Abstract
A synthetic hydrophobic peptide (core peptide; CP) containing two positively charged amino acids, lysine and arginine was derived from the transmembrane sequence of the T-cell receptor (TCR) α chain and has been shown to inhibit T-cell-mediated inflammation. In this study, we investigated the specificity of CP (10 μm) on lymphocyte function and found that it significantly inhibited interleukin-2 production in T cells and natural killer cytotoxicity by 46–58% compared to positive control. CP had no effects on B-cell proliferative responses when used at these concentrations; however, it suppressed B-cell proliferation at higher concentrations (50 μm). Inhibition by CP was not the result of membrane pore formation or cytotoxicity when examined by trypan blue, propidium iodide staining or transmission electron microscopy. CP analogues, with both lysine and arginine replaced by neutral or negatively charged amino acids, or by randomly distributing charges in the peptide sequence, had no effect on lymphocyte function. These results suggest that peptide inhibition is affected by its structure and charge interactions, and may involve common signalling molecules in T, B and natural killer cells. The potential of the immuno-inhibitory effects of CP as a novel anti-inflammatory peptide in therapy should be further explored.
Introduction
Conventional T cells play a central role in controlling the acquired cellular immune response through antigen-specific recognition by the T-cell antigen receptor (TCR). The TCR is comprised of an αβ heterodimer in non-covalent association with the non-polymorphic CD3 molecule. The disulphide-linked αβ heterodimer (αβ-Ti) is primarily involved in the recognition of antigens presented in the context of major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs), while the CD3 molecule, consisting of ε, γ, δ as well as ζ and/or η chains, is involved in coupling ligand binding to signalling pathways that result in T-cell activation, and the elaboration of a specific cellular immune response. Individual peptide chains forming the CD3 and αβ-Ti molecules have a single membrane-spanning unit, where each CD3 chain contains a negative charge while the variants TCR-α and TCR-β contain two and one positive charges in this region, respectively. Stable interactions between TCR-α and CD3-δ and between TCR-α and CD3-ε are localized to a stretch of eight amino acids within the transmembrane region of TCR-α that also contains the two charged amino acids, arginine and lysine.1
Accordingly, synthetic peptides based on this critical region of the TCR-α chain have been designed and synthesized and their effect on T-cell function has been examined. The prototypic peptide, termed core peptide (CP), has nine amino acids in its sequence. CP contains the two basic amino acids, arginine and lysine. CP has previously been shown to suppress interleukin-2 (IL-2) production in vitro, and in vivo signs of inflammation in experimental T-cell-mediated diseases (adjuvant-induced arthritis, cyclophosphamide-induced diabetes and experimental allergic encephalitis).2 The amino acid sequence of CP resembles that found in a growing family of antimicrobial peptides such as magainin and mellitin in that it contains multiple positive charges, and is amphipathic, allowing interactions with the bilayer of cell membranes.3 These peptides were recently the subject of a series of biophysical studies investigating the interactions between peptides and lipid surfaces. Peptides containing hydrophobic residues (Leu, Ile, Val) and positive charges (Lys, Arg) as part of their sequence have a propensity to form α-helical conformations. These studies concluded that such peptides could either be absorbed onto the membrane surface or be inserted into the membrane by a cluster of helical bundles.4–6 Solid-state nuclear magnetic resonance spectroscopy and circular dichroism studies indicate that CP undergoes α-helical and random coil configuration in the lipid environment.7 Despite these similarities, there is no known amino acid homology for the TCR transmembrane sequence seen in CP, indicating the unique occurrence of this peptide.
