Abstract
While Epstein–Barr virus (EBV) is known to establish latency in the memory B-cell compartment, there is controversy as to whether the memory or the naïve B cell is the initial target for infection. Here we have explored the infectability of the B-cell subsets contained in peripheral blood and tonsils, as distinguished by their surface expression of the immunoglobulin isotypes that help to define naïve and memory pools. First we show that both CD21 and major histocompatibility complex (MHC) class II molecules – respectively, the major receptor and co-receptor for EBV on B cells – are expressed at similar levels on blood and tonsillar B cells, irrespective of surface immunoglobulin class, indicating that each of the subsets demonstrate an equal potential, at least for infection. Then, following in vitro infection of total tonsillar B cells, we found that the relative frequencies of immunoglobulin (Ig)M-, IgG- and IgA-positive cells containing EBV-encoded Epstein–Barr virus nuclear antigen 5 (EBNA5) protein at 48 hr were similar to those of the starting population. However, IgD expression was uniformly decreased, probably as a consequence of cellular activation. These data indicate that recirculating B cells have both the potential for, and susceptibility to, initial infection by EBV, irrespective of the immunoglobulin isotype expressed.
Introduction
Epstein–Barr virus (EBV) is a human herpesvirus that infects and subsequently persists in more than 90% of the world's adult population. Infection is asymptomatic during childhood, but if delayed to adolescence can cause infectious mononucleosis (IM). The virus uses the complement receptor 2 (CR2; also known as CD21) as its conduit receptor for entry into susceptible cells; human leucocyte antigen (HLA)-DR is required as a co-receptor, at least for B cells.1–3 In vivo, latently infected B cells are found almost exclusively in the memory compartment, with the constituent population residing in a resting (G0) state.4–7 Consistent with the inherent longevity of the memory pool, it has been suggested that the number of B cells latently harbouring EBV remains constant over long periods of time.8
Primary infection with EBV occurs in the oro-pharynx, most probably in the palantine tonsil. Immunohistochemistry has failed to provide an unequivocal picture regarding the surface immunoglobulin (sIg) phenotype of B lymphocytes within the tonsil epithelium. Liu et al. reported that the majority were of a memory B-cell phenotype, namely IgD− CD38− CD20+, while Tang et al. reported the expression of sIgM and, to a lesser extent, sIgD; no other immunoglobulin isotypes were analysed in the latter study.9,10
EBV transforms B lymphocytes in vitro, generating immortalized, permanently growing lymphoblastoid cell lines (LCL). Brown & Miller have reported that on direct cloning of peripheral B cells following their infection with EBV, the proportion of emerging clones carrying IgM, IgG or IgA corresponded closely to the surface immunoglobulin isotype distribution in the starting population.11 This suggested, albeit indirectly, that EBV could infect and immortalize peripheral B cells independently of sIg isotype expression.
Miyawaki et al. wished to examine whether EBV might induce immunoglobulin class switching.12 Peripheral blood B lymphocytes were sorted (using a fluorescence-activated cell sorter) according to sIg expression, and subsequently infected. After 3–4 weeks, the cultures were assayed for immunoglobulin production. On infection and outgrowth of naïve IgM+/IgD+ cells, only IgM production – and never IgG or IgA – was found, indicating that the virus does not directly induce a class switch.
Despite the aforementioned studies, it remains far from clear as to whether naïve and memory cells B cells are equally susceptible to infection with EBV. Although it was recently shown that a small fraction of the memory B-cell compartment retains the expression of sIgM – and an even more minor compartment retains the expression of sIgD – a shift from IgD and/or IgM expression to downstream, switched isotypes remains a reliable, general feature of transition from a naïve to a memory phenotype. In this article we first studied the expression of CD21 and HLA-DR on peripheral blood and tonsillar B lymphocytes expressing different immunoglobulin isotypes to indicate their potential for EBV infection; then analysed tonsillar B cells shortly after exposure to EBV for sIg isotype expression and Epstein–Barr virus nuclear antigen 5 (EBNA5) positivity, the latter being an early marker of successful infection.
