Abstract
The majority of activated T lymphocytes undergo cell death at the end of a primary immune response, while a minority survive as memory cells. The mechanisms that control the decision between these two fates are unknown. In the present study we examined the response of activated T cells to interleukin-2 (IL-2) withdrawal. Within hours, the percentage of T lymphocytes in cell cycle showed a steady decrease, while the percentage arrested in G1 increased proportionally. Deprivation of IL-2 resulted in upregulation of the cell cycle inhibitor p27kip1. Comparison with resting T-cell populations revealed that the highest expression of p27kip1 occurs in activated T cells undergoing cell cycle arrest following IL-2 withdrawal. T cells deficient in p27kip1 expression showed an impaired ability to undergo cell cycle arrest in response to IL-2 deprivation. Moreover, T cells deficient in p27kip1 showed significantly more apoptosis after IL-2 withdrawal. Collectively, this study demonstrates that p27kip1 regulates both the cell cycle arrest and the apoptosis of antigen-specific T lymphocytes.
Introduction
Antigenic activation results in the clonal expansion of antigen-reactive T cells following signals initiated through the T-cell receptor (TCR) and associated costimulatory molecules.1–3 The subsequent production of cytokines, including interleukin (IL)-2, IL-4, IL-12, tumour necrosis factor (TNF) and interferon-γ (IFN-γ), plays an important regulatory role in shaping both the nature and magnitude of the immune response. While the initial frequencies of antigen-reactive T cells border on the lower limits of detection, responding lymphocytes expand by several orders of magnitude upon recognition of their specific antigen either in vivo or in vitro.2 The frequency of antigen-reactive cells then undergoes a significant decline as the immune response resolves. The clearance of activated T cells is regulated by a variety of mechanisms, including signalling through cell-surface death-inducing receptors such as Fas (CD95)4 and the TNF receptor-1 (TNFR-1).5 Declining concentrations of local growth factors also result in the apoptosis of activated T cells.6 While elimination of the majority of antigen-reactive T cells is crucial for the homeostasis of the immune system, the survival of a few antigen-reactive T cells is the basis of immunological memory. Although the mechanisms responsible for the removal of activated T cells are becoming increasingly clear, those which govern the survival of antigen-reactive memory cells remain poorly understood.
Antigenic stimulation of naive resting T lymphocytes results in the activation of multiple signalling pathways initiated through the TCR, costimulatory molecules (i.e. CD28, CD40) and growth factor (i.e. IL-2, IL-4 and IFN-γ) receptors.7,8 Signals from the IL-2 receptor (IL-2R) are mediated through Janus Kinase 3 (JAK3) signal transducer and activator of transcription 3/5 (STAT3/5) and phosphoinositide 3-kinase (PI3 kinase) pathways, initiating the transcriptional activation of genes necessary for proliferation.9–12 Important regulatory checkpoints then determine cellular activation and survival following antigenic stimulation. In T lymphocytes, such key regulatory checkpoints include: V(D)J rearrangement during thymic development; activation in response to ligation of the TCR; and susceptibility to apoptosis following activation (reviewed in ref. 13).
The mechanisms that govern cell activation function as a hierarchy, which ultimately regulates the activity of transcription factors belonging to the E2F family. In T lymphocytes, this includes three family members: E2F-1, E2F-3 and E2F-4 (reviewed in ref. 14). These molecules then heterodimerize with one of three DNA-binding proteins, termed DP1-3 (reviewed in ref. 15). In resting naive T cells, the transcription activity of these molecules is inhibited by members of the retinoblastoma (Rb) protein family, including p107, p110 and p130, all of which bind specific E2Fs.15–17 During cell activation, phosphorylation of specific residues on the Rb molecules results in the release of transcriptionally active E2F molecules. The phosphorylation of the Rb molecules is mediated by specific complexes of cyclins with cyclin-dependent kinases (CDK).18 Expression of these specific cyclin–CDK complexes occurs during distinct phases of the cell cycle, and these complexes in turn regulate the phosphorylation and activity of particular Rb and, ultimately, E2F family members. The activity of cyclin–CDK complexes is regulated by two families of inhibitory molecules: the INK-4 family; and the cip/kip family.19,20 Within the INK-4 family, expression of p16INK4a, p15INK4b, p18INK4c and p19INK4c regulate the activity of CDK4 and CDK6 primarily during early G1 (reviewed in ref. 19). The successful entry of cells into S phase involves passage through a ‘restriction’ point in late G1, controlled primarily by members of the cip/kip family.21 The members of the cip/kip family – p21cip1, p27kip1 and p57kip2 – associate with cyclin D-, E-, and A-dependent kinase complexes via their highly conserved N-terminal domains.22,23 In T cells, both p21cip1 and p27kip1 are expressed.24 Although p21cip1 and p27kip1 share a great deal of structural and functional similarity, their expression is distinctly regulated. Expression of p21cip1 is induced following DNA damage in a p53-dependent manner.25 In contrast, the expression of p27kip1 is inducible by cyclic adenosine monophosphate (cAMP),26 transforming growth factor beta (TGF-β)27,28 and growth factor withdrawal.24
The involvement of p27kip1 in maintaining G0/G1 arrest is supported by the increased cyclin–CDK activity observed in the absence of this inhibitor's expression in many cell types (reviewed in ref. 29). The importance of p27kip1 has been formally tested in animals with targeted disruptions within the gene encoding p27kip1.30–32 These animals have an increased body size, with a disproportionate increase in thymic and splenic lymphocytes. Surprisingly, the absence of p27kip1 had little effect on the kinetics or magnitude of naïve T-cell responses to proliferative stimuli.30,31
In this study, we have further examined the role of p27kip1 in cell cycle arrest following, rather than prior to, activation. In actively cycling T cells, withdrawal of IL-2 resulted in the induction of p27kip1 expression, concurrent with the induction of cell cycle arrest in G1. The importance of p27kip1 expression in response to IL-2 withdrawal was also examined in T cells from mice deficient in p27kip1. The absence of p27kip1 had little effect upon cellular proliferation, as previously reported.30,31 However, T cells deficient in p27kip1 failed to initiate cell cycle arrest and exhibited increased apoptosis in response to IL-2 withdrawal. Despite this increased apoptosis, p27kip1-deficient T cells had a normal apoptotic response to Fas (CD95) ligation. Taken collectively, the results of this study show that p27kip1 is important in regulating both the cell cycle arrest and survival of T cells deprived of IL-2.
