Abstract
Optimal T-cell activation requires both an antigen-specific and a costimulatory signal. The outcome of T-cell activation can be influenced by the nature of the costimulatory signal the T cell receives. We recently demonstrated the ability of stimulation through intercellular adhesion molecule-1 (ICAM-1), resident on the T-cell surface, to provide a second signal for T-cell activation, and have extended that work here to begin an examination of the functional outcome of this set of signals. Costimulation through ICAM-1 resulted in a greater percentage of cells having undergone more than three divisions when compared to costimulation through leucocyte function-associated antigen-1 (LFA-1). Costimulation through ICAM-1 also had an effect similar to costimulation through CD28 in its ability to down-regulate the cyclin dependent kinase inhibitor p27kip1. Costimulation through ICAM-1 provided greater protection from apoptosis than costimulation through LFA-1, especially in cells having divided more than three times. This was supported by the ability of costimulation through ICAM-1 to up-regulate the anti-apoptotic protein Bcl-2. Finally, costimulation through ICAM-1 or CD28 produced a greater number of T cells with a memory phenotype than costimulation through LFA-1.
Introduction
T cells can become activated when their T-cell antigen receptor (TCR) encounters its specific antigen in the context of a major histocompatibility complex (MHC) molecule displayed on an antigen-presenting cell (APC). During its interaction with an APC, if the T cell receives signals only through its TCR, the cell will become anergic or apoptotic.1 In addition to an antigen-specific signal, T-cell activation is dependent upon receiving a costimulatory signal,2 which results in clonal expansion and acquisition of effector functions.3,4 The role of different costimulatory molecules in determining the outcome of T-cell activation has been the focus of considerable recent research.
The best studied costimulatory molecule involved in T-cell activation is CD28, which interacts with its ligands B7.1 and B7.2 on the APC. Stimulation through CD28 synergizes with signalling through the TCR to activate phosphatidylinositol (PI)3-kinase, stabilize cytokine mRNA, and up-regulate anti-apoptotic genes.5–7 In addition to CD28, several other surface molecules possess the ability to deliver a costimulatory signal to T cells. Among these is leucocyte function-associated antigen-1 (LFA-1) on the T-cell surface. Upon interaction with its ligand, intercellular adhesion molecule-1 (ICAM-1) resident on antigen-presenting or target cells, LFA-1 on the T cell activates PI3-kinase and increases T-cell proliferation and interleukin (IL)-2 secretion.8,9 In spite of these activation signals, recent work suggests that costimulation through LFA-1 does not lead to the generation of memory T cells and favours entry into a state of anergy.10
Recent work in our laboratory has demonstrated that ICAM-1, resident on T cells, also can serve a costimulatory molecule.11 In conjunction with stimulation through the TCR, costimulation through ICAM-1 leads to activation of PI3-kinase, increased transcription from the IL-2 promoter, and increased proliferation and secretion of T helper 1 (Th1) cytokines. Despite the fact that LFA-1 and ICAM-1 are counter-receptors and each capable of delivering a costimulatory signal into T cells, the cytoplasmic tails of these two proteins are decidedly different, as are their overall cellular distribution patterns.12,13 Thus, it is of considerable interest to ascertain whether biological outcomes of second signals delivered through ICAM-1 are similar to or different from those delivered through LFA-1. In addition, the general ability of activation signals delivered through ICAM-1 to promote cell division, memory cell generation, and protect cells from apoptosis compared to other costimulatory signals has not yet been determined.
In the present study, we have investigated the functional outcome when T cells receive costimulation through ICAM-1 as compared to costimulation through either CD28 or LFA-1. Despite the similarity of these signals to increase proliferation as measured by thymidine incorporation and to induce cytokine secretion, we demonstrate that costimulation through ICAM-1 causes an increase in the number of cells that have undergone three or more divisions when compared to LFA-1. Further analysis of these populations shows that cells having undergone three or more divisions in response to ICAM-1 costimulation are protected from apoptosis at a level comparable to that induced by stimulation through CD28, and in contrast to signalling through LFA-1. In addition, costimulation through either ICAM-1 or CD28 results in a greater number of memory T cells when compared to LFA-1. Taken together, these data suggest a distinct difference between the costimulatory signals delivered through ICAM-1 and LFA-1, and support the role of ICAM-1 as a true costimulatory receptor. In some ways costimulation through ICAM-1 more closely resembles costimulation through CD28 than through LFA-1.
