Abstract
Polymorphonuclear neutrophils (PMNs) are capable of synthesizing various pro-inflammatory cytokines which may indirectly influence specific immune responses. PMNs may also have the capacity to present foreign peptides to helper T cells (Th cells). In support of this hypothesis, recent studies have shown that neutrophils, when activated by the correct combination of cytokines, can be induced to express cell surface major histocompatibility complex (MHC) Class II (DR) antigen, CD80 (B7.1) and CD86 (B7.2): molecules required for antigen presentation and subsequent T-cell activation. In this study we have used normal ‘resting’ human peripheral blood neutrophils and demonstrated, using a mild fixation and permeabilization protocol, significant cytoplasmic ‘stores’ of these molecules known to be important in antigen presentation. Cytoplasmic MHC Class II antigen was found with two out of 20 normal donors tested whereas cytoplasmic CD80 and CD86 were found to a variable extent within all normal donors. Surprisingly, we also found several other neutrophil cytoplasmic CD antigens more commonly associated with B cells, i.e. CD20, CD21 (CR2/EBV-R) and CD22 (BL-CAM). All of these antigens were confined to the ‘resting’ cell cytoplasm and were never found to be expressed on the cell surface. To exclude the possibility that these antigens were absorbed from plasma and to provide evidence for active synthesis, we used a novel whole blood in situ hybridization flow cytometry assay method to detect mRNA specific for these antigens within normal PMNs. We also conducted real-time polymerase chain reactions to confirm these findings using CD22 as a good example of an ‘inappropriately expressed’ CD antigen. These observations therefore provide support for the hypothesis that human PMNs have the potential to express molecules required for antigen presentation and cell signalling.
Introduction
Polymorphonuclear neutrophils (PMNs) play an important role in innate immunity, providing the ‘first-line’ of defence against infection. Although phagocytosis is thought to be their main function, activated PMNs indirectly regulate specific immune responses by synthesizing various pro-inflammatory cytokines [e.g. interleukins -1, -6, and -8, tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ)]1–3 and may even be capable of presenting foreign peptides to helper T cells (Th cells). In support of the latter hypothesis, numerous in vitro studies, have shown that cytokine-activated PMNs can express cell surface major histocompatibility complex (MHC) Class II (DR) antigen.4–8 Similarly, MHC Class II antigen has also been observed in vivo on circulating PMNs in active Wegener's granulomatosis,9 on PMNs isolated from cerebral spinal fluid in bacterial meningitis,10 and on synovial fluid PMNs in rheumatoid arthritis.11 Recent studies have also demonstrated CD80 (B7.1) and CD86 (B7.2) on the surface of cytokine-activated PMNs, i.e. co-stimulatory molecules required for T-cell activation normally found on the surface of ‘activated’ B cells and on dendritic antigen-presenting cells (APCs).10,12 Certain chemokine receptors (e.g. CCR6) normally associated with APCs have also been shown to be expressed on cytokine-activated PMNs13 and pre-neutrophils can be induced, by an appropriate combination of cytokines, to acquire a dendritic cell phenotype and to function as potent T-cell stimulators.14 Finally, several functional studies have shown that activated PMNs can present bacterial superantigens and small soluble antigens to Th cells, resulting in a proliferative T-cell response.12,15
If PMNs do indeed have the capacity to function as APCs then they should be able to synthesize and maintain cytoplasmic stores of MHC Class II antigen required for antigen processing within the cytoplasm prior to transport to the cell surface where peptide presentation to Th cells occurs. Although this has not yet been demonstrated, Windhagen and colleagues10 have however, recently demonstrated cytoplasmic stores of the co-stimulatory molecule CD80 within normal human peripheral blood PMNs. Using a mild fixation and permeabilization protocol, suitable for the detection of cytoplasmic CD antigens by flow cytometry, we found that normal human peripheral blood PMNs appear to express cytoplasmic CD80 (B7.1), CD86 (B7.2), CD20, CD21 (CR2/EBV-R) and CD22 (BL-CAM). Cytoplasmic MHC Class II antigen was also detected but was donor restricted. In addition, we also demonstrated mRNA specific for these CD antigens, within PMNs, using a novel whole blood in situ hybridization flow cytometry (ISH-FC) assay and by real-time polymerase chain reaction (PCR; TaqMan). These observations therefore support the hypothesis that PMNs have the capacity to synthesize and store various molecules known to play a role in cell signalling and in antigen presentation. PMNs may therefore have the potential to function as APCs.
Materials and methods
Detection of cell surface CD antigens by flow cytometry: whole blood method
Ethylenediaminetetraacetic acid (EDTA) anti-coagulated whole blood (50 μl) was incubated with fluorochrome-conjugated primary monoclonal antibody (5 μl) at 4° for 30 min. Cells were then washed in 2 ml fluorescence-activated cell sorter (FACS) Lysing solution (Becton Dickinson, Oxford, UK) to remove excess antibody and to lyse any contaminating erythrocytes. Following centrifugation at 200 g for 5 min and aspiration of the supernatant, cells were suspended in 200 μl membrane (Millipore, Watford, UK; 0·22 μm) filtered phosphate-buffered saline (PBS) for acquisition using a Becton Dickinson flow cytometer. Background staining was established using isotype-matched fluorescein isothiocyanate (FITC) -conjugated mouse immunoglobulin G (IgG; Dako Ltd, Ely, UK). Results were analysed using Becton Dickinson ‘Cell Quest’ software on an Apple Mac G3 computer. The optimum concentration for cell surface staining was determined for each antibody by pre-titration.
The various fluorochrome [RPE (Phycoerythrin) or FITC]-conjugated mouse monoclonal antibodies (clone indicated in brackets) used in this study were as follows. All antibodies were IgG1 isotype with the exception of a few, which are indicated * for IgG2a or # for IgG3.
