Abstract
Clinical reports suggest that acute ethanol intoxication is often associated with lymphopenia. Previously, ethanol was reported to invoke thymocyte apoptosis. We studied the effect of ethanol on T cell apoptosis. In addition, we evaluated the molecular mechanism of ethanol-induced T cell apoptosis. Human T cells harvested from healthy subjects after an alcohol drinking binge showed enhanced T cell apoptosis (before, 0·4 ± 0·2% versus after, 19·6 ± 2·5% apoptotic lymphocytes/field; P < 0·001). In in vitro studies, ethanol in a concentration of 50 mm and higher enhanced the apoptosis of Jurkat cells. DNA isolated from ethanol-treated Jurkat cells displayed integer multiples of 180 base pairs. Ethanol decreased Jurkat cell expression of Bcl-2, whereas ethanol increased Jurkat cell expression of Bax. Jurkat cells treated with ethanol also showed translocation of cytochrome C into cytosol. Moreover, a caspase-9 inhibitor partially inhibited ethanol-induced Jurkat cell apoptosis. In in vivo studies, after binge drinking, T cell expression of Bcl-2 also decreased. In addition, binge drinking induced the cleavage of caspase-3, suggesting activation of caspase-3 in T cells. These results suggest that ethanol promotes T cell apoptosis through the activation of intrinsic or mitochondrial pathway.
Excessive use of alcohol is a serious problem in our society. Its chronic use affects health in the form of cirrhosis, gastrointestinal haemorrhage, trauma and cancer. Moreover, chronic alcoholics are prone to develop bacterial infections.1–3 Infection in alcoholics is usually more severe and is associated with higher mortality.3,4 A similar effect of alcohol has been reported in acutely intoxicated experimental laboratory animals.5 It appears that alcohol may modulate the immune status both directly as well as through associated complications such as cirrhosis, malnutrition and neurological abnormalities.2,6 Ethanol has been demonstrated to trigger apoptosis in macrophages, neutrophils and thymocytes.7–10 Because apoptotic cells are functionally compromised it is probable that ethanol may be modulating immune function directly.
Lymphocytes are the major components of the host defence system. Both quantitative and qualitative alterations of lymphocytes may contribute to decreased host resistance to infections. Lymphopenia has been reported in most alcoholic patients with leucopenia.6,11,12 Ethanol has been reported to trigger apoptosis of thymocytes in vitro as well as in vivo.9,10,13In vitro studies suggest that alcohol activates thymocyte membranal adenylate cyclase.14 The latter raises the intracellular concentration of cyclic AMP.14 Elevated levels of cAMP have been implicated in suppressed lymphocyte function in alcoholics.14 Because agents that cause intracellular elevation of cAMP in thymocytes have also been reported to cause thymocyte apoptosis,15 we hypothesize that diminished lymphocyte function in alcoholics may be related to ethanol-induced lymphocyte apoptosis.
In vivo studies of ethanol are important, but they are associated with complexities. Ingestion of alcohol may alter the absorption of a nutrient, e.g. zinc,16 as well as elevating glucocorticoids,17 both of which have the potential to trigger the apoptosis of thymocytes.18,19
The effect of ethanol has been studied extensively in liver cells.20–22 Ethanol has been reported to induce apoptosis in liver cells both in vivo and in vitro.20–22 This effect of ethanol on liver cells is suggested to be the result of oxidative stress.20,21 As expected, ethanol-induced injury proceeds through the activation of the mitochondrial pathway.20 However, occasional reports suggest the role of FasL.21
In the present study, we evaluated the molecular mechanism involved in alcohol-induced T cell apoptosis.
Materials and methods
Binge drinking
Binge drinking as a researchable construct has been defined as five or more drinks on one occasion.23 Ten healthy male volunteers (three Caucasian and seven Asian Indian, ranging in age from 21 to 30 years) consumed five to seven drinks within 90–120 min. Blood was collected pre- and post-binge drinking. T lymphocytes were isolated from pre- and post-binge samples. Plasma ethanol levels were measured in post-binge samples. Consent was obtained from the participating subjects.
Human T lymphocytes
Lymphocytes from whole blood were isolated by a discontinuous density gradient using the Ficoll–Hypaque method. In brief, three ml of Histopaque-1119 and Histopaque-1077 and 6 ml of whole blood was layered consecutively in 15 ml conical centrifuge tubes followed by centrifugation at 700 g for 30 min at room temperature. Of two distinct bands (between plasma and red cells), the upper band was aspirated and cells were washed by the addition of 10 ml of isotonic phosphate buffered saline (PBS), followed by centrifugation for 10 min at 200 g. The supernatant was discarded and cells were again washed twice. The cells were incubated in Petri dishes in complete RPMI-1640 media for 120 min at 37° in a CO2 incubator followed by the collection of non-adherent cells. This method isolates lymphocytes and approximately 5% monocytes. However, subsequent passage of the lymphocyte eluant through a human T cell recovery column (Accurate Chemical and Scientific, Westbury, NY) removes virtually all B cells by a process of negative selection. T cells were incubated in RPMI-1640 (Life Technologies, Grand Island, NY) containing 10% fetal calf serum (FCS, heat-inactivated), 1 mm l-arginine, 1% Hepes, 0·2% NaHCO3, 50 U/ml penicillin and 50 µg/ml streptomycin (Life Technologies). In the present study, 10%-conditioned medium (lymphocyte culture supernatant) was added to the control as well as experimental variables. In our laboratory we use conditioned medium routinely to subculture Jurkat cells and macrophages, allowing better growth of these cells. The ingredients of the conditioned medium are comprised of components of the incubation medium (RPMI + FCS) and the secretory products of separated lymphocytes. To evaluate contamination of the lymphocyte population with monocytes, lymphocytes were labelled with human monoclonal anti-CD14 antibody. Less than 2% of the cells showed positive staining. The purity of lymphocytes in different experiments varied from 97 to 99% (98 ± 0·8%). T lymphocytes were used within 24 hr of isolation.
