Abstract
Several lines of evidence implicate the p38 mitogen-activated protein kinase (p38 MAPK) in the proinflammatory response to bacterial agents and cytokines. Equally, the transcription factor, nuclear factor (NF)-κB, is recognized to be a critical determinant of the inflammatory response in intestinal epithelial cells (IECs). However, the precise inter-relationship between the activation of p38 MAPK and activation of the transcription factor NF-κB in the intestinal epithelial cell (IEC) system, remains unknown. Here we show that interleukin (IL)-1β activates all three MAPKs in Caco-2 cells. The production of IL-8 and monocyte chemotactic protein 1 (MCP-1) was attenuated by 50% when these cells were preincubated with the p38 MAPK inhibitor, SB 203580. Further investigation of the NF-κB signalling system revealed that the inhibitory effect was independent of the phosphorylation and degradation of IκBα, the binding partner of NF-κB. This effect was also independent of the DNA binding of the p65 Rel A subunit, as well as transactivation, determined by an NF-κB luciferase construct, using both SB 203580 and dominant–negative p38 MAPK. Evaluation of IL-8 and MCP-1 RNA messages by reverse transcription–polymerase chain reaction (RT–PCR) revealed that the inhibitory effect of SB 203580 was associated with a reduction in this parameter. Using an IL-8–luciferase promoter construct, an effect of p38 upon its activation by both pharmacological and dominant–negative p38 construct co-transfection was demonstrated. It is concluded that p38 MAPK influences the expression of chemokines in intestinal epithelial cells, through an effect upon the activation of the chemokine promoter, and does not directly involve the activation of the transcription factor NF-κB.
Introduction
Intestinal epithelial cells (IECs) are an integral component of the enteric immune system. They have the capacity to express antigens to T cells, and they produce cytokines and chemokines in response to bacterial invasion. Through poorly understood mechanisms, they are involved in mounting an immune response against harmful pathogens, whilst suppressing a response against the normal enteric flora.1,2 As a result, there is a basal level of physiological inflammation within the lamina propria of the intestine. This inflammatory response is highly regulated, and although the precise mechanisms underlying this phenomenon remain unknown, it is probably dependent upon the co-ordination of different cell systems through elaborate signalling networks. Dysregulation of inflammation is seen in inflammatory bowel diseases (IBD) such as Crohn's disease (CD) and ulcerative colitis (UC), and is often accompanied by an increased expression of the proinflammatory cytokines interleukin (IL)-1β and tumour necrosis factor-α (TNF-α). It is also characterized by an increased infiltration with immune cells such as monocytes, neutrophils and T lymphocytes.3,4 Although the underlying aetiology of these chronic disorders is unknown, knowledge of aberrant cytokine production has led to the development and effective use of immunoregulatory therapies, best exemplified by anti-TNF monoclonal antibodies (mAbs) for CD.5
Attraction and infiltration of leucocytes into extravascular body compartments involves the expression of a subfamily of small cytokines, called chemokines. These molecules are classified into two major groups (C–C and C–X–C) on the basis of the arrangement of the two N-terminal cysteine residues. IECs have been shown to express these molecules in response to cytokines.6 Increased expression levels of chemokines have been correlated with the pathophysiology of IBD. IL-8, a potent attractant for neutrophils, has been shown to be elevated in both UC and CD.7 Other chemokines dysregulated in IBD include monocyte chemotactic factor 1 (MCP-1), MCP-3 and epithelial-cell-derived neutrophil-activating peptide 78 (ENA-78).8–11 Furthermore, the chemokine, regulated on activation, normal, T-cell expressed, and secreted (RANTES), has been shown to be involved in the progression of acute to chronic disease, in a rodent model of colitis.12
One of the key components of the inflammatory response is the multifunctional cytokine, IL-1β. Its significance in IBD is documented by the demonstration of its increased expression in affected tissues13 and the attenuation of its damaging properties by its regulatory partner the IL-1 receptor (IL-1R) antagonist.14,15 After binding of IL-1 to its receptor, IL-1R1 and interleukin receptor associated kinases (IRAK)-1 and -2 are recruited via the adapter protein, MyD88 (reviewed in ref. 16). Following their phosphorylation, downstream signalling events are initiated through TNF receptor associated factor (TRAF)-6, which appears to be a prerequisite for the activation of both the nuclear factor (NF)-κB and c-Jun N-terminal protein kinase (JNK) signalling pathways.17 TRAF-2 has been demonstrated to play a role in this process in HT-29 and IEC-6 cells.18
NF-κB is an inducible heterodimeric transcription factor that recognizes the consensus motif 5′-GGGRNNYYCC-3′, and is composed most commonly of the Rel A (p65) and NF-κB1 (p50) subunits.19 Normally sequestered in the cytoplasm by NF-κB, IL-1β stimulation results in the phosphorylation and subsequent degradation of IκBα.20 This allows NF-κB to translocate to the nucleus, where it can effect the transcription of many genes, including IL-6, IL-8, MCP-1, IL-1β and TNF-α.21 Notably, NF-κB is activated in IBD22,23 and therapies that target it have been shown to attenuate inflammatory disease activity.24–26
