Abstract
Although production of chemokines by vascular endothelial cells has been documented, there is only limited information regarding the expression of chemokines by the lymphatic endothelium. Here we used lymphatic endothelial cells (LEC) derived from experimentally induced murine lymphangiomas to investigate the pattern of chemokine expression by these cells. Histological analysis of the lymphatic hyperplasia revealed the presence of leucocytes in the tissues surrounding the lesions, suggesting the presence of chemoattractant activity. A functional chemotactic assay on human polymorphonuclear cells and on purified subpopulations of murine leucocytes using culture supernatants from LEC primary cultures confirmed the presence of chemoattractant activity. The identity of different cytokines of the CXC, CC and C subfamilies was investigated by reverse trancriptase–polymerase chain reaction on total endothelial cell RNA. Amplified fragments corresponding to KC, IP10, Mig-1, BCL, MIP-2, SLC, RANTES, MCP-1, C10, and Lptn were obtained, and confirmed by Southern blot and sequencing. In contrast, MIP-1α, MIP-1β, and MIP-1γ were not detected. Higher levels of expression were revealed by Northern blot analysis for Mig-1, MCP-1 and C10. The lymphatic endothelium-restricted production of these chemokines was also confirmed by in situ hybridization. The presence of high C10 mRNA expression levels in LEC was particularly unexpected, because the production of this molecule has been previously identified only in cells of the haematopoietic lineage. These observations represent the first detailed analysis of chemokine production by lymphatic endothelial cells and may account, in part, for the mechanism of leucocyte recruitment into the lymphatics, and of lymphocyte recirculation within the lymphatic system.
Introduction
At inflammatory sites, circulating leucocytes adhere to the endothelial cells of postcapillary venules, and emigrate from the blood into sites of tissue damage or infection. Granulocytes and monocyte/macrophages accumulate in the inflamed tissue whereas lymphocytes that encounter specific antigens are only transiently retained. Antigen-presenting cells, which have picked up antigens, and lymphocytes, resulting from activation and proliferation after interaction with cognate antigens, leave the site and migrate through the lymphatic vessels to regional lymph nodes.1–4 In contrast, granulocytes do not drain into the lymphatic system, unless the spread of infectious agents causes an acute inflammatory involvement of these vessels.
In recent years, the mechanisms regulating leucocyte migration from blood into inflamed tissues or secondary lymphoid organs, such as lymph nodes and mucosal lymphoid follicles, have been partly characterized.5–7 In contrast, little is known about the mechanisms regulating leucocyte migration from inflamed tissues to lymphatic vessels and lymph nodes and, more generally, within the lymphatic system. In the process of leucocyte extravasation, a dominant role appears to be played by chemokines: small (8000–10 000 MW) cytokines with chemotactic activity. Various combinations of chemokines retained on the endothelial cell surface have been shown to account for the recruitment and extravasation of leucocyte subtypes involved in the inflammatory/immunological process.8–10 As for the leucocytes directed towards the lymphatic circulation, in principle, the flowing of extracellular fluids might convey non-adherent cells, together with cellular debris and insoluble particles generated in the inflammatory process, into the lymphatic capillaries.9 However, evidence of specific recruitment of certain leucocyte subpopulations towards the lymphatic capillaries suggests that this process is precisely regulated. Consistent with this hypothesis is a recent observation that secondary lymphoid chemokine (SLC), a chemokine with preferential activity towards naive T cells, is expressed in the lymphatic endothelium of multiple organs.11,12
In a recent study, we showed that mice treated intraperitoneally with Freund's adjuvant develop a reversible hyperplasia of lymphatic vessels on the surfaces of the liver and of the diaphragm, which we characterized as a lymphangioma.13 These benign tumours represent a unique source of lymphatic endothelial cells, which express, among other markers, the lymphatic endothelium specific Flt-4 tyrosine kinase receptor for the VEGF-C ligand. We have now used these cells to test the hypothesis that lymphatic endothelial cells (LEC) produce chemokines which may account for an active recruitment of leucocytes into the lymphatics. We show here that LEC both in ex vivo lymphangioma preparations and in primary in vitro cultures, produce a number of C, CC and CXC chemokines which induce chemotaxis of lymphocytes, granulocytes and mononuclear cells.