Materials and methods
Animals
Female and male C57BL/6 mice (4–6 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, ME) or the National Cancer Institute (Frederick, MD). P27kip1-deficient mice were kindly donated by Dr A. Koff (Sloan Kettering, New York, NY), while OT-1 TCR transgenic mice33 were kindly donated by Dr L. Lefrancois (University of Connecticut Health Center, Farmington, CT). Animals were maintained under specific-pathogen free conditions in the SHM animal unit at the Yale School of Medicine. Female mice, heterozygous for the expression of p27kip1, were bred with male mice deficient in p27kip1. Tail DNA of the resulting progeny was screened by polymerase chain reaction (PCR) using the following primers: p27kip1F1, 5′-AAACGTGAGAGTGTCTAACGGGAG-3′; and p27kip1R1, 5′-CACCTCCTCCCATTCGTATCT-3′; encompassing the insertion site of the neo gene cassette. PCR was conducted (for 30 cycles at an annealing temperature of 56°) in the presence of 2·0 µm MgCl2. OT1 transgene-positive mice were identified by flow cytometry using a fluorescence-activated cell sorter (FACScalibur flow cytometer; Becton-Dickinson Immunocytometry Systems, Mountain View, CA) and CellQuest software (Becton-Dickinson Immunocytometry Systems) following retro-orbital plexus bleeding and dual staining for Vα2 and CD8 using antibodies from PharMingen (San Diego, CA). OT-1 × P27 kip1–/– mice were generated by crossing OT1 transgenic and P27kip1–/– mice, and their progeny were screened by PCR and FACS for p27kip1 and Va2 expression, respectively.
Activation of T lymphocytes
Single-cell spleen suspensions were prepared using a sterile ground-glass homogenizer. The resulting suspensions were washed and resuspended in Dulbecco's modified Eagle's minimal essential medium (DMEM), containing 5% fetal calf serum (FCS) (Life Technologies, Grand Island, NY), following depletion of red blood calls (RBC) by lysis with Tris-buffered ammonium chloride. Activation of splenic T cells (1 × 106/ml) was achieved in vitro, in the presence of autologous antigen-presenting cells (APC), by incubation for 2 days with either soluble hamster anti-mouse CD3ε (145–2C11) or, in the case of OT-1 transgenic T cells, 100 nm of the specific ovalbumin peptide, SIINFEKL.33 Cultured cells were extensively washed and returned to culture overnight in medium supplemented with 25 U/ml recombinant IL-2 (Cellular Products Inc., Buffalo, NY) following centrifugation over Ficoll (Sigma Chemical Co., St Louis, MO). The resulting cultures consisted almost exclusively of T cells (>95%) based on FACS, with ≈ 45% in cell cycle, based on total cellular DNA content.
Assessment of cell cycle arrest and apoptosis
Cycle cell arrest was induced following the withdrawal of IL-2. After overnight (16 hr) culture in IL-2-supplemented medium, proliferating T cells were washed extensively to remove IL-2 and returned to culture in complete medium in the presence or absence of IL-2. At various time-points, cell were harvested and their viability determined by Trypan blue exclusion and by flow cytometry following staining with fluorescein isothiocyanate (FITC)-conjugated annexin V (R&D Systems, Minneapolis, MN). In some experiments, cultures were pulsed with 1 mm bromodeoxyuridine (BrdU) during the final 30 min of culture to assess cell cycle arrest. Induction of Fas-mediated apoptosis was performed, as previously described,34 using control or CD95L-transfected 3T3 fibroblasts in six-well tissue culture wells (Becton-Dickinson). Cell death assays involved the co-culture of 3 × 106 thymic T cells on control or CD95L-transfected 3T3 cells at 37° in 5 ml of complete RPMI medium. At the time-points indicated, non-adherent T cells were isolated from adherent fibroblast monolayers by gentle aspiration.
Cell staining and FACS analysis
Harvested T-cell cultures were stained with propidium iodide (PI) at 50 µg/ml (Sigma Chemical Co.) for 45 min in 0·1% sodium citrate, 0·3% Nonidet P-40 (NP-40), and 50 mg/ml RNase (Calbiochem, San Diego, CA). To detect the incorporation of BrdU, cells were fixed in 70% ethanol and incubated overnight in 1% paraformaldehyde and 0·01% Tween. After washing, the cells were incubated with 50 U/ml DNase (Boehringer Mannheim, Indianapolis, IN) in 0·9% NaCl and 5 mm MgCl2, pH 5·0, for 30 min and then with FITC-conjugated anti-BrdU monoclonal antibody (mAb) (Becton-Dickinson). DNA synthesis or total DNA content was determined by flow cytometry using a FACScalibur flow cytometer and CellQuest software (Becton-Dickinson Immunocytometry Systems).
