Abstract
Cyclosporin A (CsA) is a potent immuno-suppressant and is approved for the treatment of various disease conditions. The molecular biological mechanism of CsA has been investigated intensively in T cells and has been shown to involve the intracellular calcineurin pathway. Recently, it was reported that CsA has capacities to affect not only T cells but also antigen-presenting cells such as B cells and dendritic cells (DCs). DCs are a master regulator of immune responses that have an integral capacity to prime naive T cells. In the present study, we investigated the biological effects of CsA on human peripheral blood DC subsets: CD11c+ myeloid and CD11c− lymphoid subsets. CsA inhibited the up-regulation of co-stimulatory molecules induced with or without microbial stimuli and CD40L on both CD11c+ and CD11c− subsets. In addition, CsA negatively regulated the endocytic activity of CD11c+ DC during the immature state. CsA inhibited the interleukin-12 (IL-12) production, but augmented the IL-10 production from the LPS-stimulated CD11c+ subset, whereas CsA reduced the interferon-α (IFN-α) production from the CD11c− subset infected with Sendai virus (SV). Both the LPS-stimulated CD11c+ subset and SV-infected CD11c− subset preferentially induced the development of IFN-γ-producing T helper-type 1 (Th1) cells. Pretreatment of these DC subsets with CsA inhibited the Th1 skewing. These findings suggested a DC-mediated mechanism of immunosupression by CsA.
Introduction
Cyclosporin A (CsA) is an immunosuppressive agent and has been widely used in a variety of disease conditions such as post-organ transplant states1 and autoimmune diseases,2–4 with the purpose of controlling or suppressing the aberrantly activated immune responses. Although therapeutic application of CsA often brings clinically favourable outcomes it sometimes causes severe immuno-compromised states in the patients, despite careful use of the agent.5,6 The immunosuppressive effect of CsA is recognized to be due primarily to its biological property of directly suppressing T cells.7,8 The molecular mechanism of the CsA effect was shown to be exhibited by virtue of interfering with the intracellular calcineurin pathway of T cells.9 On the other hand, a line of evidence has suggested recently that CsA exerts its immunological functions not only on T cells but also on antigen-presenting cells such as B cells,10 macrophages11 and dendritic cells (DCs).12,13
Dendritic cells are potent antigen-presenting cells derived from bone marrow (BM) progenitor cells14–16 and are distributed widely in the tissues of various organs.17 In addition to having an essential capacity of priming naive T cells, DCs can also regulate various immune effector cells such as B cells,18,19 natural killer (NK) cells20 and NKT cells21,22 and thus play central roles in eliciting the immune responses in the body.23
In human peripheral blood, two major subsets of DCs, CD11c+ and CD11c− subsets have been identified.24–26 The CD11c+ subset belongs to the myeloid lineage and is considered to be immature DCs,27 whereas the CD11c− subset, recognized as plasmacytoid cells,28 is the direct precursor of lymphoid DCs and can differentiate into immature DCs in response to interleukin-3 (IL-3).29 These blood DC subsets may traffic to the tissues of various organs and experience maturation processes during the trafficking. Briefly, the immature CD11c+ subset in the peripheral blood may migrate into the nonlymphoid tissues as ‘sentinel’ DCs such as Langerhans cells.24 These ‘sentinel’ DCs produce IL-12 and act as fully mature antigen-presenting cells (APCs) in response to ‘danger’ signals such as LPS and CD40 ligands.30 On the other hand, the blood CD11c− subset may migrate directly into the lymphoid tissues and produce interferon-α/β upon encountering a virus,31,32 and then acts as mature APCs.29,33 Thus, both blood DC subsets are regarded as circulating pools for the DCs in the peripheral tissues.34
In this study, we have demonstrated that CsA had discernible suppressive effects on the maturation process of both CD11c+ and CD11c− subsets of blood DCs.
