Abstract
We investigated the individual CD8+ populations with natural killer (NK) cell markers (NK-type T cell); CD56 single positive (CD56)-T cells, CD56/CD57 double positive (DP)-T cells and CD57 single positive (CD57)-T cells in the peripheral blood. All NK-type T-cell populations expressed CD122 and intermediate levels of T-cell receptor (TCR; regular CD8+ T cells are CD122− and express high levels of TCR). The number of both DP-T cells and CD57-T cells, but not CD56-T cells, gradually increased with age. All NK-type T-cell populations produced larger amounts of interferon-γ than did regular CD8+ T cells after stimulation with interleukin (IL)-2, IL-12 and IL-15. However, CD56-T cells and CD57-T cells but not DP-T cells showed a potent antitumour cytotoxity to NK-sensitive K562 cells, whereas only CD56-T cells showed a potent cytotoxity to NK-resistant Raji cells. Furthermore, although NK-type T cells produced large amounts of soluble Fas-ligands, their cytotoxic activities appeared to be mediated by the perforin/granzyme pathway. The oligoclonal or pauciclonal expansions of certain VβT cells were found in each NK-type T-cell population. The non-variant CDR3 region(s) for the TCRβ chain(s) showed CD57-T cells and CD56-T cells to be derived from distinct origins, while the DP-T cell population consisted of a mixture of the clones seen in both CD56-T cells and CD57-T cells. Our results suggest that CD57-T cells and CD56-T cells are functionally and ontogenically different populations while DP-T cells appear to originate from both CD56-T cells and CD57-T cells.
Introduction
In addition to normal CD8+ T cells without natural killer (NK) cell markers, CD8+ T cells with NK cell markers (NK-type T cells) are also present in the peripheral blood of humans.1–6 A small but substantial number of CD56 or CD57 bearing NK-type T cells (most of which express CD8) are present in peripheral blood mononuclear cells (PBMC; 2–5% and 5–10%, respectively) and these cells are abundant in the liver and bone marrow, however, they are rarely found in the lymph nodes and spleen.4,7 Because human Vα24 T cells and murine Vα14 NK1·1+ T (NKT) cells have a T-cell receptor (TCR) sequence homology8 and both human Vα24 T cells and murine Vα14 NKT cells CD1-dependently respond to α-galactosylceramide,9,10 Vα24 T cells have been regarded as human NKT cells. However, in contrast to mouse Vα14 NKT cells, human Vα24 T cells are very rarely found in the peripheral blood and in the liver.11,12 Therefore, based on the preferential location in the liver, CD161 (NKRP-1) expression, their potent interferon-γ (IFN-γ) producing capacity and interleukin (IL)-12-induced antitumour cytotoxicity,13 we propose that human CD56+ T cells are functional counterpart of mouse NKT cells, especially in T helper 1 (Th1) responses.7,11,13,14 Other researchers also reported that CD56+ T cells showed an antitumour activity.2,12,15
We recently reported that not only CD56+ T cells but also CD57+ T cells in PBMC stimulated in vitro with anti-CD3 antibody or cytokines, such as IL-2, IL-12 and IL-15, produced a large amount of interferon-γ (IFN-γ) and strongly expressed the cytoplasmic perforin/granzyme7 and also exhibited a potent cytotoxic activity to tumour cells.5,9–12 Furthermore, the proportion of the CD57+ T cells in PBMC correlated with the anti-CD3. Antibody-stimulated IFN-γ production from PBMC.7 In addition, CD57+ T cells increase with ageing7,16,17 and thereby the anti-CD3. Antibody-stimulated IFN-γ production from PBMC increased in older hosts.7 We therefore proposed that the increase in the number of CD57+ T cells with ageing may be an appropriate physiological and immunological adaptation to compensate for the dysfunction in regular T cells.5 In fact, CD56+ T cells as well as CD57+ T cells were abundantly found in tumour-infiltrating lymphocytes,18,19 and these NK-type T cells produce a large amount of IFN-γ when they are exposed to a bacterial superantigen.20 Based on these findings, NK-type T cells may thus play an important role in the Th1 immune responses of the host defence.7,11,12 Interestingly, recent flow cytometric analyses have shown that certain VβT cells in CD56+ T cells oligoclonally expand in PBMC.10,15 A few VβT cells in CD57+ T cells have also been reported to oligoclonally expand in both healthy individuals21–23 and bone marrow transplant recipients.24
However, CD56+ T cells and CD57+ T cells substantially overlap and, as a result, some CD57+ CD56+ double positive T cells are present.7 Therefore, it is important to clarify both the characteristics and the differences among CD56+ CD57− TCR+ (CD56-T) cells, CD56+ CD57+ TCR+ (DP-T) cells and CD56− CD57+ TCR+ (CD57-T) cells. In the present study, we, for the first time, demonstrate the unique features of individual NK-type T-cell populations in view of the surface phenotype, IFN-γ production, antitumour activity and TCR Vβ repertoire and show both the similarities and differences among the NK-type T-cell subsets which suggest a possible mutual relationship.