Materials and methods
Cell preparation
To obtain peripheral blood B cells, buffy coats were first obtained from healthy donors (Blood Bank, Karolinska Hospital, Stockholm, Sweden). Then, mononuclear cells were isolated on Lymphoprep gradients and macrophages depleted by plastic adherence. B cells were enriched twice by mixing the non-adherent cells with 5% sheep red blood cells in 20% fetal calf serum (FCS) (Gibco, Paisley, UK). After 30 min of incubation at room temperature, the rosetted and non-rosetted cells were separated on a Lymphoprep gradient, with the latter comprising the enriched B cells that were collected from the interface. For tonsillar B cells, tissue – obtained from routine tonsillectomy (Karolinska Hospital) – was cut into fragments and dispersed into cell suspensions. T cells were removed by E-rosetting followed by separation on Lymphoprep. The remaining cells were suspended in RPMI-1640 supplemented with 20% (FCS) (Gibco).
EBV infection
Tonsillar B cells were incubated with EBV (B95-8 strain) for 1 hr at 37°. After virus removal, the cells were incubated for 48 hr at a density of 106 cells/ml. All cultures were carried out in RPMI-1640 supplemented with 10% FCS, antibiotics and glutamine (2 mm).
Monoclonal antibodies and conjugates
The polyclonal antibodies and monoclonal antibodies (mAbs) used in the study were: mouse anti-EBNA5 (J186)13 Texas Red horse anti-mouse IgG (Vector, Burlingame, CA); fluorescein isothiocyanate (FITC)-conjugated rabbit (F(ab′)2) anti-human IgM, IgD, IgG and IgA (DAKO A/S, Glostrup, Denmark); mouse anti-human CD21 (HB-5) (a gift from Dr M. Cooper, University of Alabama, Birmingham, Alabama, USA); and R-phycoerythrin (RPE)–mouse anti-human HLA-DR (PharMingen, San Diego, CA).
Flow cytometric analysis
A total of 1 × 106 cells were incubated with saturating amounts of anti-CD21 or anti-HLA-DR mAb for 30 min. After washing, the cells were exposed to the conjugate for 30 min. The cells were washed once and then incubated with FITC-labelled rabbit anti-human IgM, IgD, IgG or IgA. All the incubations were carried out at 4°. After staining, the cells were fixed in phosphate-buffered saline (PBS) containing 1% formaldehyde and analysed in a fluorescence-activated cell sorter (FACScan) using the Cellquest software.
Immunostaining
Cytospins were fixed in cold methanol : acetone (1 : 1) and then rehydrated in PBS for 30 min. Double staining for EBNA5 and sIg was performed by incubation with anti-EBNA5 mAb for 45 min followed by three washes in PBS, then incubation for a further 45 min with Texas Red horse anti-mouse IgG to which Bisbenzimide (Hoescht 33258; Sigma, St Louis, MO) had been added at a concentration 0·4 µg/ml. Then, after three washes in PBS to remove the conjugate, FITC–rabbit antibody to human IgM, IgD, IgG, or IgA was added for 45 min, after which the cytospins were washed three times before mounting with 80% glycerol solution in PBS containing 2·5% 1,4-diazabicyclo-(2.2.2) octane (Sigma).
Results
Expression of receptors for EBV on B-cell subsets defined by immunoglobulin isotype
In order to study the potential for infectability of B cells carrying different sIg isotypes, we examined the co-expression of the receptors (CD21 and the HLA-DR) needed for infection with EBV, and class of sIg expressed, for both peripheral blood and tonsil populations.
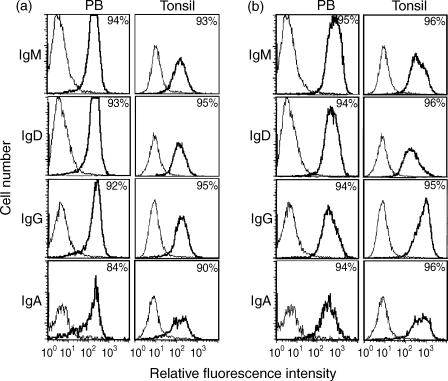
Blood and tonsillar B lymphocytes were stained and analysed by two-colour immunofluorescence for CD21 and one of the four major sIg isotypes (IgD, IgM, IgG, or sIgA). As seen in Fig. 1(a), no major differences in CD21 expression were identified between cells positive for the different sIg isotypes. The same type of analysis was performed for the expression of HLA-DR and the different sIg classes. Virtually all cells expressed HLA-DR at a similarly high level (Fig. 1b).