The bilayer of cell membranes presents a complex and heterogeneous environment that can facilitate specific interactions between membrane proteins and the interphase region of the phospholipids. This interaction can influence a number of functional processes including membrane protein assembly, topology of membrane proteins, mode of protein insertion, and anchoring into the membranes. Analyses of the structure of transmembrane proteins suggest two types of amino acids; the neutral aromatic and the charged amino acids have significant interactions with membrane proteins/lipids. The aromatic amino acid tryptophan has a preferred interaction at the lipid interface, whereas the positively charged residues interact with negatively charged phospholipids that are predominantly positioned at the cytoplasmic aspect, and thereby have potential to interact with cell signalling.8 Hierarchical dissection of CP suggests that it inhibits the early events in T-cell activation and signalling, as well as co-localizing with the TCR.9
The specificity of CP, and the role of these two positively charged amino acids in other immune cells such as B and natural killer (NK) cells, is unknown. The aim of this study was to examine the in vitro effects of CP and its analogues on the function of different lymphocyte subsets. The results indicated that CP had inhibitory effects on both T and NK cells. CP also inhibited B cells, but only at higher concentrations. Both arginine and lysine in the peptide structure were important for these inhibitory effects, as CP analogues with charge modifications did not inhibit lymphocyte function. The immunosuppressive effects caused by CP have implications for its role as a novel therapeutic agent.
Materials and methods
Peptides
All peptides were synthesized by Auspep (Melbourne, Australia) using solid-phase synthesis by fluorenyl methoxycarbonyl (FMOC) chemistry in the manual mode and were shown to be 95% pure by high-performance liquid chromatography (Table 1).
Table 1.
Immunomodulatory peptides evaluated for their specificity of action
| Peptide name | Amino acid sequence | Charge |
|---|---|---|
| Core peptide | GLRILLLKV | 2 positive |
| Peptide C | GLGILLLGV | neutral |
| Peptide E | GLDILLLEV | 2 negative |
| Peptide D | GLKILLLRV | 2 positive |
| Peptide F | GLRILLLLIKV | 2 positive |
| Peptide G | GLRLLLKV | 2 positive |
| Peptide 4 | C(ACM)GLRILLLKV | 2 positive |
| Peptide 5 | SS-GLRILKLLKV | 3 positive |
| BCR1 | IITAEGIILL | 1 negative |
| BCR2 | IITAKGIILL | 1 positive |
| BCR3 | IITAAGIILL | neutral |
Letters in bold type indicate charged amino acids.
Mice
Female 10–16-week-old CBA/H inbred mice obtained from the Animal Resource Centre (Perth, Australia) were used for the isolation of primary B cells. Mice were maintained in a specific pathogen-free environment at the Centenary Institute of Cancer Medicine and Cell Biology animal facility.
T-cell activation
Cell lines.
2B4.11 is a cytochrome c (CC) -specific murine T helper hybridoma obtained by fusing pigeon CC primed lymph node T cells with BW 5147 cells. This cell line expresses a complete TCR at the cell surface and recognizes a fragment of CC (81–104) presented by the MHC-II molecule, I-Ek. LK35.2 cells are a MHC I-Ek genotype B-lymphoma cell line and are used to present CC peptide fragments to 2B4.11 cells. CTLL-2 is an IL-2-dependent cell line used to measure IL-2 production of 2B4.11 cells following antigen presentation.
Cell lysate containing inactivated varicella zoster virus was obtained from the cell line MRC-5 (American Tissue Culture Collection, Rockville, MD). MRC-5 cells were derived from normal lung fibroblasts. Varicella zoster virus-cell lysate (obtained by trypsination after growing varicella zoster virus and MRC-5 cells together) was used at a final dilution of 1 : 20.
Antigen presentation and IL-2 bioassay.
As previously described,2 T-cell hybridoma 2B4.11 (105 cells) specific for both pigeon and moth CC and LK35.2 (105) were incubated with moth CC peptide (5 μm), in 96-well microtitre plates for 24 hr in the presence of TCR peptides (10 μm). An aliquot of each supernatant (100 μl) was removed by serial twofold dilutions using RPMI-1640 (Gibco BRL, Grand Island, NY). Diluted supernatant was then incubated with CTLL (5 × 104 cells) for 18 hr at 37° and 5% CO2 in microtitre plates. [3H]Thymidine (0·5 μCi) was added for 6 hr and CTLL were harvested onto glass filter paper mats using a Titertek™ cell harvester and measured by scintillation counting (Hewlett Packard, Canberra, Australia). Sample radioactivity was compared to standard curves and expressed as IU/ml. The amount of IL-2 present in peptide-treated samples was compared to the IL-2 level in positive controls and expressed as a percentage.
T-cell proliferation.
