Abstract
We showed in a previous study that the intranasal (i.n) delivery of bacille Calmette–Guérin (BCG) to BP2 mice (H-2q) inhibits eosinophilia and bronchial hyperreactivity in a mouse model of asthma. The present work has been performed to characterize the leucocyte lineages recruited to the lungs of mice after i.n. delivery of BCG and potentially involved in the polarization of T lymphocytes. The different antigen-presenting cells (APC) recruited to bronchoalveolar lavage (BAL) and to lung tissue of mice shortly after the delivery of BCG were analysed in parallel as well as their capacity to drive the immune response towards a T helper type 1 cytokine production. Alveolar macrophages (AM) from the BAL were CD11c+, F4/80+ and CD11b−, and in the lung tissue two major populations of potential APC were detected: one CD11c−, F4/80+, CD11b+ and I-Aq− was identified as interstitial macrophages (IM) and a second expressing CD11c+ and I-Aq+ antigens, negative for CD11b and F4/80 markers as leucocytic dendritic cells (DC). Freshly isolated DC up-regulated CD11b and CD40 antigens after overnight culture, but remained negative for CD8α antigen, suggesting a myeloid origin. Lung DC which produced high amount of interleukin (IL)-12 were potent inducers of naive CD4+ T lymphocyte priming, as assessed by interferon-γ (IFN-γ) production by these naive CD4+ T cells. Lung explants recovered long term after BCG delivery produced sustained levels of IFN-γ. Our results suggest that AM and particularly DC by secreting IL-12 shortly after BCG delivery induce the long-term persistence of IFN-γ-secreting T cells percolating in BCG-loaded lung tissue.
Introduction
Allergic asthma involves airway allergen-induced inflammation, inflammatory leucocytes, eosinophils and sometimes neutrophils, being recruited to the airways as a consequence of the presence and activation of T lymphocytes, which react with the inhaled allergens in the respiratory tract. CD4+ T lymphocytes play a major role in initiating allergic airway inflammation via the production of T helper type 2 (Th2) cytokines, which trigger the recruitment of eosinophils to the airways and, very likely, their subsequent activation.1 It has been suggested that an imbalance between Th2 and Th1 effectors drives the onset and sustained pathogenesis of asthma.2,3 Interferon-γ (IFN-γ) has been shown to inhibit the development of Th2 responses both in vitro and in vivo.4,5
We and others have shown that the immunization of mice with intracellular bacteria known to induce a strong Th1 immune response may counterbalance the allergen-induced Th2 response and reduce eosinophilia and the associated bronchial hyperreactivity (BHR).6–9 The bacille Calmette–Guérin (BCG)-loaded leucocyte lineages which direct the T cell polarization after the intranasal (i.n.) delivery of BCG have not been identified. For this reason, we explored, shortly after i.n. delivery of BCG to the airways, the lineages and number of leucocytes recruited to the lungs, their loading with BCG and the type of cytokines they induce when acting as antigen-presenting cells (APC): i.e. as a source of immunogenic signals for naive and for primed T lymphocytes.
T-cell immunity to live intracellular bacteria is triggered and maintained by professional phagocytic cells, dendritic cells (DC) and macrophages, via differential cytokine secretion and membrane display of different costimulatory molecules that act as immunogenic signals for T lymphocytes. In the respiratory tract, alveolar macrophages (AM) are the first leucocyte subset to clear inhaled soluble allergens or take up particles including cellular microorganisms. They phagocytose and secrete mediator molecules, some of which act directly on the microorganisms within their vacuoles,10 whereas others, such as interleukin (IL)-12, stimulate IFN-γ production and promote type 1 responses. DC, which also produce IL-12 following microbial stimuli, may preferentially direct the development of Th1 cells.11 Immature DC phagocytose live mycobacteria in vitro12,13 and it has been recently demonstrated that DC pulsed with mycobacteria in vitro can efficiently stimulate mycobacteria-reactive T-cells primed in vivo.14 The inhalation of bacteria or of soluble proteins results in the recruitment of DC to the airway epithelium.15 Lung DC are equipped with phagocytic receptors and have a rapid turnover rate, reflecting the continuous sampling for antigen and migration of these cells to the draining lymph nodes.16,17 These results suggest that the AM and/or DC present before BCG delivery and/or recruited, and the cytokines they produce during the first hours or days following micro-organism delivery are crucial. AM and lung DC may thus influence the outcome of the subsequent T-cell dependent immune reactivity to other unrelated immunogenic molecules. We therefore studied the time-dependent recruitment of the different APC as well as their capacity to be loaded with BCG and to secrete bioactive IL-12, in the lungs of mice shortly after i.n. immunization with BCG. Our study shows that DC recruited to the lungs during the first hours after BCG delivery allow naive T cells to secrete IFN-γ a Th1-type cytokine known to play a pivotal role in protecting against asthma.4,5 Moreover, by secreting IL-12 shortly after the BCG delivery, lung DC induce the long-term persistence of IFN-γ production by lung explants from 1 to 3 months after i.n. delivery of BCG.
Materials and methods
Mice
Male BP2 (H-2q) mice at 6–7 weeks old were obtained from the Centre d'Elevage Janvier (Le Genest, St Isle, France) and were maintained in our animal facilities in specific pathogen free-conditions.
Microorganisms and immunization
The Mycobacterium bovis BCG Pasteur vaccine strain 1173P2 was grown as dispersed bacilli in Beck–Proskauer medium supplemented with 0·05% Triton WR 1339 (Sigma, St Louis, MO) and 6% glucose. The bacteria were stored at −70° in Beck–Proskauer medium supplemented with 0·05% Triton and 6% glycerol. The number of colony-forming units (CFU) per ml was determined by plating suitable dilutions in phosphate-buffered saline (PBS) on Middlebrook 7H10 agar medium (Difco, Fisher Scientific Labs, Elancourt, France). The suspension was diluted in PBS just before its administration. Unanaesthetized BP2 mice were immunized i.n. with 106 CFU of BCG in a volume of 10 µl.
Lung cell isolation for flow cytometry analysis
BP2 mice were anaesthetized intraperitoneally with urethane (1·5 mg/g body weight) and the thoracic cavity was opened. The trachea was cannulated and the lungs of each mouse were washed eight times with 1 ml of saline each by gentle instillation. After bronchoalveolar lavages, mice were exsanguinated via the abdominal aorta and their lungs were perfused via the right ventricular cavity of the heart with 5 ml of saline (0·9% NaCl). Lungs were aseptically removed, cut into small pieces and digested for 30 min at 37° in RPMI-1640 medium (Seromed, Munich, Germany) containing collagenase (2 mg/ml) (Worthington Biochemical Corporation, Lakewood, NJ) and DNAse I (1 mg/ml; Sigma). Enzyme activity was stopped by adding 8 ml of RPMI supplemented with 10% fetal calf serum (FCS). Single cell suspension of lung pieces were obtained by pushing the digested tissues through cell-strainers (Falcon, BD, Franklin Lakes, NJ). Cells isolated from bronchoalveolar lavage (BAL) and lung tissue of 10 individual mice were washed and resuspended in RPMI medium supplemented with 10% FCS. Cells from each BAL and lung tissue were counted in haemocytometer and thereafter pooled before stabilization with StabilCyte medium (BioErgonomics Inc., St Paul MN). Under these conditions, cells from BAL and lung digests could be stored at 4° for at least 4 weeks before labelling for flow cytometric analysis.