CD2-RPE (MT190), CD3-FITC (UCHT-1), CD4-FITC (MT310), CD5-RPE(DK23), CD8-RPE (DK25), CD11b-RPE(2LPM19c), CD14 *-RPE (TUK4), CD16-RPE (DJ130c), CD18-FITC (MHM23), CD19-RPE (HD37), CD20-FITC (BLy-1), CD21-FITC (1F8), CD22-FITC (4KB128), CD45 (T29/33) and MHC Class II-FITC (CR3/43); and Control Mouse IgG1-FITC + Mouse–IgG1 RPE (X0932), were obtained from Dako Ltd, UK. Serotec Labs, Oxford, UK supplied CD32-FITC(AT10) and CD35-FITC (E11). The CD20-FITC # (H147), CD20-RPE # (H147), CD21-RPE (BU32), CD22-FITC (RFB4), CD80-FITC (3H5), CD86-FITC (24F) were purchased from Caltag-Med Systems Ltd, Touscester, UK. CD80-FITC (MEM233), CD86–FITC (BU63) were suppled by Quest Biomedical, Solihall, UK.
Detection of cytoplasmic CD antigens following fixation and permeabilization of leucocytes
EDTA anti-coagulated whole blood (50 μl) was incubated for 10 min in 2 ml FACS Lysing solution (Becton Dickinson) and following one wash at 200 g for 5 min in PBS, cells were suspended in 50 μl PBS and incubated with 5 μl fluorochrome-conjugated primary monoclonal antibody at 4° for 30 min. Cells were then washed at 200 g and suspended in 200 μl PBS for acquisition on the flow cytometer. Cell permeability was confirmed by the uptake of proidium iodide or by the binding of monoclonal antibodies specific for intermediate filaments or myeloperoxidase. Background uptake of mouse IgG was established using an appropriate FITC or phycoerythrin (PE)-conjugated mouse immunoglobuin (Dako Ltd, Ely, UK). Since this method did not significantly damage cell surface CD antigens, binding of monoclonal antibodies to permeabilized cells actually represents cell surface plus cytoplasmic staining. For this reason baseline values for cell surface staining of viable cells were always measured in parallel for comparison.
Detection of mRNA by in situ hybridization flow cytometry (ISH-FC)
This method was modified from a method originally described by Morvan and colleagues.16 Oligonucleotide probes (25–30-mers) were designed from genetic sequences obtained from the Online Mendelian Inheritance in Man (ONIM) genome database (http:/http://www.ccc.nottingham.ac.uk/mpzjlowe/onimncbi.html) and were analysed for specificity using the European Bioinformatics Institute human database (www2.ebi.ac.uk/fasta3/). Unique sequences were written in the antisense form in the 5′ to 3′ direction and synthesized by Oswell DNA service (Southampton, UK). All probes were labelled with digoxygenein (DIG) using a standard 3′ tailing method according to the manufacturer's instructions (Boehringer Mannheim, Lewes, UK).
For each test, 50 μl of whole EDTA anti-coagulated blood was incubated at room temperature overnight in 2 ml FACS Lysing solution (Becton Dickinson). Following centrifugation at 200 g for 5 min cells the supernatant was discarded and the cell pellet was washed once in 2 ml PBS prior to suspension in 20 μl PBS. To each tube was then added a single DIG-labelled oligonucleotide probe (1 μl probe diluted in 24 μl standard sodium citrate buffer; SSC) and hybridization was allowed to occur in a 37° water bath for 3 hr. The Na+ ion concentration, determined by the concentration of SSC buffer, was found to have a significant effect on the strength of probe binding. Probes were found to bind even at very high stringency (up to 0·1 × SSC). Optimum binding was found to occur at 1 × SSC. Following one wash in 2 ml of 1 × SSC buffer, cells were suspended in 5 μl FITC-conjugated rabbit, anti-DIG F(ab′)2 fragments diluted 1/20 in PBS (Boehringer Mannheim, Germany) at 4° for 30 min. Cells were then washed once in PBS and suspended in 200 μl membrane-filtered PBS for acquisition on the flow cytometer. To minimize background fluorescence, the FL1 channel was reduced from a normal value of 550 to 450 V. Binding of probe was estimated by single histogram analysis relative to the background uptake of a DIG-labelled negative probe, i.e. a sequence not recognized by the human gene database. As a positive control we used probes for CD antigens known to be expressed on the surface of human neutrophils (CD32-FcγRIIa and CD35-CR1) and two cytokines known to be synthesized by neutrophils (TNF-α and IFN-γ).
Probe sequences were as follows: negative control probe, 5′-AAGCACTCCGGGCCCGGGTGTCCCGCGAGC-3′; CD32a, 5′-ACATTCTGAGACATTGGGTCTCCA-3′; CD35, 5′-GTCTGGCACTGCACAGTGGGACCCTACC-3′; TNF-α, 5′-TTGGTCTGGTAGGAGACGGCGATGCGGCTG-3′; IFN-γ, 5′-CTTAGGTTGGCTGCCTAGTTGGCCCGAATC-3′; CD20, 5′-TGAGGATTCCTGATCTTGGGGAGGTTCTGG-3′; CD21, 5′-TCCCGTTAGGGGTCTTAGGCGGAGGTGGAC-3′; CD22, 5′-GACCACTGTCATTGAGGTGCACCGGGTGGA-3′; CD80, 5′-GACGTGACCAAGGAAGTGAAAGAAGTGGC-3′; CD86, 5′-GTTTGCAAATTGGCATGGCAGGTCTGCAGT-3′; MHC class II DRα, 5′-CTTGCGGAAAAGGTGGTCTTCCCTGGGCAG-3′; MHC class II DRβ, 5′-GTCAGCGCTGTCATGCAGGAGCCTCCAGGG-3′.