Peripheral blood lymphocytes (PBLs) from healthy donors who have been previously exposed to varicella zoster virus were isolated. After isolation (see below), they were treated with CP or its analogues for 30 min. Cells (105) were incubated with varicella zoster virus cell lysate in RPMI-1640 supplemented with 2 mm l-glutamine, 1 mm sodium pyruvate, 100 μg/ml streptomycin and 100 U/ml penicillin and cultured in 10% human serum. Cells were pulsed with thymidine on day 6, harvested on day 7 and scintillation counting was performed.
B-cell proliferation
Reagents.
Percoll was obtained from Pharmacia Biotech AB (Uppsala, Sweden). Lipopolysaccharide (LPS) from Escherichia coli was obtained from Sigma (St Louis, MO). CD40 ligand prepared from the Sf9 insect cell line transfected with baculovirus vector containing the CD40L gene and kept frozen at − 70° was a generous gift from Dr M. R. Kehry (Boehringer Ingleheim, Ridgefield, CT).10 Recombinant murine IL-4 was kindly provided by Dr R. Kastelein (DNAX, Palto Alto, CA). Rat immunoglobulin G (IgG) anti-mouse IgM was purified from b.7.6 hybridoma, a gift from Dr G. G. B. Laus (NIMR, Mill Hill, London, UK).
B-cell preparation and in vitro culture.
Small resting B cells were prepared as previously described.11 Briefly, single cell suspensions were prepared by teasing mouse spleens through a stainless steel mesh. After lysing red blood cells with hypotonic ammonium chloride solution, adherent cells were depleted by incubation on a plastic tissue culture dish. T cells were depleted by complement lysis using a mixture of CD4, CD8 and Thy-1-specific monoclonal antibodies. Small dense B cells were further purified by centrifugation on a Percoll density gradient. B cells recovered from the 65/80% Percoll gradient interface were considered to be resting B cells and were 95% B220+ and < 1% CD4+ and CD-8+ using fluorescence-activated cell sorter (FACS) analysis. Isolated naive B cells were treated with a TCR peptide (dissolved in 0·1% dimethyl sulphoxide) and stimulated with CD40 ligand and IL-4, LPS or anti-IgM and IL-4 over 3 days. Cell proliferation was measured by a thymidine incorporation assay.
NK cytotoxicity assay
Cell line.
K562 cells, derived from the leukaemic cells of a patient with chronic myeloid leukaemia in blastic crisis, were obtained from the ATCC. The cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum containing 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified incubator at 37° with 5% CO2.
Isolation of peripheral blood lymphocytes.
PBLs were prepared from the blood of healthy donors mixed with sodium heparin (1000 U/ml). Blood was separated on Ficoll–Paque (4 : 3 parts) and centrifuged at 400 g for 30 min at 4°. The layer containing platelets and PBLs was isolated and washed twice with RPMI-1640. These cells were then used for either NK cytotoxicity studies or T-cell proliferation (see above).
NK cell cytotoxicity.
Cells from the NK-sensitive leukaemia cell line K562 were used as targets. These cells were labelled for 1 hr with radioactive chromium (Cr 100 μCi per 106 cells). This method has been previously described and is a standard method for assessing NK function.12 Chromium-labelled target cells were washed twice and suspended into 96-well plates at 104 cells per well. ‘Effector cells’ or PBLs were treated with CP for 30 min and then cultured with target cells for 5 hr. Standard six replicate wells were set up for measuring spontaneous and total Cr release by K562 cells. After 5 hr, the plates were spun briefly and 100 μl of supernatant was collected, loaded into polypropylene tubes and radioactivity was measured using a gamma-counter (Hewlett Packard). Tubes were counted for 5 min each. The results were expressed as percentage specific lysis, using the following formula: % specific lysis = (sample release – spontaneous release)/(total release − spontaneous release). Assays in which spontaneous release exceeded 20% were not included.
