Abstract
Granuloma is a typical feature of tuberculosis. We evaluated the chemotaxis of selected human leucocyte subsets induced by macrophages incubated with Mycobacterium tuberculosis (MT)-derived products in vitro. The release of monocyte chemotactic protein 1 (MCP-1) and interleukin-8 (IL-8) correlated with the specific induction of strong chemotaxis towards monocytes and polymorphonuclear leucocytes (PMNs). γδ and T helper type 1 (Th1) αβ lymphocytes were chemoattracted, while T-resting, IL-2-activated and Th2 lymphocytes were unaffected. Activation with mycobacterium-derived, phosphate-containing components, modulated the chemokine receptor profile of γδ T lymphocytes as well as their pattern of cyto-chemokine production, disclosing a potential for their active participation in granuloma formation. In particular, CXCR3 and IP-10, which we found to be released by MT-pulsed alveolar macrophages, seem to represent the receptor–counter-receptor pair implicated in the chemotaxis of γδ lymphocytes. Immunohistochemical analysis and in situ hybridization revealed the in vivo presence of IL-8, MCP-1 and IL-10 in lymph node and lung tuberculous granulomas. Our results underscore the role of MT extracts in the induction of macrophage-derived chemokines responsible for the orchestrated recruitment of PMNs, monocytes, and Th1 and γδ T cells, as well as in the regulation of γδ function.
Introduction
Mycobacterium tuberculosis (MT) is a facultative intracellular pathogen of human mononuclear phagocytes,1,2 capable of causing different clinical outcomes of tuberculosis. Accumulating evidence indicates that tuberculous granulomas are mainly comprised of T cells and cells of the monocytic lineage,3–5 and represent the typical MT lesion.
The histological appearance of established granulomatous foci strongly suggests that a defined sequence of events, involving both natural and adaptive components of the immune response, is responsible for their formation. In this respect, inflammatory and chemotactic cytokines released by leucocytes or stromal cells are likely to represent a predominant mechanism whereby the recruitment of various leucocyte subsets is well regulated to the site of MT infection; in addition, the expression of particular sets of chemokine receptors provides leucocytes with an exclusive combinatorial ‘address code’ for positioning within the tissue in a multistep navigation mode.6,7 It has indeed been reported that MT-derived products can elicit strong and specific release of a host of chemokines upon phagocytosis by blood monocytes or by resident alveolar macrophages.8,9 In agreement with these findings, selected chemokines have been detected in large amounts in the bronchoalveolar lavage fluid of patients with active pulmonary tuberculosis.10,11
As a result of the complexity of the intervening events required for granuloma formation, the process is susceptible to fail at multiple steps, thus generating unsuccessful clinical outcomes: the more frequent of these being the syndrome known as latent tuberculosis, characterized by MT persistence,12 and the lethal, active tuberculosis, associated with a ‘spread’, disorganized histology.13
The relative contribution of T-cell subsets in the control of infection has been dissected in a straightforward manner in mice, using several knockout models.14,15 In humans, T lymphocytes bearing the γδ T-cell receptors (TCR) accumulate in early mycobacterial lesions.16 Moreover, mycobacteria-activated γδΤ lymphocytes secrete type I cytokines [such as tumour necrosis factor-α, interferon-γ (IFN-γ)] and kill MT-infected macrophages in vitro, suggesting their potential role in vivo in the immune response against the pathogen.17,18
The protective capacity of each leucocyte population is secondary to its migratory capability which allows it to reach the inflammatory site.
We approached the present work to establish whether chemokines released from MT-pulsed macrophages selectively govern the migration of specific leucocyte subpopulations, and in turn regulate the expression of the correlated receptors.
We show that MT-pulsed macrophages are indeed able to chemoattract polymorphonuclear cells (PMNs), monocytes, and T helper type 1 (Th1) and γδ T lymphocytes selectively; moreover, upon exposure to MT components, including the isopentenyl-pyrophosphate (IPP) antigen, γδ T-lymphocytes modulate their chemokine receptor profile and become efficient cytokine producers. We therefore postulate that γδ lymphocytes can contribute to the containment of MT infection both through their effector functions and through the recruitment of new immunocompetent cells.
Materials and methods
Chemicals and antibodies
The MT whole cell lysate from the strain H37-R (WC), containing whole cell wall, and the MT lypoarabinomannan-free culture filtrate proteins from the same strain (CF), were obtained from the Department of Microbiology, Colorado State University, Fort Collins, CO. The Mycobacterium bovis His-Ag85C Protein (BCG) was obtained from the AIDS Reagent Program (Mckesson Bioservices Corporation, Rockville, MD). The γδ T-cell clones were stimulated with IPP (Sigma, St Louis, MO) at a final concentration of 10 μm and with anti-CD3 monoclonal antibody (mAb; 5 μg/ml) (OK-T3, Ortho Diagnostics, Raritan NJ) followed by cross-linking with goat anti-mouse antiserum (Zymed, San Francisco, CA). Both anti-interleukin-8 (IL-8) and anti-monocyte chemotactic protein (MCP-1) rabbit sera were kindly provided by P. Allavena (Mario Negri Institute, Milan, Italy). Recombinant interferon γ-inducible protein 10 (IP-10) and I-309 were from R & D Systems (Minneapolis, MN).
Cell isolation and cultures
Human peripheral blood lymphocytes (PBL) were isolated from donors' buffy coats from the San Raffaele Hospital (HSR) Blood Bank, by separation on Ficoll–Hypaque (Nycomed Pharma S., Oslo, Norway), followed by two steps of plastic adherence to deplete monocytes. PBL were resuspended in RPMI-1640 medium supplemented with 2 mm l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin (Biochrom, Berlin, Germany), 10% heat-inactivated fetal calf serum (FCS; PAA Labour, Linz, Austria) (complete medium, CM) and immediately used (resting T cells). To obtain IL-2-activated T cells, PBL were cultured for 15 days in CM supplemented with recombinant IL-2 at 400 U/ml (Eurocetus, Amsterdam, the Netherlands). Adherent CD14-positive monocytes (PBM) were recovered and resuspended in CM at a final concentration of 5 × 105 cells/ml.