Jurkat cells (T cell line, human)
The human leukemia T cell line, Jurkat (EG-1) was obtained from the American Type Culture Collection (ATCC), Rockville, MD. Jurkat cells were cultured in RPMI-1640 medium supplemented with 10% FCS, 0·15% NaHCO3, 1 mm sodium pyruvate, 1% Hepes, 0·45% glucose, 50 U/ml penicillin and 50 µg/ml streptomycin in a humidified incubator with 5% CO2 in air at 37°.
Apoptosis studies
To evaluate the occurrence of necrosis and apoptosis, we used propidium iodide (Sigma Chemical Co, St Louis, MO) and Hoechst (H)-33342 (Molecular Probes, Eugene, OR) stains. Propidium iodide stains the necrosed cells, whereas H-33342 stains the nuclei of live cells and identifies apoptotic cells by increased fluorescence.24 Human T lymphocytes or Jurkat cells (105 cells/well) were prepared under control and experimental conditions. At the end of the incubation period, aliquots of methanol containing H-33342 (final concentration, 1 µg/ml) were added and incubated for 10 min at 37°. Cells (without washing) were placed on ice and propidium iodide (final concentration, 1 µg/ml) was added to each well. Cells were incubated with dyes for 10 min on ice, protected from light, and then examined under ultraviolet light using a Hoechst filter (Nikon, Melville, NY). The percentage of live, apoptotic and necrosed cells was recorded in eight random fields by two observers unaware of experimental conditions.
To determine the effect of ethanol on T cell apoptosis, equal numbers of Jurkat cells (106 cells/well) were incubated in media containing either buffer (control) or ethanol (100 mm) for 24 hr. Four sets of experiments were carried out.
To determine the dose–response effect of ethanol on Jurkat cell apoptosis, Jurkat cells (105 cells/well) were incubated in media containing either buffer (control) or variable concentrations of ethanol (25, 50, 100, 150 and 200 mm) for 24 hr. Four series of experiments were carried out.
To determine the time–course effect of ethanol on Jurkat cell apoptosis, equal numbers of Jurkat cells (105 cells/well) were incubated in media containing either buffer (control) or ethanol (100 mm) for variable periods (2, 4, 12, 24 and 48 hr). Subsequently cells were assayed for apoptosis. Three series of experiments were carried out.
To determine the dose–response effect of ethanol on freshly isolated human T cells, equal numbers of T cells (105 cells/well) were incubated in media containing either buffer (control) or variable concentrations of ethanol (25, 50, 100, 150 and 200 mm) for 24 hr. Subsequently, cells were assayed for apoptosis. Three sets of experiments were carried out.
To determine the role of caspase-9 in ethanol-induced T cell apoptosis, equal numbers of Jurkat cells (105 cells/well) were incubated in media containing either buffer (control) or ethanol (50 and 100 mm) with or without caspase-9 inhibitor (1 µm; LEHD-CHO, cat. no. 218776, Calbiochem, San Diego, CA) for 24 hr. Subsequently, cells were assayed for apoptosis. Three sets of experiments were carried out.
DNA isolation and gel electrophoresis
This is a simple method that is specific for isolation and confirmation of DNA fragments from apoptotic cells.25 Equal numbers (108 cells/Petri dish) of human T lymphocytes or Jurkat cells were prepared under control and experimental conditions (ethanol, 100 mm; 24 hr). At the end of the incubation period, cells were centrifuged at 1600 g for 10 min at room temperature and the pellets were resuspended in DNA lysis buffer (1% NP-40 in 20 mm EDTA, 50 mm Tris-HCL, pH 7·5; 10 µl/106 cells). After centrifugation, the supernatant was collected and the extraction was repeated. SDS in a final concentration of 1% was added to the supernatants before the samples were treated with RNAse A (final concentration 5 µg/µl) at 56°. This was followed by digestion with proteinase K (Promega, Madison WI) for 2 hr at 37°. After addition of 0·5 vol 10 m ammonium acetate, the DNA was precipitated with 2·5 vol ethanol, dissolved in gel loading buffer, and separated by electrophoresis in 1·6% agarose gels.
Protein extraction and Western blotting
To evaluate the effect of ethanol on the accumulation of cell death proteins such as Bcl-2, Bax and cytochrome C, and on the activation of the caspase-3, equal numbers of Jurkat cells (107 cells/Petri dish) were incubated for 16 hr under control and experimental (ethanol, 100 mm) conditions. At the end of the incubation period, cells were lysed with lysis buffer and protein was assayed using a BCA kit (Pierce, Rockford, IL). In a parallel series, cells were treated either with buffer or ethanol (50, 100 and 150 mm) for 16 hr; subsequently, cells were harvested and mitochondrial and cytosolic fractions were separated. Twenty micrograms of protein from each variable was separated on a 4–20% gradient polyacrylamide gel and blotted onto a nitrocellulose membrane using a BIO-RAD Western blotting apparatus (Hercules, CA). The nitrocellulose membranes were then processed for Bcl-2 using rabbit anti-Bcl-2 (5 µg/ml; Calbiochem, San Diego, CA), rabbit anti-Bax (1 µg/ml; PharMingen, San Diego, CA), rabbit anticytochrome C (Santa Cruz Biotechnology Inc, Santa Cruz, CA) or goat anticaspase-3 (1 µg/ml, Upstate Biotechnology, Lake Placid, NY) antibodies. The membranes were incubated further with horseradish peroxidase labelled secondary goat antirabbit (Oncor, Gaithersburg, MD) or donkey antigoat (Santa Cruz) antibodies; blots were developed using enhanced chemiluminescence (ECL, Amersham, Arlington Heights, IL). To determine loading, blots were stripped and reprobed for actin. Quantitative densitometry was performed on the identified bands by using a computer-based measurement system (IS-1000 Digital Imaging System, Alpha Innotech Corp., San Leandro, CA).