Materials and methods
Antibodies and reagents
Hybridomas expressing anti-CD3 (OKT3), anti-CD11a (HB202), and anti-CD54 (R6.5D6) were purchased from American Type Culture Collection (ATCC, Rockville, MD) and antibodies were purified from serum-free medium using protein G sepharose. Anti-CD28 (clone 28.2) was purchased from Pharmingen (San Diego, CA). Anti-p27kip1 was from Transduction Laboratories (Lexington, KY). Anti-Bcl 2 was from Oncogene Science (Boston, MA). Anti-CD11a-fluorescein isothiocyanate (FITC), anti-CD54-FITC, anti-CD27-phycoerythrin (PE), and anti-CD45RA tricolour were purchased from Caltag Laboratories (Burlingame, CA). AnnexinV-PE was purchased from Pharmingen. CFSE (5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester) was purchased from Molecular Probes (Eugene, OR). Propidium iodide was from Sigma (St. Louis, MO). Unless indicated otherwise, all other reagents and chemicals were from Sigma.
T-cell isolation
T cells were isolated from peripheral blood of multiple healthy donors as previously described.14 Briefly, whole blood diluted 1 : 1 in phosphate-buffered saline (PBS) was layered over Ficoll-Hypaque (Pharmacia, Piscataway, NJ) and spun for 30 min at 670 g followed by E-rosetting to isolate T cells. Purity was typically greater than 98% as assessed by flow cytometry. CD4+ T cells were isolated by negative magnetic separation using a CD4+ enrichment cocktail according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Cells were cultured in RPMI-1640 (Mediatech, Herndon, VA), containing 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), 50 U/ml each of penicillin and streptomycin, and 20 mm glutamine (Life Technologies, Grand Island, NY).
Cell stimulation and flow cytometry
Antibodies in PBS were attached to 12- or 96-well tissue culture plates (Midwest Scientific, St. Louis, MO) by incubation at 37° for 2 hr and washed three times with PBS prior to the addition of 4 × 105 T cells. Stimulations were performed with 1 µg/ml anti-CD3, 10 µg/ml anti-ICAM-1, 10 µg/ml LFA-1, or 2 µg/ml anti-CD28. Optimal titration of all antibodies was used to determine the minimum dose that costimulates maximal cell proliferation (data not shown) and this dose was used throughout. At the indicated times, cells were removed from the plate for analysis. To examine the generation of memory T cells, cells were gated for the CD45RA− population, except for the non-stimulated cells, and assessed for expression of CD11a and CD27 (memory T cells are designated as CD11ahi/CD27−).15 Flow cytometry was performed on a FACScan (Becton Dickinson, San Jose, CA) and data were analysed with Cell Quest (Becton Dickinson) and WinMDI software (Joe Trotter).
CFSE dilution assay
Cell division was analysed by CFSE as previously described.11 T cells were labelled with 2·5 µm CFSE for 10 min at 37° in serum-free RPMI. Prior to stimulation, cells were washed twice in RPMI containing 10% FBS to remove excess CFSE. Cell division was assessed at 3 and 5 days. To measure apoptosis, cells were labelled with 0·5 µg annexinV-PE prior to flow cytometric analysis. Dead cells were excluded from analysis by the incorporation of propidium iodide (2 µg/ml). To determine the percentage of apoptotic cells in each division group, the total number of cells in that group were divided by the annexinV+ cells within that group.