In a second experiment in order to characterize more precisely lung DC, these cells were purified and accessory and major histocompatibility complex (MHC) class II molecules were analysed by flow cytometry on freshly isolated and on overnight cultured lung DC. To purify lung DC, after BAL, cells from digested lung tissue were suspended in RPMI–FCS supplemented with 2 mm ethylenediaminetetraacetic acid (EDTA) and passed through a 25-gauge needle to break up clumps. Cells were counted and incubated for 15 min at 4° at the appropriate ratios with magnetic activated cell sorting (MACS) CD11c microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). The cells were washed, and diluted in 5 ml of PBS−0·5% FCS, and CD11c+ cells were isolated by passing the antibody-coated cell suspensions through a column on an AutoMACS magnetic cell separator (Miltenyi). Freshly purified lung DC were stabilized with StabilCyte medium or suspended in RPMI−10% FCS and incubated overnight in tissue culture dishes. The non-adherent cells and those that easily detached after the overnight culture were harvested, washed and stabilized in StabilCyte medium.
Monoclonal antibodies (mAb) for flow cytometric analysis
mAb reactive to CD11c (clone HL3, hamster immunoglobulin G; IgG), CD11b (Mac-1, M1/70, rat IgG2a), Ly-6G-Gr1 (clone RB6-8C5, rat IgG2b), CD8α (clone53-6.7, rat IgG2a), I-Aq (clone KH116, mouse IgG2b), CD80 (B7-1, clone 16-10A1, hamster IgG), CD86 (B7-2, clone GL1, rat IgG2a), CD40 (clone 3/23, rat IgG2a), were purchased from BD PharMingen (San Diego, CA) as purified immunoglobulin or immunoglobulin directly conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), Cy-Chrome or biotin. Biotin-conjugated anti-F4/80 (clone C1:A3-4, rat IgG-2b) and DEC-205 (clone NLDC-145, rat IgG2a) were purchased from Caltag Laboratories, Burlingame, CA. Streptavidin, anti-rat or anti-mouse IgG conjugated to FITC, PE or Cy-Chrome (Caltag Laboratories) were used as secondary antibodies when necessary.
Flow cytometric analysis
Cells from the BAL and from digested lung tissues were recovered 6, 24, 48, and 96 hr after the i.n. delivery of BCG and fixed in StabilCyte medium. Before flow cytometry analysis, the cells were washed in PBS containing 5% FCS and stained for 30 min at 4° with purified or directly conjugated antibodies. If necessary, cells were then washed twice with PBS–FCS and incubated with a secondary antibody. Isotype controls were used for each antibody. The cells from all time points were analysed on the same day on a FACScan flow cytometer (BD, Oxford, UK). This procedure minimized variability in staining intensities. The number of cells from the BAL expressing CD11c+ F4/80+ and negative for CD11b (AM) and from lung digests expressing either F4/80+ CD11b+ and negative for CD11c (interstitial macrophages; IM) or CD11c+ and negative for F4/80 and CD11b (DC) was calculated as: percentage of each subset as determined by FACS analysis × number of cells counted in the BAL or lung digest, and was expressed as the mean ± SEM.
To characterize more precisely the lung DCs, purified CD11c+ cells freshly isolated from lung tissue or overnight cultured in RPMI−10% FCS were analysed on the same day on a FACScan flow cytometer and expression of accessory and MHC class II molecules was compared to isotype controls.
Laser scanning image cytometry
To confirm the phenotype of lung dentritic cells and to evaluate their capacity to achieve their maturation in vitro, purified CD11c+ cells were cultured in Laboratory-Tek chambered coverglass for tissue culture (8 × 105 cells/chamber) (Nunc, Roskilde, Denmark) for 1 or 24 hr in RPMI–FCS medium supplemented with 10% granulocyte–macrophage colony-stimulating factor (GM-CSF) medium. Cells were stained with anti-MHC class II I-Aq mAb conjugated to PE and then double stained with anti-B7-2 mAb conjugated to FITC. This technique allows visualization of the same field with cells firstly monolabelled in red (I-Aq PE-conjugated) and thereafter double labelled in red and green (I-Aq PE-conjugated and B7-2 FITC-conjugated). The visualization of cells was obtained with an argon ion laser (Coherent 92-5, Palo Alto, CA) emitted beam at 488 nm, with 50 mW power reduced to 12·5 mW by an acousto-optic modulator. A 100× immersion oil objective lens allowed to obtain a laser spot diameter of 0·2 µm. The effective power at the plane of focus was determined using a field Master power meter with a LM-2 detector head (Coherent). An image cytometer ACAS 570 (Meridian, Okemos, MI) was used for cell counting.
Immunohistochemical analysis of the lung
Mice which have received BCG i.n. 48 hr previously were anaesthetized. BAL was performed and the lungs were inflated via the trachea with 1 ml of 50% Optimum Cutting Temperature (OCT; Sakura Finetek, Torrance, CA) in saline solution. One lobe was placed in an Eppendorf tube and immediately frozen in liquid nitrogen for cryostat sectioning. The frozen blocks were stored at −80° prior to use. Sections (5 µm) were cut in a cryostat at −20° and collected on glass slides coated with poly l-lysine (Sigma). They were then fixed in chloroform–acetone v/v (Merck) for 10 min, wrapped in plastic film and stored at −20° prior to use. Consecutive sections of each block were stained with the following purified or biotinylated mAb (all from Pharmingen): CD11c (clone HL3, hamster IgG), I-Aq (clone KH116, mouse IgG2b). The alkaline phosphatase–anti-alkaline phosphatase (APAAP) staining procedure was performed by incubation with rabbit immunoglobulin against rat immunoglobulin (D455, Dako A/S, Glostrup, Denmark), followed by rat APAAP antibody (D488, Dako) or alkaline phosphatase-conjugated streptavidin AP antibody (D0396, Dako) for the biotinylated primary antibodies. The reaction was developed with the substrates Fast red TR (Sigma) and naphthol AS MX phosphate (Sigma), and light haematoxylin counter-staining. Sections were stained, coded and examined in a blind fashion. At least 10 fields were analysed, and one representative field is shown.