Statistical analysis
For comparison of mean values between two groups, the unpaired t-test was used. To compare values between multiple groups, analysis of variance (anova) was applied and a Newman–Keuls multiple range test was used to calculate a q-value. All values are means ± SEM except where indicated otherwise. Statistical significance was defined as P < 0·05.
Results
In vivo binge drinking
Mean plasma concentration of ethanol was 95·5 ± 5·5 mm (range 85–109 mm). In in vivo studies, binge drinking triggered the apoptosis of lymphocytes (before, 0·4 ± 0·2% versus after, 19·6 ± 2·5% apoptotic lymphocytes/field; P < 0·001).
Effect of ethanol on lymphocyte apoptosis in vitro
Ethanol promoted (P < 0·001) Jurkat cell apoptosis (control, 1·3 ± 0·3% versus ethanol, 20·8 ± 0·6% apoptotic Jurkat cells/field; n = 4).
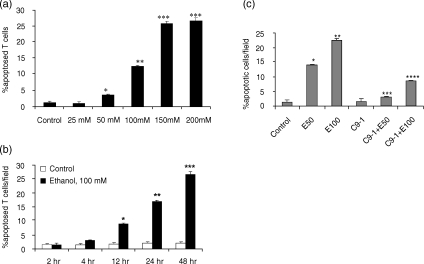
To determine the dose–response effect of ethanol on Jurkat cell apoptosis, Jurkat cells were treated with either buffer (control) or variable concentrations of ethanol (25, 50, 100, 150 and 200 mm). Ethanol enhanced Jurkat cell apoptosis in a dose-dependent manner (control 1·6 ± 0·3%; ethanol, 25 mm, 6·3 ± 0·4%; ethanol, 50 mm, 11·5 ± 0·5%; ethanol, 100 mm, 19·7 ± 0·8%; ethanol 150 mm, 28·3 ± 1·2%; ethanol, 200 mm, 29·6 ± 1·0% apoptotic Jurkat cells/field). As shown in Fig. 1, DNA isolated from ethanol-treated Jurkat cells showed DNA fragmentation (integer multiples of 180 base pairs) in the form of a ladder pattern when isolated DNA was run on agarose gel electrophoresis, whereas control Jurkat cells revealed only a small amount of DNA laddering. A similar DNA ladder pattern was observed in control and ethanol-treated T cells (freshly separated; data not shown).
Figure 1.
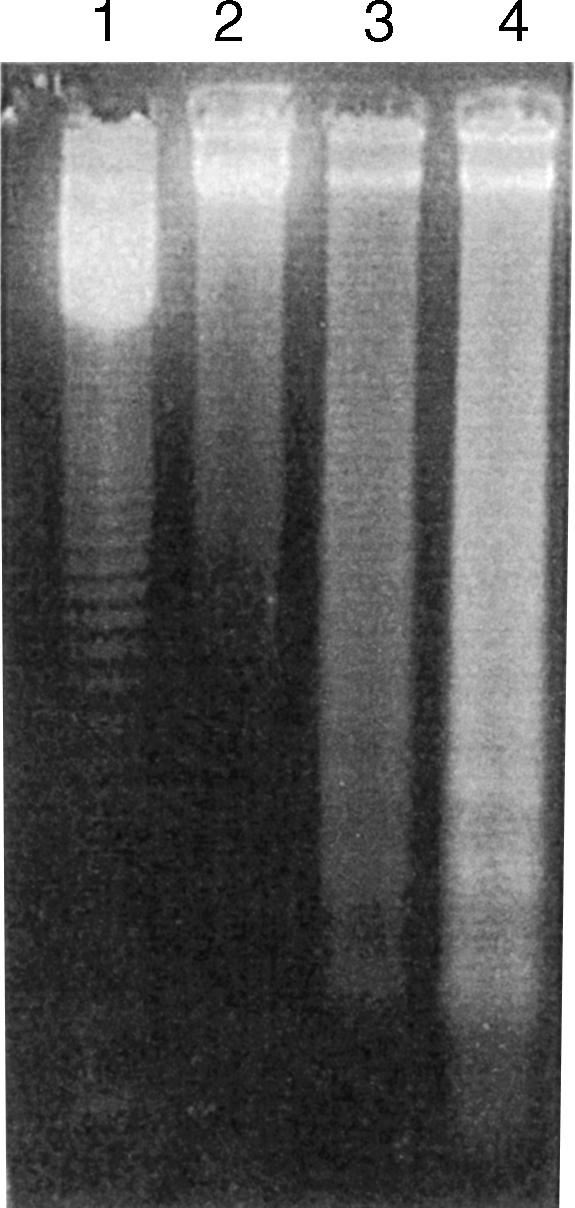
Gel electrophoresis of DNA isolated from control (lane 2) and ethanol-treated Jurkat cells (lane 3, ethanol, 50 mm; lane 4, ethanol, 100 mm). Ethanol-treated Jurkat cells showed a classic ladder pattern. Lane 1 shows molecular weight markers.