Isolation of peripheral blood mononuclear cells and PMNs using the lymphoprep barrier centrifugation method
Four millilitres of EDTA anticoagulated whole blood was poured directly onto a barrier centrifuge tube (Sigma Immunochemicals, Poole, UK) containing 3 ml Lymphoprep (Nycomed, Pharma, Norway) solution (specific gravity = 1·077) and centrifuged at 400 g for 10 min. Peripheral blood mononuclear cells (PBMCs) were recovered from the visible band which forms above the barrier at the plasma/lymphoprep interface and washed in PBS at 200 g for 10 min. Erythrocytes and PMNs were recovered from the bottom of the gradient following displacement of the polystyrene barrier using forceps. Cells were suspended in an equal volume of autologous plasma, mixed with 10 ml of 6% (w/v in PBS) Dextran 110 and allowed to sediment by approximately 50% of the initial volume at 4°. Residual erythrocytes were removed by hypotonic lysis. Using this method PMNs were found to be 99% pure as assessed by flow cytometry using forward versus side light scatter and by phenotyping using monoclonal antibodies specific for CD16 (FcγRIII) and CD35 (CR1). Following a final wash in PBS at 200 g for 5 min the cell pellet was suspended in 1 ml of RNAzol™ in an Eppendorf tube and stored at −70° prior to RNA extraction, or in PBS for flow cytometric studies.
Activation of PMNs
Purified PMNs were suspended in 5 ml sterile Iscove's medium (Gibco BRL, Life Technologies, UK) supplemented with 10% heat-inactivated fetal calf serum, l-glutamine (200 mm, Gibco BRL) and antibiotics (penicillin-streptomycin-gentamicin, Gibco BRL), then incubated with an equal volume of formyl-methionyl-leucyl-phenylalanine (fMLP; Sigma Chemical Co., Poole, UK) at 10−5 m, 10−6 m, 10−7 m and 10−8 m and incubated again for 2 hr at 37° in 5% CO2 in air. Cells were then retrieved and washed twice in PBS at 400 g for 5 min prior to RNA extraction or for flow cytometric studies. Activation was confirmed by observing the distinct change in neutrophil morphology, i.e. polarization which occurs in the presence of fMLP.
Quantification of mRNA using real-time PCR
Total RNA was prepared using RNAzol B (Biogenesis, Poole, UK) according to the manufacturer's instructions. RNA was treated with DNA-free (Ambion, Austin, TX) and reverse transcribed to cDNA using superscript II reverse transcriptase (Life Technologies). The cDNA levels of CD22 and hypoxanthine phosphoribosyl transferase (HPRT) were quantified by real-time PCR using an ABI prism 7700 sequence detector according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). The cDNA levels during the linear phase of amplification were normalized against HPRT controls. Determinations were made in triplicate and expressed as the mean ± SD.
Primers and probes used were as follows: HCD22 forward primer, 5′-TGG AGT CCA AGA CTG AGA AAT GG-3′; HCD22 reverse primer, 5′-GAC TTC CTG GGA CTC TTG AAT TTC T-3′; HCD22 TaqMan® probe, 5′-FAM-AAG GCC TTT TCC ACC TCA TAT CCA GCT CC-TAMRA-3′. To demonstrate the efficiency of these probes and primers, real-time PCR was conducted using cDNA prepared from a known B-cell line (Raji cells), from purified mononuclear cells containing 10–20% B cells and from highly purified Th1 and Th2 cells (kindly donated by Dr Duncan Thomson, Department of Immunology, Western Infirmary, Glasgow G11 6NT, UK). The results of four such experiments are expressed as a percentage of HPRT: mean ± SEM, Raji cells, 400 ± 108; PBMCs, 65 ± 42; Th1 cells, 2 ± 1; Th2 cells, 1 ± 1.
Results
Detection of cytoplasmic CD antigens within normal human peripheral blood leucocytes by flow cytometry
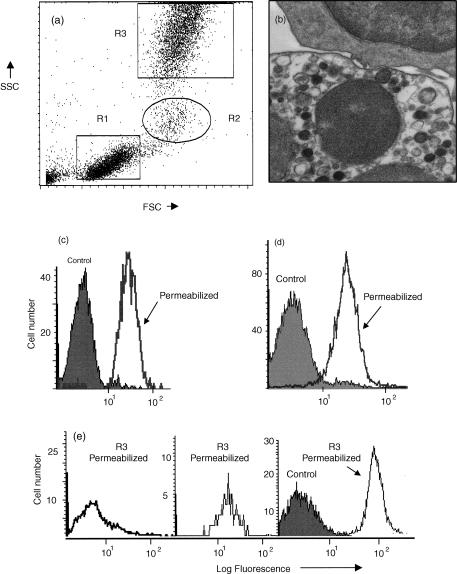
Numerous methods were used to detect cytoplasmic CD antigens including methanol/Triton X-100, enzyme digestion, saponin and several ‘two-stage’ commercial kits including ‘Fix & Perm’ (Caltag Laboratories) and ‘Leukoperm’ (Serotec). We found that simultaneous fixation and permeabilization using FACS Lysing solution (Becton Dickinson) was by far the best method for the detection of cytoplasmic CD antigens within human peripheral blood neutrophils. FACSLyse rapidly removes contaminating erythrocytes and because this solution contains a mixture of formaldehyde (20%) and di-ethylene glycol (<50%), it will also simultaneously fix and permeabilize leucocytes. This method has been used previously for the detection of intracellular enzymes, e.g. myeloperoxidase and terminal deoxynucleotide transferase.17,18 The FACSLyse solution method proved to be rapid, had no significant adverse effect on cell morphology (see Fig. 1a,b) and had minimal effect on leucocyte cell surface CD antigen expression. Efficient cell permeabilization was confirmed by the uptake of propidium iodide and by the detection of intermediate filaments and neutrophil myeloperoxidase (Fig. 1c–e). For this reason we used FACS Lyse solution as our standard method for the detection of intracellular CD antigens.
Figure 1.
Demonstration of cytoplasmic antigens using FACSLysing solution. (a) Dot-plot of forward (FSC) versus side angle light scatter (SSC) showing three main types of human peripheral blood leucocyte: R1 = lymphocytes, R2 = monocytes and R3 = neutrophils. (b) Transmission electron micrograph of leucocytes following fixation and permeabilization. Intact plasma and nuclear membranes of a lymphocyte (top cell) and a neutrophil (bottom cell) are visible. Cytoplasmic organelles are also intact. (c) Histogram analysis of neutrophils (R3) showing uptake of propidium iodide by control viable cells (solid area) compared with fixed and permeabilized cells (single line). (d) Histogram analysis of neutrophils (R3) showing binding of monoclonal anti-vimentin (intermediate filaments) by control viable cells (solid area) compared with fixed and permeabilized cells (single line). (e) Histogram analysis of lymphocytes (R1), monocytes (R2) and neutrophils (R3), showing binding of monoclonal anti-myeloperoxidase following fixation and permeabilization of cells (single line). This monoclonal antibody did not bind to viable cells (surface staining) as shown for R3 as a solid area.