Western blotting
Western blotting was performed as previously described.16 Briefly, T cells were stimulated in 12-well tissue culture plates coated with antibodies as described above. Cells were harvested at the indicated times, washed once in ice-cold PBS, and lysed for 30 min on ice in 500 µl of lysis buffer (1% Triton-X-100, 10 mm Tris, pH 8·0, 140 mm NaCl, 0·2 mm Na3VO4, 100 mm NaF, and 1·0 mm phenylmethylsulphonyl fluoride). Protein concentrations were determined by Bradford assay and equalized. Lysates were resolved on 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), and transferred to nitrocellulose membranes (Life Science Products, Denver, CO). Membranes were probed with the indicated antibodies at the dilutions recommended by the manufacturers. Proteins were visualized by enhanced chemiluminescence (ECL, Amersham, Arlington Heights, IL). Densitometry was performed as previously described17 and protein levels were normalized to actin levels taken from the same blot.
Results
Costimulation through ICAM-1 results in an increased number of cells having undergone more than three divisions compared to LFA-1
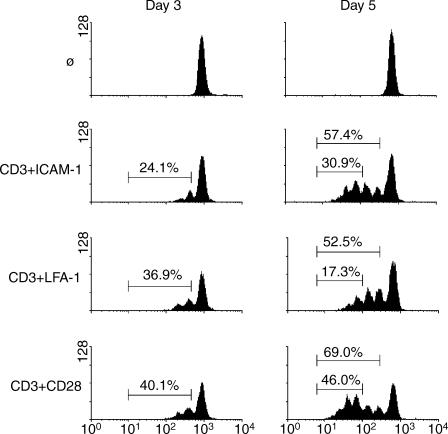
The ability of T cells to divide in response to antigen stimulation is necessary for the acquisition of effector functions. To determine the capacity of T cells to divide in response to different costimulatory signals, peripheral blood T cells were labelled with CFSE and assessed for cell division at 3 and 5 days in response to stimulation through CD3 plus costimulation through either ICAM-1, LFA-1, or CD28 (Fig. 1). Cells costimulated through either LFA-1 or CD28 consistently showed approximately equal numbers of divided cells at day 3 (36·9% and 40·1%, respectively). However, cells receiving a costimulatory signal through ICAM-1 showed fewer dividing cells (24·1%) at the same time. In contrast to 3 days, ICAM-1 costimulation for 5 days resulted in approximately the same number of total divided cells (57·4%) as LFA-1 costimulation (52·5%). Thus, any delay in the onset of proliferation at 3 days was removed by 5 days.
Figure 1.
Costimulation through ICAM-1 leads to a greater number of cells having divided three or more times compared to LFA-1. Peripheral blood T cells were labelled with CFSE and left non-stimulated or stimulated with antibodies against CD3 plus ICAM-1, LFA-1, or CD28. Cell division was assessed at day 3 (left panels) and day 5 (right panels) by dilution of CFSE. Percentages represent the total number of divided cells (left panels and top marker on the right panels) or the number of cells having divided three or more times (right panels, lower marker). Representative of five experiments.
Despite the equal total numbers of divided cells, a dramatic difference could be seen between these two populations in cells that have undergone three divisions. ICAM-1 costimulation caused 30·9% of cells to undergo three divisions compared to only 17·3% for LFA-1. CD28 costimulation at 5 days resulted in 69·0% of the cells having divided, and 46·0% having undergone three divisions. These data demonstrate that costimulation through ICAM-1 is similar to CD28 in the ability to promote sustained cell division.
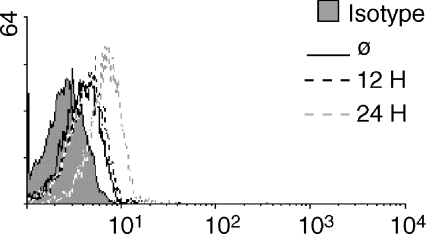
ICAM-1 is expressed at very low levels on the surface of resting T cells, but is up-regulated following exposure to inflammatory mediators such as cytokines.18 This suggests that the apparent delay in cell division following costimulation through ICAM-1 may be caused, in part, by the time needed to up-regulate ICAM-1 to a sufficient level on the T-cell surface. An increase in ICAM-1 expression may allow the T cell to overcome a signalling threshold that prohibits the immediate delivery of an effective costimulatory signal. In Fig. 2, T cells were stimulated through the TCR and analysed for surface expression of ICAM-1 at 12 and 24 hr. Following 12 hr of stimulation through the TCR, there is no change in the surface expression of ICAM-1 compared to non-stimulated cells. In contrast, there was a considerable increase in ICAM-1 expression by 24 hr.