To determine the dose–response effect of ethanol on freshly isolated human T cells, equal numbers of T cells were incubated in media containing either buffer (control) or variable concentrations of ethanol (25, 50, 100, 150 and 200 mm) for 24 hr. As shown in Fig. 2a, ethanol induced T cell apoptosis in a dose-dependent manner.
Figure 2.
(a) Dose–response effect of ethanol on T cell apoptosis. Equal numbers of T cells were incubated in media containing either buffer (control) or variable concentrations of ethanol (25, 50, 100, 150 and 200 mm) for 24 hr. At the end of the incubation period cells were stained with H-33342 and propidium iodide and evaluated for apoptosis. Results (means ± SEM) are from three sets of experiments each carried out in triplicate. *P < 0·05 compared with control and ethanol, 25 mm; **P < 0·001 compared with control and ethanol, 25–50 mm; ***P < 0·001 compared with control and ethanol, 25–100 mm. (b) Time–course effect of ethanol on Jurkat cell apoptosis. Equal numbers of Jurkat cells were incubated in media containing either buffer (control) or ethanol (100 mm) for variable periods (2, 4, 12, 24 and 48 hr). At the end of the incubation period cells were stained with H-33342 and propidium iodide and evaluated for apoptosis. Results (means ± SEM) are from three series of experiments each carried out in triplicate. *P < 0·001 compared with respective control and ethanol, 2 and 4 hr; **P < 0·001 compared with respective control and ethanol, 2, 4, 12 and 48 hr; ***P < 0·001 compared with respective control and ethanol, 2, 4, 12 and 24 hr. (c) Effect of a caspase-9 inhibitor on ethanol-induced Jurkat cell apoptosis. Equal numbers of Jurkat cells were incubated in media containing either buffer (control), ethanol (E50 and 100 mm) with or without a caspase-9 inhibitor (C9-I, 1 µm) for 16 hr. Subsequently, cells were stained with H-33342 and propidium iodide. Results (means ± SEM% apoptotic cells/field) are from three sets of experiments. To compare values between multiple groups, analysis of variance (anova) was applied and a Newman–Keuls multiple range test was used to calculate a q-value. P < 0·001 compared with control; **P < 0·001 compared with control E50; ***P < 0·001 compared with E50; ****P < 0·001 compared with E 100.
To determine the time–course effect of ethanol on Jurkat cell apoptosis, equal numbers of Jurkat cells were incubated in media containing either buffer (control) or ethanol (100 mm) for variable periods (2, 4, 12, 24 and 48 hr). Ethanol promoted Jurkat cell apoptosis in a time-dependent manner (Fig. 2b).
Role of cell death proteins in ethanol-induced T cell apoptosis
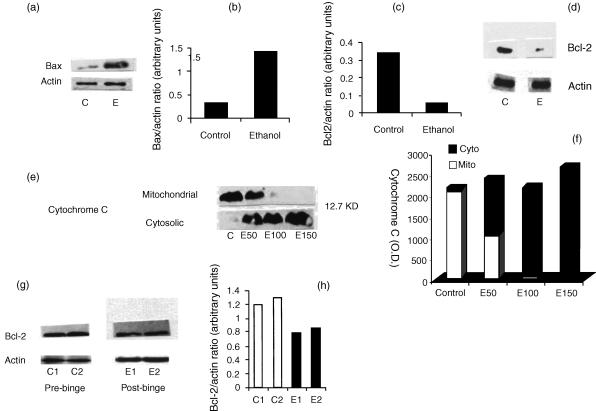
To determine the role of cell death proteins in ethanol-induced T cell apoptosis, equal numbers of Jurkat cells were incubated in media containing either buffer or ethanol (100 mm) for 16 hr. Subsequently, Western blots were developed and probed for Bcl-2 and Bax. As shown in Fig. 3a,b, ethanol increased Jurkat cell expression of Bax twofold; however, ethanol decreased Jurkat cell expression of Bcl-2 (Fig. 3d) fourfold (Fig. 3c).
Figure 3.
(a) Effect of ethanol on Jurkat cell Bax expression. Equal numbers of Jurkat cells were incubated in media containing either buffer (control, C) or ethanol (E, 100 mm) for 1 hr. At the end of the incubation period cells were prepared for Western blots and probed with anti-Bax antibody. Two sets of experiments were carried out. Representative Western blots of ethanol-treated Jurkat cells show expression of Bax. (b) Densitometric analysis showing Bax/actin ratio in control (C) and ethanol (E)-treated Jurkat cells. Ethanol increased Jurkat cell expression of Bax twofold. (c) Densitometric analysis showing Bcl-2/actin ratio in control (C) and ethanol (E)-treated Jurkat cells. (d) Effect of ethanol on Jurkat cell expression of Bcl-2. Equal numbers of Jurkat cells were incubated in media containing either buffer (control, C) or ethanol (E100 mm) for 16 hr. Subsequently, cells were lysed, protein extracted, Western blots generated and probed with anti-Bcl-2. Two sets of experiments were carried out. Representative Western blots of ethanol-treated Jurkat cells show expression of Bcl-2. (e) Effect of ethanol on mitochondrial translocation of cytochrome C. Equal numbers of Jurkat cells were incubated in media containing either buffer (control, C) or ethanol (E50, 100 or 150 mm) for 1 hr. At the end of the incubation period, mitochondrial and cyotosolic fractions were isolated, Western blots generated and probed with anticytochrome C antibody. Two sets of experiments were carried out. Representative Western blots of ethanol-treated Jurkat cells show translocation of cytochrome C. (f) Densitometric analysis showing mean cytochrome C location in cytosolic (Cyto) or mitochondrial (Mito) compartments of control and ethanol treated (E50, 100 and 150 mm) Jurkat cells. (g) Effect of binge drinking on T cell expression of Bcl-2. Equal numbers of cells isolated before (C1 and C2) and after (E1 and E2) binge drinking, were lysed, protein extracted, Western blots generated and probed with anti-Bcl-2 antibody. Representative Western blots of T cells show expression of Bcl-2. (h) Densitometric analysis showing Bcl-2/actin ratio before (C1 and C2) and after (E1 and E2) binge drinking.
