Abstract
The presence in the bone marrow of memory CD8 T cells is well recognized. However, it is still largely unclear how T-cell migration from the lymphoid periphery to the bone marrow is regulated. In the present report, we show that antigen-specific CD4 T cells, as well as antigen-specific CD8 T cells, localize to the bone marrow of immunized mice, and are sustained there over long periods of time. To investigate the rules governing T-cell migration to the bone marrow, we generated chimeric mice in which the lymphoid periphery contained two genetically or phenotypically distinct groups of T cells, one of which was identical to the host. We then examined whether a distinct type of T cell had an advantage over the others in the colonization of bone marrow. Our results show that whereas ICAM1 and CD18 molecules are both involved in homing to lymph nodes, neither is crucial for T-cell bone marrow colonization. We also observed that memory-phenotype CD44high T cells, but not virgin-type CD44−/low T cells, preferentially home to the bone marrow upon adoptive transfer to normal young mice, but not to thymectomized old recipients where an existing memory T-cell pool precludes their free access. Thus, T-cell colonization of the bone marrow uses distinct molecules from those implicated in lymph node homing, and is regulated both by the properties of the T cell and by the competitive efficacy of other T cells inhabiting the same, saturable niche. This implies that the homing potential of an individual lymphocyte is not merely an intrinsic property of the cell, but rather a property of the lymphoid system taken as a whole.
Introduction
For the induction and the accomplishment of effective immune responses, it is essential that lymphocytes traffic through lymphoid and extra-lymphoid organs in a regulated way.1,2 The migration of T cells from blood stream into tissues occurs via rolling or primary adhesion, activation, secondary adhesion, and diapedesis. Furthermore, the maintenance of both structure and function of the immune system relies on regulated T-cell homing to specific microenvironments within lymphoid tissues, representing specialized sites for cellular interactions.1,2
Integrins and chemokines are key molecular players in T-cell trafficking. Integrins are transmembrane heterodimeric proteins acting as receptors for cell adhesion molecules and extracellular matrix ligands. T lymphocytes constitutively express CD11a/CD18 (integrin αLβ2, also called LFA1) which recognizes adhesion molecules of the immunoglobulin superfamily, notably CD102 (also called ICAM2), that is constitutively expressed by endothelial cells, and CD54 (also called ICAM1), that is expressed by antigen-presenting cells (APC) and up-regulated on several cell types, including endothelial cells, during inflammation.1,2 Following activation, T cells display increased levels of CD11a/CD18 and may also express other integrins including CD11b/CD18 (integrin αMβ2, also called Mac-1)3 and CD49d/CD29 (integrin α4β1, also called VLA4);4 hence, these molecules have been used as markers to discriminate between virgin and effector/memory T cells.5
Chemokines compose a family of small proteins, released by several cell types including endothelial cells, that act as chemoattractants. As with the integrins, cell activation provokes T cells to express an altered repertoire of chemokine receptors. The functional consequences of these various changes are that memory T cells, compared with virgin T cells, have an increased capacity to bind to APC and to endothelial cells in inflamed sites, but a lower tendency to migrate to lymph nodes.6,7
Experimental studies in sheep demonstrated that naive T cells enter the lymph nodes mostly from blood and exit through the efferent lymph.8 In contrast, memory T cells are abundant in the afferent lymph, suggesting that they extravasate from blood into tissues and then home to lymph nodes, thereby patrolling the extra-lymphoid organs.8 Furthermore, memory T cells are heterogeneous and phenotypically distinct cell subsets show the tendency to home to different extra-lymphoid tissues.9–11 Recently, the differential expression of the chemokine receptor CCR7 was used to identify two groups of memory T cells in human blood, which, it was proposed, might represent two distinct subsets, specialized either in effector function in extra-lymphoid organs or in memory maintenance in lymphoid organs.12 However, this hypothesis has been challenged by two thorough studies on human CD4 and CD8 T cells, demonstrating that a single chemokine receptor is insufficient for T-cell subset definition and suggesting that effector and memory T cells cannot reliably be classified on the basis of the currently available markers.13,14
In an attempt to improve our understanding of memory T cells, we have focused on their homing and anatomical localization. Although many studies of memory T cells have focused on those derived from the spleen and/or extralymphoid organs such as the gut, we and others have observed that memory T cells are found in the bone marrow.15–21 In particular, we showed that antigen-specific CD8 T cells can still be detected in the bone marrow for several months after immunization.21 Thus the capacity of CD8 T cells to migrate to the bone marrow and assume long-term residence in this site may be an important aspect of immunological memory. Here we have investigated whether migration to the bone marrow is restricted to memory CD8 T cells or whether it is also a property of antigen-specific CD4 T cells. Additionally, we have undertaken adoptive transfer experiments to demonstrate that entry into the bone marrow is competitive, and exploiting this fact, illustrate properties of T cells that are either relevant or irrelevant to long-term bone marrow colonization.
Materials and methods
Mice
C57BL/6J (B6) mice were purchased from Charles River (Calco, Italy) and Harlan Nossan (Corezzana, Italy). B6 mice thymectomized at 4 weeks were purchased from Charles River. C57BL/6J–Itgb2tm1 Bay (ITGB2ko)22 and C57BL/6J–Icam1tm1 Bay (ICAM1ko)23 breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred and maintained at the Regina Elena Cancer Institute animal facility, according to the institutional guidelines. Sentinel mice were screened for seropositivity to Sendai virus, rodent coronavirus and Mycoplasma pulmonis by Murine Immunocomb test (Charles River) and were found negative.