Mitogen-activated protein kinases (MAPKs) are a group of protein kinases that are activated in response to a variety of extracellular stimuli and are responsible for a diverse range of biological processes, including response to stress, growth-related stimuli, inflammation and differentiation.27,28 MAPKs are comprised of three subfamilies: JNKs; extracellular signal-regulated kinases (ERKs); and p38 MAPK. Their precise roles in the regulation of inflammatory processes within the intestine are currently unknown. p38 MAPK is a stress-activated protein kinase, first discovered as a kinase activated by hyperosmolarity and endotoxin29 and later shown to be activated in response to many physical stresses such as heat and ultraviolet (UV) irradiation, as well as cytokines such as IL-1β and TNF-α. It is regulated by phosphorylation via the MAPK kinases (MAPKK) MKK3 and MKK6.30,31 Several lines of evidence indicate an important role for this molecule in the inflammatory process. These include interferon-γ (IFN-γ) production by lymphocytes;32 inducible nitric oxide synthase (iNOS) production;33,34 and Cox-2, IL-6 and TNF production by cells of the monocytic series.35,36 Inhibition of this molecule by specific pharmacological antagonists has resulted in the attenuation of inflammation seen in a number of animal models.37–39
In this report we have examined the role that p38 MAPK plays in chemokine expression by IECs, in response to IL-1β. Furthermore, owing to the potential link between p38 MAPK and NF-κB,35,40 we addressed the possible involvement of the p38 MAPK in several aspects of regulation of the latter molecule. Our findings indicate a role for p38 MAPK in the reduction of IL-8 expression, at the level of the chemokine promoter, and that p38 MAPK does not influence NF-κB activation directly. We propose that p38 MAPK inhibitors may have a supplementary role in the treatment of IBD.
Materials and methods
Cell culture
HT29 and Caco-2 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The latter cell line has been well characterized to undergo differentiation into functional enterocytes, and was therefore chosen for detailed studies.41 Both cell lines were pretreated for 2 hr using PD 98059, curcumin, SB 203580 and DRB (Calbiochem, San Diego, CA) at the appropriate concentrations before stimulation with IL-1β (Calbiochem) for the indicated lengths of time. HT-29 and Caco-2 human colonic epithelial cells were grown in M199 media containing 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT), supplemented with penicillin and streptomycin (Life Technologies, Burlington, Ont., Canada). Transient transfections with p38 MAPK (wild-type and kinase-dead) plasmids were carried out using Effectene. These plasmids were kindly donated by Dr J. Han (Scripps, La Jolla, CA).
Nuclear extracts
Caco-2 cells were seeded onto 60-mm plates and grown to confluence, then pretreated with the appropriate inhibitor for 2 hr before stimulation with IL-1β for 30 min. Cells were washed once with ice-cold phosphate-buffered saline (PBS) and scraped into 1 ml of PBS. They were then centrifuged at 4000 g and resuspended in 200 µl of Buffer A [10 mm HEPES, pH 7·9, 10 mm KCl, 0·1 mm EDTA, 0·2 mm EGTA, 1 mm dithiothreitol (DTT), 0·5 mm phenylmethylsulphonyl fluoride (PMSF)] for 15 min, before the addition of 13 µl of 10% Nonidet P-40 (NP-40). The cell suspension was then vortexed for 10 seconds before being centrifuged again at 4000 g for 30 seconds. The supernatant was removed and the nuclear pellet resuspended in 30 µl of Buffer C (20 mm HEPES, pH 7·9, 0·4 m NaCl, 1 mm EDTA, 1 mm EGTA, 25% glycerol, 1 mm DTT, 1 mm PMSF), rocked vigorously for 15 min, then centrifuged for 5 min at 4000 g. The supernatant was retained and the protein concentration determined by using the Bradford assay (Bio-Rad, Mississuagua, Ont., Canada). Samples were stored at −80° until use.
Electromobility shift assay
A synthetic κb oligonucleotide was cloned into the cloning vector, pBS (Stratagene, La Jolla, CA), using the EcoRI and HindIII sites, to create pBS–EMSAκb. To radiolabel the probe, it was excised from pBS–EMSAκb using EcoRI and HindIII, and labelled using [γ-32P]dCTP (Amersham, Montreal, Québec, Canada) and the Klenow fragment of DNA polymerase (New England Biolabs, Missisauga, Ont., Canada). The probe was then purified by running on a 5% non-denaturing gel, cutting out the fragment, and incubating the gel slice in elution buffer [(0·6 m ammonium acetate, 1 mm EDTA, 0·1% sodium dodecyl sulphate (SDS)] overnight. Ten micrograms of nuclear extract was preincubated for 15 min in binding buffer (20 mm HEPES, pH 7·9, 100 mm KCl, 10% glycerol, 1 mm DTT) containing 1 µg of poly dIdC (Amersham), 20 000 counts per minute (c.p.m.) of probe was then added and the reaction mixture incubated at room temperature for 30 min before electrophoresis on a 5% non-denaturing polyacrylamide gel in 0·25 × Tris 89 mm, boric acid 89 mm, EDTA 2 mm (TBE) at 200 V for 1·5 hr. The gel was subsequently dried for 45 min before phospho-imaging analysis was carried out using a Bio-Rad molecular imager FX (or alternatively exposed to film overnight at −80° and then developed). For supershift or cold competitor reactions, the nuclear extract was preincubated with 1 µg of anti-p65 antibody (Calbiochem), or 100-fold excess of unlabelled probe with binding buffer and poly dI-dC for 30 min before adding the radiolabelled probe. The probes used are as follows: NF-κB: 5′-AATTCGGTTACAAGGGACTTTCCGCTGA-3′ and 3′-GCCAATGTTCCCTGAAAGGCGACTTCGA-5′; and AP1: 5′-CGCTTGATGACTCAGCCGGAA-3′ and 3′-GCGAACTACTGAGTCGGCCTT-5′.