To evaluate the effect of ethanol on mitochondrial translocation of cytochrome C into the cytosol, equal numbers of Jurkat cells were treated either with buffer or variable concentrations of ethanol. Subsequently, both mitochondrial and cytosolic fractions were isolated and probed for cytochrome C. Jurkat cells treated with ethanol showed translocation of cytochrome C from the mitochondria to the cytosol (Fig. 3e,f).
To determine the role of caspase-9 in ethanol-induced T cell apoptosis, Jurkat cells were incubated in media containing either buffer (control) or ethanol (50 and 100 mm) with or without caspase-9 inhibitor. As shown Fig. 2c, ethanol promoted Jurkat cell apoptosis; however, this effect of ethanol on Jurkat cells was inhibited by an inhibitor of caspase-9.
Effect of binge drinking on cell death protein expression
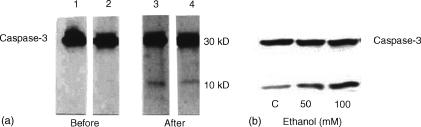
As shown in Fig. 3(g,h), binge drinking decreased T cell expression of Bcl-2. However, the effects of ethanol on Bcl-2 in peripheral blood T cells were not as marked as for the in vitro effects of ethanol on Jurkat T cells (Fig. 3d). The alcohol binge induced cleavage of caspase-3, suggesting activation of T cell caspase-3 (Fig. 4a). Similarly, Jurkat cells treated with ethanol (50 and 100 mm) showed increased cleavage of caspase-3 when compared with control cells (Fig. 4b).
Figure 4.
(a) Effect of binge drinking on caspase-3 activation. Equal numbers of T cells isolated before (lanes 1 and 2) and after (lanes 3 and 4) binge drinking were prepared for Western blots and probed for caspase-3. Representative Western blot of T cells before and after binge drinking showing activation of caspase-3. The alcohol binge induced cleavage of caspase-3 into 10 and 30 kDa proteins, suggesting activation of caspase-3. (b) Effect of ethanol on caspase-3 cleavage in Jurkat cells. Equal numbers of Jurkat cells were incubated in media containing either buffer (control, C) or ethanol (E50 and 100 mm) for 16 hr. Subsequently, cells were harvested, Western blots prepared and probed for caspase-3. Ethanol promoted cleavage of caspase-3 into 10 and 30 kDa proteins.
Discussion
The present study demonstrates that ethanol promotes the apoptosis of T cells in a dose-dependent manner. Ethanol not only decreased the Jurkat cell expression of Bcl-2 but also promoted the expression of Bax. In addition, ethanol translocated mitochondrial cytochrome C to the cyotosol in Jurkat cells. Moreover, an inhibitor of caspase-9 attenuated the ethanol-induced Jurkat cell apoptosis. In in vivo studies, binge drinking not only promoted T cell apoptosis but also attenuated Bcl-2 expression. Binge drinking also induced the cleavage of T cell caspase-3. These results suggest that ethanol enhances T cell apoptosis through the mitochondrial pathway.
Apoptosis is a regulatory process in the thymus by which thymocytes reacting with self-antigen are deleted from the population.26 Glucocorticoids have been reported to be the important inducers of thymocyte apoptosis after short-term alcohol ingestion;27 nevertheless, ethanol-fed adrenalectomized animals had fewer thymocytes than control diet-fed adrenalectomized animals.28 The latter studies suggest that ethanol, in vivo, may also be inducing thymocyte apoptosis through another mechanism independent of glucocorticoid secretion.
Although there is no doubt about the role of intracellular calcium in ethanol-induced thymocyte apoptosis,29 there are controversial reports in relation to the role of intracellular calcium on peripheral T cell apoptosis.10 Nevertheless, treatment of T cells with the calcium chelator EGTA promoted apoptosis.10 Similarly, in another study calcium chelations induced DNA fragmentation in human peripheral blood T cells,30 but this effect was found to be due to the depletion of other bivalent ions.30 Depletion of zinc in peripheral blood lymphocytes is also reported.30
Protein kinase C (PKC) has been shown to play a role in ethanol-induced thymocyte apoptosis.31 Ethanol potentiated the apoptosis triggered by suboptimal doses of phorbol myristate (PMA); moreover, H7, an inhibitor of PKC, attenuated ethanol-induced thymocyte apoptosis.10 Ethanol increases protein kinase C (PKC) activity in human peripheral blood lymphocytes.32,33 However, the ethanol-induced apoptosis was attenuated completely by the treatment of T cells with PMA. These investigators suggested that activation of PKC by PMA in T cells might induce the formation of second messengers that may interfere with the signalling pathway induced by ethanol.32,33
Because degradation of structural proteins by active caspases results in morphological cell death changes, caspases seem to be important players in the induction of apoptosis. Interleukin-converting enzyme (ICE) was the first identified human cystein–protease which cleaves aspartic acid residues.34 Activation of caspase-3 is considered to be a major event following apoptotic stimuli and is responsible for cleaving various proteins.35 Caspase-3 activation may occur either through an intrinsic (mitochondrial) or extrinsic (receptor-mediated) pathway. The extrinsic pathway proceeds (upstream) through the activation of caspase-8, whereas the intrinsic pathway proceeds through the activation of caspase-9. Peripheral (type 1) T cells have been shown to undergo autocrine cell death as a result of FasL expression leading to the cleavage of casapse-8, whereas type 2 T cells (cell lines) predominantly undergo apoptosis through cleavage of caspase-9. In the present study an inhibitor of caspase-9 attenuated ethanol-induced T cell apoptosis; moreover, ethanol suppressed Bcl-2 expression by T cells, and also induced the translocation of cytochrome c, the upstream activators of caspase-9. Finally, ethanol cleaved caspase-3 in T cells, thus suggesting propagation of the apoptotic message to the degradation stage, an irreversible phase. These findings suggest that ethanol utilizes the mitochondrial pathway in the induction of T cell apoptosis.