Figure 2.
Stimulation through the TCR increases surface expression of ICAM-1. Resting T cells were left nonstimulated (solid black line) or stimulated with anti-CD3 for 12 (dashed black line) and 24 hr (dashed grey line) and analysed for ICAM-1 surface expression by flow cytometry. The isotype control is indicated by the filled grey histogram. Representative of three experiments.
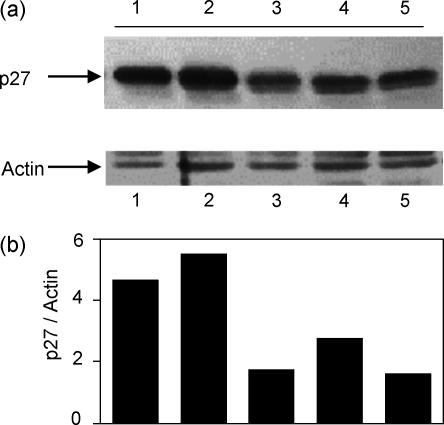
The ability of cells to divide is regulated by cyclin/cyclin-dependent kinases (cdk). The cdk inhibitory protein p27kip1 delays cell-cycle progression and is present at high levels in resting T cells.19 Recent work by others has demonstrated that costimulation through CD28 leads to a down-regulation of p27kip1 protein, an activity that promotes cell division.20 We examined whether costimulation through ICAM-1 could exert a similar effect on the cell cycle regulator p27kip1. In Fig. 3, T cells were stimulated as indicated for 5 days, and lysates were examined for p27kip1 protein levels. T cells left non-stimulated or stimulated through the TCR alone showed high levels of p27kip1 (lanes 1 and 2), whereas cells receiving a costimulatory signal through ICAM-1 or CD28 (lanes 3 and 5) showed markedly decreased amounts of p27kip1. Interestingly, cells costimulated through LFA-1 (lane 4) have a small but consistently increased amount of p27kip1 compared to ICAM-1 and CD28 costimulated cells, supporting the observation that these cells may not be as actively dividing by 5 days. Figure 3(b) represents the amount of p27kip1 in each sample normalized to actin in the same lane. These data imply that costimulation through ICAM-1 was able to initiate cell cycle progression in a manner similar to CD28.
Figure 3.
Costimulation through ICAM-1 causes down-regulation of p27kip1. (a) T cells were left non-stimulated (lane 1), stimulated with anti-CD3 alone (lane 2), anti-CD3 + anti-ICAM-1 (lane 3), anti-CD3 + anti-LFA-1 (lane 4), or anti-CD3 + anti-CD28 (lane 5) for 5 days. Whole cell lysates were resolved on SDS–PAGE, blotted, and probed to determine the relative level of p27kip1 protein (upper panel). The blot was stripped and reprobed for actin as a loading control (lower panel). (b)Densitometric ratio of p27kip1/actin on the same blot. Representative of four experiments.