Western analysis
Cells were washed once with ice-cold PBS, resuspended in homogenization buffer (20 mm MOPS, 50 mm β-glycerophosphate, 5 mm EGTA, 50 NaF, 1 mm DTT, 1 mm sodium vanadate, and 1 mm PMSF) for 30 min, sonicated for 15 seconds and centrifuged at 4000 g for 15 min. The protein concentration in the supernatant was determined by using the Bradford assay (Bio-Rad). Fifty micrograms of protein from each sample was resolved using 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) before transferring to nitrocellulose membranes (Bio-Rad). The blots were blocked in 5% skim milk in TBS-T (20 mm Tris–HCl, pH 7·4, 250 mm NaCl, 0·05% Tween-20) for 1 hr before probing for 2–4 hr using the appropriate primary antibody. The blots were washed with TBS-T three times (10 min each wash), before incubation with the appropriate secondary antibody for 1·5 hr. Following three further washes in TBS-T, they were developed using the enhanced chemiluminescence detection system (ECL; Amersham). Phospho-p38, phospho-IκB, phospho-tyrosine, phospho-JNK and IκBα antibodies were all purchased from New England Biolabs. Antibodies to p38 protein were purchased from Stressgen Biotechnologies (Victoria, BC, Canada).
MAPKAPK2 assay
This was carried out as an in vitro assay using crude lysate. Briefly, Caco-2 cells were preincubated with SB 203580 for 2 hr, stimulated with IL-1β (2 ng/ml) for 30 min and then harvested in homogenization buffer (described under Western analysis above). Ten microlitres (equal to 10 µg of protein) of cell lysate was then used in a kinase assay, using heat shock protein 27 (hsp 27) (1 µg; Stressgen Biotechnology) as the substrate and 0·5 µg of ATP (250 µm ATP, 1 µCi [γ-32P]-labelled), for 20 min at 30°. Samples were then boiled in 5× sample buffer and resolved on SDS–PAGE. After transfer to nitrocellulose membranes, the substrate bands were visualized using autoradiography. Ponceau staining was carried out to demonstrate equal loading.
Transient transfections
Transient transfections were performed using the Effectene reagent (Qiagen, Toronto, Ont., Canada) as per the manufacturer's instructions. Cells were seeded and transfected upon reaching 90% confluency. For one 35-mm well, 0·2 µg of DNA was transfected using a DNA : Effectene ratio of 1 : 25. Transfections were carried out overnight before replacing with fresh medium for 24 hr prior to conducting experiments.
Reporter gene assays
An NF-κB-dependent reporter, containing four repeats of the κb consensus sequence from the IL-1β gene cloned into the BglII site of the pGL3-promoter vector (Promega, Madison, WI), creating 4 x κb-luc, was co-transfected with a LacZ plasmid (Kindly donated by William Jia, UBC) using Effectene (Qiagen, Toronto, Ont., Canada). Cells were pretreated for 2 hr with the appropriate inhibitor, then stimulated with IL-1β for 6 hr, before being harvested. Luciferase and β-galactosidase activities were measured according to the manufacturer's instructions (Promega). Light emission was measured using a luminometer, and the results were normalized using β-galactosidase. The transfection efficiency was normalized for, by assaying for β-galactosidase activity from a constitutively expressed LacZ plasmid that was co-transfected with the reporter construct. IL-8 reporter (kindly supplied by Dr Gary Wu, Philadelphia) assays were performed and corrected in a similar manner.
ELISA assays for IL-8 and MCP-1
Cells were pretreated with the appropriate inhibitors and stimulated for 36 hr with IL-1β. The supernatants were sampled and the chemokine concentration was determined, in triplicate, by using enzyme-linked immunosorbent assay (ELISA) (BD Pharmingen, Missisaugua, Ont., Canada), as per the manufacturer's instructions.
Isolation of RNA and reverse transcription–polymerase chain reaction (RT–PCR)
Cells were pretreated for 2 hr with the appropriate inhibitor, and stimulated with IL-1β. RNA was isolated using the TRIZOL method (Life Technologies). The purity of the RNA was determined by running 1 µg of RNA on a 1% agarose gel for 1·5 hr at 75 V. One microgram of RNA was reverse transcribed, for 50 min at 37°, in a reaction mix of 20 µl containing: 0·5 µg of oligo (dT)12−18 (Amersham), 1 µl of 10 mm dNTPs, 2 µl of 0·1 m DTT and 40 U of RNA-guard (Amersham, Montreal, Quebec) in 1× first-strand buffer (Life Technologies) using 200 U of murine Moloney leukaemia virus (M-MLV) reverse transcriptase. Two microlitres of cDNA was used in each subsequent PCR reaction. For each 50-µl PCR reaction, 2 U of TAQ (PE Biosystems, Branchburg, NJ), 1 × PCR Buffer (PE Biosystems), 10 pmol of each primer, 1 µl of 10 mm dNTPs, and 3 µl of 25 mm MgCl2 were used. The PCR temperatures were 94° for 45 seconds (denaturation), 56° for 45 seconds (annealing), and 72° for 1 min (extension). Ten-microlitre aliquots of the reaction mix were resolved on a 1·5% agarose gel containing ethidium bromide. Negative controls for cDNA synthesis were run without template, and also without RT. The linearity of PCR reactions was determined in the range between 20 and 40 cycles. Densitometry was performed using Bio-Rad Quantity-One software. The sizes of the PCR products were 289 bp, 510 bp and 235 bp for IL-8, MCP-1 and actin, respectively.