Labelling of living bacilli before the phagocytosis assay
To determine the phagocytic capacity of the different APCs, fluorescent live BCG was prepared. Carboxyfluorescein-diacetate succinimidyl ester (CFDA; Molecular Probes Inc, Eugene, OR) was used to label the bacteria. The native CFDA molecule is not fluorescent. It is a non-polar molecule that spontaneously penetrates the bacterial cell membrane. CFDA is then converted to an anionic fluorescent molecule by intracellular esterases, which are present and active in live bacteria. The succinimidyl group makes possible the covalent amine binding of the molecule to any amine residue in the vicinity. The stock solution of CFDA (1 mg/ml in dimethyl sulphoxide; DMSO) was diluted 1:100 in PBS and 100 µl was added to 1 ml of BCG suspension (108 CFU/ml). This suspension was incubated in the dark at room temperature for 60 min Labelled bacteria were centrifuged for 15 min at 1600 g, washed twice and resuspended in PBS. They were able to form colonies on 7H10 medium after this treatment.
Phagocytosis assay
Alveolar macrophages were obtained from BAL 1, 6, 24 and 48 hr after the i.n. delivery of labelled BCG. The trachea was cannulated and the lungs were washed as described above. Lavage fluids from five mice were pooled and centrifuged at 4° for 5 min at 400 g. Cells were incubated 30 min at 4° with PE conjugated anti-Gr1 mAb (a neutrophil marker), and were washed before incubation at the appropriate ratio for 15 min at 4° with MACS anti-PE microbeads. The cells were then washed, and diluted in 5 ml of PBS−0·5% FCS, by passing the antibody-coated cell suspensions through a column on an AutoMACS magnetic cell separator. Accordingly, BAL cells were depleted from neutrophils (Gr1+).
After BAL, cells from lung tissue were isolated as described in lung cell isolation for flow cytometry, cells were then suspended in RPMI–FCS supplemented with 2 mm EDTA and passed through a 25-gauge needle to break up clumps. Cells were counted and incubated at the appropriate ratios with MACS CD11c or CD11b microbeads for 15 min at 4°. They were washed, and diluted in 5 ml of PBS−0·5% FCS, CD11c+ or CD11b+ cells being isolated by passing the antibody-coated cell suspensions through a column on an AutoMACS magnetic cell separator. Cytospins were prepared by centrifugation of 200 µl containing around 105 cells isolated from BAL or lung tissue 1, 6, 24 and 48 hr after the i.n. delivery of BCG. Cytospins from BAL (AM) and from purified lung cells – CD11b+ (IMs) or CD11c+ (DCs) – were stained with Diff-Quick (Baxter Dade AG, Duedingen, Switzerland). Since non-specific fluorescence decreased during storage at 4°, the labelled bacteria were counted by fluorescence microscopy 15 days after staining. Moreover, the fluorescence due to labelled bacteria bound to the surface of the cells but not ingested was excluded by adding trypan blue to the preparations. The fluorescence of externally bound bacteria was quenched by trypan blue, whereas the fluorescence of internalized bacteria, which were not in contact with trypan blue, was not affected. We counted 200–400 cells and the number of cells that were significantly phagocytic is expressed as a percentage of the whole population studied.
IL-12 production by lung cells
At 6, 24, 48 and 96 hr after i.n. delivery of BCG, AMs were harvested from BAL and then IM and DC were isolated from digested lung tissue. Cells harvested from BAL of 10 mice were pooled and depleted of neutrophils (Gr1+) as described above. IM and DC were isolated from digested lung tissue after incubation with CD11b or CD11c microbeads and passage through a magnetic column as described above. The purity of the different populations sorted by the autoMACS system was confirmed by fluorescence-activated cell sorting (FACS) analysis (90% purity). Purified cells were cultured in RPMI−10% FCS at 37° and 5% CO2, supernatants were harvested 24 hr later and assayed for immunoreactive IL-12 by enzyme-linked immunosorbent assay (ELISA). mAb C15.6 (p40 + p70) (Biosources, Camarillo, CA) or 9A5 (p70) (Perbio Sciences, Erembodegem-Aalst, Belgium) were used as the capture antibodies and the anti-IL-12 mAb C17.8 as the secondary biotinylated antibody. The binding of the secondary mAb was detected using peroxidase-conjugated streptavidin (Biosources) with TMB as substrate (KPL Laboratory, Gaithersburg, MD). Since immunoreactive IL-12 p70 was not detected in our culture conditions, the results obtained with IL12 p40 + p70 correspond to the number of pg of IL-12 p40/ml of supernatant.
Source and enrichment of naive and primed CD4+ T lymphocytes
T cells were isolated from inguinal lymph nodes of non immunized mice or from mice immunized subcutaneously (s.c) at the base of the tail with 107 CFU of BCG 4 days before recovery. Cells suspended in PBS−0·5% FCS and incubated at the appropriate ratios with MACS CD4+ microbeads for 15 min at 4° were washed, diluted in 5 ml of PBS-0·5% FCS and separated by passing the antibody-coated cell suspension through a column on an AutoMACS magnetic cell separator. Naive and primed CD4+ T cells were counted and cocultured with the different APC isolated from the lungs of naive or i.n. immunized mice.
IFN-γ and IL-5 production by T cells stimulated with IM, AM and DC isolated from lungs
The stimulatory activity of lung AM, IM and DC isolated 24, 48 and 96 hr after the i.n. delivery of BCG as described above, was assessed by coculturing these cells with CD4+ T cells from naive mice or from mice immunized s.c. with BCG and isolated as described above. CD4+ T cells were added at a ratio of 1 : 5 to the different APC populations separated from BAL and lungs of naive or of i.n. immunized mice. The mixed APC and T cell cultures were then incubated in triplicate in flat-bottomed 96-well plates (Nunc) for 72 hr at 37° and 5% CO2. The inhibition of IFN-γ production by CD4+ T lymphocytes was monitored after adding IL-12 p40 neutralizing mAb (clone C15.6) (10 µg/ml) to some wells.
IFN-γ was determined in the supernatants with a commercial ELISA kit (Biosources). Briefly, 96-well plates (Nunc) were coated with rabbit anti-mouse/-rat IFN-γ polyclonal antibody, incubated with supernatants and dilutions of rIFN-γ standard. A biotinylated anti-IFN-γ antibody (RMMG-1) was added and antibody binding was detected with steptavidin substrate, absorbance being measured at 490 nm. IL-5 was determined with an immunometric assay: 96-well plates were coated with 10 µg/ml rat anti-mouse IL-5 (TRFK-4), supernatants or dilutions of rIL-5 standard were added, followed by an acetylcholinesterase-labelled rat anti-mouse IL-5 antibody (TRFK-5) at 10 Ellman units/ml. Absorbance was measured at 405 nm. Results for IFN-γ and IL-5 production are expressed in pg/ml.