When cell surface and ‘cytoplasmic’ staining were measured in parallel (i.e. before and after permeabilization) no change in binding of the mouse IgG1 isotype control antibody was observed, nor was there a significant increase in the binding of monoclonal antibodies specific for CD2, CD3, CD4, CD5, CD8, CD11b, CD14, CD16, CD18, CD19, CD35 and CD45. A significant increase in binding of monoclonal antibodies specific for CD20, CD21, CD22, CD80 and CD86 was however, observed mainly within PMNs (gated as R3) following cell permeabilization (Fig. 2). This increase was observed with different clones of these monoclonal antibodies and occurred irrespective of the mouse IgG isotype or the type of fluorochrome used. When viewed under UV-light it was evident that fluorescence was confined to the cytoplasm of PMNs with little or no staining of the nucleus observed (see cytoplasmic CD86 in Fig. 2). We did observe a slight increase in these CD antigens within lymphocytes and monocytes obtained from a few individuals but this cytoplasmic staining was very low in comparison with levels observed with PMNs.
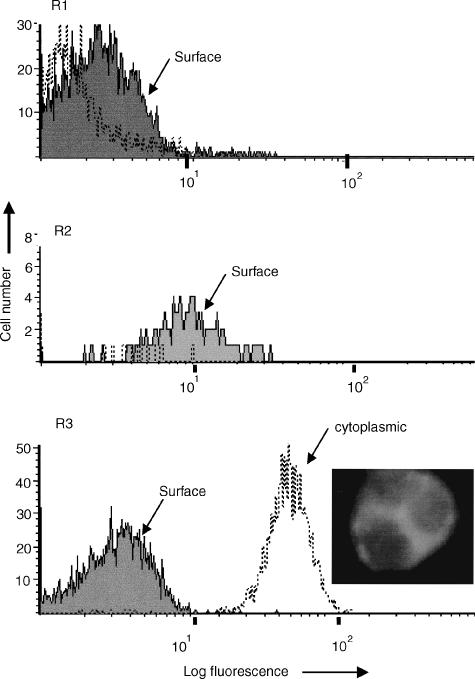
Figure 2.
Demonstration of cytoplasmic CD86 (B7.2) using FACSLysing solution. R1, histogram analysis of lymphocytes showing no significant binding of FITC-conjugated monoclonal anti-CD86 either before (solid area) or after (dotted line) permeabilization. R2, weak surface staining of monocytes for CD86 but no cytoplasmic staining observed. R3, strong staining of neutrophils for CD86 is evident (mean fluorescence intensity = 50), following fixation and permeabilization, as indicated by the dotted line. The overlay shows that surface staining (solid area) was not observed when viable cells were used in parallel. The photograph shows that when viewed under UV-light, fluorescence (FITC-anti-CD86) was observed predominantly within the cytoplasm of neutrophils with little or no nuclear staining observed. Similar results were obtained using monoclonal antibodies specific for CD20, CD21, CD22 and CD80, the only exception being that these CD antigens were not expressed on the surface of monocytes. In contrast, no significant increase in binding, following permeabilization, was observed with isotype-matched control mouse IgG1 or with monoclonal antibodies specific for CD2, CD3, CD4, CD8, CD11b, CD14, CD16, CD18, CD19, CD25, CD32, CD35, or CD45.
Normal variation
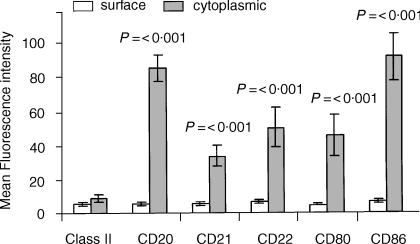
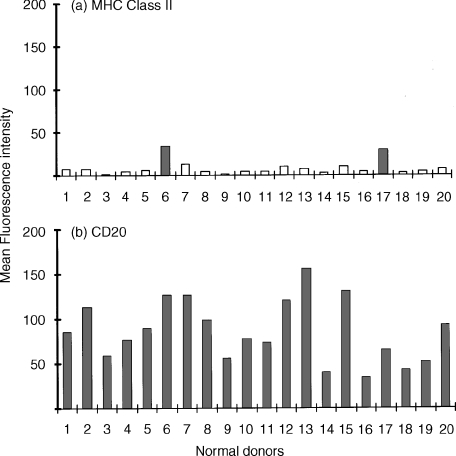
In a group of 20 normal subjects, permeabilized PMNs showed a significant increase, relative to cell surface values, in the mean fluorescence intensity of staining for CD20, CD21, CD22, CD80 and CD86 (Fig. 3). Weak expression of cytoplasmic MHC Class II antigen was also found within PMNs but this was only observed with two of 20 donors (Fig. 4a) whereas the other CD antigens, e.g. CD20 were found to a variable extent with all donors tested (Fig. 4b).
Figure 3.
Comparison of surface versus cytoplasmic staining for CD antigens following fixation and permeabilization of neutrophils: normal donors. Surface and cytoplasmic CD antigens within peripheral blood neutrophils (R3), measured in parallel using the whole blood obtained from 20 normal donors, were compared. Results derived from histogram analysis are expressed as the mean fluorescence intensity (MFI) with vertical bars indicating the mean ± SEM for each CD antigen tested. MFI results, indicating cytoplasmic staining, which were significantly higher than cell surface values are indicated by a P-value (Mann–Whitney test). All values were calculated in relation to non-specific background staining using an appropriate fluorochrome-conjugated, isotype-matched mouse immunoglobulin (Dako). The mean background value for mouse IgG (±SEM) for 20 normal donors was 5 ± 2 for normal cells and 6 ± 2 for permeabilized cells.
Figure 4.