Figure 1.
(a) Analysis of CD21 expression on peripheral blood (PB) lymphocytes and tonsillar (Tonsil) cells gated for immunoglobulin (Ig)M, IgD, IgG or IgA positivity. Cells were first stained indirectly for CD21, then stained for one of the four immunoglobulin isotypes. The numbers given represent the percentage of CD21-positive cells of the population, gated on the basis of immunoglobulin isotype. The example shown is representative of three identical experiments. (b) Analysis of human leucocyte antigen (HLA)-DR expression on PB lymphocytes and tonsillar cells gated for IgM, IgD, IgG or IgA positivity. B cells were first stained for HLA-DR and subsequently for one of the four immunoglobulin isotypes. The numbers given represent the percentage of HLA-DR-positive cells of the population, gated on the basis of immunoglobulin isotype. The example shown is representative of three identical experiments.
Ability to productively infect B cells expressing different sIg isotypes
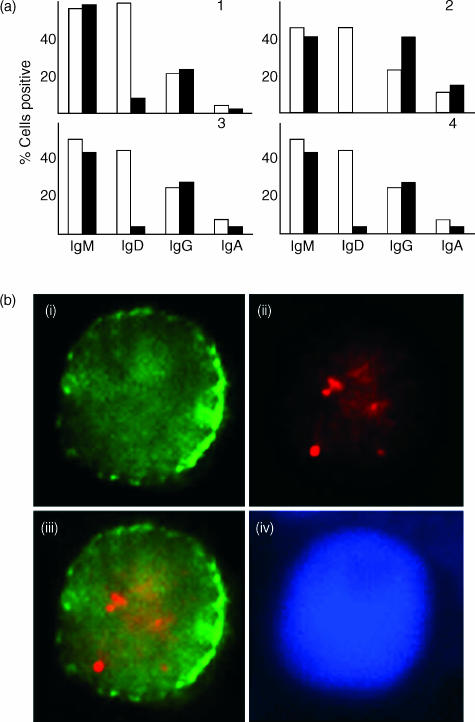
We next investigated whether cells of different sIg phenotypes were equally susceptible to EBV infection or whether there was a preference between naïve and memory B cells in this regard. To assess this, purified – but otherwise further unseparated – tonsillar B cells were infected with EBV then subjected to short-term culture. After 48 hr, the cells were harvested and double stained for EBNA5 and one of the four major immunoglobulin isotypes under study (IgD, IgM, IgG, or IgA). The relative percentage of EBNA5-positive cells among B cells of different sIg isotype following infection approximated that of the immunoglobulin isotype distribution among the starting population (Fig. 2a): Fig. 2(b) shows an example of an IgA-positive tonsillar B cell expressing EBNA5. The one exception was IgD, where there was an apparent marked reduction in the relative number of surface positive cells postinfection (Fig. 2a).
Figure 2.
(a) Distribution of immunoglobulin isotype expression on Epstein–Barr virus nuclear antigen 5 (EBNA5)-positive tonsillar B cells 48 hr after infection compared with that of the starting population. Enriched tonsillar B cells were infected with Epstein–Barr virus (EBV) for 1 hr. After 48 hr of culture, cytospins were prepared and – together with those generated from the starting population – stained for EBNA5, then for immunoglobulin isotype. Results of four individual experiments are shown for the starting population (white bar) and infected cells (black bar). (b) Example of co-expression of EBNA5 and IgA. Tonsillar B cells were infected, as described above, and double stained for EBNA5 and IgA, with cells representing the pattern observed detailed as follows: (i) IgA (green); (ii) EBNA5 (red); (iii) overlay of EBNA5 and IgA double stain; (iv) DNA staining. Original magnification: × 100.