Materials and methods
Cell staining and flow cytometric analysis
All fluoroscein isothiocyanate (FITC)-, phycoerythrin (PE)- and PC5-conjugated monoclonal antibodies (mAbs) were purchased from Immunotech (Marseille, France). The human PBMC separated by Lymphocyte Separation Medium (ICN Biochemicals Inc., Aurora, OH) were stained with PE-anti-αβTCR mAb, FITC-anti-CD57 mAb and PC5-anti-CD57 mAb. In some experiments, separated PBMC were depleted of CD4+ T cells by magnetic beads-conjugated anti-CD4 mAb (Dynal A.S., Oslo, Norway), and thereafter PBMC were stained with a combination of FITC-anti-CD57, PC5-anti-CD56 mAb and PE-anti-αβTCR mAb, a combination of FITC-anti-αβTCR mAb, PC5-anti-CD56 mAb and PE-anti-CD122 mAb, or a combination of PC5-anti-αβTCR mAb, FITC-anti-CD57 mAb and PE-anti-CD122 mAb. For the analysis of the Vβ repertoires of various T-cell subsets, PBMC depleted of CD4+ were stained with FITC-anti-CD57 mAb, PC5-anti-CD56 mAb and biotin-conjugated anti-Vβ (Vβ1, Vβ2, Vβ5·1, Vβ8, Vβ14, Vβ17 or Vβ22) mAb and then were developed with PE-streptavidin (PharMingen, San Diego, CA). The stained PBMC were analysed by a flow cytometric analyser (FACSCalibur, Becton Dickinson, Cookeysville, MD) with Cell Quest software (Becton Dickinson).
Analysis of VβT cell receptor repertoire of CD56-T cells, DP-T cells, CD57-T cells and regular-T cells
The percentages of each VβT cell population were determined in CD56-T cells, DP-T cells, CD57-T cells and regular CD8+ T cells in CD4+ T-cell-depleted PBMC as follows: % of VβnT cells in CD56-T cells = (% CD56+ CD57− VβnT cells/% CD56+ CD57−αβT cells) × 100; % of VβnT cells in DP-T cells = (% CD56+ CD57+ VβnT cells/% CD56+ CD57+αβT cells) × 100; % of VβnT cells in CD57-T cells = (% CD56− CD57+ VβnT cells/% CD56− CD57+ VβT cells) × 100; % of VβnT cells in regular-T cells = (% CD56− CD57− VβnT cells/% CD56− CD57−αβT cells) × 100. Vβn TCR mAbs that reportedly reacted with relatively larger populations of αβT cells were selected and used.
Preparation and cultivation of each T-cell subset
After the depletion of CD4+ T cells from the human PBMC were stained with FITC-anti-CD57 mAb, PE-anti-αβTCR mAb and PC5-anti-CD56 mAb, and then CD56-T cells, DP-T cells, CD57-T cells and regular-T cells were sorted by Epics Elite (Beckman Coulter, Miami, FL). The sorted cells (2 × 105 cells/well) were cultured with a RPMI-1640 medium containing 20% human serum, 100 ng/ml human IL-2 (Peprotec, London, UK), 20 ng/ml human IL-12 (Peprotec) and 5 ng/ml human IL-15 (Genzyme, Cambridge, MA) in 5% CO2 at 37° for 96 hr.
Assay for IFN-γ and soluble FAS-ligand levels
The IFN-γ and FAS-ligand levels in lymphocyte culture supernatants were evaluated using the enzyme linked immunosorbent assay kits purchased from PharMingen (San Diego, CA) and Medical & Biological Laboratories Co. (Nagoya, Japan), respectively.
Cytotoxic assays
NK-sensitive cells or NK-resistant Raji cells were labelled with Na2 (51Cr) O4 (100 µCi/106 cells) (Amersham Pharmacia Biotech, Amersham, UK) for 60 min at 37° in RPMI-1640 medium containing 10% fetal calf serum (FCS) and were washed three times with medium. The labelled targets (104/well) were incubated in a total volume of 200 µl with 105 effector cells (E/T = 10/1) in 10% FCS–RPMI-1640 in 96-well round-bottom microtitre plates. The plates were centrifuged and then incubated for 4 hr in 5% CO2 at 37°, after which the supernatants were harvested and counted in a gamma counter. The cytotoxity was calculated as a percentage of the releasable counts after subtracting the spontaneous release. The spontaneous release was less than 15% of the maximal release. In some experiments, the effector cells were preincubated with concanamycinA (10 nm) (Wako Pure Chemical Industries Ltd, Osaka, Japan) for 2 hr to inhibit the perforin-mediated cytotoxicity and then they were subjected to cytotoxic assays.
Preparation of cDNA library for specific TCRβ chains
The sorted CD56-T cells, DP-T cells, CD57-T cells and regular-T cells were lyzed with acid GTC solution (0·8 m guanidine-thiocyanate, 10 mm Tris-HCl (pH 7·6), 2 mm disodium ethylenediaminetetraacetic acid (Na2EDTA), 0·4% sodium lauryl sarcosin, 31 mm 2-mercaptoethanol, 600 mm sodium acetate (pH 4·8), 40% phenol saturated with water, 20% chloroform), and RNAs were precipitated with ethanol from the aqueous phase. The RNAs were then reverse-transcribed using 0·5 µg of oligo(dT)12−18 primers (Life Technologies Inc., Gaithersburg, MD) and 100 units of Superscript II (Life Technologies Inc) in 20 µl reaction mixtures. Aliquots (1/10) of the generated cDNAs were amplified by 35 cycles of polymerase chain reaction (PCR) with 100 pmol each of forward (5′-ACG ATT CTC CGC ACA ACA GT-3′ for Vβ1, or 5′-CCT GAA GAC AGC AGC TTC TA-3′ for Vβ2) and reverse (5′-TCA GGC AGT ATC TGG AGT CA-3′ for the common region of Cβ1 and 2) primers by 1·75 units of Expand High Fidelity PCR system (Boehringer Mannheim GmbH, Germany) in 50 µl reaction mixtures. The amplified cDNAs were subcloned into pBluescript II KS+ (Stratagene, La Jolla, CA) and sequenced with ABI Prism310 DNA Sequencer (Perkin Elmer Co., Norwalk City, CT).