Materials and methods
Media and reagents
RPMI-1640 supplemented with 2 mml-glutamine, 100 U/ml penicillin, 100 ng/ml streptomycin and heat-inactivated 10% fetal calf serum (FCS) (Irvine Scientific, Santa Ana, CA) was used for the cell culture throughout the experiments. CsA (Wako, Osaka, Japan) was dissolved in anhydrous ethanol and used at a final concentration of 50–500 ng/ml. Ethanol was diluted in parallel to serve as vehicle control. Recombinant human cytokines, granulocyte-macrophage colony-stimulating factor (GM-CSF) (used at a concentration of 100 ng/ml) and IL-3 (at 10 ng/ml) were purchased from Pepro Tech EC (London, UK). Baculovirus-expressed soluble CD40 ligand (sCD40L) was used at a concentration of 1 µg/ml. Lipopolysaccharide (LPS) (Salmonella typhimurium) (1 µg/ml) was purchased from Sigma (St Louis, MO). UV-irradiated Sendai virus (SV) (HVJ: Cantell strain, provided by Sumitomo Pharmaceuticals (Ehime, Japan)) was used at 5 haemagglutinating U/ml.35
Isolation of blood DC subsets
Peripheral blood DC subsets were isolated according to the modified protocol as described previously.24,25 Briefly, The DC-enriched population (CD4+/CD3−/CD14− cells) was obtained from peripheral blood mononuclear cells (PBMC) by negative and subsequent positive immunoselections. The CD11c+/lin−/DR+ cells (CD11c+ DCs) and CD11c−/lin−/DR+ cells (CD11c− DCs) were sorted by an EPICS ALTRA® flow cytometer (Coulter Corp., Hialeah, FL) by using PE-labelled anti-CD11c [Leu-M5: Becton Dickinson (BD), Sunnyvale (CA)], mixture of fluorescein isothiocyonante (FITC)-labelled monoclonal antibodies (mAbs) against lineage markers, CD3 (M2AB: Exalpha, Boston, MA), CD14 (FWKW-1: Exalpha), CD15 (Leu-M1: BD), CD16 (J5511: Exalpha), CD19 (SJ25C1: BD) and CD56 (NCAM16·2: BD), and phycoerythrin–cyanin 5·1 (PC5)-labelled HLA-DR (Immu-357: ImmunoTech, Marseille, France). The purity of each cell was > 98%.
Culture of DCs
The sorted CD11c+ DCs were cultured with medium alone, GM-CSF + sCD40L or LPS, while CD11c− DCs were with IL-3, IL-3 + sCD40L or SV in 96-well round-bottomed tissue culture plates at 5 × 104 cells in 200 µl of medium/well for 24 hr or 72 hr. CsA or vehicle was added into these cultures.
Analyses of DCs
In the viability assay, the sorted CD11c+ and CD11c− DCs were cultured with medium alone and IL-3, respectively, for 1 or 3 days. Viable cells were evaluated as annexin V-negative fractions using an Annexin V-FITC Apoptosis Detection Kit (Genzyme, Cambridge, MA), after cell debris was excluded by an appropriate forward scatter threshold. To analyse the expression of co-stimulatory molecules and MHC class II, the culture cells were stained with FITC-labelled anti-CD40 (5C3: PharMingen, San Diego, CA), CD80 (BB-1: Ancell, Bayport, MN), CD86 (2331: PharMingen) or HLA-DQ (Tü169: PharMingen) and then analysed by a FACScan® (BD). The expression was analysed on gated live cells. To analyse the expression of macrophage mannose receptor (MMR), freshly isolated and cultured CD11c+ DCs were stained with unconjugated anti-MMR mAb (3·29B1·10: ImmunoTech), followed by FITC-labelled goat antimouse IgG F(ab′)2 (BD). To analyse the endocytic activities, CD11c+ DCs were incubated with 0·1 mg/ml of FITC-dextran (Polysciences, Warrington, PA) at 37° for 30 or 60 min. The results were displayed as mean fluorescence intensity after subtracting the background in which cells were incubated with FITC-dextran at 4°. The production of cytokines in the culture supernatants was determined by ELISA 24 hr later (kits for IL-12 p40 + p70, IL-12 p70 and interferon-α (IFN-α) were purchased from Endogen and that for IL-10 was from ImmunoTech).
DC-T cell co-culture
CD4+/CD45RA+ naive T cells were obtained from allogeneic healthy volunteers using a CD4+ T cell Isolation Kit (Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany) followed by positive selection with CD45RA-conjugated microbeads (Miltenyi Biotec). The purity of the cells was 94% or greater by reanalysis. To analyse the Th cell polarization, each blood DC subset preincubated with various stimuli in the presence of CsA (500 ng/ml) or vehicle for 24 hr was washed three times and then co-cultured with allogeneic CD4+/CD45RA+ naive T cells (105 cells/well, DC-T ratio 1: 5) for 7 days in 96-well round-bottomed tissue culture plates. To analyse the T cell stimulatory activities, DCs precultured for 72 hr were γ-irradiated at 15 Gy and graded doses of these DCs were added to 2 × 105 allogeneic naive Th cells for 5 days. The cells were pulsed with 10 µm 5-bromo-2'-deoxyuridine (BrdU) during the last 8 hr of the culture period. A kit of Cell Proliferation ELISA, BrdU (colorimetric) (from Roche, Mannheim, Germany) was used to measure BrdU incorporation of T cells.