Peptides and immunization procedures
Peptides derived from ovalbumin (OVA 257–264) and hepatitis B virus core antigen (HBVc 128–140) were purchased from Primm (Milan, Italy). Purity was >95%, as analysed by high-performance liquid chromatography and mass spectrometry. B6 female mice were immunized by two subcutaneous injections of 50 μg OVA 257–264 and 140 μg HBVc 128–140 peptides emulsified in either complete (CFA) or incomplete Freund's adjuvant (IFA).21,24
ELISPOT
Spleen, inguinal and axillary lymph nodes, tibiae and femurs from control and primed mice were collected from 2 weeks to 2 months after immunization. Single cell suspensions were prepared from spleen and lymph nodes by mechanical disruption and passage through cell strainers and from tibiae and femurs by syringe insertion into one end of the bone and flushing with phosphate-buffered saline (PBS). For the CD8 T-cell response, we incubated cells harvested from either control or primed mice (responder cells) in anti-interferon-γ monoclonal antibody (IFN-γ mAb) precoated multiscreen plates with interleukin-2 (IL-2) at 20 U/ml and 500 000 irradiated syngeneic spleen cells, prepulsed with either OVA 257–264 peptide at 10 μg/ml or medium alone. For the CD4 T-cell response, we incubated the responder cells in anti-IFN-γ mAb-precoated multiscreen plates with 500 000 irradiated syngeneic spleen cells and either HBVc 128–140 peptide at 50 μg/ml or medium alone. Responder spleen and lymph node cells were 250 000/well, bone marrow cells were 500 000/well. In some experiments, the anti-I-Ab mAb AF6-120.1.2 was used as ascites at a 1:20 final dilution in the culture medium. After 40 hr of incubation at 37°, cells were washed and the plates were sequentially incubated with anti-IFN-γ biotinylated mAb, poly-horseradish peroxidase–streptavidin (Endogen, Woburn, MA) and 3-amino-9 ethylcarbaozle(AEC) substrate (Sigma, Milan, Italy).21,25 IFN-γ transfected mammary adenocarcinoma cell line (TSA) cells and their parental untransfected line26 were used as controls in each ELISPOT plate, after treatment with mitomycin-C (Sigma). The spots were counted using a Zeiss Axioplan microscope and the KS elispot software. We checked that each responder mouse had a normal spleen cell response to concanavalin A and a normal T-cell receptor (TCR), CD4 and CD8 staining profile of spleen, lymph node and bone marrow cells.21
Cell labelling and transfer
Cells were purified from spleen, inguinal and axillary lymph nodes of donor mice and incubated at 107/ml for 8 min at room temperature with 2·5 μm 5-(and 6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE, Molecular Probes, Eugene, OR).27 After extensive washing in 10% fetal bovine serum/PBS and PBS, cells were counted and injected either intraperitoneally or intravenously into recipient mice (25 × 106−57 × 106 cells per mouse). In the experiments with thymectomized B6 mice, a sample of cells was taken before incubation with CFSE and cell phenotype was analysed by flow cytometry, after blocking with saturating doses of anti-FcγR mAb 2.4G2 and staining with anti-TCR β phycoerythrin (PE; H57-597), anti-CD44 fluorescein isothiocyanate (IM7), anti-CD49d biotin (SG31) mAbs (Pharmingen, San Diego, CA), followed by incubation with streptavidin-RED670 (Life Technologies, Grand Island, NY).
Transferred cell analysis
One to three weeks after adoptive transfer, cells were purified from spleen, inguinal and axillary lymph nodes, and bone marrow of recipient mice, and stained with anti-TCR β PE mAb. Non-specific staining was blocked with anti-FcγR mAb 2.4G2. Cells were analysed by flow cytometry, gating on TCR+ cells and acquiring about 20 000 events per sample, except in experiments with thymectomized B6 mice when 60 000 events were acquired. In the experiments with ITGB2ko and ICAM1ko mice, spleen cell samples were also stained with anti-CD18 PE (C71/16) and anti-ICAM1 PE (3E2) mAbs (Pharmingen) and analysed by flow cytometry, to check each mouse for both donor and recipient cell expression of CD18 and ICAM1 molecules.
Statistics
Statistical analysis was performed by Student's t-test.