Materials and methods
Cells
Induction of lymphangiomas in BALB/c mice was performed as previously described.13 Histological analyses were performed on samples fixed in phosphate-buffered saline−10% formalin, embedded in paraffin, and stained with haematoxylin & eosin. Histological analyses of expression of the lymphatic endothelial cell marker podoplanin on lymphangioma sections with a rabbit polyclonal anti-podoplanin immunoglobulin were kindly conducted by Dr Kerjaschki's laboratory, as described previously.12 LEC primary cultures were prepared as previously described.13 Primary culture LEC were stained by treatment with anti-podoplanin rabbit serum and then with biotin-conjugated goat anti-rabbit immunoglobulin G (IgG), followed by reaction by the ABC kit/pk 6200 (Vector, Burlingame, CA) and diaminobenzidine (Lab Vision, Fremont, CA). The anti-podoplanin rabbit serum was obtained by genetic vaccination with a plasmid construct containing a polymerase chain reaction (PCR)-amplified podoplanin fragment (primers: 5′-GAATGTGCACGACTCAGCGCAGGG AGGGGCCA-3′ and 5′-GCTATCCGGAGCCATCTTTCTTATCTGTGGTCTG-3′) fused to the human IgG1 CH3 domain, as previously described.14 For migration assays, supernatants of LEC primary cultures were collected at day 7, after 48 hr of culture in complete medium. Human or mouse neutrophils (PMN) were purified by Ficoll–Hypaque (Sigma, St Louis, MO) density gradient centrifugation (1·077 Ficoll–Hypaque density for human leucocytes, and 1·081 for murine leucocytes), as described by Boyum.15 Resident peritoneal macrophages were obtained from mouse peritoneal washes by Ficoll–Hypaque density centrifugation. All animal studies were approved by the local Institutional Review Board. Cytofluorimetric analysis was performed on a FacsCalibur cytofluorimeter (Becton and Dickinson, Franklin Lakes, NJ). Cells were double stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3, and phycoerythrin (PE)-conjugated anti-CD45R (B220) or with FITC-conjugated anti-CD4, and PE-conjugated anti-CD8. Antibodies were purchased from Pharmingen (San Diego, CA).
Migration assays
Migration of leucocytes was assessed in 24-well transwell chambers equipped with 5-μm pore polycarbonate Transwell culture inserts (Costar, Cambridge, MA), as described elsewhere.16 Cells that had migrated into the lower chamber were counted in a ZBI Coulter Counter (Coulter Electronics LTD, Luton, UK). C10 was obtained from supernatants of Chinese hamster ovary (CHO) cells transfected with a plasmid (pCDNA3, Invitrogen, Ch Groningen, the Netherlands) containing the full-length C10 cDNA. Expression was confirmed by Western immunoblotting using goat anti-mouse C10 antibodies. Zymosan-activated serum was prepared by adding lyophilized zymosan (Sigma), reconstituted following the indications of the manufacturer, to freshly obtained human or mouse serum; it was then added to the medium at a 10% final concentration. Interleukin-8 (IL-8), used at 10−8 m as a positive control in chemotactic assays, was kindly provided by Dr Marco Baggiolini (University of Bern, Switzerland). Polyclonal goat anti-mouse C10 antibodies were purchased from R & D (Minneopolis, MN).