Statistical analysis
Differences between the groups were analysed by either Student's t-test or Spearman's rank correlation using StatView5 software package (SAS Institute Inc., Cary, NC). Differences were considered to be significant when P was < 0·05.
Results
CD57-T cells and DP-T cells increase with age, and all NK-type T cells express CD122 and intermediate levels of TCR
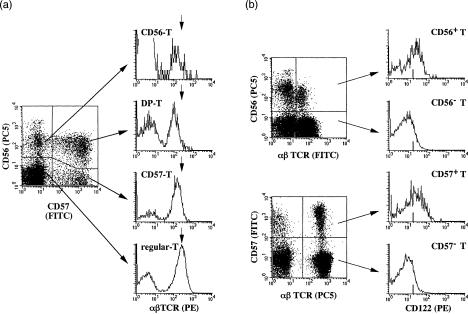
We first determined the proportions of each NK-type T-cell subsets in PBMC from 60 donors of different ages by gating αβTCR+ T cells in whole PBMC. The results showed that the proportions of CD56+ CD57− αβTCR+ (CD56-T) cells, CD56+ CD57+ αβTCR+ (DP-T) cells and CD56− CD57+ αβTCR+ (CD57-T) cells were 1·07 ± 0·86%, 1·10 ± 1·80% and 5·51 ± 4·50% (means ± SE), respectively (Table 1). The proportions of CD57-T cells and DP-T cells increased with age while neither CD56SP-T cells nor regular-T cells did (Table 1). Thereafter, surface αβTCR levels on CD56-T cells, DP-T cells, CD57-T cells and regular-CD8+ T cells were determined after gating CD56+ CD57−, CD56+ CD57+, CD56− CD57+ and CD56− CD57− populations in CD4+ T-cell depleted PBMC (by magnetic beads). All NK-type T-cell subsets (CD56-T cells, DP-T cells and CD57-T cells) expressed intermediate levels of TCR, whereas the regular-CD8+T cells expressed high levels of TCR (Fig. 1a). NK-type T cells were CD122+ while regular T cells were mainly CD122− (Fig. 1b).
Table 1.
Proportions (%) of CD56-T cells, DP-T cells, CD57-T cells and regular T cells in PBMC
| Age | CD56-T | DP-T¶ | CD57-T¶ | Regular-T | |
|---|---|---|---|---|---|
| < 20 | (n = 14) | 0·61 (± 0·50) | 0·09 (± 0·11) | 1·89 (± 0·11) | 49·84 (± 15·65) |
| 20–39 | (n = 20) | 1·22 (± 0·79)*† | 0·86 (± 1·05)*† | 5·41 (± 3·92)*† | 48·40 (± 6·18) |
| 40–59 | (n = 12) | 1·09 (± 1·05) | 1·46 (± 2·48)*† | 6·53 (± 5·50)**† | 48·67 (± 11·24) |
| 60–79 | (n = 14) | 1·39 (± 1·02)*† | 2·58 (± 2·37)**†, **‡ | 9·58 (± 3·62)**†, **‡, *§ | 40·30 (± 10·60) |
| Whole | (n = 60) | 1·07 (± 0·86) | 1·10 (± 0·80) | 5·51 (± 4·50) | 47·38 (± 11·22) |
Data shown represent the means ± SEs. ¶Significant (P < 0.01) correlated to age, and significant(*P < 0.05 and **P < 0.01) compared with †persons under 20 years of age ‡persons of 20–39 years old-or§persons of 40–59 years old, respectively.
Figure 1.
Expression of the intermediate levels of TCRs and CD122 on NKT cells. (a) TCR levels were demonstrated after gating CD4+ T-cell depleted PBMC to populations of either CD56-T cells, DP-T cells, CD57-T cells or regular T cells. Arrows in histograms indicated the peak immunofluorescence level of TCR on regular CD8+ T cells. (b) The expressions of CD122 were demonstrated after gating PBMC to CD56+ αβTCR+, CD56− αβTCR+, CD57+ αβTCR+ or CD57− αβTCR+ populations.
Comparison of IFN-γ production, soluble FAS-ligand production and antitumour cytotoxicity among NK-type T-cell populations
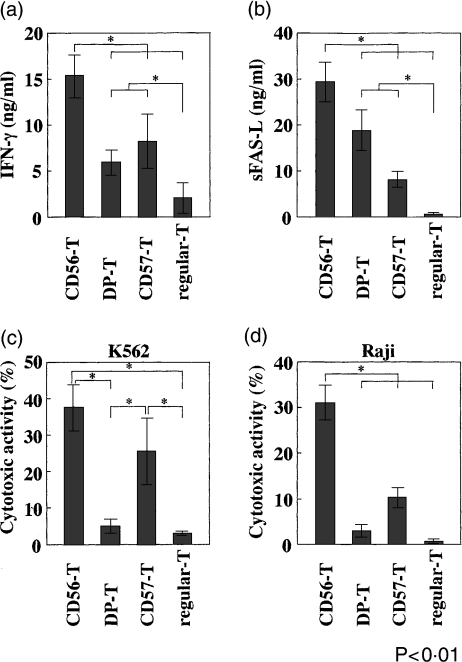
When each NK-type T-cell population purified by cell sorting was stimulated with IL-2, IL-12 and IL-15 for 96 hr, the CD56-T cells produced the largest amount of IFN-γ and soluble FAS-ligand (sFAS-L) while other NK-type T-cell populations also produced larger amounts of IFN-γ and sFAS-L than did regular CD8+ T cells (Figs 2a,b). The cytokine-stimulated CD56-T cells and CD57-T cells showed potent antitumour cytotoxities to NK-sensitive K562 cells while neither DP-T cells nor regular-T cells did (Fig. 2c). Only the CD56-T cells showed a potent cytotoxicity to NK-resistant Raji cells (Fig. 2d). Futhermore, these cytotoxic activities were completely inhibited by the concanamycin A treatment (Table 2), and K562 cells were FAS negative (data not shown).