Th1 and Th2 lines were generated from cord blood leucocytes as described.19 Briefly, human neonatal leucocytes were isolated from freshly collected, heparinized, neonatal blood by Ficoll–Hypaque density gradient centrifugation. Neonatal T-cell preparations were >70% CD45Ra-positive and 50–60% CD4-positive. Th1 and Th2 cell lines were propagated in the presence of phytohaemagglutinin (PHA) and either IL-12 and neutralizing anti-IL-4 antibodies for Th1 lines, or IL-4 and neutralizing anti-IL-12 antibodies for Th2 lines, respectively. Both were expanded in CM containing 100 U/ml of IL-2 and used on day 10. The purity of the two Th cell lines was tested through intracellular staining [and an enzyme-linked immunosorbent assay (ELISA) on the supernatants] for the two chemokines IFN-γ and IL-4: 88% of Th1 were IFN-γ-positive and 80% of Th2 were IL-4-positive (not shown).
γδ T-cell clones were propagated from peripheral blood, as described.20 Briefly, after PBM isolation, the αβ T cells were depleted by magnetic bead purification (Dynabeads, M450, Dynal, Oslo, Norway), after incubation with anti-CD4 and anti-CD8 mAbs. The resulting cell population contained more than 95% γδ T cells, as assessed by staining with the pan-γδ TCR1δ1 mAb (T Cell Sciences, Cambridge, MA). γδ T-cell clones were obtained by limiting dilution and propagated by cyclic restimulation (every 3–4 week) with irradiated allogeneic PBM and PHA (5 μg/ml), in RPMI-1640 supplemented with 10% FCS, in the presence of recombinant IL-2 (50 U/ml).
Human alveolar macrophages (AM) were obtained from non-pathological areas of lung from patients undergoing partial pneumectomy in the Surgery Department of HSR. Briefly, the lung was cut into small pieces and repeatedly perfused with RPMI-1640: the cells thus obtained were seeded on plastic wells (Costar, Cambridge, MA), those recovered after adherence (CD68-positive cells) were resuspended at a final concentration of 1 × 106/ml.
PMNs were obtained from venous blood of healthy donors, anti-coagulated with citric acid and sodium citrate, as described.21 Briefly, the cells were isolated by dextran (Sigma) sedimentation followed by Ficoll–Hypaque gradient and hypotonic lysis of erythrocytes; they displayed a CD15s (anti-Sialyl-Lex) positive phenotype.
In vitro chemotaxis assay
The chemotactic ability of the different cell types was assayed using a double-chamber system (Transwell Costar, Cambridge, MA), as previously described.22 Briefly, 105 cells, labelled with 51Cr (10 μCi/106 cells) were added to the semi-porous polycarbonate membrane bearing 3-μm pores (for PMNs and T lymphocytes) or 5-μm pores (for monocytes), in triplicate, and incubated at 37° for 3 hr in a 5% CO2 humidified incubator. Afterwards, migrated cells were recovered from the bottom chamber, lysed and the cell-associated radioactivity was determined in a gamma-counter (Packard Instruments, Downers Grove, IL). Either PBM or AM (105/well) were seeded in the lower chamber, pulsed or not with MT extracts and incubated at 37°, 5% CO2 overnight before the onset of chemotaxis assay. When indicated, anti-MCP-1 or anti-IL8 antisera were added to the lower chamber during the test. Percentage migration was calculated by measuring the counts recovered from the lower chamber and comparing them to the total input counts; results represent the mean ± SD of three independent experiments.
ELISA for chemokine measurement
Supernatants from 5 × 105 PBM or AM cultures, either pulsed or not with MT extracts, were collected after overnight incubation. The quantification of IL-8, MCP-1, IP-10 and monokine induced by interferon γ (Mig) production was performed using a sandwich ELISA as previously described.23 Briefly, for IP-10 and MCP-1, Maxi-Sorp™ ELISA plates (Nunc, Roskilde, Denmark) were coated with anti-chemokine antibody, blocked with a 2% bovine serum albumin solution, extensively washed, and test samples (PBM or AM cultures supernatants) or purified chemokines (standards) were added and incubated overnight. After extensive washings, biotinylated anti-chemokine antibodies were added, incubated for 3 hr at 37°, washed and incubated with ExtrAvidin®-coupled alkaline phosphatase (Sigma) for 30 min at room temperature. After washing, phosphatase activity on p-nitrophenyl phosphate (Sigma) (1 mg/ml in diethanolamine–HCl, pH 9·8) was allowed to occur for 1 hr at 37°. The reaction was stopped after 1 hr, and absorbance at 405 nm was measured in an ELISA reader. For IL-8 detection, the anti-IL-8 mAb WS-4 was used for coating, and a rabbit anti-IL-8 was used as detecting antibody. An anti-rabbit immunoglobulin G (IgG) coupled to alkaline phosphatase was used as secondary antibody. Detection limit was 50 pmol. IFN-γ, IL-4, IL-8 and MCP-1 were measured in overnight γδ T-cell culture supernatants (1 × 106/ml) with ELISA kits from R & D Systems, according to the manufacturer's instructions.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
This semi-quantitative assay was performed as previously described in detail.24 Briefly, 0·5 μg of total RNA extracted from γδ T-cell clones (1 × 106 for each experimental condition) was reverse-transcribed and the cDNA was amplified with specific primers (Primm s.r.l, Milan, Italy). Primer sequences for IL-4, IFN-γ, IL-8 and the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) have been reported.24 Primer sequences, amplification cycles and product size (base pairs, bp) for CCR1 and CCR2b are the following: CCR1 – forward, 5′-ACC ATG GAA ACT CCA AAC ACC ACA G, reverse, 5′-GTC AGA ACC CAG CAG AGA GTT CAT G, 40 cycles, 1100 bp; CCR2b – forward, 5′-AAC ATG CTG TCC ACA TCT CGT TCT, reverse, 5′-CGT TTA TAA ACC AGC CGA GAC TTC, 35 cycles, 1100 bp.
Surface expression of chemokine receptors
Flow cytometric analyses were performed with directly conjugated antibodies: anti-human CXCR3-fluorescein isothiocyanate (FITC), anti-human CCR3-phycoerythrin (PE), anti-human CXCR1-FITC and anti-human CXCR2-PE (R & D Systems), anti-human CCR4-PE, anti-human CCR5-PE and anti-human CXCR4-PE (Pharmingen Europe, Becton Dickinson, Heidelberg, Germany). Samples were acquired with a FACScan instrument (Becton Dickinson) and analysed using lysis software.