Western blot analysis
For Western blot analysis, anti-CD3 splenic T-cell blasts (2 × 106/lane) or peptide-activated OT-1 transgenic T cells (3 × 107/lane), cultured in either the presence or absence of IL-2 for the times indicated, were lysed in 50 µl of lysis buffer containing 0·1% NP-40, 50 mm HEPES, 300 mm NaCl, 1 mm dithiothreitol (DTT) and protease inhibitors [1 mm phenylmethylsulphonyl fluoride (PMSF), 10 µg/ml leupeptin and 10 µg/ml aprotinin]. Equivalent cell numbers were electrophoresed in 5–15% sodium dodecyl sulphate (SDS)–polyacrylamide gradient gels (Bio-Rad, Hercules, CA) and then electrophoretically transferred onto nitrocellulose membranes (Bio-Rad). Membranes were blocked in phosphate-buffered saline (PBS) containing 4% powdered milk and 0·04% Tween-20, for 1 hr at room temperature with gentle agitation. Blots were then probed with purified anti-p27kip1 mAb (PharMingen) and rat anti-mouse actin (Sigma), followed by appropriate horseradish peroxidase-conjugated secondary mAb (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were then developed using enhanced chemiluminescence (Amersham, Piscataway, NJ) and exposed on BioMax (Kodak, Rochester, NY) autoradiography film.
Results
Induction of cell cycle arrest in response to IL-2 withdrawal
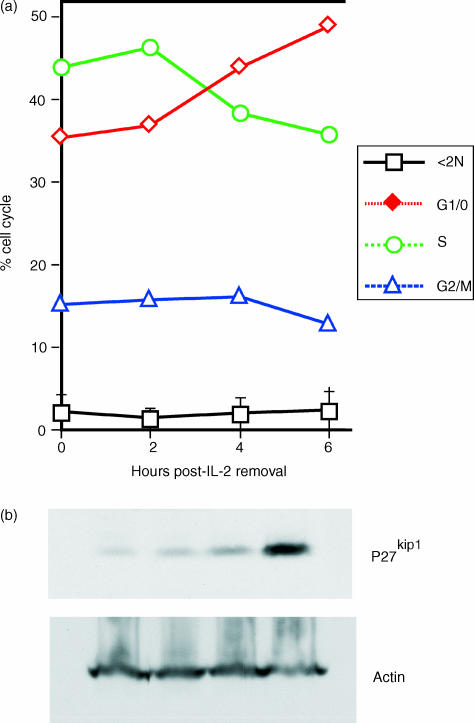
The role of cell cycle regulation in the survival of activated T cells following IL-2 withdrawal was investigated. Splenic C57BL/6 T cells were activated in vitro for 2 days in the presence of autologous APC and soluble anti-CD3ε (0·5 µg/ml). After 2 days, viable cells were isolated by centrifugation over Ficoll, washed extensively to remove the anti-TCR antibody and cultured for 16 hr in medium supplemented with 25 U/ml of recombinant IL-2. These cultures resulted in proliferating T cells, with >45% actively cycling, as determined by measurement of DNA content using FACS analysis (Fig. 1a). The ability of cells to undergo cell cycle arrest in response to IL-2 deprivation was then examined. Within the first 9 hr, IL-2 withdrawal had little or no effect on the percentage of subdiploid cells or cells in G2/M phase. In contrast, the percentage of cells in S phase steadily decreased in response to IL-2 withdrawal, with a coincident increase in the percentage of cells in G0/1, and these effects were seen as early as 4 hr.
Figure 1.
Withdrawal of interleukin-2 (IL-2) results in the induction of cell cycle arrest of activated T cells. Splenic C57BL/6 T-cell blasts were generated in vitro following activation with soluble anti-CD3 monoclonal antibody (mAb). After 2 days, cells were washed and cultured overnight in the presence of IL-2 to establish vigorous proliferation. Cell cycle arrest was induced following the removal of IL-2. (a) The percentage of T cells with subdiploid (<2 N), G0/G1, S and G2/M DNA content was then determined at 2, 4, and 6 hr after IL-2 deprivation. The data are representative of five experiments. (b) Western blot analysis of T cells undergoing cell cycle arrest in response to IL-2 withdrawal reveals the upregulation of p27kip1. Lysates were prepared from cultures identical to those in (a). Equivalent cell numbers (2 × 106/well) were then subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membranes. Blots were probed with mAb specific for p27kip1 and actin for loading control. The figure is representative of results obtained from eight independent experiments.
During T-cell activation, signalling via the IL-2 receptor ultimately results in the downregulation of the cyclin-CDK inhibitory protein, p27kip1.24,35 Therefore, we examined whether IL-2 withdrawal resulted in the reciprocal upregulation of p27kip1 (Fig. 1b). Western blot analysis of T cells from cultures identical to those examined for DNA content, in Fig. 1, revealed a steady increase in p27kip1 expression following IL-2 withdrawal, coinciding with the arrest of cells in G0/G1. Despite the induction of p27kip1, no induction of p21cip1 was observed (data not shown). The induction of cell cycle arrest, in parallel with the upregulation of p27kip1, supports a role for p27kip1 in the arrest of actively cycling T cells.
P27kip1 is highly expressed by activated T cells undergoing cell cycle arrest
Expression of p27kip1 is downregulated in T cells in response to TCR-mediated activation following production of IL-2. Signalling via the IL-2 receptor results in the downregulation of p27kip1.24,29 The rapid degradation of p27kip1, observed following cell activation, has suggested its involvement in cell cycle arrest in a variety of cell types (reviewed in ref. 21). However, it remains unclear whether p27kip1 expression is equally important in all populations of resting T cells (i.e. naive and memory subsets). To examine this, p27kip1 expression was examined during distinct phases of cellular activation in T cells from TCR transgenic mice expressing an antigen receptor specific for a defined peptide of ovalbumin.33 OT-1 trangenic T cells were then activated with specific antigenic peptide for 2 days in the presence of autologous APC. Prior to activation, 95% of T cells were in the G0/G1 phase of the cell cycle. Two days after antigenic activation and after 16 hr of culture in 25 U/ml of IL-2, 66% of the cells (versus 5%) were now in S phase. Cell cycle arrest of these actively cycling T cells was then induced by IL-2 withdrawal, resulting in 88% of the T cells arresting in G0/G1 by 36 hr (Fig. 2a).