Figure 2.
IFN-γ production, sFAS-L production, and antitumour activity of various T-cell subsets of PBMC. The indicated T-cell subsets were purified by cell sorting, and 2 × 105 cells from each subset were stimulated with IL-2, -12 and -15 in 96-well flat-bottom plates for 96 hr, and IFN-γ levels (a) and sFAS-L levels (b) in culture supernatants were determined by ELISA after culturing for 48 hr. The cytotoxicities of the cultured subsets against K562 cells (c) (E/T ratio was 10/1) and against Raji cells (d) (E/T ratio was 10/1) were also measured after culturing for 96 hr. All data represent the means ± SE from four independent experiments.
Table 2.
Effect of concanamycin A on cytotoxic activity of CD56-T cells, DP-T cells and CD57-T cells
| Cytotoxic activity (%) | |||
|---|---|---|---|
| Target | Effector | ConcanamycinA(–) | ConcanamycinA(+) |
| K562 | CD56-T | 36·8 ± 5·6 | 0·1 ± 0·1 |
| DP-T | 6·2 ± 1·2 | 0·1 ± 0·1 | |
| CD57-T | 25·6 ± 8·8 | 0·1 ± 0·1 | |
| Raji | CD56-T | 30·4 ± 4·7 | 0·1 ± 0·1 |
| DP-T | 4·1 ± 0·8 | 0·1 ± 0·1 | |
| CD57-T | 10·2 ± 2·3 | 0·1 ± 0·1 | |
Data shown represent the means ± SEs of values from triplicate samples.Data represent the findings of experiments repeated four time with similar results.
Comparison of TCR Vβ repertoires among CD56-T cells, DP-T cells and CD57-T cells
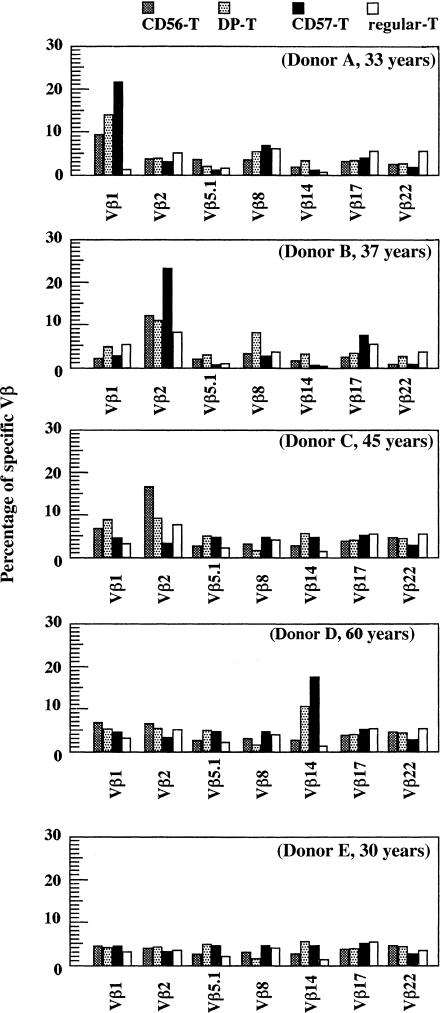
The proportions of some VβT cells were evaluated in CD56-T cell, DP-T cell and CD57-T cell populations of five healthy donors with specific 7 mAbs, that were reportedly reacted with relatively larger populations of αβT cells and were selected. (Fig. 3). The Vβ1 T cells expanded in all NK-type T-cell populations in donor A, whereas Vβ2 T cells expanded in all NK-type T-cell populations in donor B. The biased expression of specific Vβ chains was also observed in donor C (Vβ1T cells in CD56-T cells and DP-T cells but not in CD57-T cells) and donor D (Vβ14 T cells in CD57-T cells and DP-T cells but not in CD56-T cells). Whereas no oligoclonality was found with the seven Vβ specific mAbs so far tested in donor E, it does not exclude the oligoclonal expansion of other VβT cells. In contrast, no biased expansion of specific VβT cells was found in regular CD8+ T cells (Fig. 3). These results showed either the oligoclonality or pauciclonality of the CD56-T, DP-T and CD57-T cell populations in most donors.
Figure 3.
T-cell receptor β repertoires of CD56-T cells, DP-T cells CD57-T cells and regular-CD8+ T cells. PBMC from five individual healthy volunteers with indicated ages were stained as described in Materials and Methods and percentages (%) of VβT cells in each subset were demonstrated.