Reverse transcriptase (RT)–PCR and Northern blot
Total RNA was isolated using the acid guanidinium thiocyanate procedure. For RT-PCR, 2 μg of total RNA were reverse transcribed and amplified using the Gene Amp RNA-PCR kit (Perkin Elmer Cetus, Norwalk, CT). Specific sets of primers (Primm s.r.l., Milan, Italy) were used for each chemokine coding region (Table 1). The amplification procedure was performed by 30 cycles, as follows: 30 seconds at 95°; 30 seconds at 56°; and 30 seconds at 72°. For each amplification reaction, the specific negative control (amplification of a sample containing the same reagents except reverse transcriptase) was included. Identities of all PCR products were confirmed by Southern blot analysis using as probes 32P end-labelled oligonucleotides internal to the amplified fragments, and by sequencing. PCR fragments were cloned into the pGEM-Teasy plasmid vector (Promega, Madison, WI) and then sequenced using the T7 Sequencing kit (Pharmacia LBK, Uppsala, Sweden). For Northern blots, 20 μg of total RNA was used on each lane. Filters were hybridized with probes labelled with [γ-32P]dCTP (Amersham, UK) using the oligolabelling kit (Pharmacia LBK). Hybridizations were carried out overnight at 50° in 5× Saline Sodium phosphate-EDTA (1×SSPE: 150 mm NaCl, 10 mm sodium phosphate, 1 mm EDTA), 0·5% sodium dodecyl sulphate (SDS), 5× Denhardt's solution, 50% formamide, and 100 mg/ml of salmon sperm DNA. Filters were washed in 2× saline sodium citrate (SSC)/0·1% SDS at 50°, 60° and 70° for 30 min, and autoradiographed overnight.
Table 1.
Nucleotide sequence of primers used for RT/PCR and Southern blot analysis
| cDNA | Primers | Probes | |
|---|---|---|---|
| GAPDH | F | 5′-ACATGTTCCAGTATGACTCT-3′ | 5′-GAGTATGTCGTGGAGTCT-3′ |
| 570 bp | R | 5′-TGCCTTCCGGTACGGTCAC-3′ | |
| RANTES | F | 5′-GGTACCATGAAGATCTCTGCA-3′ | 5′-AGGAGTATTTCTACACCAG-3′ |
| 293 bp | R | 5′-AAACCCTCTATCCTAGCTCAT-3′ | |
| JE | F | 5′-ACCACCATGCAGGTCCCTGT-3′ | 5′-AACCTGGATCGGAACCAAAT-3′ |
| 464 bp | R | 5′-GAGTCACACTAGTTCACTGT-3′ | |
| IP-10 | F | 5′-CCCCATCAGCACCATGAAC-3′ | 5′-ATAGGGAAGCTTGAAATCAT-3′ |
| 331 bp | R | 5′-GCTTCACTCCAGTTAAGGA-3′ | |
| KC | F | 5′-GTACCATGATCCCAGCCAC-3′ | 5′-AACATCCAGAGCTTGAAGGT-3′ |
| 312 bp | R | 5′-GTCTTCTTTCTCCGTTACTTG-3′ | |
| MIP-2 | F | 5′-ACTTCAGCCTAGCGCCATGG | 5′-AACTGCGCTGTCAATGCCTG-3′ |
| 303 bp | R | 5′-AGGTCAGTTAGCCTTGCCTT-3′ | |
| C10 | F | 5′-CAGGAGGATGAGAAACTCCA-3′ | 5′-TATGCCACACAGATCCCATG-3′ |
| 362 bp | R | 5′-CTTCTCAAGCAATGACCTTG-3′ | |
| Lpnt | F | 5′-CTCAGCCATGAGACTTCTC-3′ | 5′-AAAGCAGCGATCAAGACTGT-3′ |
| 360 bp | R | 5′-TGGAGGCTGTTACCCAGTCA-3′ | |
| BLC | F | 5′-AGAATGAGGCTCAGCACAGC-3′ | 5′-ATAGATCGGATTCAAGTTACG-3′ |
| 314 bp | R | 5′-TAGTGGCTTCAGGCAGCT-3′ | |
| 6Ckine | F | 5′-ATGGCTCAGATGATGACTCT-3′ | 5′-AGCCTGGTCCTGGCTCTCTGC-3′ |
| 409 bp | R | 5′-TACTGGGCTATCCTCTTGA-3′ | |
| MIG | F | 5′-TGCCATGAAGTCCGCTGTTC-3′ | 5′-ATAAGGAATGCACGATGCTC-3′ |
| 398 bp | R | 5′-AAAGTAATGGTCTCTTATGTAG-3′ | |
| EXODUS | F | 5′-AACTCAACCACAATCATGGC-3′ | 5′-GACCAGGCTCTCTGCATC-3′ |
| 412 bp | R | 5′-GCTATCCTCTTGAGGGCTGT-3′ | |
| MIP1-α | F | 5′-AACATCATGAAGGTCTCCAC-3′ | 5′-TCATCGTTGACTATTTTGAA-3′ |
| 294 bp | R | 5′-CCAAGACTCTCAGGCATTCA-3′ | |
| MIP1-β | F | 5′-ACACCATGAAGCTCTGCGT-3′ | 5′-AGACCAGCAGTCTTTGCTCC-3′ |
| 293 bp | R | 5′-CGCTGGAGCTGCTCAGTTC-3′ | |
| MIP1-γ | F | 5′-TAAGAAGATGAAGCCTTTTCA-3′ | 5′-ACTCTTCAGATTGCTGCCTG-3′ |
| 390 bp | R | 5′-TCTCTAAAGCAAATGTTATTGT-3′ |