Results
Antigen-experienced CD4 and CD8 T-cell homing to the bone marrow
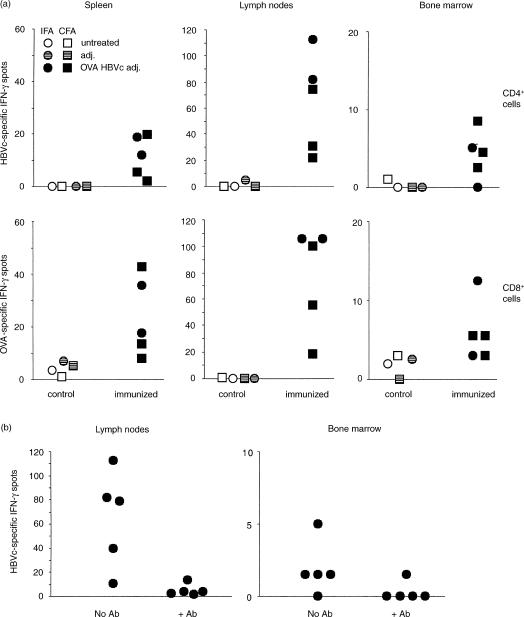
To investigate whether CD4 T cells migrate to the bone marrow after antigen immunization as CD8 T cells do,21 we injected B6 mice subcutaneously with a mixture of H2-Kb-restricted OVA 257–264 and I-Ab-restricted HBVc 128–140 peptides, emulsified in either IFA or CFA, to prime CD8 T cells and CD4 T cells, respectively.24 We then purified cells from the lymph nodes draining the immunization site, the spleen and the bone marrow, and examined their response in vitro to HBVc 128–140 by IFN-γ ELISPOT, following a 40-hr culture. Two weeks after immunization HBVc 128–140 peptide-specific T cells could be found in the bone marrow, as well as in the spleen and lymph nodes (Fig. 1a). The HBVc 128–140-specific IFN-γ response could be inhibited by adding the anti-I-Ab mAb AF6-120.1.2 to the culture medium during the 40-hr incubation (Fig. 1b). In parallel cultures, we examined each mouse for its CD8 T-cell response to OVA 257–264, and confirmed that there was a specific response among cells derived from all three lymphoid compartments (Fig. 1a and ref. 21). Hence, following this co-immunization regimen, bone marrow localization is a property of CD4 T cells as well as CD8 T cells.
Figure 1.
IFN-γ ELISPOT analysis of antigen-specific CD4 and CD8 T cells from spleen, lymph nodes and bone marrow of OVA 257–264 and HBVc 128–140 peptide immunized and control B6 mice. (a)B6 female mice were immunized by two subcutaneous injections of OVA 257–264 and HBVc 128–140 peptides emulsified in either IFA or CFA, as indicated (Adj. = adjuvant). Two weeks after immunization, cells from spleen, inguinal and axillary lymph nodes and bone marrow of control and primed mice were purified and their response to each of the two immunizing peptides was analysed by IFN-γ ELISPOT. For the CD4 T-cell response, responder cells were stimulated with irradiated APC in the presence of either HBVc 128–140 peptide at 50 μg/ml or medium alone (P = 0·05 for bone marrow spots of immunized versus control mice). For the CD8 T-cell response, the responder cells were stimulated with either OVA 257–264 peptide pulsed or unpulsed irradiated APC in the presence of IL-2 at 20 U/ml. Responder spleen and lymph node cells were 250 000/well and bone marrow cells were 500 000/well. The HBVc 128–140 and OVA 257–264 peptide specific-IFN-γ spots of individual mice are represented, after subtraction of medium background. (b) B6 female mice were immunized by two subcutaneous injections of OVA 257–264 and HBVc 128–140 peptides emulsified in IFA and analyzed within 2 months after priming as in (a). Cells were incubated either in the presence or in the absence of the anti-I-Ab mAb AF6-120.1.2 in the culture medium during the 40-hr incubation of the ELISPOT test. The anti-I-Ab mAb AF6-120.1.2 was used as ascites at a 1:20 final dilution in the culture medium; at this dose, the IFN-γ ELISPOT response to the MHC class I-restricted OVA 257–264 peptide by lymph node cells from immunized mice was not blocked. The presence or absence of the anti-I-Ab mAb AF6-120.1.2 in the culture medium is indicated on the x-axis. The HBVc 128–140 peptide specific-IFN-γ spots of individual mice are represented, after subtraction of medium background (P < 0·05 for lymph node spots in the presence vs. the absence of the anti-I-Ab mAb).
Although the number of IFN-γ spots in the bone marrow cell cultures stimulated with antigen was much lower than in the corresponding spleen and lymph node cultures, taking into account the different percentages of T cells, the calculated frequency of antigen-specific cells in either the CD8 T-cell subset or the CD4 T-cell subset was in a similar range in the three organs. On average, the antigen-specific frequency among the CD4 T cells, as calculated for the immunized mice of Fig. 1, was 1/1251 in the bone marrow and 1/1124 in the lymph nodes. Although one of the immunized mice had no detectable CD4 T-cell response in the bone marrow (Fig. 1a), we observed in other experiments that rare mice would likewise lack OVA 257–264-specific CD8 T-cell responses in the bone marrow (ref. 21 and data not shown). Hence, there is no experimental evidence to suggest that there is any substantial difference in the capacity of CD4 T cells and CD8 T cells to localize to the bone marrow. Similar to CD8 T cells,21 antigen-specific CD4 T cells could still be found in the bone marrow about 2 months after immunization (Fig. 1b and data not shown).
