zürich, Zürich, Switzerland) for the maintenance of our parasite isolates in vivo.
References
- 1.Dai WJ, Gottstein B. Nitric oxide-mediated immunosuppression following murine Echinococcus multilocularis infection. Immunology. 1999;97:107–16. doi: 10.1046/j.1365-2567.1999.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai WJ, Hemphill A, Waldvogel A, Ingold K, Deplazes P, Mossmann H, Gottstein B. A major carbohydrate antigen of Echinococcus multilocularis induces a T-cell-independent response. Infect Immun. 2001;69:6074–83. doi: 10.1128/IAI.69.10.6074-6083.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–40. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiloh MU, Nathan CF. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr Opin Microbiol. 2000;3:35–42. doi: 10.1016/s1369-5274(99)00048-x. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Hunter CA, Farrell JP. Anti-TGF-beta treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J Immunol. 1999;162:974–9. [PubMed] [Google Scholar]
- 6.Vouldoukis I, Riveros-Moreno V, Dugas B, Ouaaz F, Becherel P, Debre P, Moncada S, Mossalayi MD. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fc epsilon RII/CD23 surface antigen. Proc Natl Acad Sci USA. 1995;92:7804–8. doi: 10.1073/pnas.92.17.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–73. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdan C, Röllinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–23. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirata M, Hirata K, Kage M, Zhang M, Hara T, Fukuma T. Effect of nitric oxide synthase inhibition on Schistosoma japonicum-induced granuloma formation in the mouse liver. Parasite Immunol. 2001;23:281–9. doi: 10.1046/j.1365-3024.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomes MS, Florido M, Pais TF, Appelberg R. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J Immunol. 1999;162:6734–9. [PubMed] [Google Scholar]
- 12.Tomioka H, Sato K, Maw WW, Saito H. The role of tumor necrosis factor, interferon-gamma, transforming growth factor-beta, and nitric oxide in the expression of immunosuppressive functions of splenic macrophages induced by Mycobacterium avium complex infection. J Leukoc Biol. 1995;58:704–12. doi: 10.1002/jlb.58.6.704. [DOI] [PubMed] [Google Scholar]
- 13.Doherty TM, Sher A. Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to Mycobacterium avium infection. J Immunol. 1997;158:4822–31. [PubMed] [Google Scholar]
- 14.Kanazawa T, Asahi H, Hata H, Mochida K, Kagei N, Stadecker MJ. Arginine-dependent generation of reactive nitrogen intermediates is instrumental in the in vitro killing of protoscoleces of Echinococcus multilocularis by activated macrophages. Parasite Immunol. 1993;15:619–23. doi: 10.1111/j.1365-3024.1993.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 15.MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–50. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 16.Bresson-Hadni S, Liance M, Meyer JP, Houin R, Bresson JL, Vuitton DA. Cellular immunity in experimental Echinococcus multilocularis infection. II. Sequential and comparative phenotypic study of the periparasitic mononuclear cells in resistant and sensitive mice. Clin Exp Immunol. 1990;82:378–83. doi: 10.1111/j.1365-2249.1990.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devouge M, Ali-Khan Z. Intraperitoneal murine alveolar hydatidosis: relationship between the size of the larval cyst mass, immigrant inflammatory cells, splenomegaly and thymus involution. Tropenmed Parasitol. 1983;34:15–20. [PubMed] [Google Scholar]
- 18.Emery I, Liance M, Deriaud E, Vuitton DA, Houin R, Leclerc C. Characterization of T-cell immune response to Echinococcus multilocularis-infected C57BL/6J mice. Parasite Immunol. 1996;18:463–72. doi: 10.1111/j.1365-3024.1996.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 19.Kizaki T, Kobayashi S, Ogasawara K, Day NK, Onoe K. Immune suppression induced by protoscoleces of Echinococcus multilocularis in mice. Evidence for the presence of CD8dull suppressor cells in spleens of mice intraperitoneally infected with E. multilocularis. J Immunol. 1991;147:1659–64. [PubMed] [Google Scholar]
- 20.Treves S, Khan Z. Characterization of the inflammatory cells in progressing tumor-like alveolar hydatid cyst. 2. Cell surface receptors, endocytosed immune complexes and lysosomal enzyme content. Tropenmed Parasitol. 1984;35:231–6. [PubMed] [Google Scholar]