IFN-γ and IL-5 production by lung explants is a long-term process after BCG delivery
To evaluate the ex vivo long-term production of IFN-γ and IL-5 by lungs at 14, 28, 56, 90 and 116 days after the i.n. delivery of BCG, lungs were perfused via the right ventricular cavity, they were cut into small pieces (usually 15 pieces/lung). The pieces were randomly collected and cultured at 37° and 5% CO2 in 24-well plates (Nunc), each well containing five lung pieces and 1 ml of AIM V medium (Life Technologies) supplemented with 1% l-glutamine (Life Technologies) and gentamicin (10 µg/ml) (Sigma). Supernatants from lung explants with medium alone or stimulated with 1 µg/ml of soluble anti-CD3 mAb were harvested after 6 hr culture for IL-5 and after 24 hr culture for IFN-γ determinations. IFN-γ and IL-5 contents were measured as above described. Explants and total lung were weighted (dry weight) and results are expressed in pg of IFN-γ and IL-5 produced by total lung.
Statistical analysis
An unpaired Student's t-test was used for all analysis. Values of P < 0·05 were considered statistically significant.
Results
Recruitment of CD11c+ F4/80+ CD11b− (AM), CD11c− F4/80+ CD11b+ (IM) and CD11c+ F4/80− CD11b− (DC) to the lungs shortly after i.n. delivery of BCG
To characterize the recruitment of potential APC to BAL and lungs shortly after the i.n. delivery of BCG (from 6 to 96 hr), cells were harvested from the BAL fluid and from digested lungs. The markers F4/80, CD11c, and CD11b were used to identify, by three-colour flow cytometry, the phenotypes of the cells, those expressing Gr1 being excluded (less than 10% without any increase after BCG delivery).
The number of cells recruited to BAL after the i.n. delivery of BCG increased transiently 6 hr after BCG delivery. The majority of these cells expressed the myeloid DC marker CD11c, but were also positive for the macrophage marker F4/80, thus identifying AM (Fig. 1). Two major populations were recovered from the lung tissue, one expressing F4/80 and negative for CD11c, representing IM, the second population (lung-derived DC) expressing CD11c and being negative for F4/80 (Fig. 1). Both populations were recruited later to the lungs (48–96 hr). It should be noted that AM never expressed the myeloid lineage molecule CD11b, whereas IM expressed this molecule, pointing out major differences in surface markers among the two lung populations of monocytes/macrophages.
Figure 1.
Recruitment of CD11c+ F4/80+ CD11b− (AM), CD11c− F4/80+ CD11b+ (IM) and CD11c+ F4/80− CD11b− (DC) to the lungs of mice shortly after i.n. delivery of BCG. Cells harvested from the BAL and from digested lung tissue 6–96 hr after i.n. delivery of BCG (106 CFU) were stained with antibodies as follows: FITC- or PE-conjugated CD11c, F4/80 and CD11b. Cells expressing CD11c+ F4/80+ CD11b− (AM) or CD11c− F4/80+ CD11b+ (IM) or CD11c+ F4/80− CD11b− (DC) were analysed by FACScan flow cytometry. Rat IgG2a, rat IgG2b isotypes and hamster IgG were used as controls. The number of cells present in the three populations was calculated as: percentage of each subset as determined by FACS analysis × number of cells counted in the BAL or lung digest, and was expressed as the mean ± SEM. Values of P < 0·05 (*) were considered significant. The results shown are representative of three separate experiments.
To further characterize the cells recruited to BAL and lungs after i.n. delivery of BCG, we studied the expression of different markers on CD11c+ cells which represented more than 90% of the population in BAL and only 15–20% of the population from the lung tissue (Table 1). The myeloid marker CD11b was poorly expressed on CD11c+ cells isolated from BAL and lungs, whereas more than 90% of CD11c+ cells from BAL were F4/80+ and less than 5% from lungs expressed the β2 integrin. The expression of the MHC class II I-Aq molecule on CD11c+ cells recovered from BAL was very low (<2%) but increased in lung tissue, particularly 96 hr after BCG delivery (Table 1).
Table 1.
Characterization of CD11c+ cells recovered in BAL and digested lung tissue after BCG delivery
| Times (hours) | CD11c+(%)* | CD11c+ CD11b+ (%)† | CD11c+ F4/80† (%)b | CD11c+ MHC classII I-Aq+ (%)† | |
|---|---|---|---|---|---|
| 0 | 97·5 | 0·9 | 93·9 | 1·1 | |
| BAL | 6 | 90·3 | 1·6 | 91·3 | 1·4 |
| 24 | 95·0 | 0·7 | 93·6 | 1·2 | |
| 48 | 95·4 | 0·7 | 94·8 | 1·4 | |
| 96 | 96·6 | 1·7 | 94·0 | 1·3 | |
| 0 | 17·7 | 4·9 | 1·8 | 6·6 | |
| Lung tissue | 6 | 14·0 | 6·6 | 2·9 | 18·3 |
| 24 | 16·4 | 4·6 | 2·8 | 13·2 | |
| 48 | 19·9 | 4·3 | 1·5 | 14·4 | |
| 96 | 21·7 | 2·2 | 2·2 | 29·8 |
Data are expressed as percentage of total CD11c+ cells in BAL and digested lung tissue.
Data are expressed as percentage of CD11c+ cells expressing CD11b, F4/80 and I-Aq markers.
Data are representative of two independent experiments.
Costimulatory and MHC class II molecules displayed on the membrane of freshly isolated and cultured lung DC
The in vitro infection of DC with mycobacteria induces the up-regulation of costimulatory cell surface antigens.18 To investigate whether up-regulation of costimulatory and MHC class II molecules occurred in vivo, we analysed by flow cytometry the cell surface markers on DC freshly isolated or overnight cultured in RPMI–FCS medium. DC were purified from the lungs 6–96 hr after i.n. delivery of BCG. Freshly isolated lung DC expressed low levels of CD86, which was not up-regulated in culture, whereas the expression of CD80 was high on freshly isolated and cultured cells (Fig. 2). Most freshly isolated lung DC were CD11b− and CD40−; these two markers appeared after culture (Fig. 2). The DC marker DEC-205, which was moderately expressed on freshly isolated DC from naive mice or 6 hr after i.n. delivery of BCG, was up-regulated later, indicating a more mature phenotype of the DC. The level of expression of DEC-205 increased in cultured cells only when DC were isolated from mice that received BCG i.n. The expression of the lymphoid-related CD8α marker was very low and did not change in culture. Following overnight culture, CD11c+ cells expressed CD11b and CD40 antigens and remained negative for CD8α antigen, suggesting that they were myeloid-derived (Fig. 2). A large percentage of DC expressed moderate levels of I-Aq MHC class II and B7-2 molecules even after culture, suggesting that DCs present and/or recruited to the lung after i.n. delivery of BCG display an immature phenotype.
Figure 2.