Primers
The primers used are listed below:
IL-8 Forward: 5′-TCTGCAGCTCTGBTGTGAAGGTGCAGTT-3′;
IL-8 Reverse: 5′-TTCCTTTGACCCACGTCTCCCAA-3′;
MCP-1 Forward: 5′-TCTGTGCCTGCTGCTCATAGC-3′;
MCP-1 Reverse: 5′-GGGTAGAACTGTGGTTCAAGAGG-3′;
β-actin Forward 5′-CCAACCGCGAGAAGATGACC-3′; and
β-actin Reverse 5′-GATCTTCATGAGGTAGTCAGT-3′.
Results
Il-1β activates protein tyrosine phosphorylation and MAPKs in IECs
We first examined the effect of IL-1β stimulation on the temporal characteristics of the activation of the MAPK family members. The data indicates that all three MAPKs were activated within 10–15 min, with p38 MAPK exhibiting the earliest activation (Fig. 1a). Additionally it is apparent that the activation was sustained for at least 60 min. The lowest panel shows that proteins corresponding to MAPKs (with a molecular mass of 40–44 kDa) become tyrosine phosphorylated at the same times.
Figure 1.
Interleukin (IL)-1β stimulation of intestinal epithelial cells results in activation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal protein kinase (JNK) and p38 (a), and p38 mitogen-activated protein kinase (p38 MAPK) signalling can be inhibited using the specific pharmacological inhibitor SB 203580 (10 µm). (a) Caco-2 cells were stimulated with IL-1β (2 ng/ml) for the indicated times, before being harvested. Western immunoblotting was carried out as described in the Materials and methods. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; p-Tyr, tyrosine phosphorylation. (b) Caco-2 cells were preincubated with SB 203580 for 2 hr before being stimulated with IL-1β (2 ng/ml) for 30 min and harvested. Ten microlitres of cell lysate was then used in a kinase assay, with the heat shock protein 27 (hsp 27) as substrate. Samples were boiled in 5× sample buffer and resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). After transfer to nitrocellulose membranes, the substrate bands were visualized using autoradiography. The panel on the left is an autoradiogram and the one on the right is a Ponceau stain of the same membrane, showing co-localization of the hsp 27 band. The results shown are representative of at least three independent experiments. (c) Cells were pretreated with SB 203580 or PD 98059 (25 µm) for 2 hr, stimulation with IL-1β (2 ng/ml) for 36 hr. Supernatants were collected and analysed for the presence of IL-8 (c) or monocyte chemotactic protein 1 (MCP-1) (d) by enzyme-linked immunosorbent assay (ELISA). (Lane 1, control; lane 2, IL-1β; lane 3, IL-1β with SB 203580; lane 4, IL-1β with PD 98059.) The results shown are representative of at least three independent experiments. The results are expressed as mean ± standard error of the mean (SEM) (*P <0·01).
To confirm that the SB 203580 inhibited signalling through to the MAPKAPK2, in vitro phosphorylation reactions were performed using its substrate, hsp27. The data indicates that preincubation with the p38 inhibitor attenuated activation of this downstream target (Fig. 1b).
SB 203580 attenuates IL-8 and MCP-1 production by IL-1β-stimulated IECs
We next sought to examine the effect of the p38 MAPK inhibitor, SB 203580, on the production of an alpha-chemokine (IL-8) and a beta-chemokine (MCP-1) by IECs. This inhibitor acts by binding in the ATP-binding pocket of p38. Pretreatment of both Caco-2 cells and HT-29 with SB 203580 led to a significant reduction in IL-8 production (Fig. 1c). A similar inhibitory effect of the p38 MAPK inhibitor was observed on MCP-1 production in Caco-2 cells (Fig. 1d). HT29 cells do not produce any MCP-1 in response to IL-1β alone, as previously reported.42 PD 98059 (an inhibitor of MEK via upstream activator-dependent phosphorylation) had no effect upon the production of either chemokine in the Caco-2 cell line (lane 4). The data indicate that p38 MAPK is activated in IECs in response to IL-1β and is involved in the production of important immunoregulatory chemokines.
IκBα phosphorylation and degradation are independent of p38 MAPK
NF-κB has been reported to be a major regulator of IL-8 and MCP-1 transcription.43,44 The phosphorylation and subsequent degradation of IκBα is an integral step in the activation of NF-κB. Previously, protein kinase casein kinase 2 (CK2) has been shown to phosphorylate IκBα at both the PEST region45–47 and serine 32/36 in vitro.48 Furthermore, it has been demonstrated that the stress-induced activation of p38 MAPK activates CK2.49 Therefore, it seemed logical to explore the possibility that p38 MAPK or CK2 were involved in the phosphorylation and degradation of IκBα. However, as the data indicates, pretreatment of cells with either SB 203580 or DRB, a specific inhibitor of protein kinase CK2 at the concentrations used,50 did not prevent IL-1β-induced phosphorylation and degradation of IκBα (Fig. 2a).