Costimulation through ICAM-1 protects cells from apoptosis compared to LFA-1
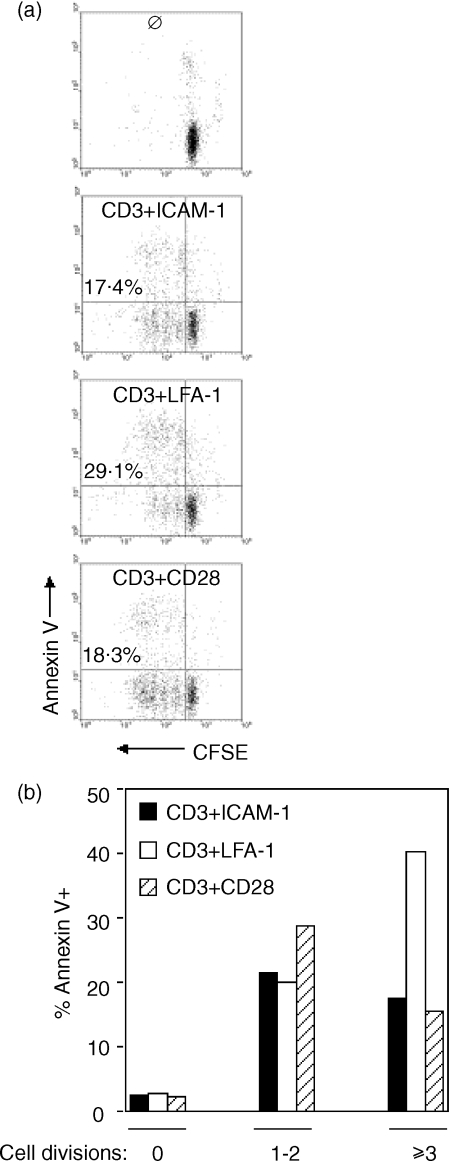
Previous reports have indicated that T cells costimulated through LFA-1 are more prone to apoptosis compared to costimulation through CD28.21 To determine the ability of costimulation through ICAM-1 to protect T cells from apoptosis, cells were costimulated through ICAM-1, LFA-1, or CD28 for 5 days and analysed for annexinV binding. In Fig. 4(a), CFSE-labelled cells costimulated through LFA-1 showed increased total numbers of apoptotic cells when compared to ICAM-1 and CD28 (29·1% compared to 17·4% and 18·3%, respectively). For reference, 40% of the cells stimulated through CD3 alone were undergoing apoptosis (not shown), a figure consistent with established values. The percentage of apoptotic cells having undergone one to two divisions was approximately equal among the three costimulatory signals (Fig. 4b). However, the percentage of annexinV+ cells was considerably higher in cells that had undergone three or more divisions following costimulation through LFA-1 compared to ICAM-1 and CD28. Thus, costimulation through ICAM-1 appears to have provided the same degree of protection from apoptosis as CD28.
Figure 4.
Cells having divided three or more times following costimulation through ICAM-1 show less apoptosis compared to costimulation through LFA-1. T cells were labelled with CFSE and stimulated as indicated. (a) Following stimulation for 5 days, cells were stained with annexinV-PE and cell division and apoptosis were analysed by flow cytometry. The total percentage of annexinV+ cells is indicated on the left side of each plot. (b) CFSE dilution was used to separate cells into three groups based on the number of divisions, and the percentage of annexinV+ cells in each group was determined. Representative of four experiments.
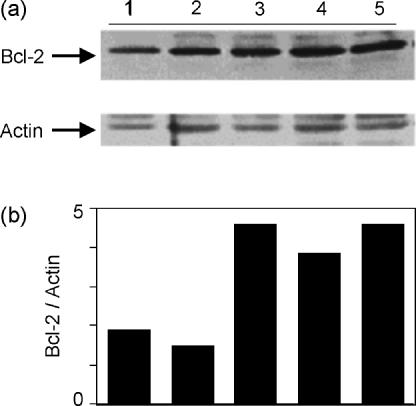
The molecular mechanisms underlying programmed cell death continue to be a focus of intense study. One protein playing a central role in various apoptotic pathways is Bcl-2, which protects cells from apoptosis by maintaining mitochondrial membrane potential.22 In Fig. 5, cells were stimulated for 5 days and lysates were analysed for Bcl-2 expression. Cells receiving a costimulatory signal through ICAM-1, LFA-1, or CD28 all showed increased levels of Bcl-2 compared to non-stimulated cells or cells stimulated through the TCR alone (Fig. 5a). Figure 5(b) depicts the level of Bcl-2 expression each normalized to actin from the same lane. These data indicate that costimulation through ICAM-1 up-regulated Bcl-2 protein levels. In addition, despite the fact that costimulation through LFA-1 made cells more prone to apoptosis (Fig. 4), these cells showed an equivalent amount of Bcl-2 to that seen with ICAM-1 or CD28 costimulation.
Figure 5.
Costimulation through ICAM-1 increases Bcl-2 protein levels compared to stimulation through the TCR alone. (a) T cells were left non-stimulated (lane 1), stimulated with anti-CD3 alone (lane 2), anti-CD3 + anti-ICAM-1 (lane 3), anti-CD3 + anti-LFA-1 (lane 4), or anti-CD3 + anti-CD28 (lane 5) for 5 days. Protein levels of Bcl-2 were determined by Western blotting. (b) Densitometric ratio of Bcl-2/actin on the same blot. Representative of four experiments.