Flow cytometric analysis of cell surface phenotype of purified lung DC freshly isolated or overnight cultured in RPMI medium. Lung DC were purified from 6 to 96 hr after i.n. delivery of BCG and stained for MHC class II I-Aq and costimulatory molecule (CD80, CD86, CD40) and DEC205, CD11b, CD8α expression. The thin open histograms represent isotype control mAb, grey histograms freshly isolated cells and bold open histograms overnight cultured cells. Data are representative of three separate experiments.
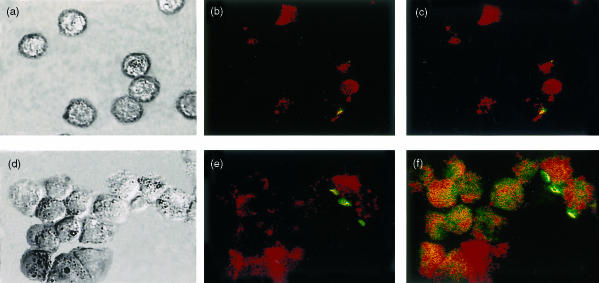
We further evaluated if purified lung DC recovered 48 hr after the i.n. delivery of BCG, would achieve in vitro differentiation if cultured in medium supplemented with GM-CSF. A laser scanning image cytometry was used to compare MHC Class II I-Aq and B7-2 antigen expression on freshly isolated DC and after culture in presence of GM-CSF. This technique allows visualization of the cells of a same field either unstained (Fig. 3a,d), stained with anti-MHC class II I-Aq–PE conjugated mAb (Fig. 3b,e) and double-stained with I-Aq–PE and B7-2–FITC conjugated mAb (Fig. 3c,f). Forty-eight hours after the i.n. delivery of BCG, freshly isolated DCs loaded or not with labelled BCG expressed different intensity in the expression of I-Aq (Fig. 3b,c), B7-2 not being expressed (Fig. 3c). After 24 hr culture in presence of GM-CSF, DC displayed a more mature phenotype, expressing higher levels of I-Aq (Fig. 3e,f) and doubly expressing I-Aq and B7-2 antigens. Indeed, the majority of cells after 24 hr of culture in presence of GM-CSF were double-labelled in red and green (Fig. 3f).
Figure 3.
Laser scanning image cytometry on lung-derived DC freshly isolated or overnight cultured with RPMI medium supplemented with GM-CSF. Lung DC were purified 48 hr after i.n. delivery of CFDA-label BCG, cells were cultured for 1 h (a, b, c) or 24 hr (d, e, f) with GM-CSF. The laser scanning image cytometry allows visualization of the cells of same field with cells either unstained (a, d), monolabelled in red (I-Aq conjugated to PE) (b, e) and then double labelled in red and green (I-Aq and B7-2 conjugated, respectively, to PE and FITC) (c, f). CD11c DCs freshly isolated expressed moderate levels of I-Aq molecule (b), the expression of B7-2 was not observed (c). After overnight culture with GM-CSF the majority of the cells were double-labelled in red and green expressing I-Aq and B7-2 molecules (f). Figures are representative of two separate experiments.
Identification and morphology of lung DCs after the i.n. delivery of BCG
Lung tissue sections were stained with anti-CD11c and MHC class II I-Aq mAb. The recruitment of positive cells was more intense in the peribronchiolar zone, even though DC were also present in the whole lung tissue (Fig. 4a,b). After BAL, DC were purified from the lung tissue, most cells presenting large nuclei, abundant cytoplasm and small cytoplasmic projections (Fig. 4c), with few cells displaying very long cytoplasmic processes (Fig. 4d). After in vivo phagocytosis, unlabelled BCG induced weak non-specific fluorescence (Fig. 4e), whereas CFDA-labelled BCG, visualized by fluorescence microscopy, were intensely fluorescent (Fig. 4f). The release of labelled bacterial constituents accounts for the abundant fluorescent labelling of a few DC.
Figure 4.
Identification and morphology of lung DC after the i.n. delivery of BCG.Frozen lung tissue sections were stained with anti-I-Aq(a) or anti-CD11c mAb(b).Positive cells in red were easily detected in the peribronchiolar area and at a lower extent in the lung tissue. Original magnification ×20. The tissue sections shown are representative of 10 individual sections. All the CD11c enriched leucocytes purified from the lung tissue after i.n. delivery of BCG presented large nuclei and abundant cytoplasm with small cytoplasmic projections, the typical morphology of lung DC(c). A few cells had very long cytoplasmic processes, as shown in this higher magnification(d). Lung DCs purified after i.n delivery of unlabelled BCG (e) or CFDA-labelled BCG (f) were analysed by fluorescence microscopy after cytospin and Diff-Quick staining. Labelled bacteria are strongly fluorescent as compared to non labelled bacteria.
Phagocytic activity of lung IM, AM and DC
The capacity of different APC to phagocytose mycobacteria is well documented in vitro12,13,18 and recently it was shown that both macrophages and DC recovered from the spleen phagocytose BCG upon i.v. injection of very high doses (108 CFU).19 However, the phagocytic capacity of the potential APC (IM, AM, DC) recruited to the lungs after i.n. delivery of BCG remained to be explored. Therefore, AM were harvested from the BAL 1, 6, 24 and 48 hr after the i.n. delivery of labelled BCG. Following exsanguination and lung perfusion with saline, IM and DC were isolated from the digested lungs. Cytospins were stained with Diff-Quick and vizualized under a fluorescence microscope, positive cells (more than two fluorescent bacilli) being counted. Cytospins obtained 1 hr after the i.n. delivery of labelled BCG showed numerous AM containing fluorescent bacilli. No fluorescent bacteria were detected in DC isolated from lung tissue at this early time point. At 6 hr, the percentage of AM showing fluorescent bacilli remained high and few DC (<20%) were loaded with fluorescent bacilli. At 24 and 48 hr, the percentage of AM and DC showing fluorescent particles was comparable (40–50%). No fluorescent bacilli were detected at any time in IM from mice immunized i.n. with labelled BCG (Fig. 5a). These results show that after its i.n. delivery, BCG is phagocytosed by AM and by DC in the lung tissue, and that IM are unable to phagocytose bacilli.
Figure 5.
Phagocytic capacity and IL-12 p40 production of AM, IM and DC isolated from BAL and lungs of mice shortly after i.n. delivery of BCG.(a) BCG bacilli were labelled with CFDA, and 1, 6, 24 and 48 hr after their i.n. delivery, AM were obtained by bronchoalveolar lavage and IM and DC were isolated from digested lung tissue, followed by incubation with MACS CD11b or CD11c microbeads and passage through a column of an AutoMACS separator. Cytospins of purified AM, IM and DC were stained with Diff-Quick and the bacilli taken up by phagocytosis were visualized by fluorescence microscopy. Two hundred to 400 cells were counted and the number of cells that were phagocytic (more than two bacilli) is expressed as a percentage of the whole population studied. The experiment is representative of three separate experiments. (b) AM, IM and DC purified from naive mice and 6, 24, 48 and 96 hr after BCG delivery, were cultured for 24 hr and supernatants were assayed by ELISA for of IL-12 p40 production. IM were not able to produce IL-12 p40 and DC produced significantly higher amounts of IL-12 than AM (P < 0·05). The experiment shown is representative of three separate experiments. Results are expressed as mean ± SEM.