T-cell colonization of bone marrow versus spleen and lymph nodes
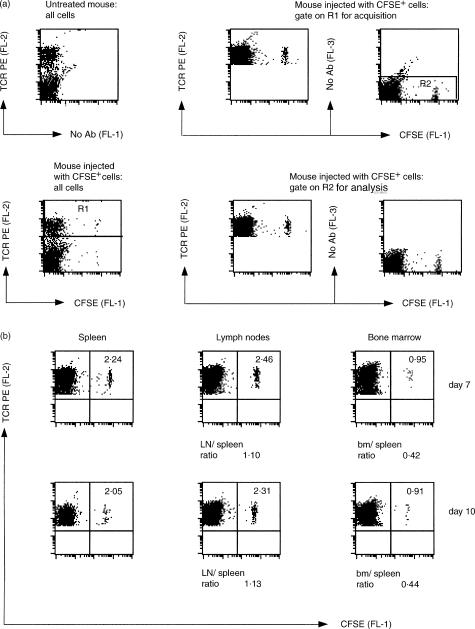
Short-term migration experiments have already shown that donor T cells can be found in the bone marrow a few hours after intravenous injection into recipient mice. Such rapid homing is mediated mostly by the interaction between the T cell VLA4 integrin and the bone marrow CD106 (also called VCAM1) adhesion molecule, with a minor role played by the CD11a/CD18 (LFA1) and its ICAM ligands.28,29 However, the potential contribution of CD11a/CD18, CD11b/CD18, or ICAM1 to interstitial migration and prolonged localization of T cells in the bone marrow has not been assessed. To this end, we performed a series of long-term migration experiments. First, we injected CFSE-labelled spleen and lymph node cells from B6 mice into age- and sex-matched recipients and analysed the distribution of donor T cells into spleen, lymph nodes and bone marrow from 1 to 3 weeks later. By performing adoptive transfer into non-irradiated recipient mice, we tested the ability of donor T cells to displace the recipient T cells in each of the three organs. Figure 2(a) shows the typical gates for cell acquisition and flow cytometry analysis used in our experiments. One week after adoptive transfer, the percentage of donor T cells found in the lymph node T-cell pool was approximately the same as that found in the spleen, whereas that in the bone marrow was about one half of this (Fig. 2b). This same pattern of T-cell distribution persisted at 10 days (Fig. 2b), and up to 19 days after transfer (data not shown), and was highly reproducible from experiment to experiment (see Table 1, below). Moreover, similar findings were obtained when we injected donor cells by either the intravenous or intraperitoneal route (data not shown). These results indicate that bone marrow seeding by donor T cells is a controlled process that does not directly parallel colonization of secondary lymphoid organs.
Figure 2.
Flow cytometric analysis of spleen, lymph node (LN) and bone marrow (bm) cells from B6 mice 7–10 days after adoptive transfer of CFSE-labelled syngeneic cells. Spleen and lymph node cells were purified from B6 mice, labelled with CFSE and injected intraperitoneally into age-and sex-matched B6 mice (25 million cells per recipient mouse). Spleen, lymph node and bone marrow cells were then purified from recipient mice, stained with anti-TCRβ PE mAb and analysed by flow cytometry. (a) The panels show the gates used for cell acquisition and analysis by flow cytometry in one typical experiment, 7 days after adoptive transfer. The two left panels show the results obtained with all spleen cells from one untreated mouse (top) and one adoptively transferred mouse (bottom). To focus the experiment on T cells, gating was set to TCR+ cells for acquisition, corresponding to the cells in the R1 region indicated in the bottom left panel. The upper middle and right panels show the results obtained with spleen cells from one adoptively transferred mouse, after gating on R1 for acquisition. To exclude from our analysis highly auto-fluorescent cells, we restricted our analysis to FL-3-negative cells, corresponding to the cells in the R2 region in the upper right panel. The bottom middle and right panels show the data acquired in the R1 region and then gated on R2 for analysis. R1 and R2 gates were always used for spleen, lymph node and bone marrow cell acquisition and analysis. (b) The panels represent the results obtained with spleen, lymph node and bone marrow cells from two representative mice, one analysed 7 days and the other 10 days after adoptive transfer, as indicated. The numbers in the upper right quadrant are the percentages of TCR+ CFSE+ cells within the total TCR+ cells. In untreated B6 mice, the percentage of TCR+ CFSE+ cells in the TCR+ subset was around 0·01. The ratio between the percentages of lymph node and spleen CFSE+ T cells and that between the percentages of bone marrow and spleen CFSE+ T cells are indicated below the panels.
Table 1.