Intracellular cytokine staining of T cells stimulated by DCs
After 7 days of co-culture, the T cells were washed and restimulated with PMA (50 ng/ml) and ionomycin (2 µg/ml) for 6 hr, and brefeldin A (10 µg/ml) was added during the final 2 hr (all from Sigma). The cells were stained with PC5-labelled anti-CD4 (ImmunoTech), and then with PE-labelled anti-IL-4 (BD) plus FITC-labelled anti-IFN-γ mAb (BD) or with PE-labelled anti-IL-10 (Caltag, Burlingame, CA) plus FITC-labelled anti-IFN-γ mAb, using a FIX and PERM kit (Caltag).
Statistical analysis
The paired Student's t-test was used for statistical analysis with a StatView statistical program (Abacus Concepts, Inc., Berkeley, CA). Differences were considered significant when tied P-values were less than 0·05.
Results
Effect of CsA on viability and maturation of blood DC subsets
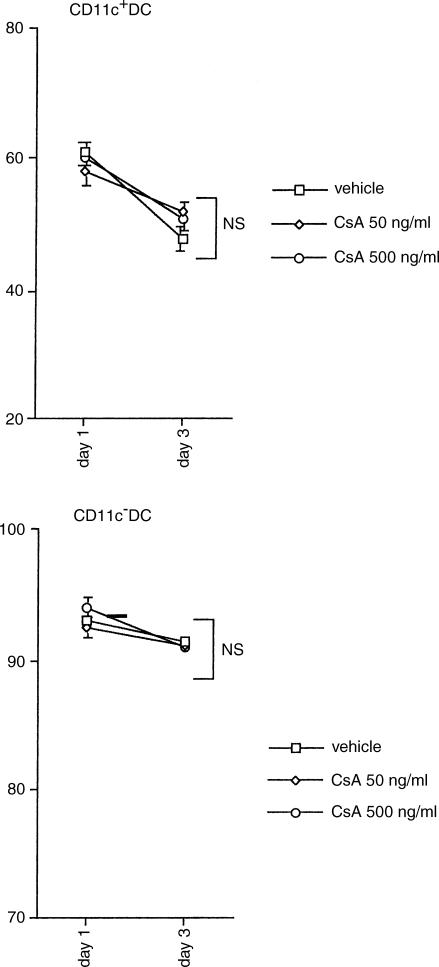
To examine the effect of CsA on viability of blood DC subsets, CD11c+ and CD11c− DCs were cultured with medium alone and IL-3, respectively, in the absence or presence of CsA. As shown in Fig. 1, Annexin V staining demonstrated that CsA did not affect significantly the cell viability of either DC subset, although spontaneous apoptosis occurred considerably in CD11c+ DC after 3-day culture with medium alone (Fig. 1).
Figure 1.
Effect of CsA on viability of DC. CD11c+ and CD11c− DC subsets were cultured with medium alone and IL-3, respectively. Both DC subsets were incubated with different concentrations of CsA for 3 days. The number of viable cells in each DC subset was evaluated as annexin V-negative fractions by flow cytometry. The data represent the means ± SEM of four independent experiments.
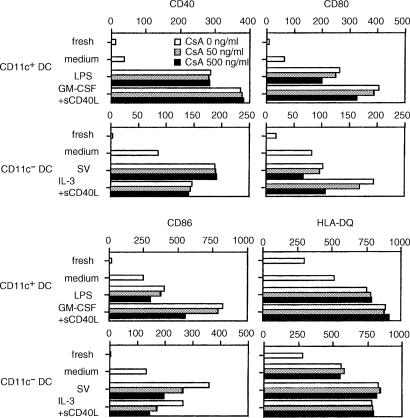
Next, to examine the effects of CsA on maturation of blood DC subsets, CD11c+ and CD11c− DCs were cultured with various agents. Three-day culture of CD11c+ DCs with LPS or GM-CSF + sCD40L and CD11c− DCs with SV or IL-3 + sCD40L up-regulated CD40, CD80, CD86 and HLA-DQ (Fig. 2). Addition of CsA substantially inhibited the up-regulation of CD80 and CD86, but showed no effect on the expression of CD40 and HLA-DQ in either DC subset. These inhibitory effects of CsA were manifested in a dose-dependent manner.