Immunohistochemistry
Immunohistochemistry was performed using purified mAbs against IL-8 (2A2 clone) and MCP-1 (3F11 clone), provided by LeukoSite Inc. (Cambridge, MA), at the final concentration of 10 μg/ml. The antibody used for the detection of IP-10 was from PeproTech EC Ltd (London, UK). Cryostatic sections derived from an axillary lymph node of a patient affected by primary tuberculosis showed typical histological features of caseating granulomas, characterized by central necrosis surrounded by histiocytic epithelioid cells, Langhans-type giant cells and lymphocytes. Sections were incubated overnight with the above mentioned antibodies and an indirect two-step immunoperoxidase method (Vecstain ABC Kit from Vector Laboratories, Burlingame, CA) was subsequently applied, with diaminobenzidine as chromogen.
In situ hybridization
A 35S-labelled BCA-1 RNA probe was generated by in vitro transcription (Boehringer Ltd, Mannheim, Germany). Tissue paraffin sections were dewaxed and re-hydrated in graded ethanol. Protein digestion was performed with 1 μg/ml proteinase K for 30 min at 37°, and blocked with 0·1 m glycine in PBS for 5 min. Tissue slides were post-fixed in 4% paraformaldehyde and dehydrated in graded ethanol before a treatment at room temperature with 0·1 m triethanolamine for 10 min.
After washes in H2O and dehydration in graded ethanol, slides were air-dried and subsequently incubated with IL-8 or MCP-1 mRNA probes. In situ hybridization was performed overnight at 45° with sense and antisense probes (106 counts per min/section) diluted in a hybridization solution [10% dextran sulphate, 50% deionized formamide, 1 mm NaCl, 20 mm Tris–HCl pH 7·5, 5 mm ethylenediamine tetraacetic acid (EDTA), 1× Denhardt solution, 100 mm dithiothreitol (DTT)]. After hybridization sections were washed with 10 mm DTT, 50% formamide, 1 mm EDTA pH 8·0, 2× sodium saline citrate for 30 min at 54°, followed by two additional washes in 2× sodium saline citrate, 10 nm DTT. Subsequently, slides were treated with 100 000 U/ml Rnase T1 (Boehringer) and 1000 U/ml Rnase A (Boehringer) for 30 min at 37°. After additional washes, tissues were dehydrated and air-dried before slide dipping in Kodak photoemulsion NTB-2, and exposed at 4° in the dark for 4 weeks. Development and fixation were performed according to Kodak protocol. The sections were counterstained with Mayer's haematoxylin.
Results
Circulating monocytes and alveolar macrophages pulsed with MT promote chemotaxis of neutrophils and monocytes
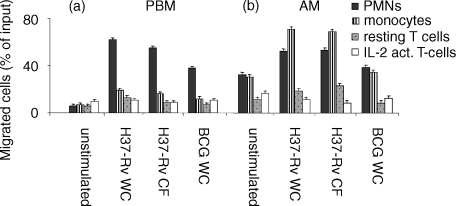
To address the question of whether soluble factors released by MT-pulsed peripheral blood macrophages induce migration of different leucocyte subsets, we seeded freshly isolated PBM from healthy donors in the lower chamber of a two-well chamber system, and incubated them overnight with various MT extracts. We utilized heat-inactivated whole cells (WC) or LAM-free culture filtrate proteins (CF) from the H37Rv (virulent) or H37Ra (avirulent) strains, and γ-irradiated whole bacilli from the attenuated M. bovis BCG (BCG WC). Selected leucocyte subpopulations were allowed to migrate in a transwell, whose lower chamber contained PBM, MT-pulsed. Incubation of adherent PBM with WC or LAM-free CF from both H37Rv (Fig. 1a) and H37Ra (not shown) and, to a lesser extent, with WC from BCG, resulted in the induction of a strong chemotactic activity towards freshly isolated PMNs, but not T cells and monocytes. Unstimulated PBM had negligible chemotactic activity towards either leucocyte subpopulation (Fig. 1a). As resting T lymphocytes have been previously reported to respond poorly to chemotactic stimuli in vitro25 we performed transmigration assays using long-term IL-2-activated, unselected T lymphocytes: they also had a negligible ability to migrate in response to chemotactic factors released by adherent PBM either spontaneously or upon induction (Fig. 1a).
Figure 1.
Peripheral blood monocytes (PBM) and alveolar macrophages (AM) pulsed with MT extracts specifically attract PMNs and monocytes. PBM (a) and AM (b) were cultured overnight in the lower chamber of a Transwell with or without MT extracts (e.g. BCG WC or H37Rv WC or H37Rv CF); after that, 51Cr-labelled PMNs, monocytes and resting and IL-2-activated T cells were seeded in the upper chamber. After 3 hr migrated cells were recovered from the lower chamber, lysed, and counted in a gamma-counter. Results are expressed as percentage of cells out of the total cell input. Data are representative of three independent experiments, each performed in triplicate.
A set of experiments identical to those described above was then performed using alveolar macrophages (AM, >95% CD68+). As shown in Fig. 1(b), under these conditions, AM spontaneously released factors that induced chemotaxis of PMNs and monocytes, suggesting that these cells have been exposed to stimulating factors in the lung microenvironment. Interestingly, incubation of AM with the various MT extracts, as well as with BCG WC, enhanced the release of a chemotactic activity for PMNs and for monocytes, suggesting that unlike PBM, AM are particularly efficient in producing leucocyte-specific chemotactic factors, both spontaneously and upon induction with mycobacterial extracts. Resting and IL-2-activated T cells were poorly attracted by the AM, as well as by PBM-produced factors, either in unstimulated or stimulated conditions.
In these experimental settings, the presence in the lower chamber of each MT product did not disclose any chemoattractive capacity (not shown).
IL-8 and MCP-1 released by MT-pulsed PBM and AM selectively drive the directional migration of PMNs and monocytes
To verify the possibility that the described differences in chemotaxis could be accounted for by the differential chemokine content present in the lower chamber, we focused on IL-8, MCP-1, IP-10 and Mig, which have been reported to be widely expressed at sites of inflammation. Indeed, high levels of IL-8 and MCP-1 were detectable in supernatants from MT-induced PBM, but not from unstimulated cells (Table 1). Conversely, unstimulated, as well as MT-induced AM cultures, released high amounts of both chemokines (Table 1), in good agreement with the results of the functional assays reported in Fig. 1. However, unstimulated, as well as MT-induced, AM cultures released moderate amounts of IP-10, while Mig could never be detected under these experimental conditions (not shown).