Adoptive transfer experiments with ITGBko and ICAM1ko mice. Percentages of donor CFSE+ cells within the total TCR+ cells from spleen, lymph nodes and bone marrow of adoptively transferred mice
| Donor | Recipient | Spleen | Lymph node | Bone marrow | Spleen: lymph node ratio | Spleen: bone marrow ratio |
|---|---|---|---|---|---|---|
| (a) ITBG2ko mouse experiments | ||||||
| B6 | B6 (n = 10) | 3·64 (1·35) | 4·80 (1·79) | 1·75 (0·73) | 1·31†† | 0·53 |
| ITBG2ko | ITBG2ko (n = 10) | 3·50 (1·32) | 3·90 (1·52) | 1·71 (0·77) | 1·11** | 0·48 |
| B6 | ITBG2ko (n = 10) | 3·95 (0·75) | 5·80 (1·55) | 1·48 (0·60) | 1·47††† | 0·37*, † |
| ITBG2ko | B6 (n = 10) | 3·61 (1·49) | 3·54 (2·03) | 2·07 (0·92) | 0·94** | 0·56 |
| (b) ICAM1ko mouse experiments | ||||||
| B6 | B6 (n = 10) | 3·68 (1·30) | 4·63 (1·41) | 2·20 (0·98) | 1·28** | 0·61 |
| ICAM1ko | ICAM1ko (n = 10) | 3·15 (1·20) | 3·28 (1·41) | 2·08 (0·79) | 1·02†† | 0·67 |
| B6 | ICAM1ko (n = 10) | 3·26 (2·51) | 2·96 (2·52) | 1·73 (1·03) | 0·87***,†† | 0·62 |
| ICAM1ko | B6 (n = 10) | 2·88 (0·77) | 3·94 (1·11) | 1·96 (0·60) | 1·37††† | 0·69 |
Spleen and lymph node cells were purified from donor mice, labelled with CFSE and injected intraperitoneally into recipient mice (35–37 million cells per mouse). After 7–19 days from adoptive transfer, spleen, lymph node and bone marrow cells were purified from recipient mice and analysed as in Fig. 2. The average percentages and standard deviations of donor CFSE+ cells within the total TCR+ cells from spleen, lymph nodes and bone marrow of adoptively transferred mice are indicated. The average ratios between the percentages of lymph node and spleen CFSE+ T cells and that between the percentages of bone marrow and spleen CFSE+ T cells are also indicated. (a) Homing of ITGB2ko and B6 mice derived T cells into spleen, lymph nodes and bone marrow of either ITGB2ko or B6 mice (summary of six experiments; total numbers of recipient mice for each group indicated in parentheses). Statistical analysis was performed by Students' t-test. The P-value was calculated for each series of data either versus the B6 donor–B6 recipient group (***P ≤ 0·001, **P ≤ 0·01, *P ≤ 0·05) or versus the ITGB2ko donor–ITGB2ko recipient group (†††P ≤ 0·001, ††P ≤ 0·01, †P ≤ 0·05). (b). Homing of ICAM1ko and B6 mice derived T cells into spleen, lymph nodes and bone marrow of either ICAM1ko or B6 mice (summary of three experiments; total numbers of recipient mice for each group indicated in parentheses). Statistical analysis was performed as in (a). The P-value was calculated for each series of data either versus the B6 donor–B6 recipient group (***P = 0·001, **P = 0·01, *P = 0·05) or versus the ICAM1ko donor-ICAM1ko recipient group (†††P ≤ 0·001, ††P ≤ 0·01,†P ≤ 0·05).
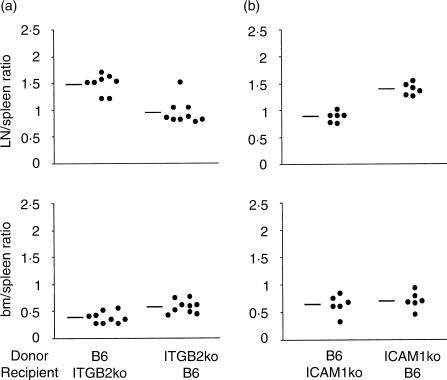
To investigate the potential roles of CD18 and ICAM1 in bone marrow lodging, we repeated the adoptive transfers using mice mutant in CD18 (ITGB2ko) or ICAM1 (ICAM1ko). The defects in the expression of the CD18 and ICAM molecules were confirmed in the respective mice by flow cytometry (data not shown). In a first set of experiments, we transferred ITGB2ko- or B6-derived cells into either ITGB2ko or B6 mice; in a second set, we transferred ICAM1ko- or B6-derived cells into either ICAM1ko or B6 mice. B6 transfers into B6 recipients were used as controls in each study. In total, we measured T-cell homing in eight experimental groups, each containing between six and 10 mice. As shown in Table 1 and Fig. 3, the donor T-cell percentage that localized to the bone marrow was about half of that found in the spleen, in all eight experimental groups. These findings mirrored those obtained with the combination of age- and sex-matched donor and recipient B6 mice (Fig. 2b). In contrast, ITGB2ko T cells had a significant disadvantage relative to B6 T cells in lymph node colonization compared to spleen colonization (ratio 0·94, compared to 1·47; Fig. 3a, Table 1), while B6 cell homing into lymph nodes in ICAM1ko recipient mice was likewise disadvantaged compared to that into the spleen (ratio 0·87 compared to 1·37; Fig. 3b, Table 1). These data are consistent with the roles reported for CD18 on T cells and ICAM1 on high endothelial cells in short-term lymph node homing.28 By contrast, although wild-type T cells had some disadvantage in entering the bone marrow compared to the spleen when transferred into ITGB2ko recipient mice, neither CD18 nor ICAM1 substantially affect bone marrow localization.
Figure 3.
Adoptive transfer experiments with ITGB2ko and ICAM1ko mice. Ratios between the percentages of lymph node and spleen CFSE+ T cells and between the percentages of bone marrow and spleen CFSE+ T cells. The data are the same as in Table 1 and, for the indicated experimental groups, the ratio between the percentages of lymph node and spleen CFSE+ T cells and that between the percentages of bone marrow and spleen CFSE+ T cells of individual mice are represented, as well as the average value of each group. (a) Homing of B6 and ITGB2ko mouse derived T cells into spleen, lymph nodes and bone marrow, respectively, of ITGB2ko and B6 mice.(b) Homing of B6 and ICAM1ko mouse-derived T cells into spleen, lymph nodes and bone marrow, respectively, of ICAM1ko and B6 mice.