Electron microscopy
PBLs treated with CP for 5 hr were washed three times before fixation in a modified Karnovsky fixative and washed in 0·1 m 3-(-4-morpholino) propane sulfonic acid (MOPS) buffer (pH 7·4). Subsequently, the cells were post-fixed in buffered 2% osmium tetroxide and dehydrated in an ethanol series prior to embedding in Spurr epoxy resin. Following polymerization (70° for 10 hr), sections were cut at 70 nm, stained with uranyl acetate and Reynold's lead citrate and examined in a Philips CM120 BioTWIN electron microscope at 100 kV to confirm the absence of cytosolic pore formation.
Statistical analysis
All experiments were performed in triplicate. Multiple comparisons were examined using anova (statview 4·5, Abacus Concepts Incorporated, 1992–5®, Berkley, CA). Results were significant for Bonferroni–Dunn P-values < 0·05.
Results
CP inhibits IL-2 production in murine T cells
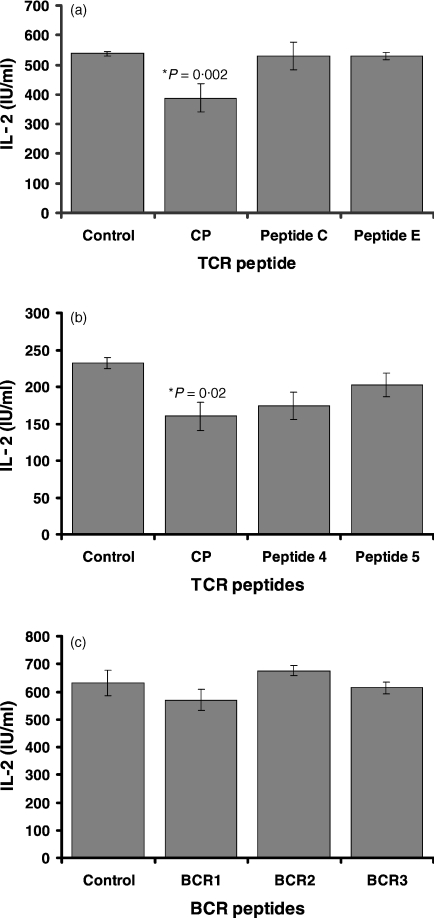
CP was tested for its ability to inhibit T-cell activation. CP reduced IL-2 production in T cells by 29% compared to positive controls (P = 0·002). CP analogues, peptides C and E, did not significantly affect T-cell IL-2 production (Fig. 1a). Similarly, peptide 5, in which an extra lysine was inserted breaking the middle hydrophobic stretch of amino acids, did not have an effect on T-cell inhibition (Fig. 1b). The peptides BCR1, its sequence derived from the transmembrane region of Igα of the BCR, and charge modification analogues BCR2 and BCR3, all had no effect on T-cell IL-2 production compared to CP (Fig. 1c). These results confirm the hypothesis that the positive charge within the peptide chain is required for T-cell inhibition, and that a similarity of amino acid sequence to the TCR is required for inhibition of T-cell function.
Figure 1.
CP and peptide 4, but not charged or structurally modified forms, inhibit antigen-induced IL-2 production by murine T cells. The cytochrome-specific T-cell hybridoma (2B4) was stimulated with antigen presented by antigen-presenting cell LK35.2 in the presence of solvent (DMSO) only or of either CP, peptide C, E, 4, 5, BCR1, BCR2 or BCR3, all of which were dissolved in DMSO. IL-2 production was measured by[3H]thymidine uptake by CTLL cells. (a) CP but not peptide C or E significantly inhibited IL-2 production by T cells, confirming previous results. (b) Both core peptide and peptide 4 inhibited IL-2 production by T cells. This effect was not seen in peptide 5. (c) BCR1, BCR2 and BCR3 had no effect on IL-2 production by T cells.
CP inhibits human T-cell activation
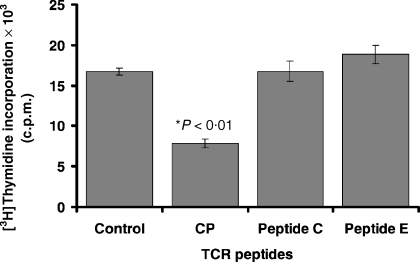
The specificity of CP and its analogues on in vitro human T-cell function was examined using inactivated varicella zoster virus cell lysate to stimulate T-cell proliferation. CP inhibited T-cell proliferation by 52% (Fig. 2). The effect on decreasing human T-cell proliferation was greater than murine T-cell IL-2 production. Neither Peptide C, nor E affected T-cell proliferation in response to inactivated varicella zoster virus antigen in previously sensitized individuals.