Costimulation through ICAM-1 causes the appearance of memory T cells
The ability of T-cell activation to lead to the generation of memory T cells is essential to providing long-term protection from previously encountered foreign antigens. Memory T cells are capable of more rapid cell division and cytokine secretion following re-encounter with specific antigen. We investigated the ability of costimulation through ICAM-1 to lead to the generation of memory T cells. In recent years it has become apparent that simple assessment of CD45RA as compared with CD45RO is insufficient to conclude with confidence that a cell population bears the memory phenotype.15 For this reason we used three markers (CD45RA, CD11a and CD27) to ascertain whether memory cells were generated. In Fig. 6, CD4+ T cells were costimulated through ICAM-1, LFA-1 or CD28 and assessed for the appearance of memory T cells (CD45RA−, CD11ahi and CD27−) by flow cytometry. Costimulation through LFA-1 generated few memory T cells (6%), supporting data previously reported by others.10 In contrast, costimulation through ICAM-1 (14%) or CD28 (15%) each generated roughly twice the number of memory cells out of this mixed population of T cells than did LFA-1. These data demonstrate that costimulation through ICAM-1 is more efficient than LFA-1 for the generation of memory T cells, and compares favourably to CD28.
Figure 6.
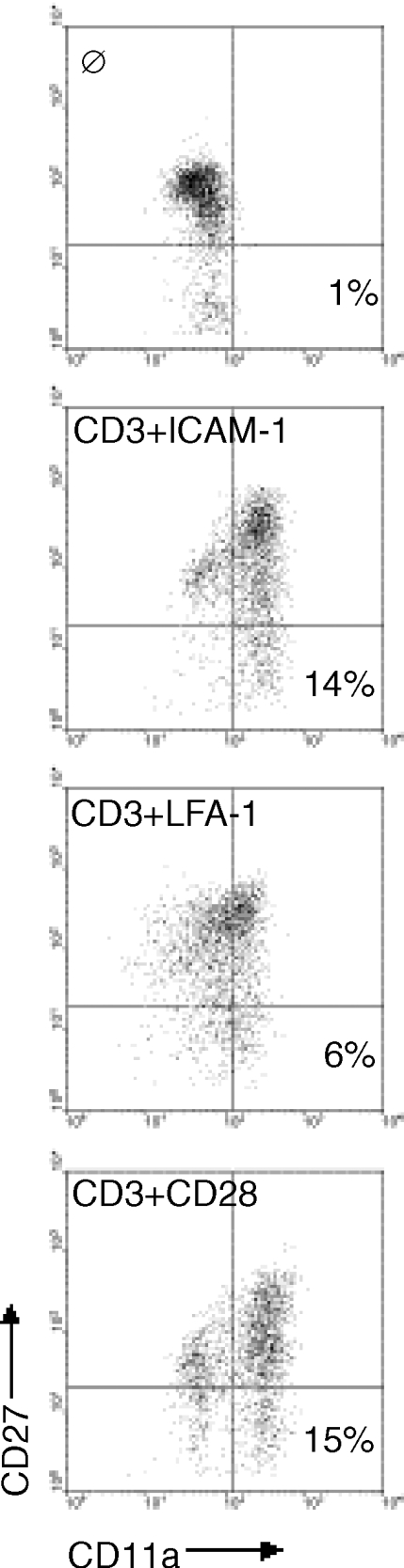
Costimulation through ICAM-1 generated an equivalent number of memory T cells compared to costimulation through CD28. T cells were stimulated as indicated for 7 days. Cells were stained for a memory cell phenotype with antibodies against CD11a, CD27, and CD45RA. Results shown were gated on CD45RA− cells prior to analysis. Representative of four experiments.