Memory- and virgin-phenotype T-cell homing to bone marrow and lymph nodes
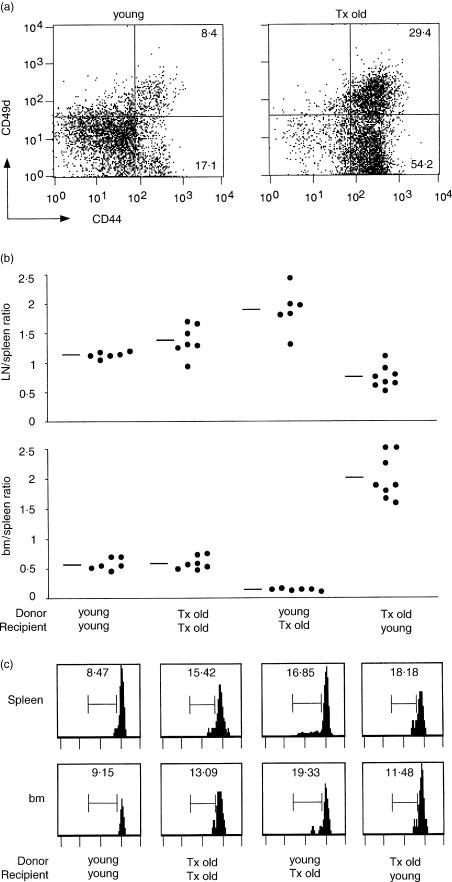
Next we analysed the influence of host phenotype on the distribution into lymphoid organs of enriched populations of either memory-phenotype CD44high or virgin-phenotype CD44−/low T cells. For this purpose, we took advantage of the peripheral increase of memory-phenotype CD44high T cells associated with aging, which in thymectomized mice, lacking the thymic output of virgin T cells, is more conspicuous than in normal mice. Hence, to obtain populations enriched in either memory- or virgin-phenotype T cells, we purified spleen and lymph node cells from thymectomized old and normal young B6 mice, respectively. Figure 4(a) shows the CD44 and CD49d expression profile of T cells derived from the two groups of mice.
Figure 4.
Adoptive transfer experiments with normal young and thymectomized old B6 mice. Enriched populations of CD44−/low and CD44high T cells were obtained by purifying spleen and lymph node cells from, respectively, normal young (2-month-old) and thymectomized old (1-year-old, thymectomized at 1 month) B6 mice. Cells were labelled with CFSE and injected intraperitoneally into either normal young or thymectomized old B6 mice (numbers of cells per recipient mouse: 25–33 million cells from normal young donors; 43–57 million cells from thymectomized old donors; different numbers of total cells from the two type of donors were injected per recipient mouse to compensate for the lower percentages of T cells found in the periphery of thymectomized old compared to young mice, see also in the text). (a) The two panels represent the phenotype of donor T cells from either young or thymectomized old B6 mice (tx = thymectomized). (b) After 7–10 days from adoptive transfer, spleen, lymph node and bone marrow cells were purified from recipient mice and analysed as in Fig. 2. The ratio between the percentages of lymph node and spleen CFSE+ T cells and that between the percentages of bone marrow and spleen CFSE+ T cells of individual mice are represented, as well as the average value of each group (summary of four experiments). Statistical analysis was performed using Students' t-test and differences were considered significant when P ≤ 0·001 (***), P ≤ 0·01 (**) and P ≤ 0·05 (*). Two series of data were considered at a time: first versus second group (*LN/spleen); first versus third group (**LN/spleen; ***bm/spleen); first versus forth group (***LN/spleen; *** bm/spleen); second versus third group (* LN/spleen; *** bm/spleen); second versus forth group (***LN/spleen; ***bm/spleen). (c) The panels represent the FL1 histograms of donor CFSE+ TCR+ cells from spleen and bone marrow of a representative mouse for each group. The scale on the x-axis is logarithmic, in arbitrary units. The numbers represent the percentage of cells in the marked region. Similar percentages were obtained when analysis was performed on lymph node cells.
We transferred each of the two cell populations into either thymectomized old or normal young B6 recipients and performed analysis of T-cell homing as before. As shown in Fig. 4(b), the transfer of memory-phenotype T cells, derived from the thymectomized old mice, to young mice produced a result strikingly different to that of other transfer experiments, in that there was preferential colonization of the bone marrow rather than the spleen (ratio 2·00, compared to 0·56 in young donor-young recipient controls). Conversely, virgin-phenotype T cells had a strongly impaired homing to the bone marrow with respect to the spleen in thymectomized old mice (ratio 0·12, compared to 0·56 in young donor–young recipient controls). The reverse pattern was evident in the case of T-cell migration into lymph nodes, where T cells derived from young mice were advantaged over those in thymectomized old recipients, whereas T cells derived from thymectomized old mice were disadvantaged in young hosts. In sum, our results indicate that in a competitive setting, memory T cells have an advantage over virgin T cells in colonization of the bone marrow, whereas virgin T cells have an advantage over memory T cells in lodging into lymph nodes. This conclusion is supported by the finding that T cells derived from thymectomized old mice do not show improved bone marrow colonization in thymectomized old recipients, where existing memory-type T cells presumably preclude their free access. Likewise, T cells from young mice have no lymph node homing advantage when transferred to young mice.
CFSE staining patterns were examined to determine whether reduced fluorescence, indicative of T-cell proliferation, correlated with the altered representation of cells in the different tissues. No correlation was evident (Fig. 4c), indicating that the diverse percentages of donor T cells found in the lymphoid organs in the four groups of mice were primarily attributable to differences in T-cell homing, not expansion.