Demonstration of cytoplasmic CD antigens by flow cytometry following fixation and permeabilization of neutrophils: normal donor variation. (a) Demonstration of cytoplasmic MHC Class II antigen within normal human peripheral blood neutrophils (R3) obtained from 20 normal donors. Results obtained from histogram analysis are expressed as the mean fluorescence intensity (MFI). Two donors (indicated by solid colour) were found to express significant levels (i.e. >background uptake of isotype matched mouse immunoglobulin, MFI = 6 ± 2) of cytoplasmic MHC Class II antigen. (b) Demonstration of cytoplasmic CD20 antigen within normal human peripheral blood neutrophils (R3) obtained from the same 20 normal donors measured in parallel. Results obtained from histogram analysis are expressed as the MFI. All normal donors were found to express significant (i.e. >background uptake of isotype-matched mouse immunoglobulin, MFI = 6 ± 2] but variable levels of cytoplasmic CD20. Essentially similar results were found for CD21, CD22, CD80 and CD86.
Detection of mRNA specific for CD antigens within normal human peripheral blood neutrophils
In situ hybridization flow cytometry (ISH-FC)
This method has previously been used to demonstrate mRNA within cultured cells and human PBMCs.16 We have modified this method for use with whole blood, thus allowing for precise and simultaneous localization of probe binding to lymphocytes, monocytes and neutrophils from the same individual. This method is rapid and requires only small volumes of blood. In all experiments, binding of specific DIG-labelled oligonucleotide probes was measured relative to a negative control probe, i.e. a sequence not specific for any protein in the human gene database. Similarly, in each experiment we included several positive probes, i.e. molecules known to be expressed on the cell surface or synthesized by PMNs.
No significant increase in binding of oligonucleotide probes, relative to the background control probe, was observed with ‘resting’ permeabilized human peripheral blood lymphocytes and monocytes (see Table 1).
Table 1.
In situ hybridization flow cytometry assay (ISH-FC): normal peripheral blood leucocytes, mean and SEM of 12 experiments
| Control probes | Test probes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | CD32 | CD35 | TNF | IFN | CD20 | CD21 | CD22 | CD80 | CD86 | IIα | II B | |
| Lymphocytes | ||||||||||||
| Mean | 10 | 10·4 | 12 | 11·4 | 11·2 | 10 | 9·4 | 10·6 | 14·2 | 9 | 11·4 | 11·6 |
| SEM | 1·6 | 1·8 | 2·5 | 3·1 | 2·3 | 1·6 | 1·8 | 1·6 | 1·8 | 1·7 | 2·6 | 3 |
| Monocytes | ||||||||||||
| Mean | 10·5 | 13·5 | 14·3 | 14 | 13·9 | 12·8 | 11·6 | 13·9 | 15·7 | 9·6 | 14 | 14·4 |
| SEM | 1 | 2·2 | 2·3 | 3·4 | 2·7 | 1·4 | 1·8 | 2·1 | 2 | 2 | 2·7 | 3·1 |
| Neutrophils | ||||||||||||
| Mean | 16·8 | 31·6 | 36·9 | 32·8 | 34 | 38·5 | 31·5 | 33·9 | 34·5 | 31·3 | 38·7 | 33·5 |
| SEM | 2 | 3·5 | 4·1 | 6·5 | 5 | 3·6 | 4·2 | 3 | 4·5 | 3 | 4 | 4·1 |
This table shows detection of mRNA specific for various CD antigens using DIG-labelled oligonucleotide probes. Results are expressed as the mean fluorescence intensity of binding (MFI) using normal human peripheral blood leucocytes obtained from 12 different normal donors. The three main leucocyte populations were analysed separately: Region 1 = lymphocytes, R2 = monocytes and R3 = neutrophils.
In contrast, significant (all P values <0·001; Mann–Whitney test) binding of all probes, relative to background, was observed with PMNs (see Table 1). A typical example of positive probe binding, to permeabilized PMNs is shown in Fig. 5(a). When viewed under UV-light it was evident that fluorescent probes were binding to the cell cytoplasm (Fig. 5b) and staining was not observed when cells were pretreated with RNase enzyme (Fig. 5c). The scatter plot shown in Fig. 6 shows the full range of probe binding to PMNs. On average, the test probes produced mean fluorescence intensity values comparable to those observed with positive control probes specific for CD32, CD35, TNF-α and for IFN-γ (see Table 1).
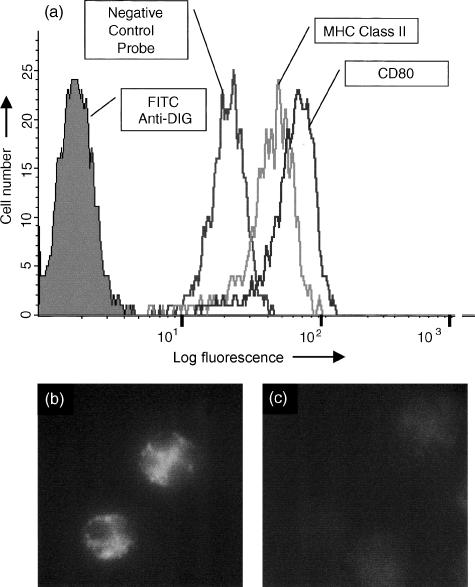
Figure 5.
In situ hybridization flow cytometry (ISH-FC) assay using fixed and permeabilized human peripheral blood neutrophils. (a) Histogram overlay showing binding of secondary antibody alone (FITC-anti-DIG), negative control probe and probes specific for MHC Class II B-chain and for CD80 (B7.1). From this type of histogram analysis the binding of specific DIG-labelled oligonucleotide probes can be expressed as the mean fluorescence intensity (MFI) and can be compared directly with the negative control probe which indicates background fluorescence. (b) When these cells were viewed under UV-light it was evident that probes were binding to the cytoplasm of neutrophils with little or no nuclear staining observed. (c) Following pretreatment of cells with RNase, no significant binding of the probes was observed.
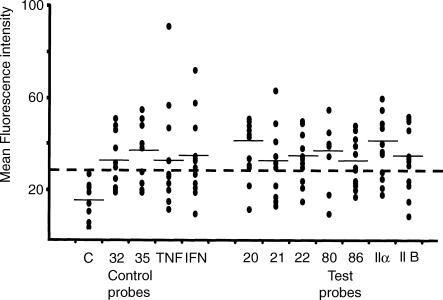
Figure 6.