Figure 2.
Effect of CsA on maturation of DCs. CD11c+ and CD11c− DC subsets were cultured with medium alone and IL-3, respectively. Then, CD11c+ and CD11c− DC subsets were cultured with different stimuli indicated in the presence or absence of CsA for 72 hr. The expression levels of CD40, CD80, CD86 and HLA-DQ on each DC subset were analysed by flow cytometry. Results were shown as ΔMFI, which is calculated by subtraction of MFI with the isotype-matched control from that with each mAb. The results shown here are representative of four independent experiments.
Effect of CsA on the expression of macrophage mannose receptor (MMR) and receptor-mediated endocytic activity of CD11c+ DC
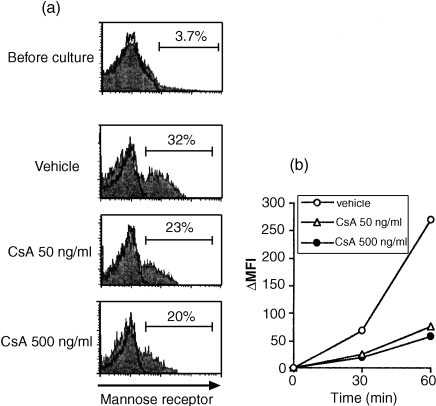
Expression of macrophage mannose receptors (MMR) and receptor-mediated endocytic activity are regarded as properties of myeloid DCs at immature states.36 Freshly isolated CD11c+ DCs, which were recognized to be in a very immature state, expressed only low levels of mannose receptors (Fig. 3a), whereas after 1-day culture with medium alone the DCs up-regulated the expression of this molecule spontaneously. Treatment with CsA inhibited this up-regulation of MMR during 1-day culture in a dose-dependent manner.
Figure 3.
Effect of CsA on MMR expression and endocytic activity of CD11c+DCs. CD11c+ DC subset was cultured with medium alone in the presence of different concentrations of CsA for 24 hr. (a) Expression of MMR was measured before and after the culture. Percentage of positive cells was indicated in the figure. (b) After incubation with FITC-dextran at 37° for 30 or 60 min, dextran uptake was analysed by flow cytometry. The results are shown as MFI, from which the background fluorescence (incubated at 4°) is subtracted. The results shown in this figure are representative of four independent experiments.
In addition, CsA suppressed the dextran uptake activity of CD11c+ DCs after 1-day culture (Fig. 3b). The effect was manifested even at low the concentration of CsA (50 ng/ml). Thus, the inhibitory effect of CsA on endocytotic activity was apparently displayed in parallel with down-regulation of MMR.
Effect of CsA on T cell stimulatory capacity of DC
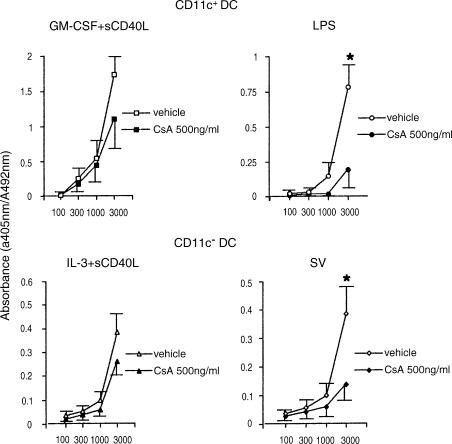
We next evaluated whether the inhibitory effect of CsA on phenotypical maturation of DCs was related to the alteration of the T cell stimulatory capacity. Pretreatment of LPS-stimulated CD11C+ DCs or SV-infected CD11C− DCs with CsA substantially suppressed the allostimulatory activity (Fig. 4). The inhibitory effects were demonstrated markedly at high DC/T cell ratios (P < 0·05). However, the inhibitory effect of CsA was shown to be subliminal when CD11c+ DCs and CD11c− DCs were stimulated with GM-CSF + sCD40L and IL-3 + sCD40L, respectively.
Figure 4.