Figure 2.
Cell cycle and Western blot analysis demonstrates higher expression of p27kip1 in resting T cells subsequent to activation. (a) DNA content analysis of naïve, activated and arrested OT-1 T cells. Naïve T cells from OT-1 T-cell receptor (TCR) transgenic mice were activated in the presence of peptide for 2 days in vitro, and then cultured in medium supplemented with interleukin-2 (IL-2). After 24 hr, cells were washed and returned to culture in the presence (activated: +IL-2) or absence (–IL-2) of IL-2. Cellular DNA content was determined, at the indicated time-points, by flow cytometry. (b) Replicate cell samples from panel (a) were examined for expression of p27kip1. Equivalent cell numbers (3 × 106/well) were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto nitrocellulose membranes. Blots were then probed with monoclonal antibody (mAb) specific for p27kip1, followed by reprobing with anti-actin as a loading control. Results shown in the figure are representative of three separate experiments.
The expression of p27kip1 in these T-cell populations was then examined by SDS–polyacrylamide gel electrophoresis and Western blotting. Analysis of the DNA content in naïve versus previously activated T cells induced to undergo cell cycle arrest revealed similar percentages (95% versus 88%) of cells in G0/G1. However, significantly higher p27kip1 expression was detected in T cells that had arrested after prior antigenic activation, even though this population contained a lower overall percentage (7%) of T cells in G1/G0. Activation of T cells leads to the downregulation of p27kip1 expression in polyclonal T-cell populations.24 Surprisingly, a significantly higher expression of p27kip1 was observed in the resting population which had undergone activation, in comparison to resting naïve T cells which had not encountered antigen (Fig. 2b). While these experiments confirm the expression of p27kip1 in resting T-cell populations,24 the significantly higher expression of p27kip1 observed in activated T cells after IL-2 withdrawal compared to unstimulated cells suggests that p27kip1 plays an important role in the cell cycle arrest of antigen-specific lymphocytes after activation.
T cells deficient in p27kip1 fail to undergo cell cycle arrest in response to IL-2 withdrawal
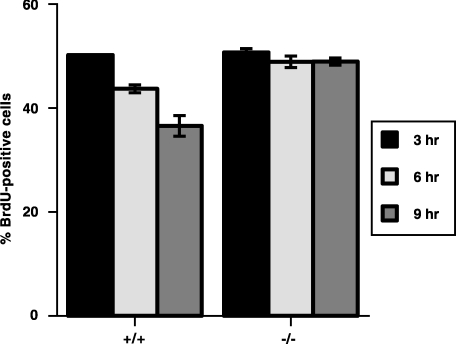
The upregulation of p27kip1 observed in response to IL-2 withdrawal suggested a functional role for p27kip1 in the arrest of T cells following activation. To test this, normal and p27kip1-deficient OT-1 splenic T cells were activated in vitro in the presence of peptide for 48 hr. T-cell blasts were washed extensively and returned to culture in medium supplemented with 25 U/ml of recombinant IL-2. Equivalent proliferative responses were observed in control and p27kip1-deficient T cells, as previously reported.30,31 After 16 hr, the ability of p27kip1–/– T-cell blasts to undergo cell cycle arrest was examined following the removal of exogenous IL-2. T cells were returned to culture for 3, 6 or 9 hr, without IL-2. Cell cycle arrest was evaluated based upon BrdU incorporation (Fig. 3). During the final 30 min of each culture, T cells were pulsed with 100 µm BrdU. The percentage of cycling cells was measured by flow cytometry (using a live gate to identify viable cells) and staining with FITC-conjugated anti-BrdU mAb. In response to IL-2 withdrawal, normal T cells began to undergo cell cycle arrest within 3 hr, as shown by decreased BrdU incorporation. By 9 hr, the percentage of T cells incorporating BrdU had decreased by 30%, consistent with the induction of cell cycle arrest. In contrast, T cells deficient in p27kip1 failed to undergo cell cycle arrest in response to IL-2 withdrawal, revealing only a slight (<5%) reduction in the percentage of BrdU incorporation over 9 hr. The continued incorporation of BrdU by p27kip1-deficient T cells in response to IL-2 withdrawal, despite incorporation similar to control T cells prior to IL-2 deprivation, further suggests that p27kip1 plays its most important role during the cell cycle arrest of, as opposed to activation of, T lymphocytes.
Figure 3.
T cells deficient in the expression of p27kip1 exhibit impaired cell cycle arrest in response to interleukin-2 (IL-2) withdrawal. The importance of p27kip1 in T-lymphocyte cell cycle arrest was examined based on the incorporation of bromodeoxyuridine (BrdU). T-cell blasts from control (+/+) and p27kip1-deficient (–/–) OT-1 T-cell receptor (TCR) transgenic mice were generated as described in the legend to Fig. 2. Following overnight culture in medium supplemented with IL-2 (25 U/ml), blasts were washed and replaced in culture in the absence of IL-2 for 3, 6 and 9 hr. Thirty minutes prior to the end of each culture, cells were pulsed with 100 nm BrdU. After 30 min, cells were washed, fixed and stained with fluorescein isothiocycanate (FITC)-conjugated anti-BrdU monoclonal antibody (mAb). The percentage of cells in cell cycle was then determined by flow cytometry, based on a live gate.