In situ hybridisation flow cytometry (ISH-FC) assay: binding of control and test probes to normal human peripheral blood neutrophils. Results derived from histogram analysis, as shown in Figure 5(a), were expressed as the mean fluorescence intensity (MFI). These MFI values were plotted as a scatter diagram to show the full range of binding to neutrophils (R3) observed with 12 normal donors. Some values were identical and appear as a single dot. The average MFI value is indicated by a horizontal bar. Values greater than the upper limit for binding of the negative probe, as indicated by the dotted line, were considered significant. Control oligonucleotide probes used in all experiments were as follows: negative control probe, a sequence not recognized in human gene database (this DIG-labelled probe was used to indicate background fluorescence in this assay); probes specific for CD32 (FcγRII a-isoform) and CD35 (CR1) (cell receptor molecules known to be expressed on the surface of human neutrophils); and probes specific for TNF-α and IFN-γ (cytokines known to be synthesized by activated neutrophils).
Real-time PCR (TaqMan)
Since the in situ hybridization flow cytometry (ISH-FC) method described above is relatively new, we sought confirmation of these findings using a more conventional PCR method.
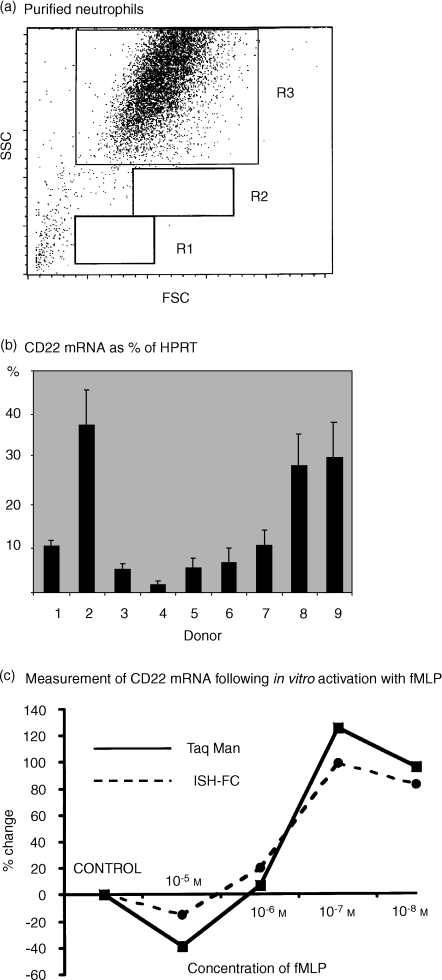
Pure neutrophils were obtained using the barrier centrifugation method (Fig. 7a), RNA extracted and transcribed into cDNA. Real-time PCR was then performed using probes and primers specific for CD22. In all experiments a housekeeping gene (HPRT) was used as a standard. On average, normal human peripheral neutrophils expressed mRNA for CD22 at a level of 14% of HPRT with the highest individual value being 38% (Fig. 7b). When neutrophils were activated in vitro using fMLP, it was apparent that an increase in CD22 mRNA of up to 130% could be induced using concentrations between of 10−7 and 10−8 m. Furthermore, this change was demonstrable, using neutrophils from the same donor, by real-time PCR and by ISH-FC, with both methods producing a virtually identical dose–response curve (see Fig. 7c). Curiously, cytoplasmic protein levels, as assessed by the binding of monoclonal antibodies to permeabilized cells, appeared to fall as the mRNA increased. For example, at concentrations of 10−7 m fMLP the mean fluorescence intensity of staining for the following PMN cytoplasmic CD antigens decreased relative to control values determined in control cultures containing no fMLP: CD20 (60% decrease), CD21 (15% decrease), CD22 (61% decrease) and MHC Class II antigen (34% decrease).
Figure 7.
Detection of mRNA specific for CD22 within normal and activated human peripheral blood neutrophils. Comparison of real-time PCR (TaqMan) and ISH-FC. (a) Dot-plots of forward versus side light scatter of human peripheral blood neutrophils isolated by the barrier method. Neutrophils gated as region 3 were all shown to express cell surface CD16, CD32 and CD35. Cells isolated by this method were virtually 100% pure as indicated by the lack of cells appearing in the lymphocyte (R1) and monocyte (R2) regions. (b) Using real-time PCR (TaqMan), mRNA for CD22 was detected to a variable extent in all of nine normal donors tested. Vertical bars indicate the mean ± SEM of triplicate measurements. Results are expressed as a percentage of a housekeeping gene (HPRT). (c) Highly purified human peripheral blood neutrophils were activated in vitro using various concentrations of fMLP. mRNA specific for CD22 was then detected in parallel using real-time PCR (TaqMan) and by ISH-FC). Results were expressed as the percentage change in mRNA relative to control cells which were incubated with culture medium alone.
Discussion
Increasing evidence suggests that human PMNs have an important role to play in regulating specific immune responses and in antigen presentation.12,15 In this study we have demonstrated, at the protein and mRNA level, significant ‘cytoplasmic stores’ of various co-stimulatory molecules known to be important in antigen presentation within normal ‘resting’ peripheral blood PMNs, i.e. CD80 (B7.1) and CD86 (B7.2). In a few cases cytoplasmic MHC Class II antigen was also detected. These observations therefore agree well with the findings of others who have detected these antigens on the surface of activated neutrophils.4–12 Somewhat surprisingly we also found significant cytoplasmic expression of CD20, CD21(CR2/EBV-R) and CD22(BL-CAM); molecules which are normally expressed on the surface of B cells.19 It seems unlikely that the detection of these PMN cytoplasmic antigens was an artefact because the molecules were demonstrated using several different clones of monoclonal antibodies and were detected irrespective of the mouse IgG isotype or fluorochrome-conjugate used. Furthermore, mouse monoclonal antibodies specific for a wide range of T-cell, large granular lymphocytes and monocyte CD antigens did not show any increase in binding following cell permeabilization. All of the cytoplasmic CD antigens found within PMNs in this study are usually found on the surface of B cells but it is worth noting that several other B-cell CD antigens were not evident within PMNs, i.e. CD19, CD32 (FcγRII) and CD35 (CR1).