Effect of CsA on allostimulatory capacity of DCs. CD11c+ DCs were cultured with GM-CSF + sCD40L or LPS in the presence or absence of CsA (500 ng/ml), and CD11c− DCs were cultured with IL-3 + sCD40L or SV in the presence or absence of CsA (500 ng/ml). Graded doses of the cultured DCs (stimulator cells) were irradiated and co-cultured with allogeneic naive CD4+ T cells. T cell proliferation was measured by BrdU incorporation. The results are shown as means of triplicate cultures. Vertical bars represent SEM of triplicate cultures (*P < 0·05). Representative data of three experiments are shown.
Effect of CsA on cytokine production of DC
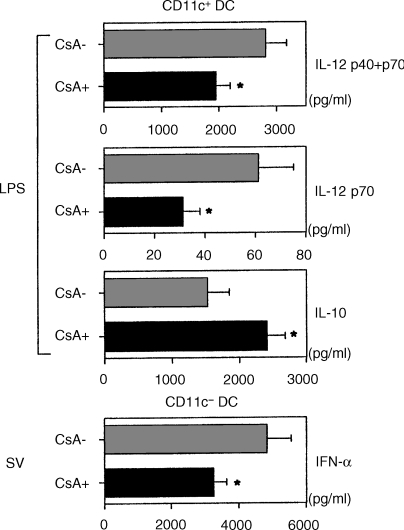
It was reported that DCs have ability to produce different types of cytokines in response to various stimuli.37,38 LPS (1 µg/ml) induced the production of total IL-12 (p40 + p70), bioactive IL-12 (p70) and IL-10 from CD11c+ DCs (Fig. 5). Addition of CsA inhibited the production of both IL-12 p40 + p70 and IL-12 p70 significantly, while it facilitated the production of IL-10 from CD11c+ DCs. On the other hand, CD11c− DCs infected with SV produced large amounts of IFN-α, as described previously.31–33 Addition of CsA significantly reduced its production from CD11c− DCs.
Figure 5.
Effect of CsA on cytokine production of DCs.After 24 hr-culture with LPS or SV in the presence or absence of CsA (500 ng/ml), supernatants of DCs were measured for IL-12 p40 + 70, IL-12 p70, IL-10 and IFN-α by ELISA. Statistical significance is indicated using paired Student's t-test (*P < 0·05). The data represent the means ± SEM of three independent experiments.
Effect of CsA on DC-mediated Th polarization
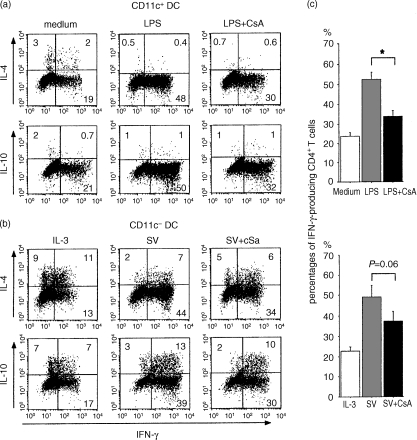
DC-mediated Th cell development is regulated mainly by DC-derived cytokines.23,25 Thus, we evaluated the CsA effect on DC-mediated Th polarization. LPS-stimulated CD11c+ DCs preferentially induced the production of IFN-γ producing T-helper cells (Th1 cells) (Fig. 6a). The frequency of IFN-γ-producing Th cells was decreased significantly by addition of CsA into the pretreatment DC culture with LPS (Fig. 6a,c). On the other hand, SV-infected CD11c− DCs induced IFN-γ-producing Th1 cells and IL-10/IFN-γ double-producing Th cells (Fig. 6b), consistent with the findings of a previous study.29 The induction of both types of Th cells was slightly inhibited by pretreatment of the DCs with CsA (Fig. 6b,c).
Figure 6.
Effect of CsA on DC-mediated Th polarization. (a,b) CD11c+ and CD11c− DCs were preincubated with LPS and SV, respectively, in the presence or the absence of CsA (500 ng/ml). These DCs were washed extensively and then co-cultured with allogeneic naive CD4+ T cells for 7 days. The T cells were restimulated with PMA and ionomycin for 6 hr. Brefeldin A was added to the cultures for the last 2 hr, and then intracellular IFN-γ, IL-4 and IL-10 of T cells was analysed by flow cytometry. The percentages of the respective cytokine-producing T cells are indicated in each dot blot profile. (c) Percentages of IFN-γ-producing Th cells are shown (upper; CD11c+ DCs, lower; CD11c− DCs). Data represent the means ± SEM of five independent experiments. Statistical significance is indicated using paired Student's t-test (*P < 0·05). Data are presented as means ± SEM.