Table 1.
Chemokine production by peripheral blood monocytes (PBM) or alveolar macrophages (AM) pulsed with various mycobacterial extracts*
| Supernatant source† | IL-8 (pm) | MCP-1(pm) | IP-10(pm) |
|---|---|---|---|
| Medium | nd‡ | nd | nd |
| PBM alone | nd | 5·72 ± 2 | nd |
| PBM + WC | 3050 ± 1030 | 445 ± 7·2 | nd |
| PBM + CF | 2498 ± 34·1 | 485 ± 89·8 | nd |
| PBM + BCG | 272 ± 5·4 | 250 ± 8·5 | nd |
| AM alone | 1053 ± 24·5 | 568 ± 20·4 | 20·8 ± 4 |
| AM + WC | 3589 ± 868 | 734 ± 29·7 | 32·1 ± 0·9 |
| AM + CF | 1067 ± 27 | 458 ± 33·1 | 15·2 ± 0·9 |
| AM + BCG | 1089 ± 34·8 | 482 ± 24·2 | 21·07 ± 0·6 |
PBM or AM, obtained as described in the materials and methods section, were seeded into round-bottom microwell plates and separately pulsed with mycobacterial extracts (H37Rv WC, H37Rv CF or BCG WC) ON at 37 °.
Supernatants from either pulsed or not pulsed PBM or AM were collected and tested, by a sandwich ELISA assay, for their contents in interleukin-8 (IL-8), and monocyte chemotactic protein (MCP-1). Three independent experiments were performed for protein determination. Data are expressed as mean chemokine concentrations (pM), (±SD) of triplicate wells.
Concentrations not detectable.
IL-8 and MCP-1 released by MT-pulsed PBM and AM are involved in the specific, directional migration of PMNs and monocytes
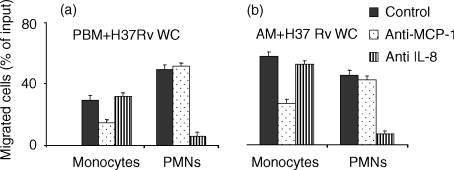
We adressed the question of whether the elevated amounts of IL-8 and MCP-1 released in the conditioned medium were specifically involved in attracting their prototypic leucocyte targets, i.e. PMNs and monocytes, respectively. To verify this, we performed our transmigration assays using either PBM (Fig. 2a) or AM (Fig. 2b) pulsed with H37 Rv WC, which resulted in the most potent stimulus among the mycobacterial extracts tested, in the presence of neutralizing anti-IL-8 and anti-MCP-1 antisera. The neutralizing anti-IL-8 antiserum largely abrogated the migration of PMNs induced by MT-conditioned supernatants (Fig. 2a,b), but did not affect migration of monocytes. Conversely, monocyte chemotaxis was markedly, although not completely, reduced by anti-MCP-1 antiserum, whereas chemotaxis of PMN was substantially unaffected, suggesting that MCP-1 is not primarily involved in the induction of their migration (Fig. 2a,b).
Figure 2.
Either IL-8 or MCP-1 are responsible for the PMNs and monocyte chemoattraction induced by PBM and AM pulsed with H37Rv WC. PBM or AM were cultured overnight without or with the MT extract in the lower chamber of a Transwell system, afterwards PMNs and monocytes were allowed to migrate in the upper chamber (a,b). Where indicated, anti-IL-8 or anti-MCP-1 antisera where added overnight to the lower chamber. The assay is detailed in the legend to Fig. 1.
MT-pulsed AM induced chemotaxis of γδ T cells
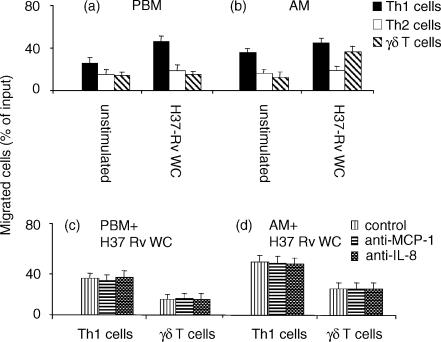
Since typical mycobacterial granulomatous lesions are circumscribed by a T cells ‘valluum’ and in our hands unselected T lymphocytes did not display any chemotactic potential, we attempted to determine the migratory capability of selected T-lymphocyte subsets and in particular polarized Th cells and γδΤ cells. Again, we performed our assays utilizing either PBM or AM H37Rv WC-pulsed. Unstimulated PBM (Fig. 3a) and AM (Fig. 3b) released chemotactic factors that selectively enhanced Th1 chemotaxis; their efficacy was moderately increased upon incubation with H37Rv WC. In none of these experimental conditions did Th2-polarized T cells express a significant migratory pattern (Fig. 3a,b). In comparison to Th1 T cells, γδ lymphocytes did not display a substantial chemotactic pattern towards unstimulated PBM or AM, which instead was inducible by incubation of AM only with H37-Rv WC. Neutralizing anti-IL-8 and anti-MCP-1 antisera did not affect migration of either Th1 or γδ T cells (Fig. 3c,d), thus indicating the non-involvement of these chemokines.
Figure 3.
Th1 and γδ T lymphocytes chemoattraction is not mediated by IL-8 and MCP-1. Th1 and γδ T lymphocytes were allowed to migrate in a transwell, whose lower chamber contained PBM (a) or AM (b), both unstimulated or stimulated with H37-Rv-WC. In inhibition experiments, PBM (c) or AM (d) were pulsed overnight with H37Rv WC, and the migration was evaluated in the presence or in the absence of neutralizing antisera against either IL-8 or MCP-1 in the lower chamber. The percentage of migration/inhibition was calculated as detailed in the legend to Fig. 1.