Cytoplasmic CD80 has been previously demonstrated within normal human PMNs10 but as far as we are aware this study represents the first report of cytoplasmic CD20, CD21, CD22, CD86 and MHC Class II antigen within normal human PMNs. The detection of these molecules therefore provides strong support for the hypothesis that human PMNs can actively synthesize immunoregulatory molecules3 and have the potential to act as APCs.12,15 While CD20, CD21, CD22, CD80 and CD86 were found to a variable extent within PMNs from all 20 normal subjects studied, cytoplasmic MHC Class II antigen was found in only two normal donors. The reason for this donor restriction is not clear but many studies have shown that, cytokine-driven, up-regulation of MHC Class II antigen, on PMNs varies considerably within the normal population.6–10 Individuals with high levels of cytoplasmic CD antigens may therefore belong to the ‘high-responder’ group as described by Gosselin and colleagues.6 Increased expression of cytoplasmic MHC Class II (DR) antigen may reflect the potential ability of an individual to present foreign or even self-antigens to Th cells. Since increased or inappropriate expression of MHC Class II antigen has been associated with the development of autoimmune disease20 it would be of interest to measure intracellular MHC Class II antigen in various patient groups. These studies are currently underway in our laboratory.
To exclude the possibility that PMN cytoplasmic antigens were simply absorbed from human plasma and to provide evidence for the hypothesis that these cells can actively synthesize these CD antigens, we attempted to detect mRNA specific for these antigens using an ISH-FC.16 Relative to background uptake of a non-specific oligonucleotide probe, we found significant binding of probes specific for CD20, CD21, CD22, CD80 and CD86 within the cytoplasm of normal human PMNs. Considerable donor variation was observed but in general the levels of mRNA detected by this assay were comparable to those for CD antigens known to be expressed on the surface of PMNs or for cytokines known to be actively synthesized by these cells. Significant mRNA levels, specific for these CD antigens, were detected within PMNs obtained from the majority (66–83%) of individuals tested, which agrees well with the detection of cytoplasmic CD antigens at the protein level. The only exception to this was MHC Class II antigen where mRNA was found in about 66% of donors but protein was found in only 10%. This observation suggests that synthesis of MHC Class II antigen by PMNs, like activated T cells, may be regulated at a post-transcriptional level.21 Up-regulation of MHC Class II antigen may therefore be a key factor involved in antigen presentation by PMNs, with cytoplasmic protein being detected only when cells are activated by the appropriate combination of cytokines.12
Because the ISH-FC assay has not previously been used for the detection of mRNA within neutrophils we decided to compare this with the more conventional real-time PCR (TaqMan) method22 using cDNA isolated from highly purified neutrophils. In these experiments we used TaqMan probes and primers specific for CD22. This antigen was selected because CD22 is exclusive for B cells, and a cytoplasmic form of this molecule has been found within immature and malignant B cells.23 In addition, CD22 was never found on the surface of PMNs but was present, to a variable extent, within the PMN cytoplasm of all 20 normal donors tested and mRNA for CD22 was found within 75% of normal donors tested using the ISH-FC assay. Using real-time PCR, mRNA specific for CD22 was found to a variable extent (mean 14%, range 5–38% of HPRT) using PMN isolated from nine normal donors. When PMNs were activated in vitro using fMLP, mRNA for this antigen was observed to increase in a dose-dependent manner. This increase in mRNA was observed using both methods resulting in virtually identical dose–response curves with optimum enhancement of mRNA occurring between 10−7 and 10−8 m fMLP.
Curiously, all cytoplasmic CD antigen protein levels were observed to fall dramatically following incubation with fMLP, suggesting that cytoplasmic stores are rapidly depleted following cell activation. No significant change in cell surface expression of these CD antigens was observed following activation by fMLP. This observation would agree well with others who have also reported that PMNs contain significant cytoplasmic stores of molecules required for rapid deployment within sites of inflammation.24–26
The ISH-FC assay has the advantage of visualizing probe binding to a particular cell type within a heterogeneous population of cells, thus allowing for simultaneous detection of mRNA within lymphocytes, monocytes and neutrophils in each blood sample. In addition to the main advantage of specific cell localization, this assay also has several other desirable features. For example, this method employs a single DIG-labelled oligonucleotide probe and does not require a special FAM/TAMRA-labelled TaqMan probe and PCR amplification primers, nor does it require expensive TaqMan PCR detection equipment. No cell isolation, RNA purification and reverse transcription methods are required and the danger of amplifying sequences from contaminating cells is eliminated. Furthermore, because the ISH-FC assay is rapid, relatively inexpensive and requires only small volumes of blood (an important consideration when dealing with patients) it is possible to run multiple probes in parallel in a single experiment. On the negative side, this method does not employ any PCR amplification, which means that probes will only hybridize with cells containing relatively high levels of undegraded mRNA within the cytoplasm and it is limited to the study of cells in suspension.
The findings reported in this study therefore support the hypothesis that human PMNs have significant cytoplasmic stores of antigens which are normally expressed on the surface of activated B cells and dendritic APCs. PMNs therefore have the capacity actively to synthesize important immunoregulatory molecules e.g. CD20, CD21 and CD22, and because they have stores of CD80, CD86 and, in some cases, MHC Class II antigen, they have the potential to become functionally active APCs. Mobilization of intracellular stores followed by transport to the cell surface may occur within sites of inflammation where the appropriate cytokines, required for cell activation, are present.11 Active synthesis of CD antigens may occur following activation as evidenced by the rapid increase in mRNA observed following stimulation of cells with fMLP. Active synthesis would be required to replace cell surface receptors lost following ligand–receptor interactions, for release of these molecules into the fluid-phase as soluble immunoregulatory molecules or simply to replenish depleted stores of these cytoplasmic antigens.