Discussion
In this study, we obtained some new insights into the immunosuppressive effects of CsA. It was shown that CsA inhibited phenotypical maturation of both CD11c+ and CD11c− peripheral blood DCs with microbial stimuli or CD40L in vitro, as evaluated by the expression level of co-stimulatory molecules. This inhibitory effect of CsA was exhibited without affecting the cell viability of both DCs subsets. It is interesting to note that the inhibitory effect of CsA was limited to the expression of CD80 and CD86. The inhibitory effect of CsA was also manifested in functional aspects of the DC maturation. Indeed, CsA regulated negatively endocytic activity of immature CD11c+ DCs. On the other hand, CsA definitely inhibited the T cell stimulatory capacities of both DC subsets matured with microbial stimuli, although CsA did not show discernible effects on the allostimulatory effects of both subsets stimulated with sCD40. The basis of this discrepancy is currently unknown. Collectively, these findings suggest that CsA might exert inhibitory roles on DCs of different lineages at multiple maturation stages in vivo.
Recent accumulating evidence suggests that cytokine production of DCs depends either on DC subsets or on stimuli that DC receive.37 We showed, in the present study, that LPS induced CD11c+ DCs to produce IL-12 and IL-10, while infection of CD11c− DCs with SV induced the production of large amounts of IFN-α. Remarkably, CsA suppressed IL-12 production significantly, but augmented IL-10 secretion from LPS-stimulated CD11c+ DCs. On the other hand, CsA exhibited a negative regulatory effect on IFN-α production by SV-infected CD11c− DCs. In light of the roles of IL-10,39–41 IL-1242 and IFN-α,33,43–45 the alteration by CsA of cytokine production from DCs may lead to the modulation of immune responses at least partly through influencing the development of effector T helper cells (Th cells).
A line of evidence indicates that Th cell development is regulated mainly by DC-derived cytokines, such as IL-1242 or IFN-α.33,44,45 This study showed that both LPS-stimulated CD11c+ DCs and SV-infected CD11c− DCs skewed naive Th cells towards IFN-γ-producing Th1 cells. It was demonstrated that CsA significantly inhibited the Th1 polarization induced by LPS-stimulated CD11c+ DCs. This may be due partly to the CsA effect that inhibited IL-12 production from CD11c+ DCs, because IL-12 is a potent inducer of Th1 cells.42 In addition, in light of the inhibitory roles of IL-10 in the development of Th1 cells40 the enhancement by CsA of IL-10 production from LPS-stimulated CD11c+ DCs may also contribute to the suppression of Th1 skewing. On the other hand, CsA affected SV-infected CD11c− DCs to interfere slightly with the development of Th1 cells and IL-10/IFN-γ double-producing Th cells. This could be attributable to the inhibitory effect of CsA on IFN-α production from the SV-infected CD11c− DCs, because IFN-α is known to induce Th1 cells.44 The limited effect of CsA on the SV-infected CD11c− DCs, however, might presumably be the reflection of the phenomenon that CsA could not abolish totally the IFN-α production from the SV-infected CD11c− DCs. Since Th1 cells are functionally immunogenic or protective against invading pathogens, the inhibition of DC-mediated Th1 polarization may constitute an immunosuppressive mechanism of CsA. On the other hand, it has been established that IL-10 not only inhibits Th1 development but also exerts negative regulatory roles for a wide variety of immune cells.40,46 Hence, the augmentation by CsA of IL-10 production from LPS-stimulated CD11c+ DCs may also contribute to the induction of immunosuppressive state.
Several studies have demonstrated recently that CsA affected the biological properties of DCs. For example, murine Langerhans cells and BM-derived DCs showed down-regulation of CD40 and B7 in the presence of CsA.12,13 Furthermore, CsA inhibited the allogeneic T cell stimulatory capacity of Langerhans cells.47 On the other hand, CsA impaired differentiation of immature DCs from monocytes and CD40L-induced maturation of monocyte-derived DCs.48 The current study findings described above were partly in accordance with these documented studies. However, the present findings appeared to be novel, in that they revealed the direct effects of CsA on naturally circulating blood DCs. In addition, this may be the first study that described the CsA effects on human lymphoid DCs (CD11c− DCs).