Chemokine and chemokine receptor expression are modulated in γδ T-cell clones in response to mycobacterial antigens
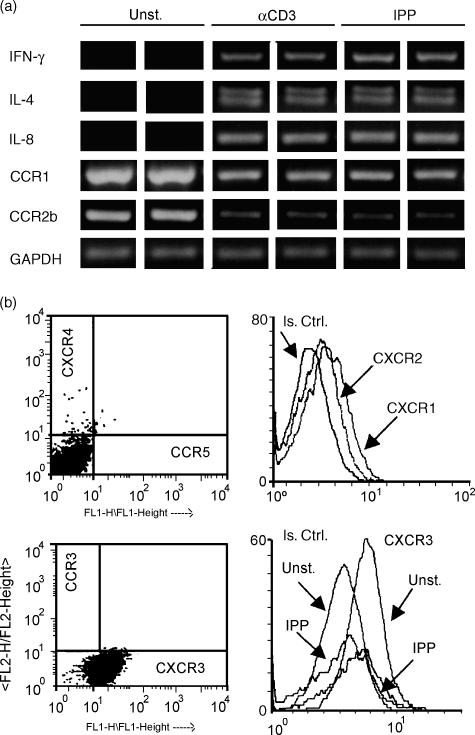
γδ T lymphocytes bearing the Vγ9Vδ2 TCR are able to kill mycobacteria-infected macrophages through a granule-dependent mechanism17 and to release type I cytokines in response to mycobacterial antigens.26 In particular, it has been shown that they specifically recognize mycobacterial non-peptidic phosphate compounds, including IPP.27–29 In addition, γδ cells have been described as playing a regulatory role in the immune response to pathogens through the secretion of chemokines.30 Thus, we investigated the production of IL-8 and MCP-1 by γδ T-cell clones in response to IPP, as well as to CD3 stimulation, which has been previously described.24 We could demonstrate by RT-PCR a selective expression of IL-8 (Fig. 4a), but not MCP-1 (not shown), upon treatment with either stimulus; both IL-4 and IFN-γ were also induced, underlining a Th0 profile in IPP-activated γδ T cells (Fig. 4a). These data were confirmed by measuring the secreted proteins in culture supernatants from four γδ T-cell clones. While unstimulated cells yielded undetectable levels of cytokines, the mean value (±SD) from IPP-stimulated cell cultures was 1390 (±245·3) pg/ml for IFN-γ, 93·7 (±29·8) pg/ml for IL-4 and 92·6 (±40·6) pg/ml for IL-8. MCP-1 was undetectable also in IPP-stimulated conditions. Having documented chemotaxis of γδ T-cell clones towards MT-pulsed AM supernatants (Fig. 3b), we investigated the profile of chemokine receptors combining RT-PCR and cytofluorimetric analyses. Constitutive expression of CCR1 and CCR2b was down-modulated upon IPP stimulation (Fig. 4a), in agreement with data obtained by others through ribonuclease protection assays.31 Flow cytometric analyses of γδ T-cell clones revealed negligible expression of CCR5 and CXCR4, which are receptors for macrophage inflammatory protein 1α (MIP-1α), macrophage inflammatory protein 1β (MIP-1β), and regulated on activation normal T-cell expressed and secreted (RANTES) and for stromal cell-derived factor 1 (SDF-1), respectively;32 and modest surface labelling of the IL-8 receptors CXCR1 and CXCR2 (Fig. 4b). CXCR3, receptor of the IFN-γ-inducible ligands IP-10, Mig and interferon-inducible T-cell α chemoattractant (ITAC),32 was instead expressed at high levels. Constitutive CXCR3 expression was stable throughout the culture period: in fact, when γδ T-cell clones were analysed at different time points (2–3 weeks) after restimulation, comparable levels of the receptor were detected (not shown); on the other hand, CXCR3 expression was down-modulated by IPP stimulation (Fig. 4b). Together, these findings indicate that IPP shapes the pattern of chemokine receptors on circulating γδ cells, thus modulating their potential to enter the sites of inflammation.
Figure 4.
Modulation of cytokine and chemokine receptor expression by IPP in γδ T-cell clones. (a) The data of RT-PCR analyses performed with RNA extracted after 6 hr of cell culture. Message RNA from each sample was reverse-transcribed in duplicate and the cDNA was amplified with the specific primers. (b) Flow cytometric analyses of: CXCR4 versus CCR5; CXCR1 and CXCR2; CCR3 versus CXCR3 chemokine receptors on unstimulated γδ T clones; to assess the modulation of CXCR3, cells were either unstimulated or stimulated with IPP (50 μm) for 24 hr. Data shown in (a) and (b) are representative of experiments performed with two different γδ T-cell clones. Is. Ctrl. represents the fluorescence obtained with irrelevant isotype controls.
Comparative analysis of chemokine receptor expression by Th1 and Th2 lymphocytes, used in our experimental settings, showed that, in agreement with previous reports,32 the latter had a lower level of CXCR3 expression (mean fluorescence intensity was 19·5 versus 36·6 in Th1 cells), but higher level of CCR4, the chemokine receptor for thymus and activation regulated chemokine (TARC)32,33 (mean fluorescence intensity was 12 versus 3·7 in Th1 cells).
γδ T lymphocytes specifically migrate in response to IP-10
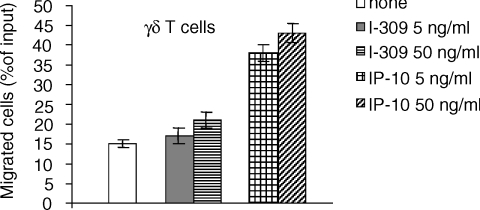
To assess whether the expression of CXCR3 chemokine receptor was instrumental to the active chemotaxis of γδ T lymphocytes, we evaluated their chemotactic response toward a gradient of IP-10, one of its natural ligands, which we found to be present in the supernatants of AM (Table 1). IP-10 strongly chemoattracted γδ T-cell clones in a dose-dependent manner, while I-309, chemoattractant for Th2 type lymphocytes,34 did not (Fig. 5).
Figure 5.
γδ T cell migration in response to recombinant chemokines. γδ T lymphocytes were allowed to migrate in a transwell, whose lower chamber contained either I-309 or IP-10 (5 and 50 ng/ml). Results are expressed as percentage of cells out of the total cell input. Data are representative of two independent experiments, each performed in triplicate using two different γδ T-cell clones.