Figure 2.
CP inhibits in vitro human T-cell activation. Human T-cell activation was measured using PBLs cultured with an antigen (varicella zoster virus cell lysate) for 7 days and cells were counted using thymidine uptake. CP, but not peptide C or E, inhibited T-cell proliferation. This suggested that CP (derived from the conserved region of mouse TCR-α transmembrane region) was not species specific, and was capable of inhibiting human T-cell function.
CP inhibits B-cell proliferation only at higher concentrations
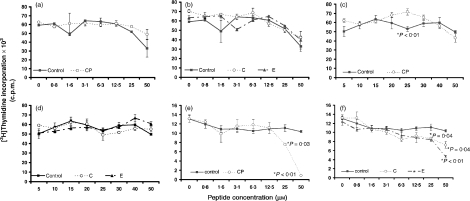
CP and its analogues (1–10 μm) did not affect B-cell proliferation when naïve B cells were stimulated with polyclonal B-cell mitogens (anti-IgM, LPS and CD40 ligand) (Fig. 3a–f). However, at higher concentrations (up to 50 μm), CP inhibited B-cell proliferation in a dose-dependent manner.
Figure 3.
TCR transmembrane peptides inhibited B-cell proliferation in the presence of polyclonal B-cell activators only at high concentrations. (a) CP did not alter B-cell proliferation in the presence of CD40 ligand and IL-4. (b) Peptides C and E did not alter B-cell proliferation in the presence of CD40 ligand and IL-4. (c) CP inhibited B-cell proliferation when stimulated with LPS in a dose-dependent manner. (d) Peptides C and E did not inhibit B-cell proliferation when stimulated with LPS.(e) CP inhibited B-cell proliferation when stimulated with anti-IgM and IL-4 in a dose-dependent manner. (f) Peptide E also inhibited B-cell proliferation when stimulated with anti-IgM and IL-4.
CP inhibits NK cytotoxicity
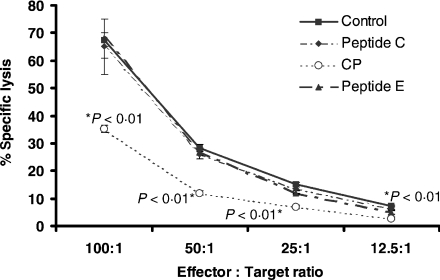
To assess the effect of CP on NK cells, we used the NK cytotoxicity assay. At an effector to target ratio (E : T) of 100 : 1, CP significantly inhibited NK function by 46–58% for the range of E : T examined (100 : 1, 50 : 1, 25 : 1 and 12·5 : 1: Bonferroni–Dunn P = 0·003, < 0·0001, < 0·0001 and < 0·001, respectively). CP also demonstrated a significant difference to peptides C and E (Bonferroni–Dunn P = 0·004 and P = 0·003, respectively). Peptides C and E had no effect on NK cell function (Fig. 4). These results suggest that the positive charge within the synthetic peptide chain present in CP is important for inhibitory effects on NK cell cytotoxicity.
Figure 4.
CP, but not peptides C or E, inhibited direct NK cytotoxicity. Direct NK cytotoxicity was assessed using the standard NK-sensitive K562 assay. PBLs were treated with peptides for 30 min before they were incubated with chromium-labelled K562 cells for 5 hr. Supernatant was collected and measured for radioactivity. CP significantly inhibited NK lysis for the range of E : T ratios examined (100 : 1, 50 : 1, 25 : 1 and 12·5 : 1).