Figure 2.
(a) Interleukin (IL)-1β-induced degradation of IκB is independent of p38 mitogen-activated protein kinase (p38 MAPK) regulation in Caco-2 cells. Cells were starved overnight before preincubation with the appropriate inhibitor for 2 hr. Cells were stimulated with IL-1β (2 ng/ml) for 30 min, then harvested. Cells were lysed, resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotted with the appropriate antibody. The results are representative of at least four independent experiments. DRB, a specific inhibitor of protein kinase CK2; SB, SB 203580. (b) IL-1β-induced translocation and DNA binding of nuclear factor (NF)-κB is independent of p38 MAPK regulation in Caco-2 cells. Cells were preincubated overnight for 2 hr before being stimulated with IL-1β (2 ng/ml) for 30 min. Nuclear extracts were prepared and assayed for κB binding by using electromobility shift assays (EMSAs). (c) Specificity of κB binding was determined by antibody supershifting or competition with cold probe. Nuclear extracts were preincubated with an anti-Rel A antibody or 100-fold excess of cold unlabelled probe, before adding radiolabelled probe. The results are representative of at least three independent experiments.
NF-κB DNA binding and trans-activation are independent of p38 MAPK activation
NF-κB is regulated by its ability to translocate to the nucleus and bind to its consensus sequence. Furthermore, it has been shown that activation of NF-κB is accompanied by the CK2-induced phosphorylation of the p65 subunit,51 which has been mapped to the serine 529 site of Rel A.52 Therefore, we investigated the potential roles of p38 MAPK and CK2 on this aspect of NF-κB activation. Electromobility shift assays (EMSAs) were performed on nuclei isolated from Caco-2 cells. Pretreatment of cells with SB 203580 or DRB did not prevent nuclear translocation or the ability of NF-κB to bind to its consensus sequence (Fig. 2b). It can be seen that there was no effect upon the bound probe signal with either of the two inhibitors. In order to confirm that in fact p65 was the major Rel family member binding to the NF-κB consensus sequence, a supershift assay was performed, by preincubating nuclear extracts with the anti-p65 antibody before addition of the probe. As seen, the Rel A band was almost entirely shifted up with the addition of the antibody (Fig. 2c).
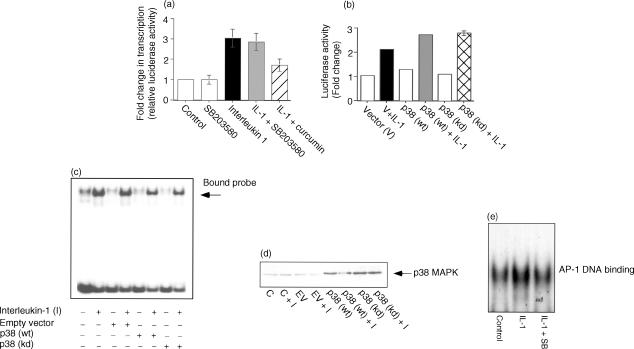
DNA binding alone does not confer transcriptional activation, as NF-κB requires multiple trans-activating phosphorylations in order to be functionally active once bound to DNA.53 To investigate the possibility that p38 MAPK may be phosphorylating and regulating the trans-activation of p65, we performed reporter assays with a synthetic NF-κB promoter fused to a luciferase gene. The data indicates that there is a threefold increase in trans-activation upon exposure of Caco-2 cells to IL-1β (Fig. 3a). Pretreatment with SB 203580 did not attenuate the trans-activation of NF-κB. Curcumin was found to attenuate trans-activation, in accordance with a previous report.54 This data excludes an effect of p38 MAPK upon NF-κB activation in this cell system.
Figure 3.
Interleukin (IL)-1β-induced trans-activation of nuclear factor (NF)-κB is independent of p38 mitogen-activated protein kinase (p38 MAPK) regulation in Caco-2 cells. Cells were co-transfected with the NF-κB reporter 4x κB-luc and LacZ for 12 hr before the medium was replaced with fresh serum-containing medium for 24 hr. (a) Cells were preincubated with the appropriate inhibitor for 2 hr, before being stimulated with IL-1β (2 ng/ml) for 6 hr. Cells were harvested and the luciferase activity was determined. Results were normalized for transfection efficiency using LacZ activity. Results are representative of at least five independent experiments. (b) Caco-2 cells were transfected with the indicated plasmids using Effectene and then stimulated with IL-1β (p38wt = p38 wild-type, p38kd = p38 kinase-dead). (c) DNA binding was carried out as described in the Materials and methods. (d) Expression of both the native and transfected p38 MAPK is shown. (e) Activator protein-1 DNA binding in the presence or absence of IL-1β and SB 203580 is shown.
In order to substantiate these findings, we investigated the effects of transient transfections of the p38 MAPK constructs upon NF-κB trans-activation. The data indicated (Fig. 3b) that despite the small changes observed in the DNA-binding study (Fig. 3c), this is not translated into a reduction in the trans-activation effect. We believe that this complements the data obtained with the SB 203580 inhibitor, and establishes that p38 MAPK is not involved at any step of NF-κB activation within the Caco-2 cell system.