Nuculeotide sequences of the cells expressing Vβ1 and Vβ2
The oligoclonal expansion of Vβ1 T cells was considered in all Vβ1 NK-type T-cell populations but not in the regular CD8+ T-cell population from donor A (Fig. 3). Therefore, to further analyse the origin of these Vβ1 bearing T cells, 12 cDNA clones for Vβ1 chains from each CD8+ T-cell populations were examined for donor A (Table 3). The nucleotide sequences for Vβ1 from CD56-T cells mainly contained two types of sequences of the CDR3 region, and all 12 sequences for Vβ1 from CD57-T cells completely converged on a single type of CDR3 region but were not identical to either of the two types of CD56-T cells. These results suggested that the Vβ1 CD56-T cell population mainly consisted of two Vβ T-cell clones and Vβ1 CD57-T cells consisted of a single Vβ T-cell clone and that Vβ1 CD56-T cells and CD57-T cells were derived from different origins. Furthermore, the CDR3 region of DP-T cells mainly contained two types of nucleotide sequences, and one was identical to that used by seven out of 12 CD56-T cell clones and the other agreed with that of CD57-T cells. These results also suggested that the Vβ1 DP-T cell population mainly consisted of a mixture of the two cell clones that individually originated from the CD56-T cells and CD57-T cells. All 12 sequences of cDNA clones for Vβ1 chains from regular CD8+ cell population showed diverse CDR3 regions and none of them were identical to the CDR3 regions of the NK-type T cell populations (data not shown).
Table 3.
Vβ1 junctional sequences of CD56-T cells, DP-T cells and CD57-T cells from donor A
| Source | Clone | Vβ1 | N-Dβ-N | Jβ | Jβ | Frequency | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD56-T | K56115 | K56120 | GCC | AG | T | CAG | GGG | GC | G | AAC | AC | T | 1·1 | 4/12 | ||||||||||
| K56132 | K56192 | A | S | Q | G | A | N | T | ||||||||||||||||
| K56117 | GCC | AGC | AGC | CAG | CAT | CGA | TGG | GGC | CC | T | AAT | TCA | 1·6 | 1/12 | ||||||||||
| A | S | S | R | H | R | W | A | P | N | S | ||||||||||||||
| K56113 | K56118 | GCC | AGC | AGC | CAG | CAT | CGA | TGG | GGC | CC | T | AAT | TCA | 2·2 | 7/12 | |||||||||
| K56122 | K56145 | A | S | S | V | G | G | R | H | T | G | |||||||||||||
| K56165 | K56172 | |||||||||||||||||||||||
| K56189 | ||||||||||||||||||||||||
| DP-T | KDP123 | KDP144 | GCC | AGG | AGC | GTA | GGC | GGG | AGG | C | AC | ACC | GGG | 2·2 | 4/12 | |||||||||
| KDP155 | KDP192 | A | S | S | V | G | G | R | H | T | G | |||||||||||||
| KDP110 | GCC | AGC | AGC | GGC | TCC | CGG | GAC | GG | C | TCC | TAC | 2·7 | 1/12 | |||||||||||
| A | S | S | G | S | R | D | G | S | Y | |||||||||||||||
| KDP168 | GCC | AGC | GGG | GCG | AAC | ACT | 1·1 | 1/12 | ||||||||||||||||
| A | S | G | A | N | T | |||||||||||||||||||
| KDP102 | KDP113 | GCC | AGC | AGC | GGG | CCG | ACC | TCT | CCT | AGC | GGG | AGT | TT | C | ACC | GGG | 2·2 | 6/12 | ||||||
| KDP114 | KDP132 | A | S | S | G | P | T | S | P | S | G | S | F | T | G | |||||||||
| KDP177 | KDP181 | |||||||||||||||||||||||
| CD57-T | K57101 | K57103 | GCC | AGC | AGC | GGG | CCG | ACC | TCT | CCT | AGC | GGG | AGT | TT | C | ACC | GGG | 2·2 | 6/12 | |||||
| K57106 | K57109 | A | S | S | G | P | T | S | P | S | G | S | F | T | G | |||||||||
| K57111 | K57115 | |||||||||||||||||||||||
| K57135 | K57158 | |||||||||||||||||||||||
| K57167 | K57174 | |||||||||||||||||||||||
| K57179 | K57191 | |||||||||||||||||||||||
On the other hand, the oligoclonal expansion of Vβ2 T cells was found in CD57-T cell population in donor B while it was not observed in donor A (Fig. 3). Therefore, to investigate whether CD57-T cells were originally composed of a single cell clone or multiple cell clones, we extended the sequencing analyses to Vβ2 T cells in the CD57-T cells from donor A and donor B (Table 4). The results showed that the nucleotide sequences of the six Vβ cDNA clones from donor B converged on a single CDR3 region. Although the six cDNA clones for the Vβ2 chain from donor A showed a substantial variability of the CDR3 sequences, the specific sequence of CDR3 mainly appeared (three out of six clones), thus suggesting that the expanded Vβ2 T cells in donor B may be the result of the clonal expansion of a single Vβ2 clone. In contrast to NK-type T cells, all six cDNA clones for the Vβ2 chains from regular CD8+ cells showed diverse CDR3 regions that were not identical to any CDR3 regions of the CD57-T cell populations from both donors A and B (data not shown).
Table 4.