IL-2 withdrawal results in the increased apoptosis of p27kip1-deficient T cells after activation
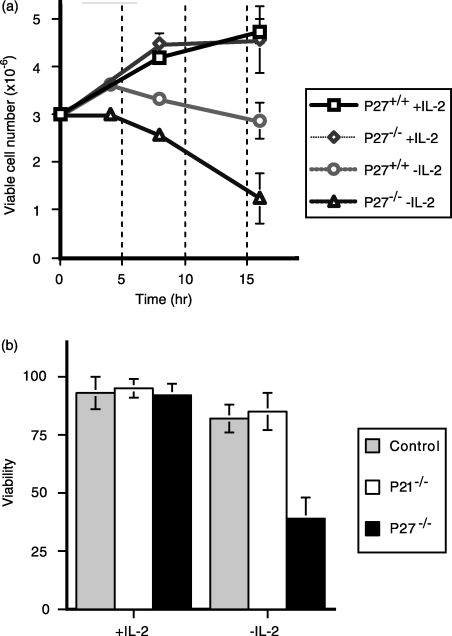
Given the apparent importance of p27kip1 in cell cycle arrest, we examined the consequences of p27kip1 deficiency for T-cell viability in response to IL-2 withdrawal (Fig. 4a). After activation with peptide for 48 hr, p27kip1+/+ and p27kip1–/– OT-1 T-cell blasts were washed and cultured for 16 hr in the presence of 25 U/ml recombinant IL-2 to establish vigorous proliferation. These T-cell blasts were then washed and returned to culture in either the presence or absence of exogenous IL-2. In the presence of IL-2, p27kip1–/– OT-1 T cells proliferated almost identically to p27kip1+/+ OT-1 T cells for the entire course of the assay. In the absence of IL-2, the viability of p27kip1+/+ OT-1 T-cell cultures remained relatively constant over the course of the assay. In contrast, IL-2 withdrawal resulted in a significant loss of viable p27kip1–/– OT-1 T cells, detectable by 3 hr, with 60% of the cells undergoing apoptosis by 16 hr. Therefore, the impaired ability of p27kip1–/– cells to undergo cell cycle arrest correlated with an increased susceptibility to apoptosis following IL-2 deprivation.
Figure 4.
The inability to upregulate p27kip1 in response to interleukin-2 (IL-2) withdrawal is accompanied by increased apoptosis. T-cell blasts from control and p27kip1-deficient mice were generated with anti-CD3 antibody, as described in the legend to Fig. 1. (a) Following culture overnight in IL-2 (25 U/ml)-supplemented medium, blasts were washed and added to culture medium in either the presence or absence of IL-2. After culture for 4, 8 and 16 hr, T-cell blasts were harvested and the number of viable cells was determined based upon cell counts and exclusion of Trypan Blue. The graph depicts the mean ± standard error of the mean (SEM) of triplicate counts obtained at each time-point. The figure is representative of data obtained from eight independent experiments.
Impaired expression of p27kip1, but not p21cip1, correlates with increased apoptosis in response to IL-2 withdrawal. T-cell blasts from C57BL/6, p27kip1–/– and p21cip1–/– mice were generated as described in the Materials and methods. Cells were then washed and returned to culture in the presence of IL-2. After 24 hr, cells were washed and cultured for a further for 16 hr in the presence or absence of IL-2. The percentage of viable cells was then determined by cell counts and staining with fluorescein isothiocyanate (FITC)-conjugated annexin. The data shown are representative of three independent experiments.
The increased apoptosis of p27kip1-deficient T cells is specific for IL-2 withdrawal
Collectively, our findings suggested that the expression of p27kip1 regulated the arrest and subsequent survival of T cells following activation. However, it remained unclear whether this role was specific to p27kip1 or a property common to all cyclin inhibitory proteins. We therefore next examined the role of the closely related cyclin inhibitory protein, p21cip1, in T cells following IL-2 withdrawal (Fig. 4b). Comparison of activated T cells from control (C57BL/6), p21cip–/– and p27kip1–/– revealed no significant differences in cellular proliferation or viability at 48 hr in the presence of IL-2. However, in response to IL-2 withdrawal, significant apoptosis was observed in p27kip1–/– (65 ± 12%) T cells, and not in p21cip1–/– (15 ± 4%) T cells, compared with wild-type controls (18 ± 6%).
The apoptosis of activated T cells is known to involve several distinct pathways, including TNF-α5 and Fas,4 and is dependent on essential growth factors.6 Indeed, the co-culture of thymocytes with Fas ligand (FasL)-expressing 3T3 fibroblasts results in the induction of Fas-mediated apoptosis of ≥ 50% of T cells in 5 hr.34 To evaluate whether p27kip1 plays a significant role in other forms of apoptosis, we next examined the susceptibity of p27kip1–/– T cells to Fas-mediated apoptosis. It is noteworthy that co-culture with 3T3–FasL cells, but not 3T3 control cells, resulted in equivalent levels of apoptosis in both p27kip1+/+ and p27kip1–/– T cells (data not shown). Consequently, the expression of p27kip1 appears to selectively protect T cells from IL-2 withdrawal, but not all apoptotic stimuli.
Discussion
In this study we examined the expression and function of p27kip1 in T cells induced to undergo cell cycle arrest. In response to IL-2 withdrawal, T cells initiated cell cycle arrest and upregulated p27kip1. The proliferative responses of p27kip1–/– T cells were equivalent to those of wild-type T cells, in either the presence or absence of exogenous IL-2. However, p27kip1+/+, but not p27kip1–/–, T cells initiated cell cycle arrest in response to IL-2 withdrawal, and the inability of p27kip1–/– T cells to undergo cell cycle arrest in response to IL-2 withdrawal was associated with increased apoptosis. These results support an important role for p27kip1 expression in the fate of activated T cells following IL-2 withdrawal, which is to control whether cells undergo cell cycle arrest or apoptosis.