We also found that untreated thymectomized old mice, compared to young mice, had lower percentages of T cells in both spleen and lymph nodes, but not in bone marrow. Specifically, TCR+ cells comprised 13·6 ± 1·8% of spleen cells and 22·4 ± 3% of lymph node cells in thymectomized old mice, compared to 26·6 ± 1·7% and 68·3 ± 12·5%, respectively, in young mice. In contrast, both thymectomized old mice and young mice had about 5% TCR+ cells in the bone marrow (5·3 ± 0·3% and 5·6 ± 0·2%, respectively). Since thymectomized mice experience a selective loss in peripheral virgin T cells while maintaining the memory T-cell pool,30 such observations confirm the bone marrow as a primary site for migration and localization of memory T cells.
Discussion
The present report extends our previous findings on CD8 T cells21 by showing that both antigen-specific CD4 and CD8 T cells migrate to the bone marrow after immunization and could still be found there several weeks later. This finding, together with the observation that memory-phenotype T cells preferentially home to the bone marrow rather than to lymph nodes, suggest that bone marrow is an important site of memory T-cell trafficking and prolonged localization. Although activated T cells up-regulate LFA1 and can express Mac-1,3 our results demonstrate that these integrins are not involved in sustained memory T-cell lodging in the bone marrow, in agreement with previous short-term studies.28 The finding of memory-phenotype T cells in the bone marrow after adoptive transfer seemingly excludes the possibility that T-cell localization in the bone marrow is the result of the local presence of antigen. Moreover, because LFA1 and ICAM1 are not required for prolonged T-cell lodging into the bone marrow, it is unlikely that antigen presentation and APC–T-cell interactions sustain T cells in the bone marrow ‘niche’.
The advantage shown by memory type cells over virgin cells in migration to the bone marrow bears obvious parallels with memory T-cell homing into extra-lymphoid organs. It is interesting that the interaction between VLA4 and VCAM1 is involved in effector/memory T-cell migration both to inflamed tissues and to bone marrow.29 T-cell homing into peripheral tissues has commonly been considered to be induced by inflammation, whereas migration of memory T cells to the bone marrow is considered to be a constitutive component of their recirculation within the lymphoid system. However, recent reports have shown high levels of constitutive memory T-cell lodging into extra-lymphoid organs in the absence of overt inflammation,31,32 and it has been demonstrated that epithelial cells produce homeostatic chemokines able to attract T lymphocytes at the body surfaces (reviewed in ref. 33). Building on this, an interesting model proposes that T lymphocytes traffic into extra-lymphoid organs through two main pathways with two distinct protective functions.33 The first, constitutive pathway regulates T-cell homing to skin and gut, large epithelial surfaces relentlessly exposed to pathogens and constitutively patrolled by T cells. The second pathway controls T-cell migration to internal organs, such as the kidney or liver, which are usually protected from pathogen attack and infiltrated by T cells only in the case of inflammation.33 In this context, we propose that memory T cells involved in the second pathway also recirculate through the bone marrow, and that this may be important for their long-term survival/proliferation, in a similar manner to the mechanisms reported for plasma cells.21,34–37
A candidate chemokine involved in the selective attraction of memory T cells to the bone marrow is stromal cell-derived factor 1α (SDF-1α), a potent chemoattractant for haemopoietic progenitor cells.38 Indeed, although both memory and virgin T cells express the SDF-1α receptor CXCR4,2,10,13 the percentage of cells showing a functional response to SDF-1α is higher in the memory T-cell subset than in the virgin T-cell subset, as indicated by the results of an in vitro assay for transendothelial migration.39
Our results on the different bone marrow colonization capabilities of T cells derived from thymectomized old mice and young donors, respectively, suggest that migration to the bone marrow is associated with a memory T-cell phenotype rather then with recent antigen activation. Thus, it is unlikely that T cells migrate to the bone marrow only during a restricted time window after priming. In contrast, a conceivable scenario is that, among the T cells constantly circulating in the lymphoid periphery, those with memory phenotype preferentially migrate to the bone marrow. We speculate that these cells persist in the bone marrow for enough time to receive survival/proliferation signals which might be relevant for the maintenance of long-term memory. At least for memory CD8 T cells, IL-15 and IL-7 might provide these signals, as they are expressed at high levels in the bone marrow and have been involved in CD8 T-cell homeostasis.40 Most probably, the cells also return to the periphery from the bone marrow as they are displaced by other memory T cells competing for the same enclosed ‘niche’. Indeed, the clear impact of the recipient phenotype on the capability of cells to enter the bone marrow indicates that the bone marrow compartment for T cells is limited and saturable. In sum, our results support the idea that colonization of specific lymphoid environments is favoured by the expression of a memory phenotype and inhibited by the competition with other rival T cells.41–44 This implies that the homing potential of an individual lymphocyte is not merely an inherent property of the cell, but rather a property of the lymphoid system as a whole.
Acknowledgments
We thank G. Bertini, G. Cortese and P. Piccoli for conscientious care of mice, A. Lupo and L. Minervino for technical assistance, and P. Giacomini, M. Giovarelli and G. Forni for reagents. We thank all the members of the Laboratory of Pathophysiology (G. Bronzi, S. Cascioli, M. Cippitelli, D. Del Bello, D. Di Bona, F. Di Modugno, C. Fionda, P. Nisticò) for helpful discussion and encouragement, U. D'Oro and A. Fanelli for reading the manuscript and A. Hayday for giving precious and stimulating comments. A special thank to P. Matzinger for her generous intellectual support.