In situ detection of transcribed IL-8 and MCP-1 mRNA in granulomatous lesions from patients with active tuberculosis
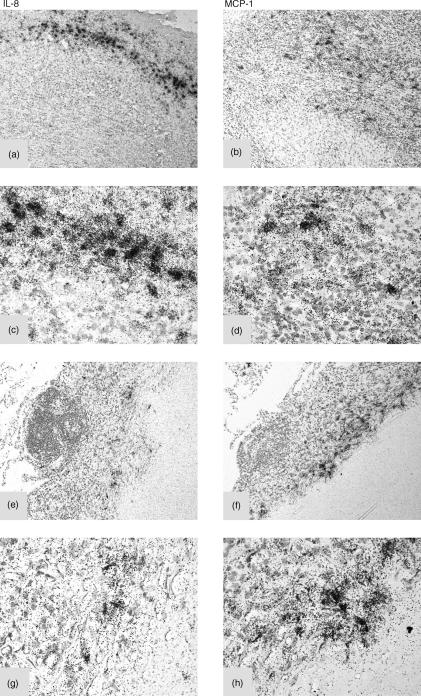
Since our in vitro data demonstrated the production of IL-8 and MCP-1 by PBM and especially by AM challenged with tubercular antigens, we searched for the production of these chemokines in vivo. We selected a patient suffering from active, uncontrolled tuberculosis and performed in situ hybridization on paraffin sections of lymph node (Fig. 6a) and lung (Fig. 6e) presenting granulomatous lesions, using specific probes for IL-8 and MCP-1 transcripts. The presence of a strong specific signal for both chemokine-encoding transcripts in the areas of the granulomatous lesions was evident (Fig. 6). This indicated that these chemokine genes are actively transcribed by inflammatory cells in lesions characterized by the presence of mycobacteria.
Figure 6.
In situ expression of mRNA for IL-8 and MCP-1 in lymph nodes and lung from a patient with active tuberculosis. In situ hybridization with specific RNA probes for IL-8 and MCP-1 was performed on paraffin sections from lymph nodes (a–d) or lung (e–h) of a patient affected by active tuberculosis. Both lymph node and lung give a positive signal for IL-8 (a, c, e, g) and for MCP-1 (b, d, f, h), respectively. (Original magnifications: 200× for a,b,e,f and 400× for c,d,g,h).
Immunohistochemical analysis demonstrates the presence of IL-8, MCP-1 and IP-10 in MT-positive granulomatous lesions in affected lungs and lymph nodes
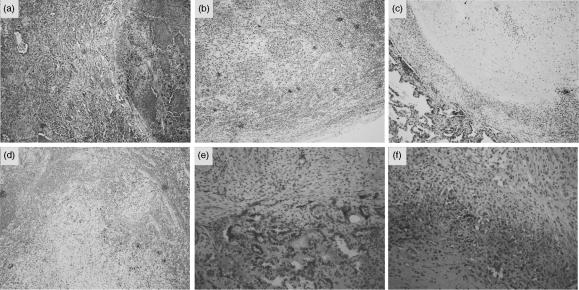
To obtain evidence for the active release of the aforementioned chemokines within lesions in the course of progressive tuberculosis infection, we performed histological analysis and immunohistochemistry in granulomas detected in the lungs and in the axillary lymph nodes from a patient with active tuberculosis. IL-8, MCP-1 and IP-10 were detected in cryosections from the lung and the draining lymph node. A prominent, spread staining for IL-8 was consistently found inside the areas of the lesion (Fig. 7a,b), while, very little staining for IL-8 was detected in the so-called lymphocytic valluum, suggesting that MT infection, rather than a general inflammatory response, was actively sustaining the production of this chemokine by the macrophage-derived epithelioid cells. Notably, PMNs could only occasionally be observed in the tuberculous lesion, in spite of the high amount of IL-8 produced locally. We observed a specular localization for IP-10, which was mainly confined in the areas of the alveolar epithelium in the lung (Fig. 7c) and in close proximity to the infiltrating elements in the necrotic areas of the lymph node (Fig. 7d). Staining with anti-MCP-1 antibody appeared weaker than IL-8 (Fig. 7e,f): it localized within the areas corresponding to the alveolar macrophages in the lung (Fig. 7e) and in the areas surrounding the necrosis in the lymph node (Fig. 7f). These findings provide evidence for the presence and the specificity of the investigated chemokines.
Figure 7.
Immunohistochemical expression of IL-8, MCP-1 and IP-10 in tuberculosis granulomatous lesions of affected lung and lymph nodes. (a, b) immunoreactivity for IL-8 is evident in the stroma and in the epithelioid elements, both in the lung (a) and in the lymph node (b), while surrounding lymphocytes are essentially free from label. Positive cells are stained in dark (original magnification 100×). (c, d) Immunohistochemistry for IP-10 displays a main localization in the alveolar macrophages in the lung (c)as well in macrophages-like elements in the lymph node (d) (original magnification 100×). (e, f) In comparison with IL-8, immunohistochemistry for MCP-1 displays a similar but weaker staining on analogous structures (original magnification 200×).
Discussion
In this work we show that γδ T lymphocytes respond to chemoattractants released by macrophages-pulsed with MT components, which in turn regulate cytokine production and chemokine receptor expression by γδ T cells themselves. Thus, γδ lymphocytes have the potential to reach the sites of MT-driven inflammation and to contribute to granuloma formation. The latter represents the final, favourable outcome at the site of MT infection,4 originated from a well-ordered temporal and spatial sequence of events. If this subtle, ordered sequence of events is altered, the histological outcome is not represented by the well-organized granuloma, but by a diffuse, disseminated shape.13
Our findings indicate that the prototypic chemokines IL-8 and MCP-1 are efficiently and persistently produced and released in response to MT infection both in vitro and in vivo, and are involved in the recruitment of the predominant innate immunity effector cells found in MT lesions, namely monocytes and granulocytes. However, PMNs are only rarely seen in MT granulomas35 and our in vitro studies do not support the idea that IL-8 exerts strong chemotactic activity towards leucocyte subpopulations other than PMNs. We propose that PMNs are indeed continuously attracted to areas of MT infection, but their rapid death after extravasation and contact with MT-derived material prevent their detection in such areas. Along this line of thoughts, necrotic or apoptotic death of PMNs is likely to contribute massively to the extensive tissue damage which is typical of the progressive outcomes of tuberculosis. Alternatively, despite the high levels of IL-8, the specular presence of PMNs is less documented possibly because of down-modulation of CXCR1- and CXCR2-specific IL-8 receptors in the course of pulmonary TB infection.36
In our assay PMNs were attracted comparably by MT-pulsed PBM and AM. Monocytes were instead more potently attracted by MT-pulsed AM than MT-pulsed PBM. These differences could be only partially explained by a different content of MCP-1 in either supernatant, because the neutralizing anti-MCP-1 antiserum determined a comparable inhibition of monocyte chemotaxis. The redundant role of MCP-1 production in clearing MT infection has been underlined by analysing mice carrying a targeted deletion of the MCP-1 gene.37 Moreover, it has been reported that osteopontin, released by AM during MT infection in vivo, is a powerful chemoattractant for monocytes.5 This raises the possibility that macrophages undergoing differentiation in the lung microenvironment selectively acquire the ability to produce alternative chemotactic substances which contribute to the peculiar features of granulomas induced by MT infection in the lung.