Although the activation of resting naive T cells has been studied extensively, relatively little is known about the mechanisms governing cell cycle arrest following activation. As a consequence, many of the molecular and biochemical mechanisms thought to regulate cell cycle arrest in T cells have been extrapolated from events observed during activation. Antigenic stimulation of T lymphocytes results in the synthesis of IL-2 following the activation of several signalling cascades.11,36 Following their activation, de novo protein synthesis allows naive T cells to progress from G0 to G1.37 However, the continued progression of T cells into S phase is largely dependent on the presence of IL-2.38 The ability of IL-2 to promote cell cycle progression into S phase involves the downregulation of the cyclin–CDK inhibitor, p27kip1, allowing cells to progress through a checkpoint in late G1.24 IL-2 receptor signalling results in the rapid downregulation of p27kip1 at the post-translational level, thereby supporting a critical role for p27kip1 in regulating progression from G1 to S.39,40 However, T cells from animals deficient in p27kip1 have failed to support this hypothesis, demonstrating little to no significance for p27kip1 during primary T-cell activation.30,31 Many explanations could be advanced to account for this. One possibility is that other inhibitors, belonging to either the INK-4 or the cip/kip family, could substitute in the absence of p27kip1. The fact that members of the INK-4 family are preferentially expressed in early G1 suggests that they are unlikely candidates for regulating a late G1 checkpoint.21 Although we have been unable to detect expression of p57kip2 in T cells ex vivo (J.W Huleatt, I.N. Crispe, unpublished observations), p21cip1 is detectable, particularly in thymocytes. While p21cip1 also regulates the activity of cyclin D–CDK2 complexes, its expression appears to be regulated by mechanisms distinct from p27kip1. In contrast to p27kip1, IL-2 treatment induces the upregulation of p21cip1,24 which is consistent with the idea that p21cip1 can function as both a cyclin–CDK inhibitor or cell cycle progression factor, depending on its level of expression.29
Alternatively, p27kip1 may play a minor role regulating the G0 to S progression of naïve T cells in response to activation. Indeed, Zhang et al. reported that p27kip1 expression had no effect on initial TCR signalling, while p27kip1–/– T-cell blasts exhibit increased proliferation when restimulated in the presence of cytokines, including IL-2.41 p27kip1 expression is downregulated by IL-2. The cell cycle progression of activated T cells may then depend on the cytokine-mediated degradation of cellular p27kip1 levels. Therefore, the increased proliferation by activated p27kip1–/– T cells in the presence of cytokines may reflect the necessity of wild-type T cells to downregulate p27kip1. Indeed, T cells from secondary lymphoid tissues, upon isolation, predominantly reside in the G0 phase.35 Cells in G0 are characterized by the absence of cyclin protein expression, precluding CDK complex formation and CDK-dependent activities. Hence, the functional significance of cyclin-inhibitory proteins, such as p27kip1 in naïve T cells, remains unclear. The function of p27kip1 has also been examined in T cells from p27kip1 transgenic mice.42 The ability of T cells to proliferate decreased as the levels of the p27kip1 transgene expression were increased. However, the functional potential of T cells from these mice is not clear as the etopic transgene expression under the lck-proximal promoter additionally resulted in a severe block in thymic development at the CD4− CD8− stage.
Much of our present understanding of cell cycle arrest is based on studies using established cell lines. Although many such cell lines are capable of undergoing cell cycle arrest, they do so in the G1, not the G0, phase of the cell cycle, and thus do not fully represent the populations of resting T cells found in vivo. Indeed, we observed lower expression of p27kip1 among naïve T-cell populations, despite the fact that >95% of the cells reside in G0/G1 of the cell cycle (Fig. 2 and data not shown). It is noteworthy that polyclonal naive T cells have been shown to express a higher level of p27kip1 upon isolation.35 It is possible that the higher expression of p27kip1 observed among polyclonal T-cell populations is related to their ability to recognize a broader range of endogenous antigens. Hence, the higher expression of p27kip1 observed among resting polyclonal T cells may relate to the higher frequency of previously activated T cells in these animals. Based upon the results of this study, we postulate that the expression of p27kip1 regulates the arrest of T cells following, rather then prior to, activation.
The results of this study also support a link between cell cycle regulation and T-cell survival following activation. Cell cycle regulation is involved in T-lymphocyte development, differentiation and apoptosis (reviewed in ref. 13). The differentiation of antigen-responding T cells into memory lymphocytes is believed to correlate with the number of cell divisions following antigenic activation.43 Similarly, upregulation of p27kip1 expression during TCR-β rearrangement has been implicated during TCR selection.44,45
The apoptosis of T lymphocytes is controlled by a variety of mechanisms. Antigenic activation results in the increased expression of both Fas and FasL, in addition to susceptibility to activation-induced cell death (AICD) (reviewed in ref. 46). Similarly, the induction of ‘propriocidal’ apoptosis of T cells in vitro results from prolonged ligation of the TCR.46 The ability of exogenous IL-2 to augment propriocidal apoptosis has been used to support the possibility that cell death occurs preferentially in the S phase of the cell cycle.47 Signalling through Fas and other members of the TNF-R1 family results in the activation of caspases, nuclear fragmentation and apoptosis (reviewed in ref. 46). Although we are unable to detect any role for p21cip1 in IL-2 withdrawal, expression of p21cip1 inhibits the apoptosis of macrophages in response to growth factor deprivation.48 The upregulation of p21cip1 presumably results in cell cycle arrest in G1, and thereby resistance to this form of apoptosis. In contrast, it is interesting to note that T cells in S phase exhibit preferential resistance to Fas-mediated apoptosis.49
The results of this study suggest that IL-2 withdrawal, like propriocidal apoptosis, leads to the preferential apoptosis of T cells during S phase. In response to IL-2 withdrawal, upregulation of p27kip1 expression coincides with the induction of cell cycle arrest. However, in the absence of p27kip1, T cells failed to initiate cell cycle arrest and exhibited significantly more apoptosis within a few hours of IL-2 deprivation. The increased apoptosis observed in p27kip1-deficient T cells in response to IL-2 withdrawal suggests that the processes which regulate cellular proliferation and apoptosis are closely linked. In the absence of p27kip1 expression, increased CDK2/4 activity would result in continuous phosphorylation of Rb family members. The continued phosphorylation of Rb would result in more free E2F molecules, resulting in increased gene transcription as observed during cell activation. (reviewed in ref. 14). It is noteworthy that E2F-1 activity is involved during proliferation and apoptosis.50–53 Interestingly, while the apoptotic function of E2F-1 is dependent on DNA binding, it is apparently independent of transcriptional trans-activation.54 The importance of Rb during apoptosis is supported by the ability of Rb overexpression to inhibit E2F-mediated apoptosis55 and by the fact that phosphorylated Rb is cleaved during apoptosis,56 in response to both CD95 ligation57 and growth factor withdrawal-induced apoptosis.58 Consequently, the apoptosis observed following IL-2 withdrawal in p27kip1-deficient T cells probably reflects the dysregulation of specific CDK activities, which in turn regulate Rb phosphorylation and function.59,60
Acknowledgments
We thank Dr L. Lefrancois for the OT-1 mice and Dr F. Carbone for permission to use them. The p27kip-1 deficient mice were provided by Dr A. Koff. J.W.H was supported by a fellowship from the Arthritis Foundation. This work was supported by NIH grant 1R01GM56689 to I.N.C.