Discussion
EBV infects and establishes lifelong latency in B lymphocytes. In latently infected healthy persons, these cells are of a resting phenotype and found in the memory compartment.4,5,7 In this study we addressed the question of whether there might be a difference in the ability of EBV to infect the naïve B cell, predominantly found in the IgM+/IgD+ population, versus the memory B cell that is most typically characterized by a lack of IgD expression and a tendency towards carrying a switched isotype such as IgG or IgA.
We first investigated the expression of the receptors necessary for infection (i.e. CD21 and HLA-DR) on B lymphocytes expressing different immunoglobulin isotypes. Each of the receptors can be readily detected on both peripheral blood and tonsillar B cells, irrespective of whether they express surface immunoglobulin of the IgM, IgD, IgG, or IgA class. This is in line with Brown & Miller's previous finding that peripheral B cells expressing different classes of sIg can be successfully transformed, as indicated by the establishment of clones seemingly retaining immunoglobulin isotype expression at similar frequencies as in the starting population.11
We then analysed directly the susceptibility to EBV infection of tonsillar B cells that were positive for sIgM, sIgG, sIgA or sIgD. With the exception of IgD, our data show no major differences in the sIg isotype expression among the EBNA5-positive cells 48 hr after infection compared to the starting population. The apparent discrepancy regarding IgD probably reflects the known downregulation of this immunoglobulin isotype from the cell surface that follows B-cell activation.14 Indeed, we have shown previously that simple virus binding to the B cell is sufficient to induce a rapid phenotypic shift away from IgD expression in resting B cells. Irradiated, non-transforming virions were also effective in bringing about such change.15
Thorley-Lawson has suggested that the naïve B cell constitutes the primary target for EBV infection and that the subsequently transformed lymphoblast then follows the normal differentiation pathway engaged during a T-dependent antigen response, eventually becoming a long-lived memory cell.16 This is partly based on the analysis of different subpopulations isolated from tonsil.17 The IgD+ naïve subpopulation expressed EBV-encoded mRNA from EBV genes associated with the lymphoblastoid phenotype. These cells were interpreted as newly infected cells.
By contrast, Kurth et al. have investigated, at the single-cell level, EBV-infected B cells in the tonsils of patients with IM.18 They studied the EBV gene-expression pattern, V-gene rearrangements and clonal expansion. The majority of the infected cells had mutations in the V genes, compatible with patterns found in memory and/or germinal centre cells. Furthermore, no intraclonal diversity was found, indicating that the clone had expanded after the somatic hypermutation which occurs during a germinal centre reaction.
That newly infected cells isolated from tonsil of persons with persistent infections are found in the IgD+ fraction may not be inconsistent with the data from Kurth et al. on primary EBV-infected cells in tonsils from patients with IM.18 Not much is known about reactivation of virus production. As the majority of latently infected persons actively shed virus, viral replication must be an ongoing process and the site where the reactivation occurs may be different from the one of the primary infection.19
Recently, Borza & Hutt-Fletcher obtained evidence for an alternate replication of the virus in B cells and in epithelial cells, which changes the tropism of EBV.20 Virus released from epithelial cells (E-EBV) had a higher tropism for B cells, whereas virus produced by B cells (B-EBV) more efficiently infected epithelial cells. This may indicate a strategy of the virus for spreading to new hosts. It also raises the possibility that virus shed from epithelial cells infects B cells of the mucosal lymphoid tissue.
The question whether the primary B-cell target for EBV infection that becomes eventually responsible for the lifelong EBV latency is pre- or postgerminal centre is a matter of debate. Our findings indicate that both types are equally susceptible. We therefore suggest that the most probable scenario is that the B cells present at the site of virus entry, or release, are equally infectable, independently of the phenotype or differentiation status of the lymphocyte.
Acknowledgments
The work was supported by Cancerfonden (Sweden). We thank Mia Löwbeer and Karin Mattsson for excellent technical assistance. The work of J.G. is supported by a Medical Research Council (UK) Programme Grant; J.G. is an MRC non-clinical research professor.