Notably, MT-pulsed PBM most effectively attracted PMNs and Th1 cells in chemotactic assays, whereas monocytes and the other T-lymphocyte subsets were only marginally induced to migrate under these conditions. Polymorphonuclear leucocyte chemotaxis was mediated by the abundant production of IL-8 by MT-pulsed monocytes, as demonstrated by the almost complete inhibition obtained with the neutralizing anti-IL-8 antiserum.
Of interest, γδ T lymphocytes displayed substantial chemotaxis towards AM- but not PBM-derived conditioned medium that contained detectable amounts of IP-10, one of the ligands for CXCR3.32 The latter was constitutively expressed at high levels on the surface of Vγ9Vδ2 cell clones derived from peripheral γδ T lymphocytes, outlining the novel finding that IP-10/CXCR3 paired interaction may effectively contribute to the γδ migratory pattern. Expression of CXCR3 has also recently been reported on freshly isolated γδ thymocytes, which mediated their migration to all three ligands, i.e. IP-10, I-Tac and Mig-1.38 In our experimental conditions Mig was never detected (not shown), suggesting that the interaction between IP-10 and CXCR3 may effectively contribute to migration of γδ cells. Our present finding of a selective response of γδ cells toward recombinant IP-10 indeed demonstrates that CXCR3 is functional and further supports the role of IP-10/CXCR3 interaction in chemotaxis induced by MT-pulsed AM. This notion is supported by the novel finding that IP-10 is expressed in the tuberculosis-affected lung and the lymph node. This interaction is likely to be functional also for migration of Th1 cells, because they have been reported to preferentially express the CXCR3 receptor [ref. 32 and our present data]. The RT-PCR analyses, which demonstrated constitutive expression of CCR1 and CCR2b by γδ cells, are consistent with a previous description of γδ T lymphocytes migrating to CC chemokines.39 However, inhibition experiments with anti-MCP-1 antiserum (not shown) ruled out the possibility that this chemokine, one of the ligands of CCR1 and CCR2, was involved in our experimental system in the migration of γδ and Th1 lymphocytes, which have also been reported to express functional receptors for MCP-1.32 Moreover, albeit γδ cell clones expressed moderate levels of the IL-8 receptors CXCR1 and CXCR2, the addition of anti-IL-8 mAb failed to inhibit their migration, supporting the hypothesis that IP-10 may be driving γδ chemotaxis. The down-modulation of CCR1, CCR2b and CXCR3 by stimulation with the mycobacterial antigen IPP suggests that γδ T lymphocytes might be recruited at the site of granuloma formation to home and secrete, upon TCR engagement, a number of soluble factors, including Th0/Th1 cytokines and chemokines, such as RANTES, MIP-1α and MIP-1β.24,31 Of note, our data indicate that peripheral blood γδ T lymphocytes contribute selectively upon IPP stimulation to amplify the production of IL-8, but not MCP-1. Indeed, amplification of IL-8 expression may be increased by the interaction between the activation marker CD30, present on γδ cells,24 and CD30L, constitutively expressed by PMNs.
Together, our work recapitulates the potential cellular players involved in the early phases of granuloma formation, by coupling functional in vitro chemotactic assays to the spectrum of cyto-chemokines secreted by PBM and AM challenged with various mycobacterial preparations.
Focusing on γδΤ lymphocytes, we show here that these cells have the requisites to be recruited to the site of granuloma formation where they may be prompt to exhibit regulatory functions in response to specific recognition of mycobacterial antigens via secretion of selected cyto-chemokines, including IL-8 and IP-10. Of note, however, γδ knockout mice display a more pyogenic form of granulomatous lesions,14 suggesting that γδ T cells may effectively contribute with a complex regulatory role to the formation of a successful granuloma. Indeed, γδ cells also produce IFN-γ, which in turn promotes the production of MCP-1, which is active on monocytes and other mononuclear cells, and inhibits that of CXC chemokines with an early-late-region motif, active on neutrophils.34
We propose that γδ T lymphocytes are efficient players in the protective rather than in the effector phase and support the hypothesis that they could exert a role in containing the dysregulated entry of PMNs, thus limiting their effect on tissue degradation, as well as on the recruitment of effector cells.
Acknowledgments
This work was supported by grants from Istituto Superiore di Sanità (National Tuberculosis Project # 81), to R. Pardi and from Istituto Superiore di Sanità (AIDS-related Infections Project). We thank Elena Dal Cin for her helpful, technical assistance.