Besides apoptosis, caspase activation is also linked to necrosis.36 Hypoxia has been demonstrated to induce tubular cell necrosis; however, this effect of hypoxia was inhibited by caspase inhibitors.36 Usually activation of poly(ADP-ribose) polymerase (PARP) by DNA breaks catalyses poly(ADP-ribosyl)ation and results in depletion of NAD+ and ATP, thus inducing necrosis. Herceg and Wang studied capsase-resistant PARP-transfected fibroblasts and showed that PARP cleavage prevents the induction of necrosis and propagates apoptosis.37 The conditions associated with depletion of ATP are often associated with necrosis.
Bcl-2 has been demonstrated to act as an antioxidant.38 Mitochondrial caspase-3 activation has also been reported to be blocked by Bcl-2·39 Several investigators have suggested that Bcl-2 and Bcl-xL (another member of Bcl-2 family) may be pore-forming proteins identical to bacterial toxin.40 In some systems Bcl-2 has been shown to regulate mitochondrial intracellular Ca++ and the loss of membrane potential produced by pro-apoptotic stimuli.41 p28 Bap31, an integral endoplasmic reticulum protein, binds to caspase-1 or -8 and forms a complex with either Bcl-2 or Bcl-xL.42 The ratios of Bax: Bcl-2 and Bax: Bcl-xL are important determinants of apoptosis. A high ratio favours cell death and a low ratio promotes cell survival.40
Interleukin (IL)-4 plays an important role in the regulation of lymphocyte apoptosis.31,43,44 IL-4 has been demonstrated to inhibit spontaneous as well as ethanol-induced lymphocyte apoptosis.10 IL-4 promotes expression of Bcl-2 by lymphocytes.45 The Bcl-2 family has been demonstrated to play an important role in the induction of apoptosis in a variety of cells.46,47 Meßmer et al. demonstrated that Bcl-2 transfected macrophages show protection from nitric oxide (NO)-induced apoptosis.47 Similarly, Bcl-2 transfected hepatic cells were resistant to the apoptotic effect of ethanol.46 In a model of alcoholic liver disease, ethanol-induced elevation of IL-6 has been suggested to provide a protective effect to liver cells by promoting the expression of Bcl-2.22 This effect of IL-6 was confirmed further by the occurrence of liver injury in IL-6 (–/–) versus IL-6 (+/+) mice and associated expression of Bcl-2.22 In the present study, ethanol both in in vitro and in in vivo studies suppressed Bcl-2 expression. On the other hand, ethanol promoted T cell expression of Bax, a cell death-promoting gene. Thus, it appears that ethanol tilted the balance (between cell survival and death) towards death.
The extrinsic apoptosis pathway involves interaction of Fas and FasL on the surface of the cells followed by downstream activation of caspase- 8 and caspase-3. Fas antigen is expressed on T cells and its cross-linking with adjacently expressed FasL leads to autocrine cell death of T cells.48 Recently, Aggarwal and Gupta demonstrated that increased expression of Fas/FasL expression caused T cell deficiency in ageing humans.49 However, Fas expression is usually low in resting B cells which do not express FasL, and thus they do not undergo autocrine cell death.50 Although Bcl-2 family proteins participate predominantly in the intrinsic pathway, at times their overexpression modulates Fas-mediated apoptosis. For example, paclitaxel induces apoptosis via the Fas/FasL pathway; however, overexpression of Bcl-xL prevented paclitaxel-induced apoptosis; it appears that Bcl-xL prevented the transcription of FasL.51 Free radicals have been demonstrated to mediate apoptosis by both extrinsic (Fas/FasL) and intrinsic pathways.52 In an animal model of autoimmunity, production of peroxynitrite by infiltrating macrophages correlated with apoptosis of Fas (+) T cells within the retina.52 We have reported recently the role of nitric oxide in Fas/FasL-mediated macrophage apoptosis.53 Thus, it appears that free radicals may be working either in downstream signal transduction and/or cross-talk − a requirement for transcription of Fas/FasL.
We conclude that ethanol directly promotes T cell apoptosis. This effect of ethanol is mediated through the activation of the mitochondrial pathway.
Acknowledgments
This work was supported by a grant RO1D12111 from the National Institutes of Health.