Transcripts of interest and the expected size of amplified fragments are indicated in the first column. Forward (F) and reverse (R) primers for PCR amplification are reported in the second column. Oligonucleotides used as probes in Southern blot analysis of the transcripts are indicated in the third column.
Western blotting
Cell samples and supernatants of LEC primary cultures, or mouse peritoneal macrophages incubated for 12 hr in the presence or absence of Escherichia coli endotoxin (lipopolysaccharide; LPS) were collected separately, and treated with the protease inhibitors phenyl methyl sulphonyl fluoride (1 mm), aprotinin (10 μg/ml), and leupeptin (10 μg/ml) (Sigma). Samples were then concentrated by Centricon (Millipore/Amicon, Bedford, MA), and aliquots were run on reducing 12% SDS–polyacrylamide gel electrophoresis, then blotted onto Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham), and incubated with goat anti-mouse C10 antibodies (R & D). Detection was performed by ECL (Amersham) after incubation with a secondary biotin-conjugated mouse anti-goat IgG (R & D).
In situ hybridization
Probes were generated by linearized pGEM-Teasy Vector plasmids containing the different chemokine templates. SP6 and T7 RNA polymerases (Promega) were used for the synthesis of the anti-sense and sense RNA probes, respectively, and labelled with Dig-UTP using the Dig RNA labelling mix (Boehringer Mannheim, Mannheim, Germany). Hybridizations were performed as described.17 Unbound probe was digested with RNAase A (20 μg/ml) for 30 min at 37°. Post-hybridization washes (twice in 2× SSC, twice in 0·2× SSC, and once in 0·1× SSC, each for 15 min) were performed at 37°. Hybridization was detected with the Dig Nucleic Acid Detection kit (Boehringer Mannheim) using Fast Red as a substrate, and counter-stained with Harris solution (Sigma).
Results
In vivo and in vitro cell migration
We have previously demonstrated expression of lymphatic endothelium-specific Flt-4 antigen by experimental microvessel hyperplasia.13 To define further the lymphatic nature of the hyperplasia, we investigated expression of the recently proposed LEC marker podoplanin12 both on histological samples of our tumour and on tumour-derived cell cultures. As shown in Fig. 1, almost all cells in the tumour and tumour-derived cultures expressed the podoplanin antigen. Production of chemotactic factors by LEC was suggested by observations of histological sections of our lymphatic vessel tumour, which consistently showed the presence of leucocytes in the tissues surrounding the lesion, including neutrophils and mononuclear cells.13 This was evident in most of the haematoxylin & eosin-stained samples of lymphangioma growing on the surface of the liver (Fig. 2). The production of chemotactic factors by LEC was confirmed by studying the chemoattractant activity of LEC primary culture supernatants on human PMN and purified murine leucocyte subpopulations (PMN, lymphocytes and monocyte/macrophages). As shown in Fig. 3(a), supernatants of LEC primary cultures were active on human PMN as well as on all three murine leucocyte subpopulations. The supernatants had an activity comparable to that of the positive control zymosan-activated serum, or the recombinant chemokines human IL-8 and murine C10. Immunofluorescence analysis of the markers CD3, CD4, CD8 and B220 on migrated lymphocytes indicated that LEC supernatants were active on both B and T (CD4+ and CD8+) cells (Fig. 3b).