Discussion
Previous work from others suggests that the costimulatory signal, the cytokine environment, and possibly the site of antigen encounter may all play a role in governing the phenotype of a particular T cell following activation.23–25 In the present study, we show that a costimulatory signal through ICAM-1 compares favourably to CD28 and is more effective than a signal through LFA-1 at promoting sustained cell division, protecting cells from apoptosis, and generating cells with a memory phenotype. Previous work from our laboratory has shown that costimulation through either ICAM-1 or LFA-1 results in the secretion of the Th1 cytokines IL-2 and interferon-γ,11 suggesting that the cytokine secretion potential may not be responsible for the differences shown here between LFA-1 and ICAM-1.
The ability of T cells to divide in response to antigen stimulation is clearly pivotal to the acquisition of effector functions. What is less clear, however, is whether a minimum number of divisions are required for acquisition of these functions. Several recent studies report that the plasticity of T cells to become polarized toward either a Th1 or Th2 phenotype, and the generation of effector vs. central memory T cells, may be dependent upon the number of divisions these cells undergo in response to stimulation.26,27 The data presented here indicate that costimulation through ICAM-1 provides a longer window for cell division than costimulation through LFA-1. Thus, our work supports the supposition that the number of cell divisions may influence the generation of memory cells.
One hallmark of a second signal is the ability to protect T cells from undergoing apoptosis. Although all the mechanisms have yet to be defined, it is likely that this protection involves both the up-regulation of anti-apoptotic factors and the down-regulation or degradation of pro-apoptotic factors. Previous work from other laboratories has shown that costimulation through CD28 provides such protection by the up-regulation of anti-apoptotic factors, whereas costimulation through LFA-1 does not. In the present work, we have demonstrated that costimulation through ICAM-1 caused an up-regulation of an important anti-apoptotic factor (Bcl-2) and thus, may provide a greater level of protection from apoptosis than costimulation through LFA-1, particularly in cells having divided three or more times. It is likely that other factors are involved in regulating this prevention of apoptosis, and further analysis of apoptotic pathways following costimulation through ICAM-1 are currently underway in our laboratory.
The role of ICAM-1 in the immune system had long been thought to be simply that of mediating intercellular adhesion and functioning as a counter-receptor to deliver a second signal to T cells through LFA-1. However, recent findings plus those presented here, suggest that reconsideration of the utility of ICAM-1 in mediating the immune response is in order. The costimulatory ligands B7.1, B7.2, LFA-1 and ICAM-1 are all expressed on dendritic cells, whereas B cells do not express the CD28 ligands B7.1 and B7.2 prior to B-cell activation. Therefore, it is possible that a T cell interacting with an antigen-presenting B cell may receive a costimulatory signal through ICAM-1 and not CD28, in contrast with the situation that occurs when a T cell interacts with a dendritic cell. In fact, cellular distribution of second signal-delivering molecules may provide a level of recognition to the T cell receiving the signal. That is, second signals delivered through CD28 or LFA-1 could come from any of the many cells expressing their counter-receptors. These include leukocytes, endothelial cells, and epithelial cells. In contrast, second signals delivered to the T cell through ICAM-1 could only come from leukocytes capable of expressing LFA-1, the counter-receptor for ICAM-1. Thus, receipt of the second signal through ICAM-1 could inform the T cell that it is interacting with a leucocyte. Finally, ICAM-1 is present at low levels on naïve and resting T cells, and is up-regulated by proinflammatory cytokines. Up-regulation of ICAM-1 on activated T cells raises the possibility that costimulation through ICAM-1 may be more prominent at later times in an immune response, or possibly that stimulation through ICAM-1 at the initiation of an immune response may have different effects from stimulation through ICAM-1 at a later time. Certainly, the previous role for ICAM-1 on T cells as merely an adhesion molecule has underestimated its importance during an immune response.
Acknowledgments
The authors are grateful to Mrs Muriel Hannig for her generous support of our work.