References
- 1.Adams HG, Jordan C. Infections in the alcoholic. Med Clin North Am. 1984;68:179–200. doi: 10.1016/s0025-7125(16)31249-4. [DOI] [PubMed] [Google Scholar]
- 2.MacGregor RR. Alcohol and immune defense. JAMA. 1986;256:1474–1479. [PubMed] [Google Scholar]
- 3.Young CL, MacGregor RR. Alcohol and host defenses: infectious consequences. Infect Med. 1980;6:163–75. [Google Scholar]
- 4.Capps JA, Coleman GH. Influence of alcohol on prognosis of pneumonia in Cook County Hospital: a statistical report. JAMA. 1923;80:750–2. [Google Scholar]
- 5.Louria DB. Susceptibility to infection during experimental alcohol intoxication. Trans Assoc Am Physicians. 1963;76:102–10. [Google Scholar]
- 6.Liu YK. Leukopenia in alcoholics. Am J Med. 1973;54:605–10. doi: 10.1016/0002-9343(73)90118-6. [DOI] [PubMed] [Google Scholar]
- 7.Singhal PC, Reddy K, Ding G, Kapasi A, Franki N, Ranjan R, Nwakoby IE, Gibbons N. Ethanol-induced macrophage apoptosis: the role of TGF-β. J Immunol. 1999;162:3031–6. [PubMed] [Google Scholar]
- 8.Singhal PC, Patel P, Nahar N, et al. Ethanol-induced neutrophil apoptosis is mediated through nitric oxide. J Leuk Biol. 1999;66:930–6. doi: 10.1002/jlb.66.6.930. [DOI] [PubMed] [Google Scholar]
- 9.Ewald SJ, Shao H. Ethanol increases apoptotic cell death of thymocytes in vitro. Alcohol Clin Exp Res. 1993;17:359–65. doi: 10.1111/j.1530-0277.1993.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 10.Slukvin II, Jerells TR. Different pathways of in vitro ethanol-induced apoptosis in thymocytes and splenic T and B lymphocytes. Immunopharmacology. 1995;31:43–57. doi: 10.1016/0162-3109(95)00032-4. [DOI] [PubMed] [Google Scholar]
- 11.Lindenbaum J, Hargrove L. Thrombocytopenia in alcoholics. Ann Intern Med. 1968;68:526–32. doi: 10.7326/0003-4819-68-3-526. [DOI] [PubMed] [Google Scholar]
- 12.McFarland W, Libre EP. Abnormal leukocyte response in alcoholism. Ann Intern Med. 1963;59:865–77. doi: 10.7326/0003-4819-59-6-865. [DOI] [PubMed] [Google Scholar]
- 13.Saad AJ, Jerrels TR. Flow cytometric and immunohistochemical evaluation of ethanol-induced changes in splenic and thymic lymphoid cell populations. Alcohol Clin Exp Res. 1991;15:796–803. doi: 10.1111/j.1530-0277.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson JP, Sullivan TJ, Kelly JP, Parker CW. Stimulation of alcohols by cyclic AMP metabolism in human leukocytes: possible role of cyclic AMP in the anti-inflammatory effects of ethanol. J Clin Invest. 1977;60:284–9. doi: 10.1172/JCI108776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McConkey DJ, Orrenius S, Jondal M. Agents that elevate cAMP stimulate DNA fragmentation in thymocytes. J Immunol. 1990;145:1227–30. [PubMed] [Google Scholar]
- 16.Ghishan FK, Patwardhan R, Greene HL. Fetal alcohol syndrome. Inhibition of placental zinc transport as a potential mechanism for fetal growth retardation in the rat. J Clin Lab Med. 1982;100:45–52. [PubMed] [Google Scholar]
- 17.Tabakoff B, Jaffe RC, Ritzmann RF. Corticosterone concentrations in mice during ethanol drinking and withdrawal. J Pharm Pharmacol. 1978;30:371–4. doi: 10.1111/j.2042-7158.1978.tb13259.x. [DOI] [PubMed] [Google Scholar]
- 18.Beach RS, Gershwin ME, Makishima RK, Hurley LS. Impaired immunologic ontogeny in postnatal zinc deprivation. J Nutr. 1980;110:805–15. doi: 10.1093/jn/110.4.805. [DOI] [PubMed] [Google Scholar]
- 19.Screpanti I, Morrone S, Meco D, et al. Steroid sensitivity of thymocyte subpopulations during intrathymic differentiation. Effects of 17 beta-estradiol and dexamethasone on subset expressing T cell antigen receptor or IL-2 receptor. J Immunol. 1989;142:3378–83. [PubMed] [Google Scholar]
- 20.Higuchi H, Kurose I, Kato S, Miura S, Ishii H. Ethanol-induced apoptosis and oxidative stress in hepatocytes. Alcohol Clin Exp Res. 1996;20(Suppl. 9):340A–46A. [PubMed] [Google Scholar]
- 21.Zhou Z, Sun X, Kang YJ. Ethanol-induced apoptosis in mouse liver. Fas- and cytochrome c-mediated caspase-3 activation pathway. Am J Pathol. 2001;159:329–38. doi: 10.1016/S0002-9440(10)61699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong F, Kim WH, Tian Z, Jargua B, Ishac E, Shen X, Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x (L) proteins. Oncogenes. 2002;21:32–43. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- 23.Conrod PJ, Stewart SH, Pihl RO. Validation of a measure of excessive drinking frequency per year that BAL exceeds 0·08% Subst Use Misuse. 1997;32:587–607. doi: 10.3109/10826089709027314. [DOI] [PubMed] [Google Scholar]
- 24.Singhal PC, Sharma P, Kapasi AA, Reddy K, Franki N, Gibbons N. Morphine enhances macrophage apoptosis. J Immunol. 1998;160:1886–93. [PubMed] [Google Scholar]
- 25.Herrmann H, Lorenz HM, Voll R, Grunke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–7. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+ and CD8+ TCR thymocytes in vivo. Science. 1990;250:17201722. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 27.McConkey DJ, Hartzell P, Nicotera P, Orrenius S. Calcium activated DNA fragmentation kills immature lymphocytes. FASEB J. 1989;3:1843–9. doi: 10.1096/fasebj.3.7.2497041. [DOI] [PubMed] [Google Scholar]