IL-12 production by IM, AM and DC shortly after the i.n. BCG delivery
IL-12 is a potent inducer of T-lymphocyte differentiation and initiates the development of the Th1 phenotype of naive T cells. We therefore analysed the capacity of isolated APC from naive and immunized mice to produce IL-12. AM and DC isolated from naive mice produced low amounts of IL-12 and 6 hr after BCG delivery there is no increase of IL-12 production by these APC harvested, respectively, from the BAL and the lung tissue. At 24, 48 and 96 hr after the in vivo infection and in absence of additional ex vivo antigen stimulation, DC produced large amounts of IL-12 p40 (Fig. 5b). It has to be noted that the production of IL-12 by AM increased slightly 24 and 48 hr after delivery of BCG; this could not be caused by DC contamination because in our experiment the majority of AM producing IL-12 are F4/80+ and this marker is poorly expressed on lung DC. IM, which failed to phagocytose BCG in vivo, also failed to produce IL-12 under our experimental conditions (Fig. 5a,b). No immunoreactive IL-12 p70 was detected in our experimental conditions.
IFN-γ production by T cells stimulated with IM, AM and DC
We next investigated the stimulatory capacity of lung APC isolated shortly after the i.n. delivery of BCG. IM, AM and DC were isolated as described above from 24 to 96 h after i.n. BCG delivery. These APC were overlaid with syngeneic naive or immune CD4+ T cells isolated from the inguinal lymph nodes and purified with a MACS separator. After 72 hr of coculture, supernatants were assayed for IFN-γ content. Co-cultures of AM with naive CD4+ T cells produced low levels of IFN-γ. DC purified from lung tissue strongly initiated IFN-γ secretion by naive CD4+ T lymphocytes, particularly when they were isolated 48 hr after i.n. delivery of BCG (Fig. 6a). When AM were used to stimulate immune CD4+ T cells, IFN-γ production was increased, but not to the same extent as when DC were used as APC (P < 0·05; Fig. 6b). The levels of IFN-γ produced by CD4+ T cells closely correlated with IL-12 produced by APC. IM, which failed to phagocytose BCG and to produce IL-12, did not induce IFN-γ production by CD4+ T cells (Fig. 6a,b). The lymph node cells from immunized mice did not produce IFN-γ in the absence of APC stimulation, similarly APC from mice that had not been immunized with BCG failed to stimulate IFN-γ production by CD4+ T cells (data not shown). Finally, the addition of IL-12 p40 neutralizing mAb to APC/CD4+ T lymphocyte cocultures prevented IFN-γ production (Fig. 6c).
Figure 6.
IFN-γ production by CD4+ T cells stimulated with APC (IM, AM or DC) isolated from the lungs of mice, shortly after i.n. delivery of BCG. Purified CD4+ T cells from lymph nodes of naive or BCG-immunized mice were cocultured for 3 days with AM, IM or DC isolated 24, 48 and 96 hr after i.n. delivery of BCG from BAL and lungs, respectively. A 1 : 5 ratio of APC/CD4+ T lymphocytes was used. Supernatants were assayed by ELISA for IFN-γ content. When APC were cocultured with CD4+ T cells from naive mice, IMs induced no IFN-γ production, AM very low levels, whereas DC allowed the production of high amounts of IFN-γ by these CD4+ T cells (a). When APC were cocultured with immune CD4+ T cells isolated from BCG-immunized mice, the IFN-γ production was found only if CD4+ T cells were stimulated with AM or DC. AM were less efficient than DC for initiating the production of IFN-γ by CD4+ T cells (P < 0·05), whereas IM failed to stimulate the production of IFN-γ(b). The addition of anti-IL-12 p40 mAb completely abrogated the production of IFN-γ(c). The experiment shown is representative of two separate experiments. Results are expressed as mean ± SEM.
As expected, the stimulation of APC/CD4+ T cell cocultures with soluble anti-CD3 mAb enhanced IFN-γ production whatever the source of APC (AMs or DC) but this production was higher when DC, rather than AM, were used as APC (data not shown). The production of IL-5 at the limit of detection (<20 pg/ml) when APC/CD4+ T cells were cocultured without restimulation was significantly enhanced in the presence of anti-CD3 (data not shown).
Production of IFN-γ and IL-5 by lung explants after stimulation in vitro with anti-CD3 mAb
Lung explants were prepared from 14 to 116 days after i.n. delivery of BCG and the ex vivo production of IFN-γ and IL-5 was compared to that of lung explants prepared from control mice. Initially, a time course for the production of cytokines by those explants stimulated ex vivo by anti-CD3 mAb was studied and the secretion of IL-5 and IFN-γ peaked, respectively, after 6 and 24 hr culture. These intervals were used for monitoring IL-5 and IFN-γ production by the lung explants. As shown in Fig. 7(a), the lung explants collected from 28 to 90 days after BCG delivery clearly secreted higher amounts of IFN-γ than lung explants from control mice (P < 0·05). The production of IFN-γ peaked at 28 days (>7000 pg/lung) but remained at a substantial levels until 90 days after i.n. delivery of BCG (≥3000 pg/lung; Fig. 7a). As expected, BP2 mice with a Th2 background produced high levels of IL-5 (Fig. 7b); the large amounts of IFN-γ produced by explants from BCG immunized mice did not inhibit the production of IL-5. This ex vivo technique for the study of cytokine secretion allowed demonstration of the fact that, for the first time, IFN-γ can be produced in situ after i.n. delivery of BCG, whereas in earlier studies IFN-γ was not found in BAL and only evidenced after in vitro restimulation of cells isolated from spleen or lymph nodes.6,9
Figure 7.
Long-term production of IFN-γ and IL-5 by lung explants exposed to anti-CD3 mAb. Lung explants were prepared from 14 to 116 days after i.n. delivery of BCG. After stimulation with soluble anti-CD3 mAb, the secretion of IL-5 and IFN-γ peaked, respectively, at 6 and 24 hr culture period. These intervals were used for monitoring IL-5 and IFN-γ production by the explants. From 28 to 90 days the production of IFN-γ was significantly higher in BCG-immunized than in control mice (P < 0·05)(a). There were no significant differences in the production of IL-5 among the two groups of mice(b). Results are expressed as mean ± SEM.