CP did not cause cellular membrane pore formation
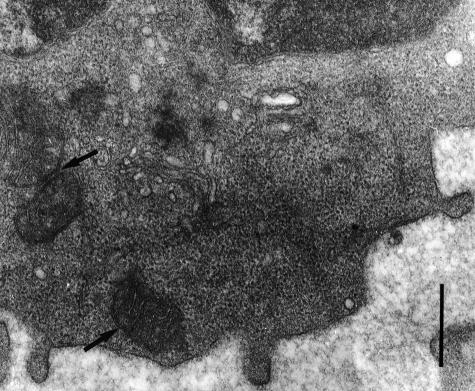
Previous trypan blue staining and propidium iodide staining by FACS analysis of CP (10–50 μm)-treated lymphocytes showed that > 95% cells were viable and not significantly permeabilized. Since CP has biophysical properties similar to antimicrobial peptides with the ability to form pores, cells treated with CP were examined by transmission electron microscopy for the presence of membrane pores. Electron microscopy was also used to assess signs of cellular toxicity after peptide treatment. Peptide-treated PBLs (10–50 μm) demonstrated no significant structural changes in the cytosolic and nuclear cell membranes compared to controls. Intracellular organelles, such as the mitochondria, remained intact (Fig. 5). These features suggest that CP inhibited NK and T-cell function without causing cellular toxicity or pore formation in the cell membranes.
Figure 5.
Electron microscopy examination for pore formation. PBLs treated with CP for 5 hr were fixed with glutaraldehyde, post-fixed with osmium tetroxide, dehydrated, polymerized and sectioned for electron microscopy. Sections did not reveal cytosolic membrane pore formation in the lymphocytes after treatment with CP. Intracellular organelles, such as mitochondria (arrows) remained intact. Bar = 500 nm.
Discussion
In this study, the effect of TCR transmembrane peptides on NK and B-cell function were examined. We confirmed that CP inhibited IL-2 production in murine T cells, and now have shown that it also inhibited human T-cell activation. This was not unexpected because the transmembrane sequence of TCR-α is totally conserved between species ranging from rodents to humans. It appears that the presence and position of positive charges in the peptide chain is important for suppressing T-cell and NK cell activation. However, charge alone is not sufficient for this inhibitory effect and the peptide sequence that bears similarities with the transmembrane region of the receptor is important. The similarities of inhibition by CP on T and NK cells at the low peptide concentration suggest that activation of both these cells may occur via a mechanism involving the presence of positive charges as well as similarities in structure in the transmembrane region. At higher concentrations, CP also inhibited B-cell function suggesting that other factors, such as signal transduction molecules, may be involved. CP analogues, with different or randomly distributed electrostatic charge, did not have a significant effect on lymphocyte function. The inhibitory effects of CP on NK and B cells add to our current knowledge of the immunosuppressive effects of CP on T-cell function.
CP inhibited both T-cell and NK cell function when used at low concentrations (10 μm). The inhibitory effects on NK cells were statistically significant for all E : T ratios examined, including the lowest E : T ratio of 12·5 : 1. It is known that charge interactions in the transmembrane region play an important role for dimeric chains in the conventional TCR.13,14 The ligand-binding receptor unit contains two positive charges from the basic amino acids lysine and arginine in the transmembrane region which interact with the negative charge aspartic acid of the CD3 chains (TCR-α/CD3-δ and TCR-α/CD3-ε).15 These charge interactions are essential for TCR assembly and signal transduction. Early events in T-cell activation involve the phosphorylation of ITAM sequences on the ζ chain, recruitment of p56lck tyrosine kinase and the activation the Syk/ZAP-70 family of protein tyrosine kinases (PTKs).16–18 These functional effects on T cells are consistent with our findings that CP inhibits the phosphorylation of the ζ chain in T cells (X. M. Wang et al., in press). Similar to the TCR, charge interactions also play an important role within the transmembrane region of the NK cell receptors. However, unlike the TCR, NK cells express a heterogeneous set of receptors, and their cytolytic function is regulated by the integration of signals from both inhibitory and activating receptors. NK-activating receptors, such as KIR, CD94, NK2GC, ILT1, are similar to the TCR in the presence