References
- 1.MacDonald HR, Nabhotz M. T cell activation. Annu Rev Cell Biol. 1986;2:231–53. doi: 10.1146/annurev.cb.02.110186.001311. [DOI] [PubMed] [Google Scholar]
- 2.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion vs functional clonal inactivation. Annu Rev Immunol. 1989;7:445–80. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–83. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 4.Itoh N, Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993;268:10932–7. [PubMed] [Google Scholar]
- 5.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Goeddel DV. The two different receptors of tumor necrosis mediate distinct cellular responses. Proc Natl Acad Sci USA. 1991;88:9292–6. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbar ANNJ, Borthwick RG, Wickremasinghe P, et al. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol. 1996;26:294–9. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 7.Weiss A, Shields R, Newton M, Manger B, Imboden J. Ligand–receptor interactions required for commitment to the activation of the interleukin 2 gene. J Immunol. 1987;138:2169–76. [PubMed] [Google Scholar]
- 8.Samelson LE, Patel MD, Weissman AM, Harford JB, Klausner RD. Antigen activation of murine T cells induces tyrosine phosphorylation of a polypeptide associated with the T cell antigen receptor. Cell. 1986;46:1083–90. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- 9.Park SY, Saijo K, Takahashi T, et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–82. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 10.Gaffen SL, Lai SY, Xu W, Gouilleux F, Groner B, Goldsmith MA, Greene WC. Signaling through the interleukin 2 receptor beta chain activates a STAT-5-like DNA-binding activity. Proc Natl Acad Sci USA. 1995;92:7192–6. doi: 10.1073/pnas.92.16.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedmann MC, Migone TS, Russell SM, Leonard WJ. Different interleukin 2 receptor beta-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proc Natl Acad Sci USA. 1996;93:2077–82. doi: 10.1073/pnas.93.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–89. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 13.Reiner SL, Seder RA. Dealing with the evolutionary pawnshop: how lymphocytes make decisions. Immunity. 1999;11:1–11. doi: 10.1016/s1074-7613(00)80076-x. [DOI] [PubMed] [Google Scholar]
- 14.Slansky JE, Farnham PJ. Introduction to the E2F family: protein structure and gene regulation. Curr Top Microbiol Immunol. 1996;208:1–30. doi: 10.1007/978-3-642-79910-5_1. [DOI] [PubMed] [Google Scholar]
- 15.Dyson N. The regulation of E2F by pRb-family members. Genes Dev. 1998;12:2245–62. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 16.Wienberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen SE, Buckmaster C, Chen TT, Feramisco JR, Wang JY. Inhibition of DNA synthesis by RB: effects on G1/S transition and S-phase progression. Genes Dev. 1998;12:2278–92. doi: 10.1101/gad.12.15.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan DO. Cyclin-dependent kinases: engines, clocks and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–91. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 19.Peter M, Herskowitz I. Joining the complex: cyclin-dependent kinase inhibitory proteins and the cell cycle. Cell. 1994;79:181–4. doi: 10.1016/0092-8674(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 20.Jacks T, Weinberg RA. The expanding role of cell cycle regulators. Science. 1998;280:1035–6. doi: 10.1126/science.280.5366.1035. [DOI] [PubMed] [Google Scholar]
- 21.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–63. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A. Cyclin-binding motifs are essential for the function of p21cip1. Mol Cell Biol. 1996;16:4673–82. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Y, Hurwitz J, Massague J. Cell cycle inhibition mediated by functionally independent CDK and PCNA domains in p21cip1. Nature. 1995;375:159–61. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 24.Nourse J, Firpo E, Flanagan WM, et al. Interleukin-2 mediated elimination of p27kip1 cyclin dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–3. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 25.Macleod KF, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. p53-dependent and independent expression of p21cip1 during cell growth, differentiation and DNA damage. Genes Dev. 1995;9:935–44. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 26.Kato J, Matsuoka M, Polyak K, Massague J, Sherr CJ. Cyclic AMP-induced cell cycle arrest mediated by and inhibitor (p27kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–96. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 27.Toyoshima H, Hunter T. P27kip1, a novel inhibitor of G1 cyclin-cdk protein kinase activity, is related to p21cip1. Cell. 1994;78:74–8. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 28.Polyak K, Kato J, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27kip1, a cyclin-cdk inhibitor, links transforming growth factor beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 29.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY. Mice lacking p27kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia and pituitary tumors. Cell. 1996;85:707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 31.Kiyokawa H, Kineman RD, Manova-Todorova KO, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27kip1. Cell. 1996;85:721–32. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 32.Fero ML, Rivkin M, Tasch M, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorgenesis and female sterility in p27kip1 deficient mice. Cell. 1996;85:733–44. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 33.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 34.Hughes DPM, Crispe IN. A soluble inhibitory isoform of murine Fas generated by alternative splicing. J Exp Med. 1995;182:1395–401. doi: 10.1084/jem.182.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon TK, Buchholz MA, Ponsalle P, Chrest FJ, Nordin AA. The regulation of p27kip1 expression following the polyclonal activation of murine G0 T cells. J Immunol. 1997;158:5642–8. [PubMed] [Google Scholar]