Abbreviations
- BCG-WC
attenuated Mycobacterium bovis Bacillus Calmette– Guérin
- CF
M. tuberculosis lypoarabinomannan-free culture filtrate proteins from the strain H37-R
- IL-8
interleukin-8
- IPP
isopentenyl-pyrophosphate
- MCP-1
monocyte chemotactic protein 1
- MT
Mycobacterium tuberculosis
- PBM
peripheral blood monocytes
- PMNs
polymorphonuclear leucocytes
- Th1
T helper 1
- Th2
T helper 2
- WC
heat-inactivated whole cell lysate from the MT strain H37-R
References
- 1.Bloom BR, Murray CJ. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–64. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 2.Schorey JS, Carroll MC, Brown EJ. A macrophage invasion mechanism of pathogenic mycobacteria. Science. 1997;277:1091–3. doi: 10.1126/science.277.5329.1091. [DOI] [PubMed] [Google Scholar]
- 3.Fenton MJ, Vermeulen MW. Minireview. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64:683–90. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–12. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 5.Nau GJ, Guilfoile P, Chupp GL, Berman JS, Kim SJ, Kornfeld H, Young RA. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc Natl Acad Sci USA. 1997;94:6414–19. doi: 10.1073/pnas.94.12.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 7.Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–60. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasahara K, Tobe T, Tomita M, et al. Selective expression of monocyte chemotactic and activacting factor/monocyte chemoattractant protein 1 in human blood monocytes by Mycobacterium tuberculosis. J Infect Dis. 1994;170:1238–47. doi: 10.1093/infdis/170.5.1238. [DOI] [PubMed] [Google Scholar]
- 9.Friedland JS, Remick DG, Shattock R, Griffin GE. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocytes cell lines. Eur J Immunol. 1992;22:1373–8. doi: 10.1002/eji.1830220607. [DOI] [PubMed] [Google Scholar]
- 10.Sadek MI, Sada E, Toossi Z, Schwander SK, Rich EA. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol. 1998;19:513–21. doi: 10.1165/ajrcmb.19.3.2815. [DOI] [PubMed] [Google Scholar]
- 11.Kurashima K, Mukaida N, Fujimura M, Yasui M, Nakazumi Y, Matsuda T, Matsushima K. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med. 1997;155:1474–7. doi: 10.1164/ajrccm.155.4.9105097. [DOI] [PubMed] [Google Scholar]
- 12.Parrish NM, Dick JD, Bishai WR. Mechanism of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–12. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]
- 13.Dannenberg AM, Jr, Rook G. TuberculosisPathogenesis, Protection and Control. Bloom BR: ASM Press; 1998. pp. 459–83. [Google Scholar]
- 14.D'Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–21. [PubMed] [Google Scholar]
- 15.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–80. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenger S, Modlin RL. T-cell-mediated immunity to Mycobacterium tuberculosis. Curr Opin Microbiol. 1999;2:89–93. doi: 10.1016/s1369-5274(99)80015-0. [DOI] [PubMed] [Google Scholar]
- 17.Dieli F, Troye-Blomberg M, Ivanyi J, Fournié JJ, Bonneville M, Peyrat MA, Sireci G, Salerno A. Vγ9/Vδ2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur J Immunol. 2000;30:1512–18. doi: 10.1002/(SICI)1521-4141(200005)30:5<1512::AID-IMMU1512>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Ladel CH, Blum Dreher A, Reifenberg K, Kaufman SHE. Protective role of γ/δ T cells and α/β T cells in tuberculosis. Eur J Immunol. 1995;25:2877–81. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 19.Rogge L, Papi A, Presky DH, et al. Antibodies to the IL-12 receptor beta 2 chain mark human Th1 but not Th2 cells in vitro and in vivo. J Immunol. 1999;162:3926–32. [PubMed] [Google Scholar]
- 20.Ferrarini M, Heltai S, Toninelli E, Sabbadini MG, Pellicciari C, Manfredi AA. Daudi lymphoma killing triggers the programmed death of cytotoxic Vγ9/Vδ2 T lymphocytes. J Immunol. 1995;154:3704–12. [PubMed] [Google Scholar]
- 21.Pellegatta F, Yan Zu Radaelli A, Zocchi MR, Ferrero E, Chierchia S, Gaja G, Ferrero ME. Drug induced in vitro inhibition of neutrophil-endothelial cells adhesion. Brit. J Pharmacol. 1996;118:471–6. doi: 10.1111/j.1476-5381.1996.tb15427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrero E, Bondanza A, Leone BE, Manici S, Poggi A, Zocchi MR. CD14+CD34+ peripheral blood mononuclear cells migrate across endothelium and give rise to immunostimulatory dendritic cells. J Immunol. 1999;160:2675–83. [PubMed] [Google Scholar]
- 23.Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark-Lewis I, MoSeries B. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP-10 and Mig. Eur J Immunol. 1998;28:961–72. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Biswas P, Rovere P, De Filippi M, et al. Engagement of CD30 shapes the secret ion of cytokines by human γδ T cells. Eur J Immunol. 2000;30:2172–80. doi: 10.1002/1521-4141(2000)30:8<2172::AID-IMMU2172>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptors expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 27.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human γδ T-cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 28.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner V. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 29.Burk MR, De Mori L, Libero G. Human V gamma 9-V delta 2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol. 1995;25:2052–8. doi: 10.1002/eji.1830250737. [DOI] [PubMed] [Google Scholar]
- 30.Mak TW, Ferrick DA. The γδΤ cell-bridge: linking innate and acquired immunity. Nature Med. 1998;4:764–5. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 31.Cipriani B, Borsellino G, Poccia F, Placido R, Tramonti D, Bach S, Battistini L, Brosnan CF. Activation of C-C chemokines in human peripheral γδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood. 2000;95:39–47. [PubMed] [Google Scholar]
- 32.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 33.D'Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–15. [PubMed] [Google Scholar]
- 34.Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–7. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- 35.Kasahara K, Sato I, Ogura K, Takeuchi H, Kobayashi K, Adachi M. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J Infect Dis. 1998;178:127–37. doi: 10.1086/515585. [DOI] [PubMed] [Google Scholar]
- 36.Meddows-Taylor S, Desmond JM, Tiemessen CT. Dysregulated production of interleukin-8 in individuals infected with human immunodeficiency virus type 1 and Mycobacterium tuberculosis. Infect Immun. 1999;67:1251–60. doi: 10.1128/iai.67.3.1251-1260.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackay CR. Chemokine receptors and T cell chemotaxis. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romagnani P, Annunziato F, Lazzeri E, et al. Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) alphabeta+ CD8+ single-positive T cells, TCRgammadelta+ T cells, and natural killer-type cells in human thymus. Blood. 2001;97:601–7. doi: 10.1182/blood.v97.3.601. [DOI] [PubMed] [Google Scholar]
- 39.Roth SJ, Diacovo TG, Brenner MB, Rosat JP, Buccola J, Morita CT, Springer TA. Transendothelial chemotaxis of human alpha/beta and gamma/delta T lymphocytes to chemokines. Eur J Immunol. 1998;28:104–13. doi: 10.1002/(SICI)1521-4141(199801)28:01<104::AID-IMMU104>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]