Vβ2·1 junctional sequences of CD57-T cells from donor A and B
| Donor | Source | Clone | Vβ2·1 | N-Dβ-N | Jβ | Jβ | Frequency | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | CD57-T | K57203 | K57205 | GCT | AGA | AAG | GTT | GGA | CAG | ATT | C | CC | TAC | AAT | 2·1 | 3/6 | ||
| K57206 | A | R | K | V | G | Q | I | P | Y | |||||||||
| K57202 | GCT | A | CC | GAC | GGG | GGG | A | TG | AAC | ACT | 1·1 | 1/6 | ||||||
| A | T | G | G | G | M | N | T | |||||||||||
| K57209 | GCT | AG | G | CAC | GGC | GAT | TAT | TAC | G | AT | GGC | TAC | 1·2 | 1/6 | ||||
| A | R | H | G | D | Y | Y | D | G | Y | |||||||||
| K57210 | GCT | A | CG | ACT | AGC | GGA | TAC | AAT | 2·1 | 1/6 | ||||||||
| A | T | T | S | G | Y | N | ||||||||||||
| B | CD57-T | A57201 | A57204 | GCT | AGA | CCG | TAT | CCG | GGA | CTA | GCG | GGA | GG | C | AAT | GAG | 2·1 | 6/6 |
| A57205 | A57208 | A | R | P | Y | P | G | L | A | G | G | N | E | |||||
| A57209 | A57210 | |||||||||||||||||
Discussion
In the present study, we demonstrated that although all NK-type T cells express CD122 and intermediate TCR (which are lower than those of regular T cells), three populations of NK-type T cells are functionally distinct populations. Both DP-T cells and CD57-T cells but not CD56-T cells increased with age. All NK-type T-cell populations produced larger amounts of IFN-γ than did regular CD8+ T cells. The DP-T cells exhibited a lower cytotoxicity to tumours than did other NK-type T-cell subsets. However, whereas DP-T cells produced a smaller amount of sFAS-L than did CD56-T cells, the DP-T cells produced a larger amount of sFAS-L than did CD57-T cells. An oligoclonal or a pauciclonal VβT cells expanded in CD57-T cells and CD56-T cells, and the nucleotide and amino acid sequence analysis of their Vβ CDR3 regions revealed that expanded CD57-T cells used a single CDR3 region of TCRβ and CD56-T cells used semivariant CDR3 regions of TCRβ which were different from that of CD57-T cells. In addition, the expanded VβT cells in DP-T cells used semivariant CDR3 regions of TCRβ, which were a mixture of the invariant CDR3 regions of TCRβ used by CD57-T cells and CD56-T cells.
Recently, CD56 has been reported to be a marker of cytolytic effector function of circulating CD8+ T cells after cytokine- or CD3-stimulation.15 We also recently found that CD56+ T cells (including both CD56+ CD57− and CD56+ CD57+ cells) stimulated with anti-CD3 antibody or cytokines exhibited a stronger antitumour cytotoxicity against NK-resistant Raji cells than did CD57-T cells, whereas the IFN-γ production and cytotoxicity against NK-sensitive K562 cells did not significantly differ between the two subsets.7 However, we have herein shown that the CD56 expression alone on T cells is not an absolute indicator for either the antitumour cytotoxicity or IFN-γ production capacity because both CD56 and CD57 expressing DP-T cells produced a lower amount of IFN-γ than CD56-T cells and also exhibited the lowest degree of antitumour cytotoxicity among all NK-type T-cell subsets. As a result, unless DP-T cells are separated from the CD56+ T-cell population, the antitumour cytotoxicity and IFN-γ production of CD56-T cells will be underestimated.
It is also of particular interest to note that, the expanded Vβ1 CD56-T cells from donor A consisted of two main Vβ1 clones while the Vβ1 CD57-T cells consisted of a single Vβ1 clone. This is in marked contrast to regular CD8+ T cells which display markedly diverse Vβ CDR3 regions. Although such a use of the invariant CDR3 regions has been reported in CD57-T cells,21 no Vβ CDR3 region analysis in CD56-T cells has yet been reported. Furthermore, expanded Vβ1 DP-T cells were mainly composed of two cell clones, one showing the same CDR3 region that used by seven out of 12 Vβ1 clones in CD56-T cells and another showing the same CDR3 region as that used by all Vβ1 CD57-T cell clones. Although we do not rule out the possibility that DP-T cells may differentiate into CD57-T cells and CD56-T cells after losing either CD57 or CD56, the fact that DP-T cells were very rare, but nevertheless a substantial number of CD57-T cells and CD56-T cells were present in young donors suggests that both Vβ1 CD56-T cells and Vβ1 CD57-T cells may differentiate into Vβ1 DP-T cells after acquiring CD57 and CD56, respectively. Nevertheless, the function of DP-T cells in vitro was not exactly intermediate between that of CD57-T cells and CD56-T cells. Thus, it now appears to be clear that CD57-T cells and CD56-T cells are obviously different NK-type T-cell populations when they are separated from DP-T cells.