Recent progress in DC biology has suggested that DCs play critical roles in various diseases. In the murine system, host residual DCs were shown to be essential in the pathophysiology of graft versus host disease (GVHD) after allogeneic bone marrow transplantation.49 Induction of tolerance versus autoimmunity might be determined by resting versus activated DCs.50 On the other hand, we have reported recently that selective recruitment of CD11c+ DCs from peripheral blood to salivary glands and the subsequent induction of Th1 polarization might be crucial steps in the genesis of primary Sjögren's syndrome.51 Furthermore, a recent study implicated the pivotal roles of IFN-α-producing plasmacytoid cells (CD11c− DCs) in lupus erythematosus.52,53 Thus, the findings obtained in this study may support the idea of introducing CsA to various disease conditions with the purpose of targeting DCs.
In conclusion, we unravelled a variety of effects of CsA on peripheral blood DCs. CsA inhibited maturation of both CD11c+ and CD11c− DCs and modulated cytokine production from these DCs, resulting in the inhibition of Th1 development. These findings will provide a better understanding of the biological effects of CsA and may promise a further extended application of this agent to various clinical situations.
Acknowledgments
This work was supported by a grant from the Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
References
- 1.First International Congress on Cyclosporine. Transplant Proc; 16–19 May 1983; Houston, Texas. 1983. pp. 2207–3187. [Google Scholar]
- 2.Alexander AG, Barnes NC, Kay AB. Trial of cyclosporin in corticosteroid-dependent chronic severe asthma. Lancet. 1992;339:324–8. doi: 10.1016/0140-6736(92)91646-p. [DOI] [PubMed] [Google Scholar]
- 3.Sketris I, Yatscoff R, Keown P, Canafax DM, First MR, Holt DW, Schroeder TJ, Wright M. Optimizing the use of cyclosporine in renal transplantation. Clin Biochem. 1995;28:195–211. doi: 10.1016/0009-9120(95)91341-y. [DOI] [PubMed] [Google Scholar]
- 4.Tugwell P, Bombardier C, Gent M, et al. Low-dose cyclosporin versus placebo in patients with rheumatoid arthritis. Lancet. 1990;335:1051–5. doi: 10.1016/0140-6736(90)92630-z. [DOI] [PubMed] [Google Scholar]
- 5.Dummer JS, White LT, Ho M, Griffith BP, Hardesty RL, Bahnson HT. Morbidity of cytomegalovirus infection in recipients of heart or heart–lung transplants who received cyclosporine. J Infect Dis. 1985;152:1182–91. doi: 10.1093/infdis/152.6.1182. [DOI] [PubMed] [Google Scholar]
- 6.Cockfield SM, Preiksaitis JK, Jewell LD, Parfrey NA. Post-transplant lymphoproliferative disorder in renal allograft recipients. Clinical experience and risk factor analysis in a single center. Transplantation. 1993;56:88–96. doi: 10.1097/00007890-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989;337:473–5. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Farmer JD, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–42. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 10.Jirapongsananuruk O, Leung DY. The modulation of B7.2 and B7.1 on B cells by immunosuppressive agents. Clin Exp Immunol. 1999;118:1–8. doi: 10.1046/j.1365-2249.1999.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasowska BA, Zheng XX, Strom TB, Kupieck-Weglinski JW. Adjunctive rapamycin and CsA treatment inhibits monocyte/macrophage associated cytokines/chemokines in sensitized cardiac graft recipients. Transplantation. 2001;71:1179–83. doi: 10.1097/00007890-200104270-00029. [DOI] [PubMed] [Google Scholar]
- 12.Lee JI, Ganster RW, Geller DA, Burckart GJ, Thomson AW, Lu L. Cyclosporine A inhibits the expression of costimulatory molecules on in vitro-generated dendritic cells: association with reduced nuclear translocation of nuclear factor kappa B. Transplantation. 1999;68:1255–63. doi: 10.1097/00007890-199911150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Salgado CG, Nakamura K, Sugaya M, et al. Differential effects of cytokines and immunosuppressive drugs on CD40, B7–1, and B7–2 expression on purified epidermal Langerhans cells. J Invest Dermatol. 1999;113:1021–7. doi: 10.1046/j.1523-1747.1999.00785.x. [DOI] [PubMed] [Google Scholar]
- 14.Reid CD, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992;149:2681–8. [PubMed] [Google Scholar]
- 15.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strunk D, Egger C, Leitner G, Hanau D, Stingl G. A skin homing molecule defines the langerhans cell progenitor in human peripheral blood. J Exp Med. 1997;185:1131–6. doi: 10.1084/jem.185.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 18.Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Briere F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185:941–51. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wykes M, MacPherson G. Dendritic cell–B-cell interaction: dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology. 2000;100:1–3. doi: 10.1046/j.1365-2567.2000.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Nieda M, Koezuka Y, et al. Analysis of human V alpha 24+ CD4+ NKT cells activated by alpha-glycosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2000;164:4458–64. doi: 10.4049/jimmunol.164.9.4458. [DOI] [PubMed] [Google Scholar]