Figure 1.
Immunohistochemistry of lymphangioma sections and lymphangioma-derived cell cultures. Paraffin embedded sections of lymphangioma grown on the surface of the liver (a), or normal liver tissue (b) were stained with antibodies specific for podoplanin. Lymphangioma-derived cell cultures were stained with antibodies specific for podoplanin (c), or control rabbit serum (d). Bars: 20 μm (a, b, and insets); 100 μm (c, d).
Figure 2.
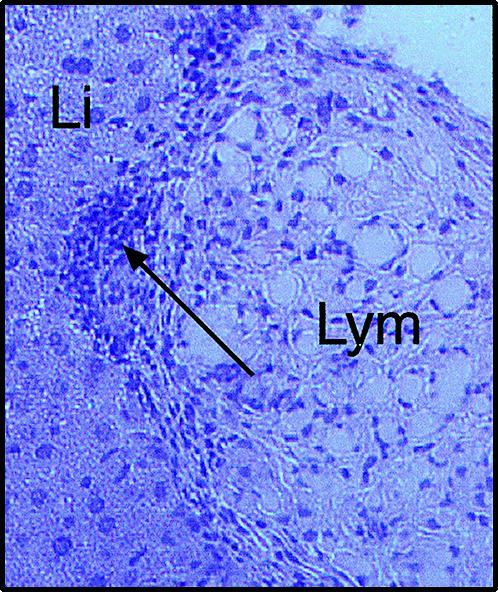
Histological section of a lymphatic hyperplasia growing on the surface of the liver, stained with haematoxylin & eosin. Recruitment of leucocytes is apparent in the tissues surrounding the lesion (arrow). Li, liver; Lym, lymphangioma.
Figure 3.
(a) Chemotactic response of purified leucocytes to culture supernatants of lymphangioma-derived lymphatic endothelial cells (LEC Supn), control medium (Control Supn), 10% zymosan-activated serum (ZAS) or control chemokines hu-IL-8 (10−8 m) or mu-C10 (undiluted supernatant of murine C10-transfected CHO cells). Chemotactic assays were performed with purified human or murine peripheral blood granulocytes (PMN), or murine lymphocytes or peritoneal resident macrophages, as indicated. Bars represent means ± SE of four to six experiments. *P < 0·01 (paired t-test), compared to control medium. (b) Cytofluorimetric analysis of murine lymphocytes migrated into LEC primary culture-conditioned medium.
Expression of chemokines
To establish the profile of chemokines potentially expressed by LEC, we performed a screening by specific RT-PCR of defined regions of the corresponding mRNAs. RNA was obtained from LEC either immediately after tumour explantation or from cultured cells. We investigated 13 well-known chemokines belonging to the three different families CXC, CC and C. Ten of the 13 chemokines investigated corresponding to all three families were amplified from lymphangioma-derived RNA: KC, IP-10, BCL, MIP-2 and Mig-1 for type CXC, 6C-kine/SLC, RANTES, MCP-1/JE and C10 for type CC and lymphotactin for type C (Fig. 4a). Similar results were obtained with RNA extracted from LEC primary cultures. Interestingly, the CC chemokines MIP-1α, MIP-1β and MIP-1γ,18–20 known to be produced by cells of the monocyte/macrophage lineage, were not detected. Both GAPDH and β-actin fragments were also amplified as internal controls for each reaction. To evaluate the relative expression level of each chemokine, the same amplified fragments were used as probes in Northern blot analysis of total lymphangioma-derived RNA (Fig. 4b). Positive expression was confirmed for most, but not all, chemokines that were detected by RT-PCR (i.e. KC was not detectable). The identified signals, which corresponded to the expected size of chemokine mRNAs, revealed the existence of different expression levels. Mig-1 was the CXC chemokine with the highest expression level, while MCP-1/JE and C10 had the highest levels amongst the CC-subfamily. Interestingly, the signal for chemokine C10 indicated an extremely high expression of this molecule by LEC, which led us to focus our attention on this chemokine. As shown in Fig. 5, Western blot analysis showed expression of C10 at the protein level in LEC primary culture supernatants. In contrast, C10 was not detected in the culture medium of either unstimulated or E. coli endotoxin (LPS)-stimulated mouse peritoneal monocyte/macrophages, used as negative controls21 in the assay. Release of C10 by LEC was further confirmed in functional assays because LEC supernatant-induced transwell migration of lymphocytes and PMN was significantly inhibited by anti-C10 antibodies (Fig. 6).