References
- 1.Fingeroth JD, Weis JJ, Tedder TF, Strominger JL, Biro PA, Fearon DT. Epstein–Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci USA. 1984;81:4510. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frade R, Barel M, Ehlin-Henriksson B, Klein G. gp140, the C3d receptor of human B lymphocytes, is also the Epstein–Barr virus receptor. Proc Natl Acad Sci USA. 1985;82:1490. doi: 10.1073/pnas.82.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Spriggs MK, Kovats S, Turk SM, Comeau MR, Nepom B, Hutt-Fletcher LM. Epstein–Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71:4657. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 5.Ehlin-Henriksson B, Zou JZ, Klein G, Ernberg I. Epstein–Barr virus genomes are found predominantly in IgA-positive B cells in the blood of healthy carriers. Int J Cancer. 1999;83:50. doi: 10.1002/(sici)1097-0215(19990924)83:1<50::aid-ijc10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Miyashita EM, Yang B, Babcock GJ, Thorley-Lawson DA. Identification of the site of Epstein–Barr virus persistence in vivo as a resting B cell. J Virol. 1997;71:4882. doi: 10.1128/jvi.71.7.4882-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph AM, Babcock GJ, Thorley-Lawson DA. EBV persistence involves strict selection of latently infected B cells. J Immunol. 2000;165:2975. doi: 10.4049/jimmunol.165.6.2975. [DOI] [PubMed] [Google Scholar]
- 8.Khan G, Miyashita EM, Yang B, Babcock GJ, Thorley-Lawson DA. Is EBV persistence in vivo a model for B cell homeostasis? Immunity. 1996;5:173. doi: 10.1016/s1074-7613(00)80493-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu YJ, Barthelemy C, de Bouteiller O, Arpin C, Durand I, Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity. 1995;2:239. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Hori S, Osamura RY, Tsutsumi Y. Reticular crypt epithelium and intra-epithelial lymphoid cells in the hyperplastic human palatine tonsil: an immunohistochemical analysis. Pathol Int. 1995;45:34. doi: 10.1111/j.1440-1827.1995.tb03377.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown NA, Miller G. Immunoglobulin expression by human B lymphocytes clonally transformed by Epstein–Barr virus. J Immunol. 1982;128:24. [PubMed] [Google Scholar]
- 12.Miyawaki T, Butler JL, Radbruch A, Gartland GL, Cooper MD. Isotype commitment of human B cells that are transformed by Epstein–Barr virus. Eur J Immunol. 1991;21:215. doi: 10.1002/eji.1830210132. [DOI] [PubMed] [Google Scholar]
- 13.Finke J, Rowe M, Kallin B, Ernberg I, Rosen A, Dillner J, Klein G. Monoclonal and polyclonal antibodies against Epstein–Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987;61:3870. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monroe JG, Havran WL, Cambier JC. B lymphocyte activation: entry into cell cycle is accompanied by decreased expression of IgD but not IgM. Eur J Immunol. 1983;13:208. doi: 10.1002/eji.1830130306. [DOI] [PubMed] [Google Scholar]
- 15.Hu CP, Aman P, Masucci MG, Klein E, Klein G. B cell activation by the non-transforming P3HR-1 substrain of the Epstein–Barr virus (EBV) Eur J Immunol. 1986;16:841. doi: 10.1002/eji.1830160720. [DOI] [PubMed] [Google Scholar]
- 16.Thorley-Lawson DA. Epstein–Barr virus: exploiting the immune system. Nature Rev Immunol. 2001;1:75. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 17.Joseph AM, Babcock GJ, Thorley-Lawson DA. Cells expressing the Epstein-Barr virus growth program are present in and restricted to the naive B-cell subset of healthy tonsils. J Virol. 2000;74:9964. doi: 10.1128/jvi.74.21.9964-9971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurth J, Spieker T, Wustrow J, Strickler GJ, Hansmann LM, Rajewsky K, Kuppers R. EBV-infected B cells in infectious mononucleosis: viral strategies for spreading in the B cell compartment and establishing latency. Immunity. 2000;13:485. doi: 10.1016/s1074-7613(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 19.Yao QY, Rickinson AB, Epstein MA. A re-examination of the Epstein–Barr virus carrier state in healthy seropositive individuals. Int J Cancer. 1985;35:35. doi: 10.1002/ijc.2910350107. [DOI] [PubMed] [Google Scholar]
- 20.Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein–Barr virus. Nat Med. 2002;8:594. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]