To confirm that there was no involvement of p38 MAPK upon NF-κB DNA binding, we transiently transfected Caco-2 cells with both the wild-type and kinase-dead versions of p38 MAPK, and investigated their influence upon DNA binding in response to stimulation with IL-1β. These interventions were found to have only a marginal effect upon this parameter (Fig. 3c). The control Western immunoblot (Fig. 3d) shows that the p38 MAPK protein was overexpressed. In contrast to the data obtained for NF-κB, we were able to show a reduction in activator protein 1 (AP-1) DNA binding using SB 203580 (Fig. 3e).
IL-8 and MCP-1 messages are regulated by p38 in IECs
The next step in the investigation led us to examine the role of the p38 MAPK inhibitor on the relative amounts of both IL-8 and MCP-1 message. A time-course investigation using semiquantitative RT–PCR was carried out, and after resolving the PCR products the bands were quantified by densitometry and corrected for by the intensity of the actin band. The data indicates (Fig. 4) that there was a reduction in the message for both of the chemokines investigated. The magnitude of this reduction was ≈ 40% for IL-8 and 50% for MCP-1. In order to verify or exclude a role for message stability being the mechanism for reduction of chemokine expression, RT–PCR assays were repeated using the method of Wang et al.55 The possibility of message stability being the mechanism for reduction of chemokine expression was excluded by our findings (Fig. 4c). More specifically, whereas Wang et al. were able to show an almost 80% reduction in lipopolysaccharide (LPS)-stimulated messages for both TNF-α and IL-6 in human monocytes within 20 min, our findings showed negligible changes, even as late as 120 min into the assay.
Figure 4.
The p38 mitogen-activated protein kinase (p38 MAPK) inhibitor, SB 203580, inhibits interleukin (IL)-1β-induced IL-8 and monocyte chemotactic protein 1 (MCP-1) mRNA expression. (a) Cells were preincubated with the appropriate inhibitor for 2 hr, before being stimulated with IL-1β for 3 hr. Total RNA was isolated, and semiquantitative reverse transcriptase–polymerase chain reaction (RT–PCR) was performed using 1 µg of RNA. PCR products were run on a 2% agarose gel containing ethidium bromide. (b) Densitometry was performed using Bio-Rad Quantity One Software. The results depicted are representative of three independent experiments. (c) Reduction in IL-8 message is independent of message stability. Cells were stimulated with IL-1β for 3 hr and then cultures were adjusted so that they contained 10 µg/ml of actinomycin D (Act D), either alone or together with 10 µm SB 203580 (SB). The samples were then harvested at the indicated time-points, total RNA extracted and semiquantitative PCR performed as described above. The data shown are representative of three independent experiments.
P38 is involved in activation of the IL-8 promoter
Our findings did not confirm an effect on chemokine message stability, and thus further studies were performed with the previously characterized IL-8 luciferase promoter56 construct to determine whether p38 was involved at this level. The data clearly indicate that using SB 203580 resulted in a significant attenuation of promoter activation (Fig. 5a). The data also indicate that the p38 kinase-dead (p38KD) construct reduces IL-8 promoter activation when compared to cells transfected with the empty vector (EV). Unexpectedly there was also a smaller reduction of activation using the wild-type (p38WT) construct when compared with the empty vector (Fig. 5b). The explanation for this is not clear and may be a result of the activation of counter-regulatory pathways by a constitutively active p38 signal.
Figure5.
Interleukin (IL)-1β-induced trans-activation of the IL-8 promoter is dependent on p38 mitogen-activated protein kinase (p38 MAPK) regulation in Caco-2 cells. (a) Cells were co-transfected with the IL-8 promoter and LacZ for 12 hr before the medium was replaced with fresh serum-containing medium for 24 hr. Cells were preincubated with the appropriate inhibitor for 2 hr, before being stimulated with IL-1β (2 ng/ml) for 6 hr. (b) Alternatively, the IL-8 promoter was co-transfected with the p38 MAPK constructs (p38WT = p38 wild-type, p38KD = p38 kinase-dead) and then stimulated with IL-1β. Cells were harvested and the luciferase activity determined. Results were normalized for transfection efficiency using LacZ activity. Results are representative of at least three independent experiments.