Another notable finding is that Vβ2 CD57-T cells of donor B use an invariant CDR3 region of the TCRβ chain while Vβ2 CD57-T cells of donor A use semivariant CDR3 regions. Considering that Vβ2 CD57-T cells were clonally expanded in donor B but not in donor A, the each CD57-T cell subset is thus indicated to originally be composed of some different Vβ clones while the expansion of certain Vβ CD57-T cell subsets in individuals may result from the expansion of a single Vβ clone. This situation may also be less tightly applicable for CD56-T cells. Because we did not analyse Vα, we cannot rule out the possibility that expanded CD57-VβT cells with a single TCRβ transcript use different TCR Vα chains. However, considering that Vα gene rearrangement takes place after Vβ gene rearrangement and N-region diversification in Vβ CDR3 is much higher than that of Vα CDR3, the expanded CD57-T cells are suggested to originate from a single or, at least, from a small number of Vα/Vβ T cell clones. These findings suggest that human NK-type T cells do not likely recognize many foreign antigens by their TCR but do recognize a limited set of antigens, including self-antigens. Anfossi et al. also suggested that CD8+ T cells with NK and/or memory phenotype which increase with age may be autoreactive.25 In fact, activated NK-type T cells injure not only tumours but also vascular endothelial cells.20
A previous report showed that the CD57-T cells increased in persons with a previous cytomegalovirus (CMV) infection while oligoclonal expansion of certain VβT cells of CD57-T cells was not the result of the CMV infection because the oligoclonality of CD57-T cells was also observed in CMV seronegative persons.21 CD57-T cells have also been reported to increase in certain diseases, such as human immunodeficiency virus (HIV) infection,26 rheumatoid arthritis27 and organ transplantations.28–30 However, CD57-T cells may inhibit both the inflammation of rheumatoid arthritis31 and also the replication of HIV.32 Furthermore, not only CD56-T cells but also CD57-T cells are abundantly found in tumour infiltrating lymphocytes.18,19 Considering that the elderly individuals in this study are healthy, we prefer to speculate the increase of CD57-T cells in various diseases and the increase of CD57-T cells as well as NK cells with ageing7,16 may simply reflect the NK cell-like effector function or a regulatory function of CD57-T cells in the host rather than their antigen specificity.
The antitumour cytotoxicities of NK-type T cells appear to be mainly mediated through the perforin/granzyme pathway because their cytotoxicities were almost completely blocked by concanamycinA which has been reported to specifically inhibit the perforin/granzyme pathway but not the FAS/FAS-L signalling pathway of antitumour cytotoxicities,33 even though NK-type T cells produced large amounts of sFAS-L by cytokine stimulation as demonstrated in this study. In addition, K562 cells in this study do not express FAS. However, because activated mouse NK1·1+ T cells cause hepatocyte injury Fas/Fas-ligand dependently but kill tumours Fas/Fas-ligand independently,34 and activated human NK-type T cells attack vascular endothelial cells,20 a possibility is raised that human NK-type T cells may also use the Fas/Fas-ligand system to injure nontumour cells.
Finally, as in the case of mouse NK1·1+ T cells and CD8+ CD122+ T cells (increasing with age),35–37 human NK-type T cells also express CD122 and intermediate levels of TCR, thus supporting the theory that these NK-type T cells in both mice and humans are functional counterparts and are distinct from regular T cells.7,11,14 In addition, human NK-type T cells more vigorously proliferate after stimulation with IL-2, IL-12 and IL-15 than after stimulation with IL-2 and IL-12 alone (our unpublished observation) probably because CD122 is a common receptor for both IL-2 and IL-15.38,39
Abbreviations
- CD56-T
CD56 single positive T
- DP-T
CD56/CD57 double positive T
- CD57-T
CD57 single positive T
- regular-T
CD56/CD57 double negative T
References
- 1.Abo T, Balch CM. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1) J Immunol. 1981;127:1024. [PubMed] [Google Scholar]
- 2.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480. [PubMed] [Google Scholar]
- 3.Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169:2233. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abo T, Watanabe H, Iiai T, Kimura M, Ohtsuka K, Sato K, et al. Extrathymic pathways of T-cell differentiation in the liver and other organs. Int Rev Immunol. 1994;11:61. doi: 10.3109/08830189409061717. [DOI] [PubMed] [Google Scholar]
- 5.Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983;131:1789. [PubMed] [Google Scholar]
- 6.Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183. [PubMed] [Google Scholar]
- 7.Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, Hiraide H, Sekine I. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103:281. doi: 10.1046/j.1365-2567.2001.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4− 8− T cells in mice and humans. J Exp Med. 1994;180:1097. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense. the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 12.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+ CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314. [PubMed] [Google Scholar]
- 13.Satoh M, Seki S, Hashimoto W, Ogasawara K, Kobayashi T, Kumagai K, Matsuno S, Takeda K. Cytotoxic gammadelta or alphabeta T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol. 1996;157:3886. [PubMed] [Google Scholar]