Discussion
This study was undertaken to identify, shortly after the delivery of BCG to the airways, which APC are recruited and their role in Th1/Th2 regulation of immune responses, a key element of these experiments involving the study of the in vivo T-cell priming properties of DC. FACS analysis showed that monocyte/macrophages recruited to the BAL (AM) and those recruited to the lungs (IM) expressed different markers and played different roles. The majority of cells recovered from the BAL expresses the cell surface marker CD11c, which has been described as a specific marker for mouse DC.20 These cells also expressed the macrophage marker F4/80, which has been shown in some subsets of lung or spleen DC.13,21 However, cells present in the BAL very shortly after i.n. delivery of BCG had the capacity to phagocytose bacilli, but were unable to stimulate naive T cells, identifying them as AM rather than DC. Recently, after administrating fluorescent beads into the mouse airway, Byersdorfer et al.22 identified cells displaying CD11c+ and F4/80+ in the BAL fluid, and speculated that they represent a subset similar to the Langerhans cells. However, their capacity to stimulate naive CD4+ T cells was not studied; our present results demonstrate that only CD11c+ isolated from lung tissue have the capacity to stimulate these T cells. AM expressing CD11c and F4/80 markers and negative for the CD11b marker represent a unique subset in the airways, by contrast to IM recovered from the lung tissue, which never expressed the CD11c marker and were CD11b+ F4/80+. Moreover, AM are well positioned in the alveolar area to take up bacilli rapidly; IM located in the lung tissue failed to do so, and in a previous study9 we have shown that BCG could be recovered in the AM 26 weeks after i.n. administration.
The third subset of potential APC recovered from the lung shortly after i.n. delivery of BCG expressing CD11c marker and negative for the F4/80 marker might represent DC. The influx of DC into the lungs after i.n. administration of BCG is consistent with the concept that the airway DC network extension is dependent upon inflammatory stimuli.15In vitro studies on the growth of DC from precursors suggest that DC cultured in GM-CSF + IL-4 are specialized for antigen uptake but are ineffective as activators of T cells.23 However, it is not known precisely how applicable this in vitro model is to DC populations in vivo, and particularly to those located in subepithelial and interstitial compartments, such as lung DC. This study therefore sought to characterize in detail the phenotype of lung DC isolated from the perfused lung tissue and to determine to what degree their function can be modulated by BCG, a potential promoter of DC maturation and stimulator of Th1 immune responses. To do so, we used histological, flow and scanning image cytometric approaches and thus characterized more precisely the DC recruited to the lungs of mice. Forty-eight hr after BCG delivery, cells expressing CD11c and MHC class II markers were detected essentially in the peribronchiolar area of lung sections; the FACS analysis revealed DC expressing similar markers. Purified lung DC freshly isolated expressed moderate levels of membrane I-Aq MHC class II and CD86 (B7-2) molecules, which are considered to be markers of a mature phenotype, when abundantly expressed.24,25 In accordance with our results, in a recent study26 lung DC collected after the intratracheal instillation of macromolecules showed the same immature phenotype with low-level expression of B7-2 molecule. The elevated expression of I-A and B7-2 molecules is the hallmark of a mature phenotype of DC,24,25 which were not up-regulated after 24 hr culture in RPMI medium alone. However, when purified CD11c cells were cultured in presence of GM-CSF, the majority of the cells displayed a more mature phenotype, indicating that DC freshly isolated from lung tissue can achieve their maturation in vitro at least in the presence of GM-CSF. Epidermis-derived Langerhans cells, which presented similarities to lung DC,27 initiate their maturation process when cultured with GM-CSF.28In vivo, at the steady state, lung DC remain relatively immature but, upon contact with immunogenic molecules, they evolve towards a mature state. DC maturation induced their migration out of the immunogen-exposed site into the interstitial afferent lymphatics, towards the T-cell area of regional lymph nodes.29 After 24 hr of culture, lung DC up-regulated the costimulatory molecule CD40 and the myeloid CD11b marker, suggesting that they are myeloid-derived DC.
In the spleen, three populations of DC have been described: CD4− CD8α+ DEC205+ CD11b−; CD4+ CD8α− DEC205− CD11b+ and CD4− CD8α− DEC205− CD11b+.30 Our lineage phenotypic analysis showed a population of CD8α− DEC205+ CD11b− when the expression of these markers was analysed on freshly isolated DC. After culture, this population expressed DEC205+ and CD11b+and remained negative for the CD8α expression. Lung DC thus do not correspond to a strict lymphoid or myeloid lineage as it was described in the spleen.30 Moreover, altogether the procedures used for cell isolation, for evaluating the phagocytic activity and the developmental state of the DC must all be considered in analysing lung DC subtypes. In a recent study22 the phenotype of lung DC was evaluated only on cells taking fluorescent beads, while in another report, lung DC phenotyping was performed on a FITC-bead-negative population.31 In our study, we analysed the phenotype of DC (CD11c+) isolated from lung tissue after i.n. delivery of BCG, without discrimination between cells loaded or not with BCG. However, this lung-recovered population of DC displaying an immature phenotype is highly specialized for uptake of mycobacteria as shown in vivo in our study and in vitro by others.12,32
It has been shown that M. tuberculosis-infected DC among DC generated from mouse bone marrow progenitors are more potent APC than macrophages,18 and similar results were recently obtained in vivo with spleen DC following i.v. administration of M. bovis BCG.19 The functional activity of AM and lung DC after i.n. delivery of BCG, particularly in terms of their ability to activate type 1- or type 2-mediated T-cell responses, still remains to be explored. IL-12 plays a key role in the control of mycobacterial infection33 stimulating IFN-γ production by natural killer and T cells, leading to the development of a type 1 immune response.34 In this study, in vivo BCG-loaded lung DC produced more IL-12 p40 than did AM, and the failure to detect IL-12 p70 in our model may be due to the production of much less immunoreactive IL-12 p70 than of IL-12 p40. However, it has been shown that M. tuberculosis-infected DC present among DC generated from mouse bone marrow progenitors produced IL-12 p70.18 These differences may result from the complexities of the in vivo interactions of lung DC with BCG bacilli, as compared to DC generated in vitro and infected with M. tuberculosis. Mycobacteria-free interstitial macrophages did not secrete IL-12 p40, suggesting that the phagocytic uptake of BCG bacilli is an essential step for triggering IL-12 secretion.