- 22.Kadowaki N, Antonenko S, Ho S, Rissoan MC, Soumelis V, Porcelli SA, Lanier LL, Liu YJ. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. 2001;193:1221–6. doi: 10.1084/jem.193.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Inaba M, Inaba K, et al. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol. 1999;163:1409–19. [PubMed] [Google Scholar]
- 25.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–9. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 26.Kohrgruber N, Halanek N, Groger M, Winter D, Rappersberger K, Schmitt-Egenolf M, Stingl G, Maurer D. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J Immunol. 1999;163:3250–9. [PubMed] [Google Scholar]
- 27.O'Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–93. [PMC free article] [PubMed] [Google Scholar]
- 28.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL) -3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–26. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–37. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 31.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 32.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 33.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 34.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–3. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 35.Uno K, Nakano K, Maruo N, et al. Determination of interferon-alpha-producing capacity in whole blood cultures from patients with various diseases and from healthy persons. J Interferon Cytokine Res. 1996;16:911–8. doi: 10.1089/jir.1996.16.911. [DOI] [PubMed] [Google Scholar]
- 36.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha. II. Functional analysis. Blood. 1997;90:1458–70. [PubMed] [Google Scholar]
- 37.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic–polycytidylic acid double-stranded RNA, respectively, stimulate CD11c− type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J Immunol. 2001;166:2291–5. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Rich BE, Inobe J, Chen W, Weiner HL. Induction of Th2 cell differentiation in the primary immune response: dendritic cells isolated from adherent cell culture treated with IL-10 prime naive CD4+ T cells to secrete IL-4. Int Immunol. 1998;10:1017–26. doi: 10.1093/intimm/10.8.1017. [DOI] [PubMed] [Google Scholar]
- 40.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–35. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 42.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 43.Zou W, Borvak J, Wei S, Isaeva T, Curiel DT, Curiel TJ. Reciprocal regulation of plasmacytoid dendritic cells and monocytes during viral infection. Eur J Immunol. 2001;31:3833–9. doi: 10.1002/1521-4141(200112)31:12<3833::aid-immu3833>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 44.Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med. 1993;178:1655–63. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demeure CE, Wu CY, Shu U, Schneider PV, Heusser C, Yssel H, Delespesse G. In vitro maturation of human neonatal CD4 T lymphocytes. II. Cytokines present at priming modulate the development of lymphokine production. J Immunol. 1994;152:4775–82. [PubMed] [Google Scholar]
- 46.Hsu DH, de Waal Malefyt R, Fiorentino DF, et al. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250:830–2. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 47.Dupuy P, Bagot M, Michel L, Descourt B, Dubertret L. Cyclosporin A inhibits the antigen-presenting functions of freshly isolated human Langerhans cells in vitro. J Invest Dermatol. 1991;96:408–13. doi: 10.1111/1523-1747.ep12469772. [DOI] [PubMed] [Google Scholar]
- 48.Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van Kooten C. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. Eur J Immunol. 2000;30:1807–12. doi: 10.1002/1521-4141(200007)30:7<1807::AID-IMMU1807>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 49.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–5. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 50.Garza KM, Chan SM, Suri R, Nguyen LT, Odermatt B, Schoenberger SP, Ohashi PS. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000;191:2021–7. doi: 10.1084/jem.191.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozaki Y, Amakawa R, Ito T, et al. Alteration of peripheral blood dendritic cells in patients with primary Sjogren's syndrome. Arthritis Rheum. 2001;44:419–31. doi: 10.1002/1529-0131(200102)44:2<419::AID-ANR61>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 52.Vallin H, Perers A, Alm GV, Ronnblom L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J Immunol. 1999;163:6306–13. [PubMed] [Google Scholar]
- 53.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]