- 36.Thomis DC, Berg LJ. Peripheral expression of Jak3 is required to maintain T lymphocyte function. J Exp Med. 1997;185:197–206. doi: 10.1084/jem.185.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajchenbaun F, Ando K, DecCaprio JA, Griffin JD. Independent regulation of human D type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268:4113–9. [PubMed] [Google Scholar]
- 38.Firpo EJ, Koff A, Solomon MJ, Roberts JM. Inactivation of a cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Mol Cell Biol. 1993;14:4889–99. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal D, Hauser P, McPherson F, Dong F, Garcia A, Pledger WJ. Repression of p27kip1 synthesis by platelet-derived growth factor in BALB/c 3T3 cells. Mol Cell Biol. 1996;16:4327–36. doi: 10.1128/mcb.16.8.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hengst L, Reed SI. Translational control of p27kip1 accumulation during the cell cycle. Science. 1996;271:1861–4. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Lawless VA, Kaplan MH. Cytokine-stimulated T lymphocyte proliferation is regulated by p27kip1. J Immunol. 2000;165:6270–7. doi: 10.4049/jimmunol.165.11.6270. [DOI] [PubMed] [Google Scholar]
- 42.Tsukiyama T, Ishida N, Shirane M, Minamishima YA, Hatakeyama S, Kitagawa M, Nakayama K, Nakayama K. Down-regulation of p27kip1 expression is required for development and function of T cells. J Immunol. 2001;166:304–12. doi: 10.4049/jimmunol.166.1.304. [DOI] [PubMed] [Google Scholar]
- 43.Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283:1745–8. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman ES, Passoni L, Crompton T, Leu TM, Schatz DG, Koff A, Owen MJ, Hayday AC. Productive T-cell receptor B-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10:948–62. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Dordai DI, Lee J, Desiderio S. A conserved degradation signal regulates RAG-2 accumulation during cell division and links V(D)J recombination to the cell cycle. Immunity. 1996;5:575–89. doi: 10.1016/s1074-7613(00)80272-1. [DOI] [PubMed] [Google Scholar]
- 46.Lenardo M, Chan K, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis – immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 47.Boehme SA, Lenardo MJ. Ligand-induced apoptosis of mature T lymphocytes (propriocidal regulation) occurs at distinct stages of the cell cycle. Leukemia. 1993;7(Suppl. 2):S45–S49. [PubMed] [Google Scholar]
- 48.Boehme SA, Lenardo MJ. Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur J Immunol. 1993;23:1552–60. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- 49.Xaus J, Cardo M, Valledor AF, Soler C, Lloberas J, Celada A. Interferon gamma induces the expression of p21cip1/waf1 and arrests macrophage cell cycle, preventing induction of apoptosis. Immunity. 1999;11:103–13. doi: 10.1016/s1074-7613(00)80085-0. [DOI] [PubMed] [Google Scholar]
- 50.Dao T, Huleatt JW, Hingorani R, Crispe IN. Specific resistance of T cells to CD95-induced apoptosis during S phase of the cell cycle. J Immunol. 1997;159:4261–7. [PubMed] [Google Scholar]
- 51.Kowalik TF, DeGregori J, Schwarz JK, Nevins JR. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J Virol. 1995;69:2491–500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WG, Jr, Livingston DM, Greenberg ME. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–61. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 53.DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci USA. 1997;94:7245–50. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shan B, Farmer AA, Lee WH. The molecular basis of E2F-1/DP-1-induced S-phase entry and apoptosis. Cell Growth Differ. 1996;7:689–97. [PubMed] [Google Scholar]
- 55.Hsieh JK, Fredersdorf S, Kouzarides T, Martin K, Lu X. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–52. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- 56.Haas-Kogan DA, Kogan SC, Levi D, Dazin P, T'ang A, Fung YK, Israel MA. Inhibition of apoptosis by the retinoblastoma gene product. EMBO J. 1995;14:461–72. doi: 10.1002/j.1460-2075.1995.tb07022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fattman CL, An B, Dou QP. Characterization of interior cleavage of retinoblastoma protein in apoptosis. J Cell Biochem. 1997;67:399–408. [PubMed] [Google Scholar]
- 58.Tan X, Martin SJ, Green DR, Wang JYJ. Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J Biol Chem. 1997;272:9613–6. doi: 10.1074/jbc.272.15.9613. [DOI] [PubMed] [Google Scholar]
- 59.Gottlieb E, Oren M. P53 facilitates pRb cleavage in IL-3-deprived cells: novel pro-apoptotic activity of p53. EMBO J. 1998;17:3587–96. doi: 10.1093/emboj/17.13.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janicke RU, Walker PA, Lin XY, Porter AG. Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J. 1996;15:6969–78. [PMC free article] [PubMed] [Google Scholar]
- 61.Tan X, Wang JY. The caspase-Rb connection in cell death. Trends Cell Biol. 1998;8:116–20. doi: 10.1016/s0962-8924(97)01208-7. [DOI] [PubMed] [Google Scholar]