In conclusion, the findings reported here therefore lend further support to the hypothesis that PMNs do indeed have the potential to play an active role in immunoregulation and in antigen presentation. The role of these cells in the specific immune system has therefore been vastly underestimated and requires further investigation.
References
- 1.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 2.Yeaman GR, Collins JE, Currie JK, Guyre PM, Wira CR, Fanger MW. IFN-γ is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160:5145–53. [PubMed] [Google Scholar]
- 3.Newburger PE, Subrahmanyam YVBK, Weissman SM. Global analysis of neutrophil gene expression. Curr Opin Hematol. 2000;7:16–20. doi: 10.1097/00062752-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto S, Takei M, Moriyama M, Imanishi H. Enhancement of Ia like antigen expression by interferon gamma in polymorphonuclear leukocytes. Chem Pharm Bull. 1987;35:436–9. doi: 10.1248/cpb.35.436. [DOI] [PubMed] [Google Scholar]
- 5.Buckle AM, Jayaram Y, Hogg N. Colony stimulating factors and interferon-gamma differentially affect cell surface molecules shared by monocytes and neutrophils. Clin Exp Immunol. 1990;81:339–45. doi: 10.1111/j.1365-2249.1990.tb03342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gosselin EJ, Wardwell K, Rigby WFC, Guyre P. Induction of MHC Class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-γ, and IL-3. J Immunol. 1993;151:1482–90. [PubMed] [Google Scholar]
- 7.Smith WB, Guida L, Sun Q, et al. Neutrophils activated by granulocyte-macrophage colony-stimulating factor express receptors for interleukin-3 which mediate Class II expression. Blood. 1995;86:3938–44. [PubMed] [Google Scholar]
- 8.Lei L, Altstaedt J, Von Der Ohe M, Proft T, Gross U, Rink L. Induction of interleukin-8 in human neutrophils after MHC Class II cross-linking with superantigens. J Leuk Biol. 2001;70:80–6. [PubMed] [Google Scholar]
- 9.Iking-Konert C, Vogt S, Radsak M, Wagner C, Hansch GM, Andrassy K. Polymorphonuclear neutrophils in Wegener's granulomatosis acquire characteristics of antigen presenting cells. Kidney Int. 2001;60:2247–62. doi: 10.1046/j.1523-1755.2001.00068.x. [DOI] [PubMed] [Google Scholar]
- 10.Windhagen A, Maniak S, Gebert A, Ferger I, Wurster U, Heindenreich F. Human polymorphonuclear neutrophils express a B7-1-like molecule. J Leuk Biol. 1999;66:945–52. doi: 10.1002/jlb.66.6.945. [DOI] [PubMed] [Google Scholar]
- 11.Cross A, Bucknall RC, Moots RJ, Edwards SW. Expression of MHC Class II molecules by synovial fluid neutrophils. American College of Rheumatology, Annual Scientific Meeting, San Francisco. 2001. Abstract Number 1501. http://www.rheumatology.org.
- 12.Radsak M, Iking-Konert C, Stegmaier S, Andrassy K, Hansch GM. Polymorphonuclear neutrophils as accessory cells for T-cell activation: major histocompatibility complex Class II restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;101:521–30. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashiro S, Wang J, Yang D, Gong W, Kamohara H, Yoshimura T. Expression of CCR6 and CD83 by cytokine activated human neutrophils. Blood. 2000;96:3958–63. [PubMed] [Google Scholar]
- 14.Oehler L, Majdic O, Pickl WF, et al. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J Exp Med. 1998;187:1019–28. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanger NA, Liu C, Guyre PM, et al. Activation of human T cells by major histocompatibility complex class II expressing neutrophils. Proliferation in the presence of superantigen, but not tetanus toxoid. Blood. 1997;89:4128–35. [PubMed] [Google Scholar]
- 16.Morvan PY, Picot C, Dejour R, Gillot E, Genetet B, Genetet N. In situ hybridisation and cytofluorometric analysis of cytokine mRNA during in vitro activation of human T cells. Eur Cytokine Network. 1994;5:469–80. [PubMed] [Google Scholar]
- 17.Tay SP, Cheong SK, Hamidah NH, Ainoon O. Flow cytometric analysis of intracellular myeloperoxidase distinguishes lymphocytes, monocytes and granulocytes. Malaysian J Pathol. 1998;20:91–4. [PubMed] [Google Scholar]
- 18.Syrjala MT, Tiirikainen M, Jansson SE, Krusius T. Flow cytometric analysis of terminal deoxynucleotidyl transferase. A simplified method. Am J Clin Pathol. 1993;99:298–303. doi: 10.1093/ajcp/99.3.298. [DOI] [PubMed] [Google Scholar]
- 19.Sandilands GP, Perry M, Wootton M, Hair J, More IAR. B-cell antigens within normal and activated human T cells. Immunology. 1998;96:424–33. doi: 10.1046/j.1365-2567.1999.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottazzo GF, Pujol-Borrell R, Hanafusa T, Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983;12(2)(8359):1115–19. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- 21.Caplen HS, Salvadori S, Gansbacher B, Zier K. Post-transcriptional regulation of MHC class II expression in human T cells. Cellular Immunol. 1992;139:98–107. doi: 10.1016/0008-8749(92)90103-v. [DOI] [PubMed] [Google Scholar]
- 22.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–94. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 23.Farahat N, van der Plas D, Praxedes M, Morilla R, Matutes E, Catovsky D. Demonstration of cytoplasmic and nuclear antigens in acute leukaemia using flow cytometry. J Clin Pathol. 1994;47:843–9. doi: 10.1136/jcp.47.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–21. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 25.McCourt M, Wang JH, Sookhai S, Redmond HP. Activated human neutrophils release hepatocyte growth factor/scatter factor. Eur J Surg Oncol. 2001;27:396–403. doi: 10.1053/ejso.2001.1133. [DOI] [PubMed] [Google Scholar]
- 26.Garrison S, Hojgaard A, Patillo D, Weis JJ, Weis JH. Functional characterization of Pactolus, a beta-integrin-like protein preferentially expressed by neutrophils. J Biol Chem. 2001;276:35500–11. doi: 10.1074/jbc.M104369200. [DOI] [PubMed] [Google Scholar]