The activation of naive T cells requires two signals provided by APC. The first signal is delivered through the T-cell receptor upon engagement of MHC molecules loaded with appropriate peptides. The second signal involves cross-linking of CD28 and other receptors on the T cell by costimulatory molecules such as B7-1, B7-2, CD40 expressed and up-regulated by the APC. Upon antigen stimulation, CD4+ T cells can be subdivided into Th1 or Th2 cells. Recent study suggest that the decision to differentiate into Th1 or Th2 cells occurs shortly after stimulation of naive CD4+ T cells by antigen-pulsed DC.35 The DC recruited to the lungs early post-BCG delivery promote the activation of effectors dominated by type 1 immune cytokines, as shown by short-term production of IFN-γ by naive and immune CD4+ T cells and ex vivo long-term production by lung explants. Thus, early events occurring after i.n. delivery of BCG may play a key role in regulating the balance between Th1- and Th2- mediated immune responses. The activation of Th1 rather than of Th2 responses by AM and DC after BCG delivery shown in this study, probably accounts for the protective effect observed on airway allergen challenge in other studies.6,9 Thus, despite the Th2-dominant background of BP2 mice, APC may contribute to the development of Th1 response shortly after i.n. delivery of BCG: probably because intracellular BCG acts as a strong stimulus on AM and DC, inducing the high IFN-γ production by activated CD4+ T cells. No IL-5 associated with the Th2-type response was detected when CD4+ T cells isolated from secondary lymphoid organs were co-cultured with lung DC; however, after anti-CD3 stimulation, the production of IL-5 is clearly enhanced (data not shown). Likewise, upon anti-CD3 stimulation, T-cells of the lung explants produced high amounts of IL-5; however, differences among naive mice and those receiving BCG i.n. were not observed. Nevertheless, by influencing the initiation of a Th1 immune response, through IL-12 production, AM, and particularly DC, recruited to the lungs of mice at the early stage after i.n. BCG delivery, induced long-term IFN-γ production by CD3 cells present in lung explants. AM and DC once loaded with BCG are indeed expected to shape an immune response which could protect against those complex stimuli which result in allergen-driven asthma.
Acknowledgments
We thank Stephanie Riveron for technical assistance, and Mai Lebastard for provinding us with the GM-CSF medium.
This work was supported by Procter & Gamble.
References
- 1.Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar lavage T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 2.Kline JN, Hunninghake GW. T-lymphocyte dysregulation in asthma. Proc Soc Exp Biol Med. 1994;207:243–53. doi: 10.3181/00379727-207-43813a. [DOI] [PubMed] [Google Scholar]
- 3.Krug N, Frew AJ. The Th2 cell in asthma: initial expectations yet to be realized. Clin Exp Allergy. 1997;27:142–50. [PubMed] [Google Scholar]
- 4.Gajewski T, Fitch F. Anti-proliferative effect of IFN-gamma in immune regulation. Part I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–52. [PubMed] [Google Scholar]
- 5.Lack G, Bradey KL, Hamelmann E, Renz H, Loader J, Leung OJ, Larsen G, Gelfand GW. Nebulized IFN-γ inhibits the development of secondary allergic responses in mice. J Immunol. 1996;157:1432–9. [PubMed] [Google Scholar]
- 6.Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-bacillus Calmette–Guérin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–9. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herz U, Gerhold K, Gruber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998;102:867–74. doi: 10.1016/s0091-6749(98)70030-2. [DOI] [PubMed] [Google Scholar]
- 8.Yeung VP, Gieni RS, Umetsu DT, DeKruyff RH. Heat killed Listeria monocytogenes as an adjuvant converts established Th2-dominated immune responses into Th1-dominated responses. J Immunol. 1998;161:4146–52. [PubMed] [Google Scholar]
- 9.Nahori M-A, Lagranderie M, Lefort J, et al. Effects of Mycobacterium bovis BCG on the development of allergic inflammation and bronchial hyperresponsiveness in hyper-IgE BP2 mice vaccinated as newborns. Vaccine. 2001;19:1484–95. doi: 10.1016/s0264-410x(00)00345-5. [DOI] [PubMed] [Google Scholar]
- 10.Nathan CF, Hibbs JB., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 11.Reis e Souza C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–29. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inaba K, Inaba M, Naito M, Steinman RM. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette–Guérin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–88. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzales-Juarrero M, Orme IM. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infect Immun. 2001;69:1127–33. doi: 10.1128/IAI.69.2.1127-1133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demangel C, Bean AGD, Martin E, Feng CG, Kamath AT, Britton WJ. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis bacillus Calmette–Guérin-infected dendritic cells. Eur J Immunol. 1999;29:1972–9. doi: 10.1002/(SICI)1521-4141(199906)29:06<1972::AID-IMMU1972>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.McWilliam AS, Napoli S, Marsh AM, et al. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–32. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and seady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153:256–61. [PubMed] [Google Scholar]
- 17.Lambrecht BN, Prins J-B, Hoogsteden HC. Lung dendritic cells and host immunity to infection. Eur Respir J. 2001;18:692–704. [PubMed] [Google Scholar]
- 18.Bodnar KA, Serbina NV, Flynn JA. Fate of Mycobacterium tuberculosis within murine dendritic cells. Inf Imm. 2001;69:800–9. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao X, Lo-Man R, Guermonprez P, et al. Dentritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J Immunol. 2002;168:1294–301. doi: 10.4049/jimmunol.168.3.1294. [DOI] [PubMed] [Google Scholar]
- 20.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–71. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leenen PJ, Radosevic K, Voerman JS, Salomon B, van Rooijen N, Klatzmann D, van Ewijk W. Heterogeneity of spleen mouse dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover. J Immunol. 1998;160:2166–73. [PubMed] [Google Scholar]
- 22.Byersdorfer CA, Chaplin DD. Visualization of early APC/T cell interactions in the mouse lung following intranasal challenge. J Immunol. 2001;167:6756–64. doi: 10.4049/jimmunol.167.12.6756. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and down regulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierre P, Shannon TJ, Gatti E, Hull M, Meltzer J, Mizra A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–92. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, Witmer-Pack M, Inaba M, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–60. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dentritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollard AM, Lipscomb MF. Characterization of murine lung dendritic cells: similarities to Langerhans cells and thymic dendritic cells. J Exp Med. 1990;172:159–67. doi: 10.1084/jem.172.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witmer-Pack MD, Valinsky J, Olivier W, Steinman RM. Quantitation of surface antigens on cultured murine epidermal Langerhans cells: rapid and selective increase in the level of surface MHC products. J Invest Dermatol. 1988;90:387–94. doi: 10.1111/1523-1747.ep12456460. [DOI] [PubMed] [Google Scholar]
- 29.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 30.Shortman K. Dendritic cells: multiple subtypes, multiple origins, multiple functions. Immunol Cell Biol. 2000;78:161–5. doi: 10.1046/j.1440-1711.2000.00901.x. [DOI] [PubMed] [Google Scholar]
- 31.Masten BJ, Yates JL, Pollard Koga AM, Lipscomb MF. Characterization of accessory molecules in murine lung dendritic cell function. Roles for Cd80, Cd86, Cd54, and CD40L. Am J Respir Cell Mol Biol. 1997;16:335–42. doi: 10.1165/ajrcmb.16.3.9070619. [DOI] [PubMed] [Google Scholar]
- 32.Henderson RA, Watkins SC, Flynn JA. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–43. [PubMed] [Google Scholar]
- 33.DeJong R, Altare F, Haagen IA, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–8. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 34.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 35.Tollener KM, Luther SA, Sze DM, Choy RK, Taylor DR, MacLennan ICM, Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]