Figure 4.
Analysis of chemokines expressed by LEC. (a) RT-PCR of transcripts of CXC, CC and C chemokines. Amplified fragments evidenced by ethidium bromide staining were identified by hybridization with sequence-specific internal oligonucleotides. Numbers indicate the size in base pairs of each band. (b) Northern blot analysis of mRNA obtained from lymphangioma-derived cells. All bands correspond to the mRNA expected size.
Figure 5.
Western blot analysis of C10 production by LEC. C10 protein was found in supernatants of LEC primary cultures (lane a), but not in culture supernatants of unstimulated (lane b) or LPS-treated (lane c) mouse macrophages. Relative molecular masses are indicated.
Figure 6.
Effect of anti-C10 antibodies on LEC-induced leucocyte migration. Purified mouse lymphocytes or PMN were added on top of transwell filters and culture medium (control), or LEC primary culture supernatants (LEC supn) with or without 20 μg/ml of anti-C10 antibodies (αC10) were added to the lower compartment of migration chambers. Bars represent means ± SE of three experiments. *P < 0·05 (paired t-test), compared to LEC supn.
These results, and those of the chemotactic assays, were in accordance with the hypothesis that secretion of chemokines is a functional property of LEC. To confirm that chemokine production was a general characteristic of these cells, in situ hybridizations were performed on lymphangioma sections using specific anti-sense and sense RNA probes for the CXC, CC and C chemokines with the highest expression levels: Mig-1, MCP-1/JE and C10, and lymphotactin. As shown in Fig. 7, only the anti-sense probes showed positive hybridization, and this was restricted to the lymphangioma lesion. Of note, C10 was also detected by us in intact lymph nodes (not shown), suggesting a similar pattern of expression between lymphangioma-derived cells and lymph node cells.
Figure 7.
In situ hybridization of lymphangioma lesions growing on the surface of the liver. Lymphangioma sections were hybridized with Digoxigenin-labelled antisense and sense RNA probes corresponding to the indicated chemokines, and developed with an alkaline phosphatase-conjugated anti-digoxigenin antibody and Fast Red.
Discussion
The production of chemokines by both immune and non-immune cells supports the contention that chemokines play important roles in different processes.22 In particular, the production of chemokines by blood vessel endothelial cells in response to several stimuli (e.g. inflammatory cytokines, hypoxia, bacterial LPS) is well documented.10,23 Most of the information available is related to cells of vascular origin, while limited documentation exists in relation to lymphatic endothelial cells. However, at least for 6C-kine/SLC, it was established that it is produced by the endothelial cells of secondary lymphoid organs, based on the morphology and location of the vessels producing the chemokine.11 Moreover, this chemokine has recently been shown to participate in the migration of dendritic cells to regional lymph nodes.24 Similarly, we detected expression of C10 in the lymph vessels of intact lymph nodes, thus confirming production of a second chemokine by unstimulated lymph node endothelial cells. The RT-PCR analysis indicated a complex profile of chemokine expression by LEC, which may include other members not investigated here and even specific chemoattractants not yet identified. With the exception of the MIP-1α, MIP-1β and MIP-1γ, known to be secreted mainly by cells of the monocyte/macrophage lineage,18,23 the expression of chemokines belonging to all three subfamilies was detected and confirmed by Northern blot and in situ hybridization analyses. Some of these (e.g. BCL, 6C-kine/SLC, C10) were reported to attract T or B lymphocytes preferentially. Interestingly, the expression of KC, a CXC chemokine that together with MIP-2α and MIP-2β18,25 performs the functions of IL-8 in the mouse, was not confirmed by the Northern blot, suggesting a very low level of expression. Also RANTES, a CC chemokine with potent chemotactic and activating properties for basophils, eosinophils and natural killer cells26 showed a low level of expression.