- 14.Kawarabayashi N, Seki S, Hatsuse K, Ohkawa T, Koike Y, Aihara T, et al. Decrease of CD56(+) T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 16.Miyaji C, Watanabe H, Minagawa M, Toma H, Kawamura T, Nohara Y, Nozaki H, Sato Y, Abo T. Numerical and functional characteristics of lymphocyte subsets in centenarians. J Clin Immunol. 1997;17:420. doi: 10.1023/a:1027324626199. [DOI] [PubMed] [Google Scholar]
- 17.Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- 18.Okada T, Iiai T, Kawachi Y, Moroda T, Takii Y, Hatakeyama K, Abo T. Origin of CD57+ T cells which increase at tumour sites in patients with colorectal cancer. Clin Exp Immunol. 1995;102:159. doi: 10.1111/j.1365-2249.1995.tb06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takii Y, Hashimoto S, Iiai T, Watanabe H, Hatakeyama K, Abo T. Increase in the proportion of granulated CD56+ T cells in patients with malignancy. Clin Exp Immunol. 1994;97:522. doi: 10.1111/j.1365-2249.1994.tb06120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ami K, Ohkawa T, Koike Y, Sato K, Habu Y, Iwai T, Seki S, Hiraide H. Activation of human T cells with NK cell markers by staphylococcal enterotoxin A via IL-12 but not via IL-18. Clin Exp Immunol. 2002;128:453. doi: 10.1046/j.1365-2249.2002.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang EC, Moss PA, Frodsham P, Lehner PJ, Bell JI, Borysiewicz LK. CD8high CD57+ T lymphocytes in normal, healthy individuals are oligoclonal and respond to human cytomegalovirus. J Immunol. 1995;155:5046. [PubMed] [Google Scholar]
- 22.Morley JK, Batliwalla FM, Hingorani R, Gregersen PK. Oligoclonal CD8+ T cells are preferentially expanded in the CD57+ subset. J Immunol. 1995;154:6182. [PubMed] [Google Scholar]
- 23.Batliwalla F, Monteiro J, Serrano D, Gregersen PK. Oligoclonality of CD8+ T cells in health and disease: aging, infection, or immune regulation? Hum Immunol. 1996;48:68. doi: 10.1016/0198-8859(96)00077-8. [DOI] [PubMed] [Google Scholar]
- 24.Gorochov G, Debre P, Leblond V, Sadat-Sowti B, Sigaux F, Autran B. Oligoclonal expansion of CD8+ CD57+ T cells with restricted T-cell receptor beta chain variability after bone marrow transplantation. Blood. 1994;83:587. [PubMed] [Google Scholar]
- 25.Anfossi N, Pascal V, Vivier E, Ugolini S. Biology of T memory type 1 cells. Immunol Rev. 2001;181:269. doi: 10.1034/j.1600-065x.2001.1810123.x. [DOI] [PubMed] [Google Scholar]
- 26.Sadat-Sowti B, Debre P, Mollet L, Quint L, Hadida F, Leblond V, Bismuth G, Autran B. An inhibitor of cytotoxic functions produced by CD8+ CD57+ T lymphocytes from patients suffering from AIDS and immunosuppressed bone marrow recipients. Eur J Immunol. 1994;24:2882. doi: 10.1002/eji.1830241145. [DOI] [PubMed] [Google Scholar]
- 27.Dupuy d'Angeac A, Monier S, Jorgensen C, Gao Q, Travaglio-Encinoza A, Bologna C, et al. Increased percentage of CD3+, CD57+ lymphocytes in patients with rheumatoid arthritis. Correlation with duration of disease. Arthritis Rheum. 1993;36:608. doi: 10.1002/art.1780360506. [DOI] [PubMed] [Google Scholar]
- 28.Fregona I, Guttmann RD, Jean R. HNK-1+ (Leu-7) and other lymphocyte subsets in long-term survivors with renal allotransplants. Transplantation. 1985;39:25. [PubMed] [Google Scholar]
- 29.Leroy E, Calvo CF, Divine M, Gourdin MF, Baujean F, Ben Aribia MH, et al. Persistence of T8+/HNK-1+ suppressor lymphocytes in the blood of long-term surviving patients after allogeneic bone marrow transplantation. J Immunol. 1986;137:2180. [PubMed] [Google Scholar]
- 30.Maher P, O'Toole CM, Wreghitt TG, Spiegelhalter DJ, English TA. Cytomegalovirus infection in cardiac transplant recipients associated with chronic T cell subset ratio inversion with expansion of a Leu-7+ TS-C+ subset. Clin Exp Immunol. 1985;62:515. [PMC free article] [PubMed] [Google Scholar]
- 31.Arai K, Yamamura S, Seki S, Hanyu T, Takahashi HE, Abo T. Increase of CD57+ T cells in knee joints and adjacent bone marrow of rheumatoid arthritis (RA) patients: implication for an anti-inflammatory role. Clin Exp Immunol. 1998;111:345. doi: 10.1046/j.1365-2249.1998.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith PR, Cavenagh JD, Milne T, Howe D, Wilkes SJ, Sinnott P, Forster GE, Helbert M. Benign monoclonal expansion of CD8+ lymphocytes in HIV infection. J Clin Pathol. 2000;53:177. doi: 10.1136/jcp.53.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678. [PubMed] [Google Scholar]
- 34.Nakagawa R, Nagafune I, Tazunoki Y, Ehara H, Tomura H, Iijima R, et al. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J Immunol. 2001;166:6578. doi: 10.4049/jimmunol.166.11.6578. [DOI] [PubMed] [Google Scholar]
- 35.Ohteki T, Okuyama R, Seki S, Abo T, Sugiura K, Kusumi A, et al. Age-dependent increase of extrathymic T cells in the liver and their appearance in the periphery of older mice. J Immunol. 1992;149:1562. [PubMed] [Google Scholar]
- 36.Tsukahara A, Seki S, Iiai T, Moroda T, Watanabe H, Suzuki S, et al. Mouse liver T cells: their change with aging and in comparison with peripheral T cells. Hepatology. 1997;26:301. doi: 10.1002/hep.510260208. [DOI] [PubMed] [Google Scholar]
- 37.Takayama E, Seki S, Ohkawa T, Ami K, Habu Y, Yamaguchi T, Tadakuma T, Hiraide H. Mouse CD8+ CD122+ T cells with intermediate TCR increasing with age provide a source of early IFN-gamma production. J Immunol. 2000;164:5652. doi: 10.4049/jimmunol.164.11.5652. [DOI] [PubMed] [Google Scholar]
- 38.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 39.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]