of a positively charged amino acid (lysine or arginine) in the transmembrane region, and the association of the receptor unit with immunoreceptor tyrosine-based activation motifs (ITAMS), in contrast to their inhibitory receptor.19 Other similarities include the recruitment of signal transduction molecules such as the recruitment of p56lck kinase with cross-linking CD16, and the activation of Syk and ZAP-70 tyrosine phosphorylation with the cross-linking of FcεRIγ and ζ receptors on NK cells.20–22 Cross-linking of KIR–DAP-12 complexes on NK cells also results in the recruitment of ZAP-70 and Syk protein kinases.23 The similarities between T and NK cells suggest that CP may prevent the early activation events in NK cells in a manner analogous to T cells, possibly via positive charge interactions with lipid phosphate groups as predicted by de Planque et al.8
CP did not affect B-cell function at low concentrations (1–10 μm). However, with higher doses, CP had an effect on B-cell function in a dose-dependent manner. These differences may be explained by reviewing the structural similarities of the different types of receptors on the B cells examined. Fewer structural similarities exist between the transmembrane region of the BCR and TCR, as compared to TCR and NK cell-activating receptors. The BCR is a complex consisting of membrane-bound immunoglobulin molecules (mIg) that mediates binding to antigen and the mIg-associated Ig-α/Ig-β. The transmembrane region of mIg contains neutral amino acids while the Ig-α has an aspartic acid (negative charge) within this region. The LPS receptor is a glycosylphosphatidylinositol-anchored protein and does not have a transmembrane sequence.2 CD40 is a member of the tumour necrosis factor receptor (TNFR) family that include TNFR2, CD30 and CD40. CD40 contains a neutral charge in its transmembrane region.24 The high concentration may allow a collective charge effect on recruitment of similar signal transduction molecules and pathways, such as Syk/ZAP-70 of PTKs by the BCR.
Phase I studies in humans suggest that CP is an effective topical treatment in many T-cell-mediated dermatological disorders, including atopic dermatitis, lichen planus, psoriasis and allergic contact dermatitis.25 The characterization of the specific pathways of immunosuppression by CP explains and supports these in vivo findings. Furthermore, it shows the importance of structure and charge in the design of therapeutic peptides. The immunosuppressive effects of CP provide fertile soil for further investigation as a potential therapeutic agent.
Acknowledgments
We would like to thank Dr Philip Hodgkin and Dr Jhagural Hasbold for their generosity and assistance with B-cell proliferation techniques.
References
- Manolios N, Kemp O, Li ZG. The T cell antigen receptor alpha and beta chains interact via distinct regions with CD3 chains. Eur J Immunol. 1994;24:84–92. doi: 10.1002/eji.1830240114. [DOI] [PubMed] [Google Scholar]
- Manolios N, Collier S, Taylor J, Pollard J, Harrison LC, Bender V. T-cell antigen receptor transmembrane peptides modulate T-cell function and T cell-mediated disease. Nat Med. 1997;3:84–8. doi: 10.1038/nm0197-84. [DOI] [PubMed] [Google Scholar]
- Bechinger B. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim Biophys Acta. 1999;1462:157–83. doi: 10.1016/s0005-2736(99)00205-9. [DOI] [PubMed] [Google Scholar]
- Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;1462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K. Why and how are peptide–lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta. 1999;1462:1–10. doi: 10.1016/s0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- Tossi A, Sandri L, Giangaspero A. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers. 2000;55:4–30. doi: 10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ali M, De Planque MRR, Huynh NT, Manolios N, Separovic F. Biophysical studies of a transmembrane peptide derived from the T cell antigen receptor. Lett Pept Sci. 2002;8:227–33. [Google Scholar]
- de Planque MR, Kruijtzer JA, Liskamp RM, Marsh D, Greathouse DV, Koeppe RE, 2nd, de Kruijff B, Killian JA. Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane alpha-helical peptides. J Biol Chem. 1999;274:20839–46. doi: 10.1074/jbc.274.30.20839. [DOI] [PubMed] [Google Scholar]