C10 was originally identified as a transcript induced in bone marrow cells upon stimulation with granulocyte–macrophage colony-stimulating factor, IL-3 or IL-4.21 Our observation provides the first evidence that C10 is also expressed in cells different from those of haematopoietic lineage. The biological significance of this molecule is still not well understood and details of its mechanism of action are still limited by the fact that its receptor has not been identified. Structurally, C10 belongs to the subfamily of CC chemokines. It is, however, unique among them because it contains an extra second exon encoding a 16-amino-acid sequence, which is inserted in the protein N-terminal region known to be involved in receptor activation in other chemokines.27 Recently, murine C10 was reported to share significant sequence homology with the human chemokine HCC-2.28 C10 has been reported to be active on mouse peritoneal exudate cells and human peripheral blood mononuclear cells, and to be chemotactic for both B and CD4+ T helper cells.21,27 By showing that C10 is highly expressed by LEC, our results suggest that this cytokine plays a relevant function in leucocyte recruitment into the lymphatic system.
The variety of potential interactions between chemokines released by LEC, on one side, and the various leucocyte subpopulations on the other, suggests a complex regulation of leucocyte recruitment and recirculation within the lymphatic system. LEC from different locations, such as mucosal lymphoid tissue and lymph node afferent/efferent vessels, may differ in the release of specific chemokines. In addition, leucocytes vary the expression of their chemokine receptors upon stimulation by inflammatory/immunological stimuli.10,29 Hence, different chemokine profiles released by endothelial cells of the lymphatic vessels combined with a varied pattern of expression of specific receptors on leucocytes according to their functional state may regulate cell migration from extravascular tissues into the lymphatic network. The same mechanism is likely to lead to lymphocyte recirculation outside the blood, by directing particular subpopulations to the efferent lymphatic vessels of the lymph nodes and other secondary lymphoid organs.
Acknowledgments
This study was supported, in part, by grants provided to A. Dobrina by the Italian Ministero dell'Università e della Ricerca Scientifica e Tecnologica (Cofin) and Ministero della Sanità. We are indebted to Dr Dontscho Kerjaschki for kindly investigating expression of podoplanine antigen on our lymphangioma histological preparations. We are indebted to Paolo Macor and Francesca Vita for skilful technical assistance.
References
- 1.Mackay CR, Marston W, Dudler L. Altered patterns of T cell migration through lymph nodes and skin following antigen challenge. Eur J Immunol. 1992;22:2205–10. doi: 10.1002/eji.1830220904. [DOI] [PubMed] [Google Scholar]
- 2.Geppert TD, Lipsky PE. Antigen presentation at the inflammatory site. Crit Rev Immunol. 1998;9:313–62. [PubMed] [Google Scholar]
- 3.Hay JB, Cahill RNP, Trnka Z. The kinetics of antigen-reactive cells during lymphocyte recruitment. Cell Immunol. 1974;10:145–58. doi: 10.1016/0008-8749(74)90158-0. [DOI] [PubMed] [Google Scholar]
- 4.Hall JG, Morris B. The origin of cells in the efferent lymph from a single node. J Exp Med. 1965;121:901–10. doi: 10.1084/jem.121.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 6.Bargatze RF, Jutila M, Butcher EC. Distinct roles of l-selectins and integrins α4 β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 7.Bradley LM, Watson SR. Lymphocyte migration into tissue: the paradigm derived from CD4 subsets. Curr Opin Immunol. 1996;8:312–20. doi: 10.1016/s0952-7915(96)80118-x. [DOI] [PubMed] [Google Scholar]