Discussion
In this report we assessed the role of IL-1β-induced p38 MAPK activation in NF-κB activation and chemokine production in IECs. Importantly, we report for the first time that the IL-1β-induced p38 MAPK and NF-κB activations are dissociated events in this system. Furthermore, we also showed that the IL-8 and MCP-1 chemokine expression attenuating effects of a specific p38 MAPK inhibitor, SB 203580, are probably caused by a reduction in activation of the IL-8 promoter and not by any component of the NF-κB signalling cascade investigation. Specifically, no effects were observed at either the DNA-binding or trans-activation levels of the latter molecule. Much attention has been given to the enhanced transcriptional activation of NF-κB observed in IBD, as well as its role in proinflammatory mechanisms in IECs.2,22,23 In contrast, although a role for p38 MAPK in the production of IL-8 in response to invasion of IECs with Salmonella typhimurium has been previously demonstrated,57 its precise role in functional responses to IL-1β has, to our knowledge, never been investigated. Significantly, several reports have indicated that p38 MAPK is involved in the trans-activation of NF-κB. This may occur via indirect mechanisms, including modulation of the DNA-binding function and phosphorylation of the associated TATA-binding protein, required for transcriptional activation. Additionally, a hypothesized (but unproven) second signalling pathway involving MAPKs, which may allow fine-tuning of transcriptional responses within the nucleus, has been invoked to explain this effect.58–60 Perhaps the most compelling evidence for cross-talk between these two integral signalling pathways is derived from data in cardiac myocytes, where there is evidence of an interaction between MKK6 and IKKβ.61 With particular reference to the IEC system, our study clearly contrasts these observations by showing that p38 MAPK plays a role distinct from that of regulating NF-κB activation. p38 MAPK did not significantly influence the ability of NF-κB to bind to DNA, or to phosphorylate IκBα, nor did it affect the ability of translocated p65 to activate a target gene. This demonstration of an effect that is independent of the activation of NF-κB may have therapeutic implications for conditions such as IBD. Phosphorylation of the p65 subunit of NF-κB has been demonstrated by several laboratories and the molecules involved in this response reported to include CK251 and PI3-kinase.62 Furthermore, a recently identified serine 471 site on c-Rel has been found to be critical for its trans-activation function in response to TNF;63 however, the protein kinase responsible for this remains unknown. Additional evidence for the functional modification of NF-κB by other signalling molecules exists. The trans-activation function of NF-κB may be modulated by the small GTP protein, Ras, PKCζ, as well as by PKB and PTEN.64–67 Collectively, these observations indicate regulation of NF-κB at multiple levels, which may be responsible for directing specific functions in different tissues. Regulation of mRNA transcript levels is probably one of the major determinants of cytosolic levels of protein. The transcripts of many short-lived cytokines and proto- oncogenes are often characterized by cis AU-rich elements (ARE) found in their 3′ untranslated region, and it is these AREs that mediate their subsequent destabilization and decay.68,69 IL-8 and MCP-1 both feature AU-rich repeats,70,71 which provide stimulus-specific regulation of their decay through the MKK6/p38 pathway. Knockout mice that have AREs deleted from the TNF-α gene exhibit increased levels of TNF-α and develop chronic inflammatory arthritis and Crohn's-like IBD. Predictably, peritoneal macrophages from these animals were found to be unresponsive to modulation by the p38 pathway using SB 203580 at concentrations similar to those used in our study (10 µm).72
Recent work in other systems has also shown a role for p38 in chemokine production. These observations include the down-regulation of IL-1β-induced MCP-1 in renal mesangial cells73 and TNF-induced MCP-1 in endothelial cells.74 Furthermore β1-integrin-stimulated IL-8 production by natural killer (NK) cells was similarly attenuated.75
It is recognized that the transcription factor NF-κB has functions other than simply those involved in immune regulation, such as regulation of cell survival76 as well as normal epithelial cell turnover within the colon.77 Therefore, it will be necessary to refine therapy to prevent only the deleterious aspects of its activation that are involved in chronic inflammation. The use of agents that work at alternative sites within the inflammatory cascade, as in the case of the p38 MAPK inhibitor in this study, merits further scrutiny. Indeed, a preliminary report indicates that inhibition of this pivotal protein kinase may be useful in the management of human CD,78 where p38 MAPK has been shown to be activated.79
Acknowledgments
This study was supported by grants from the Crohn's and Colitis Foundation of Canada (to B.S.) and the North-western Society for Intestinal Research (to B.S. and U.P.S.) and funds from the Geraldine Dow Foundation to B.S. K.P. is supported by a Michael Smith Graduate Studentship. A.R. was supported by a Canadian Association of Gastroenterology Studentship. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked, ‘advertisement’ in accordance with 18 US.C. Section 1734 solely to indicate this fact.