- Wang XM, Djordjevic JT, Bender V, Manolios N. T cell antigen receptor (TCR) transmembrane peptides colocalize with TCR, not lipid rafts, in surface membranes. Cell Immunol. 2002;215:12–19. doi: 10.1016/s0008-8749(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Kehry MR, Castle BE. Regulation of CD40 ligand expression and use of recombinant CD40 ligand for studying B cell growth and differentiation. Semin Immunol. 1994;6:287–94. doi: 10.1006/smim.1994.1037. [DOI] [PubMed] [Google Scholar]
- Hodgkin PD, Yamashita LC, Coffman RL, Kehry MR. Separation of events mediating B cell proliferation and Ig production by using T cell membranes and lymphokines. J Immunol. 1990;145:2025–34. [PubMed] [Google Scholar]
- D'orazio JA, Stein-Streilein J. Human natural killer (NK) cells present staphylococcal enterotoxin B (SEB) to T lymphocytes. Clin Exp Immunol. 1996;104:366–73. doi: 10.1046/j.1365-2249.1996.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DP, Puck JM, Pettey CL, Cho M, Coligan J, Woody JN, Terhorst C. Isolation of cDNA clones encoding the 20K non-glycosylated polypeptide chain of the human T-cell receptor/T3 complex. Nature. 1986;321:431–4. doi: 10.1038/321431a0. [DOI] [PubMed] [Google Scholar]
- van den Elsen P, Shepley BA, Borst J, Coligan JE, Markham AF, Orkin S, Terhorst C. Isolation of cDNA clones encoding the 20K, T3 glycoprotein of human T–cell receptor complex. Nature. 1984;312:413–18. doi: 10.1038/312413a0. [DOI] [PubMed] [Google Scholar]
- Manolios N, Letourneur F, Bonifacino JS, Klausner RD. Pairwise, cooperative and inhibitory interactions describe the assembly and probable structure of the T-cell antigen receptor. EMBO J. 1991;10:1643–51. doi: 10.1002/j.1460-2075.1991.tb07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn SJ, Forbush KA, Pan XC, Perlmutter RM. Activated p56lck directs maturation of both CD4 and CD8 single-positive thymocytes. J Immunol. 2001;166:2209–17. doi: 10.4049/jimmunol.166.4.2209. [DOI] [PubMed] [Google Scholar]
- Martelli MP, Lin H, Zhang W, Samelson LE, Bierer BE. Signaling via LAT (linker for T-cell activation) and Syk/ZAP70 is required for ERK activation and NFAT transcriptional activation following CD2 stimulation. Blood. 2000;96:2181–90. [PubMed] [Google Scholar]
- Ilangumaran S, Arni S, van Echten-Deckert G, Borisch B, Hoessli DC. Microdomain-dependent regulation of Lck and Fyn protein-tyrosine kinases in T lymphocyte plasma membranes. Mol Biol Cell. 1999;10:891–905. doi: 10.1091/mbc.10.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biassoni R, Cantoni C, Falco M, et al. The human leukocyte antigen (HLA)-C-specific ‘activatory’ or ‘inhibitory’ natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med. 1996;183:645–50. doi: 10.1084/jem.183.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'shea JJ, Weissman AM, Kennedy IC, Ortaldo JR. Engagement of the natural killer cell IgG Fc receptor results in tyrosine phosphorylation of the zeta chain. Proc Natl Acad Sci USA. 1991;88:350–4. doi: 10.1073/pnas.88.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo TW, Kurosaki T, Kanakaraj P, Ravetch JV, Perussia B. Physical and functional association of p56lck with Fc gamma RIIIA (CD16) in natural killer cells. J Exp Med. 1993;177:1475–80. doi: 10.1084/jem.177.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, da Silva AJ, Ackerly M, Levine H, Rudd CE, Anderson P. Association of a 70-kDa tyrosine phosphoprotein with the CD16: zeta:gamma complex expressed in human natural killer cells. Eur J Immunol. 1993;23:1872–6. doi: 10.1002/eji.1830230821. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–7. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- Bajorath J, Aruffo A. Construction and analysis of a detailed three-dimensional model of the ligand binding domain of the human B cell receptor CD40. Proteins. 1997;27:59–70. [PubMed] [Google Scholar]
- Gollner GP, Muller G, Alt R, Knop J, Enk AH. Therapeutic application of T cell receptor mimic peptides or cDNA in the treatment of T cell-mediated skin diseases. Gene Ther. 2000;7:1000–4. doi: 10.1038/sj.gt.3301183. [DOI] [PubMed] [Google Scholar]