References
- 1.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 2.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 3.McFarland HI, Nahill SR, Maciaszek JW, Welsh RM. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J Immunol. 1992;149:1326–33. [PubMed] [Google Scholar]
- 4.Andersson EC, Christensen JP, Marker O, Thomsen AR. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–45. [PubMed] [Google Scholar]
- 5.Sanders ME, Makgoba MW, Sharrow SO, Stephany D, Springer TA, Young HA, Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-γ production. J Immunol. 1988;140:1401–7. [PubMed] [Google Scholar]
- 6.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 7.Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–4. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 8.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–17. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackay CR, Marston WL, Dudler L, Spertini O, Tedder TF, Hein WR. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992;22:887–95. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–70. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 11.D'Ambrosio D, Iellem A, Colantonio L, Clissi B, Pardi R, Sinigaglia F. Localization of Th-cell subsets in inflammation: differential thresholds for extravasation of Th1 and Th2 cells. Immunol Today. 2000;21:183–6. doi: 10.1016/s0167-5699(00)01590-5. [DOI] [PubMed] [Google Scholar]
- 12.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 13.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–9. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 15.Vavassori M, Maccario R, Moretta A, et al. Restricted TCR repertoire and long-term persistence of donor-derived antigen-experienced CD4+ T cells in allogeneic bone marrow transplantation recipients. J Immunol. 1996;157:5739–47. [PubMed] [Google Scholar]
- 16.Slifka MK, Whitmire JK, Ahmed R. Bone marrow contains virus-specific cytotoxic T lymphocytes. Blood. 1997;90:2103–8. [PubMed] [Google Scholar]
- 17.Price PW, Cerny J. Characterization of CD4+ T cells in mouse bone marrow. I. Increased activated/memory phenotype and altered TCR Vbeta repertoire. Eur J Immunol. 1999;29:1051–6. doi: 10.1002/(SICI)1521-4141(199903)29:03<1051::AID-IMMU1051>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda MJ, Schmitz JE, Seth A, et al. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and cell-associated viral RNA levels in distinct lymphoid compartments of SIVmac-infected rhesus monkeys. Blood. 2000;96:1474–9. [PubMed] [Google Scholar]
- 20.Feuerer M, Beckhove P, Bai L, et al. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–8. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 21.Di Rosa F, Santoni A. Bone marrow CD8 T cells are in a different activation state than those in lymphoid periphery. Eur J Immunol. 2002;32:1873–80. doi: 10.1002/1521-4141(200207)32:7<1873::AID-IMMU1873>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O'Brien WE, Beaudet AL. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993;151:1571–8. [PubMed] [Google Scholar]
- 23.Sligh JE, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–33. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacabanne V, Viguier M, Romieu R, et al. Differential presentation of endogenously processed cytotoxic T lymphocyte epitopes by mouse hepatocarcinoma cell lines induced by SV40 large T antigen. Int Immunol. 1998;10:463–72. doi: 10.1093/intimm/10.4.463. [DOI] [PubMed] [Google Scholar]
- 25.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJD, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 26.Allione A, Consalvo M, Nanni P, et al. Immunizing and curative potential of replicating and nonreplicating murine mammary adenocarcinoma cells engineered with interleukin (IL) -2, IL-4, IL-6, IL-7, IL-10, tumor necrosis factor α, granulocyte-macrophage colony-stimulating factor, and γ-interferon gene or admixed with conventional adjuvants. Cancer Res. 1994;54:6022–6. [PubMed] [Google Scholar]
- 27.Ferreira C, Barthlott T, Garcia S, Zamoyska R, Stockinger B. Differential survival of naive CD4 and CD8 T cells. J Immunol. 2000;165:3689–94. doi: 10.4049/jimmunol.165.7.3689. [DOI] [PubMed] [Google Scholar]
- 28.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA) -1-deficient mice. J Exp Med. 1999;189:1467–78. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koni PA, Joshi SK, Temann U, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–53. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Rosa F, Ramaswamy S, Ridge JP, Matzinger P. On the lifespan of virgin T lymphocytes. J Immunol. 1999;163:1253–7. [PubMed] [Google Scholar]
- 31.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 32.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–17. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 33.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 34.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–4. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 35.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–72. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 36.Agace WW, Higgins JMG, Sadasivan B, Brenner MB, Parker CM. T lymphocyte–epithelial cell interactions: integrin αE (CD103) β7, LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12:563–8. doi: 10.1016/s0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 37.Cassese G, Lindenau S, de Boer B, et al. Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur J Immunol. 2001;31:2726–32. doi: 10.1002/1521-4141(200109)31:9<2726::aid-immu2726>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 38.Whetton AD, Graham GJ. Homing and mobilization in the stem cell niche. Trends Cell Biol. 1999;9:233–8. doi: 10.1016/s0962-8924(99)01559-7. [DOI] [PubMed] [Google Scholar]
- 39.Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001;2:515–22. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- 40.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–56. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 41.Spangrude GJ, Scollay R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes. Kinetics and phenotype of progeny. J Immunol. 1990;145:3661–8. [PubMed] [Google Scholar]
- 42.Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66:533–40. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 43.Di Rosa F, Matzinger P. Long-lasting CD8 T cell memory in the absence of CD4 T cells or B cells. J Exp Med. 1996;183:2153–63. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]