References
- 1.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–56. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 2.Chambers CM, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 3.Croft M, Dubey C. Accessory molecule and co-stimulation requirements for CD4 T cell response. Crit Rev Immunol. 1997;17:89–118. doi: 10.1615/critrevimmunol.v17.i1.40. [DOI] [PubMed] [Google Scholar]
- 4.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell co-stimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 5.Ward SG, Westwick J, Hall ND, Sanson DM. Ligation of CD28 receptor by B7 induces formation of D-3 phosphoinositides in T lymphocytes independently of T cell receptor/CD3 activation. Eur J Immunol. 1993;23:2572–7. doi: 10.1002/eji.1830231029. [DOI] [PubMed] [Google Scholar]
- 6.Umlauf SW, Beverly B, Lantz O, Schwartz RH. Regulation of interleukin 2 gene expression by CD28 costimulation in mouse T-cell clones. both nuclear and cytoplasmic RNAs are regulated with complex kinetics. Mol Cell Biol. 1995;15:3197–205. doi: 10.1128/mcb.15.6.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boise L, Minn A, Noel P, June C, Accavitti M, Lindsten T, Thompson C. CD28 costimulation can promote survival by enhancing the expression of Bcl-xL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman L, Pullen L, Miller J. Functional consequences of costimulation by ICAM-1 on IL-2 gene expression and T cell activation. J Immunol. 1998;160:3259–68. [PubMed] [Google Scholar]
- 9.Ni H-T, Deeths M, Li W, Mueller D, Mescher M. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J Immunol. 1999;162:5183–9. [PubMed] [Google Scholar]
- 10.Ragazzo JL, Ozaki ME, Karlsson L, Peterson PA, Webb SR. Costimulation via lymphocyte function-associated antigen 1 in the absence of CD28 ligation promotes anergy of naïve CD4+ T cells. Proc Natl Acad Sci USA. 2001;98:241–6. doi: 10.1073/pnas.011397798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chirathaworn C, Kohlmeier JE, Tibbetts SA, Rumsey LM, Chan MA, Benedict SH. Stimulation through intercellular adhesion molecule-1 provides a second signal for T cell activation. J Immunol. 2002;168:5530–7. doi: 10.4049/jimmunol.168.11.5530. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Rad Biol Med. 2000;28:1379–86. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 13.Lub M, van Kooyk Y, Figdor C. Ins and outs of LFA-1. Immunol Today. 1995;16:479–83. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 14.Tibbetts SA, Chirathaworn C, Nakashima M, Jois DSS, Siahaan TJ, Chan MA, Benedict SH. Peptides derived from ICAM-1 and LFA-1 modulate T cell adhesion and immune function in a mixed lymphocyte culture. Transplantation. 1999;68:685–92. doi: 10.1097/00007890-199909150-00015. [DOI] [PubMed] [Google Scholar]
- 15.De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry. Identification of human naïve T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–8. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- 16.Chirathaworn C, Tibbetts SA, Chan MA, Benedict SH. Cutting edge. Cross-linking of ICAM-1 on T cells induces transient tyrosine phosphorylation and inactivation of cdc2 kinase. J Immunol. 1995;155:5479–82. [PubMed] [Google Scholar]
- 17.Rumsey LM, Teague RM, Benedict SH, Chan MA. MIP-1α induces activation of phosphatidylinositol-3 kinase that associates with Pyk-2 and is necessary of B-cell migration. Exp Cell Res. 2001;268:77–83. doi: 10.1006/excr.2001.5272. [DOI] [PubMed] [Google Scholar]
- 18.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–88. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 19.Nourse J, Firpo E, Flanagan WM, et al. Interleukin-2-mediated elimination of the p27kip1 cyclin dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–3. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 20.Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–6. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- 21.Appleman LJ, van Puijenbroek AAFL, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–36. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 22.Palmer EM, Farrokh-Siar L, van Seventer JM, van Seventer GA. IL-12 decreases activation-induced cell death in human naïve Th cells costimulated by intercellular adhesion molecule-1. I. IL−12 alters caspase processing and inhibits enzyme function. J Immunol. 2001;167::749–58. doi: 10.4049/jimmunol.167.2.749. [DOI] [PubMed] [Google Scholar]
- 23.Salomon B, Bluestone JA. Cutting Edge. LFA–;1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J Immunol. 1998;161:5138–42. [PubMed] [Google Scholar]
- 24.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 25.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 26.Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–15. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 27.Lauvau G, Vijh S, Kong P, Horng T, Kerksiek K, Serbina N, Tuma RA, Pamer EG. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001;294:1735–9. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]