- 8.Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 9.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 10.MoSeries B, Loetscher M, Piali L, Loetscher P. Lymphocyte responses to chemokines. Int Rev Immunol. 1998;16:323–44. doi: 10.3109/08830189809043000. [DOI] [PubMed] [Google Scholar]
- 11.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–63. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriehuber E, Breiteneder-Geleff S, Groeger M, Soleiman A, Schoppmann SF, Stingl G, Kerjaschki D, Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancardi S, Stanta G, Dusetti N, et al. Lymphatic endothelial tumors induced by intraperitoneal injection of incomplete Freund's adjuvant. Exp Cell Res. 1999;246:368–75. doi: 10.1006/excr.1998.4270. [DOI] [PubMed] [Google Scholar]
- 14.Benvenuti F, Burrone OR. Anti-idiotypic antibodies induced by genetic immunisation are directed exclusively against combined VL/VH determinants. Gene Ther. 2001;8:1555–61. doi: 10.1038/sj.gt.3301546. [DOI] [PubMed] [Google Scholar]
- 15.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 1968;21(Suppl. 97):77–81. [PubMed] [Google Scholar]
- 16.Bleu CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komminoth P, Roth J, Saresmaslani P, Schroedel S, Heitz PU. Overlapping expression of immunohistochemical markers and synaptophysin mRNA in pheochromocytomas and adrenocortical carcinomas. Implications for the differential diagnosis of adrenal gland tumours. Lab Invest. 1995;72:424–31. [PubMed] [Google Scholar]
- 18.Wolpe SD, Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989;3:2565–73. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- 19.Wang JM, Sheery B, Fivas MJ, Kelvin DJ, Oppenheim JJ. Human recombinant macrophage inflammatory protein-1 alpha and beta and monocyte chemotactic and activating factor utilise common and unique receptors on human monocytes. J Immunol. 1993;150:3022–9. [PubMed] [Google Scholar]
- 20.Ziegler SF, Tough TW, Franklin TL, Armitage RJ, Alderson MR. Induction of macrophage inflammatory protein-1 beta gene expression in human monocytes by lipopolysaccharide and IL-7. J Immunol. 1991;147:2234–9. [PubMed] [Google Scholar]
- 21.Schall TJ, Bacon KB. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994;6:865–73. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 22.Mantovani A, Bussolino F, Introna M. Cytokine regulation of endothelial cell function: from molecular level to the bedside. Immunol Today. 1997;18:231–40. doi: 10.1016/s0167-5699(97)81662-3. [DOI] [PubMed] [Google Scholar]
- 23.Saeki H, Moore AM, Brown MJ, Hwang ST. Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–5. [PubMed] [Google Scholar]
- 24.Oquendo P, Alberta J, Wen DZ, Graycar JL, Derynck R, Stiles CD. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet A-granule proteins. J Biol Chem. 1989;264:4133–7. [PubMed] [Google Scholar]
- 25.Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–92. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orlofsky A, Lin YE, Prystowsky MB. Selective induction of chemokine C10 by IL-4 in mouse macrophages. J Immunol. 1994;152:5084–91. [PubMed] [Google Scholar]
- 27.Berger MS, Taub DD, Orlofsky A, Kleyman TR, Coupaye-Gerard B, Eisner D, Cohen SA. The chemokine C10: immunological and functional analysis of the sequence encoded by the novel second exon. Cytokine. 1996;8:439–47. doi: 10.1006/cyto.1996.0060. [DOI] [PubMed] [Google Scholar]
- 28.Pardigol A, Forssmann U, Zucht HD, Loetscher P, Shulz-Knappe P, Baggiolini M, Forssmann WG, Magert HJ. HCC-2, a human chemokine: gene structure, expression pattern, and biological activity. Proc Natl Acad Sci USA. 1998;95:6308–13. doi: 10.1073/pnas.95.11.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu Y, Shaw S. Lymphocyte interactions with extracellular matrix. FASEB J. 1991;5:2292–9. doi: 10.1096/fasebj.5.9.1860621. [DOI] [PubMed] [Google Scholar]