- 28.Jerrells TR, Marietta CA, Weight FF, Eckardt MJ. Effect of adrenalectomy on ethanol-associated immunosuppression. Int J Immunopharmacol. 1990;12:435–42. doi: 10.1016/0192-0561(90)90027-k. [DOI] [PubMed] [Google Scholar]
- 29.Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- 30.Treves S, Trentini PL, Ascanelli M, Ascanelli M, Buci G, Di Virgilio F. Apoptosis is dependent on intracellular zinc and independent of intracellular calcium in lymphocytes. Exp Cell Res. 1994;211:339–43. doi: 10.1006/excr.1994.1096. [DOI] [PubMed] [Google Scholar]
- 31.Illera VA, Perandones CE, Stunz LL. Apoptosis in splenic B lymphocytes: regulation by protein kinase C and IL-4. J Immunol. 1993;151:2965–73. [PubMed] [Google Scholar]
- 32.DePetrillo PB, Liou CS. Ethanol exposure increases total protein kinase C activity in human lymphocytes. Alcohol Clin Exp Res. 1973;17:351–4. doi: 10.1111/j.1530-0277.1993.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 33.Perandones CE, Illera VA, Peckham D, Stunz LL, Ashamn RF. Regulation of apoptosis in vitro in mature murine spleen T cells. J Immunol. 1993;151:3521–9. [PubMed] [Google Scholar]
- 34.Alnemri ES, Livingston DJ, Nicholson DW, Salvesn S, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 36.Edelstein CL, Shi Y, Schrier RW. Role of caspases in hypoxia-induced necrosis of rat proximal tubular cells. J Am Soc Nephrol. 1999;10:1940–9. doi: 10.1681/ASN.V1091940. [DOI] [PubMed] [Google Scholar]
- 37.Herceg Z, Wang ZQ. Failure of poly (ADP-ribose) polymerase cleavage by caspases leads to induction of necrosis and enhanced apoptosis. Mol Cell Biol. 1999;19:5124–33. doi: 10.1128/mcb.19.7.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granville DJ, Carthy CM, Hunt DWC, MaManus BM. Apoptosis: molecular aspect of cell death and disease. Lab Invest. 1998;78:893–913. [PubMed] [Google Scholar]
- 39.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome C from mitochondria: a primary site for bcl-2 regulation of apoptosis. Science. 1997;275:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 40.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–6. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 41.Rowan S, Fisher DE. Mechanisms of apoptotic cell death. Leukemia. 1997;11:457–65. doi: 10.1038/sj.leu.2400626. [DOI] [PubMed] [Google Scholar]
- 42.Ng FW, Nguyen M, Kwan T, Branton PE, Nicholson DW, Cromlish JA, Shore GC. p28 bap31, a bcl-2/bcl-Xl- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol. 1997;139:327–38. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Migliorati G, Nicoletti I, Crocicchio F, Pagliacci C, D'Adamio F, Ricardi C. Heat shock induces apoptosis in mouse thymocytes and protects them from glucocorticoid-induced cell death. Cell Immunol. 1992;143:348–56. doi: 10.1016/0008-8749(92)90031-j. [DOI] [PubMed] [Google Scholar]
- 44.Migliorati G, Nicoletti I, D'Adamio F, Spreca A, Pagliacci C, Ricardi C. Dexamethasone induces apoptosis in mouse natural killer cells and cytotoxic T lymphocytes. Immunology. 1994;81:21–6. [PMC free article] [PubMed] [Google Scholar]
- 45.Dancescu M, Rubio-Trujillo M, Biron G, Bron D, Delespesse G, Sarfati M. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates bcl-2 expression. J Exp Med. 1992;17:351–4. doi: 10.1084/jem.176.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B, Zhang D, Ren H. Resistance of Bcl-2 adenovirus vector to HepG(2) cell apoptosis induced by ethanol. Cung Hua Kan Tsang Ping Tsa Chih. 2000;8:215–7. [PubMed] [Google Scholar]
- 47.Meßmer UK, Reed JC, Brune B. Bcl-2 protects macrophages from nitric oxide-induced apoptosis. J Biol Chem. 1996;271:20192–6. doi: 10.1074/jbc.271.33.20192. [DOI] [PubMed] [Google Scholar]
- 48.Von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–28. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 49.Aggarwal S, Gupta S. (1999) Increased activity of caspase 3 and caspase 8 in anti-Fas-induced apoptosis in lymphocytes from ageing humans. Clin Exp Immunol. 1999;117:285–90. doi: 10.1046/j.1365-2249.1999.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Onel KB, Tucek-Szabo CL, Ashany D, Lacy N, Nicollic-Zugic J, Elkon KB. Expression and function of the murine CD95/FASR/APO-1 receptor in relation to B cell ontogenia. Eur J Immunol. 1995;25:2940–7. doi: 10.1002/eji.1830251034. [DOI] [PubMed] [Google Scholar]
- 51.Biswas RS, Cha HJ, Hardwick JM, Srivastav RK. Inhibition of drug-induced Fas ligand transcription and apoptosis by Bcl-XL. Mol Cell Biochem. 2001;225:7–20. doi: 10.1023/a:1012203110027. [DOI] [PubMed] [Google Scholar]
- 52.Liversidge J, Dick A, Gordon S. Nitric oxide mediates apoptosis through formation of peroxynitrite and Fas/Fas ligand interactions in experimental autoimmune uveitis. Am J Pathol. 2002;160:905–16. doi: 10.1016/S0002-9440(10)64913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singhal PC, Bhaskaran M, Patel J, et al. Role of P38 mitogen-activated protein kinase phosphorylation and Fas–FasL interaction in morphine-induced macrophage apoptosis. J Immunol. 2002;168:4025–33. doi: 10.4049/jimmunol.168.8.4025. [DOI] [PubMed] [Google Scholar]