References
- 1.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobin C, Sartor RB. The I kappa B/NF-kappa B system: a key determinant of mucosalinflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- 3.Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 4.Van Deventer SJ. Tumour necrosis factor and Crohn's disease. Gut. 1997;40:443. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 6.Yang SK, Eckmann L, Panja A, Kagnoff MF. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 7.Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue JA, Mueller C. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994;144:997. [PMC free article] [PubMed] [Google Scholar]
- 8.Reinecker HC, Loh EY, Ringler DJ, Mehta A, Rombeau JL, MacDermott RP. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995;108:40. doi: 10.1016/0016-5085(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 9.Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996;178:201. doi: 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M, Baggiolini M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol. 1999;155:331. doi: 10.1016/S0002-9440(10)65128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papadakis KA, Targan SR. The role of chemokines and chemokine receptors in mucosal inflammation. Inflamm Bowel Dis. 2000;6:303. doi: 10.1002/ibd.3780060408. [DOI] [PubMed] [Google Scholar]
- 12.Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AE, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552. doi: 10.4049/jimmunol.166.1.552. [DOI] [PubMed] [Google Scholar]
- 13.Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease – enhanced production during active disease. Gut. 1990;31:686. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cominelli F, Nast CC, Duchini A, Lee M. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology. 1992;103:65. doi: 10.1016/0016-5085(92)91096-m. [DOI] [PubMed] [Google Scholar]
- 15.Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434. [PubMed] [Google Scholar]
- 16.O'Neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650. [PubMed] [Google Scholar]
- 17.Greene C, O'Neill L. Interleukin-1 receptor-associated kinase and TRAF-6 mediate the transcriptional regulation of interleukin-2 by interleukin-1 via NFkappaB but unlike interleukin-1 are unable to stabilise interleukin-2 mRNA. Biochim Biophys Acta. 1999;1451:109. doi: 10.1016/s0167-4889(99)00079-8. [DOI] [PubMed] [Google Scholar]
- 18.Jobin C, Holt L, Bradham CA, Streetz K, Brenner DA, Sartor RB. TNF receptor-associated factor-2 is involved in both IL-1 beta and TNF-alpha signaling cascades leading to NF-kappa B activation and IL-8 expression in human intestinal epithelial cells. J Immunol. 1999;162:4447. [PubMed] [Google Scholar]
- 19.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination. the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990;344:678. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 22.Rogler G, Brand K, Vogl D, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 25.Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan F, Polk DB. Aminosalicylic acid inhibits IkappaB kinase alpha phosphorylation of IkappaBalpha in mouse intestinal epithelial cells. J Biol Chem. 1999;274:36631. doi: 10.1074/jbc.274.51.36631. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 28.Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 30.Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 31.Enslen H, Brancho DM, Davis RJ. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 2000;19:1301. doi: 10.1093/emboj/19.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rincon M, Enslen H, Raingeaud J, et al. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 1998;17:2817. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Da Silva J, Pierrat B, Mary JL, Lesslauer W. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J Biol Chem. 1997;272:28373. doi: 10.1074/jbc.272.45.28373. [DOI] [PubMed] [Google Scholar]
- 34.Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15::1914. [PMC free article] [PubMed] [Google Scholar]
- 36.Pouliot M, Baillargeon J, Lee JC, Cleland LG, James MJ. Inhibition of prostaglandin endoperoxide synthase-2 expression in stimulated human monocytes by inhibitors of p38 mitogen-activated protein kinase. J Immunol. 1997;158:4930. [PubMed] [Google Scholar]
- 37.Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther. 1996;279:1453. [PubMed] [Google Scholar]
- 38.Jackson JR, Bolognese B, Hillegass L, Kassis S, Adams J, Griswold DE, Winkler JD. Pharmacological effects of SB 220025, a selective inhibitor of P38 mitogen-activated protein kinase, in angiogenesis and chronic inflammatory disease models. J Pharmacol Exp Ther. 1998;284:687. [PubMed] [Google Scholar]
- 39.Badger AM, Griswold DE, Kapadia R, et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:175. doi: 10.1002/1529-0131(200001)43:1<175::AID-ANR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 41.Vachon PH, Beaulieu JF. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology. 1992;103:414. doi: 10.1016/0016-5085(92)90829-n. [DOI] [PubMed] [Google Scholar]
- 42.Warhurst AC, Hopkins SJ, Warhurst G. Interferon gamma induces differential upregulation of alpha and beta chemokine secretion in colonic epithelial cell lines. Gut. 1998;42:208. doi: 10.1136/gut.42.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554. [PubMed] [Google Scholar]
- 44.Roebuck KA, Carpenter LR, Lakshminarayanan V, Page SM, Moy JN, Thomas LL. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-kappaB. J Leukoc Biol. 1999;65:291. doi: 10.1002/jlb.65.3.291. [DOI] [PubMed] [Google Scholar]
- 45.Lin R, Beauparlant P, Makris C, Meloche S, Hiscott J. Phosphorylation of IkappaBalpha in the C-terminal PEST domain by casein kinase II affects intrinsic protein stability. Mol Cell Biol. 1996;16:1401. doi: 10.1128/mcb.16.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarz EM, Van Antwerp D, Verma IM. Constitutive phosphorylation of IkappaBalpha by casein kinase II occurs preferentially at serine 293: requirement for degradation of free IkappaBalpha. Mol Cell Biol. 1996;16:3554. doi: 10.1128/mcb.16.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoonbroodt S, Ferreira V, Best-Belpomme M, Boelaert JR, Legrand-Poels S, Korner M, Piette J. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J Immunol. 2000;164:4292. doi: 10.4049/jimmunol.164.8.4292. [DOI] [PubMed] [Google Scholar]
- 48.Taylor JA, Bren GD, Pennington KN, Trushin SA, Asin S, Paya CV. Serine 32 and serine 36 of IkappaBalpha are directly phosphorylated by protein kinase CKII in vitro. J Mol Biol. 1999;290:839. doi: 10.1006/jmbi.1999.2912. [DOI] [PubMed] [Google Scholar]
- 49.Sayed M, Kim SO, Salh BS, Issinger OG, Pelech SL. Stress-induced activation of protein kinase CK2 by direct interaction with p38 mitogen-activated protein kinase. J Biol Chem. 2000;275:16569. doi: 10.1074/jbc.M000312200. [DOI] [PubMed] [Google Scholar]
- 50.Zandomeni R, Zandomeni MC, Shugar D, Weinmann R. Casein kinase type II is involved in the inhibition by 5,6-dichloro-1-beta-d-ribofuranosylbenzimidazole of specific RNA polymerase II transcription. J Biol Chem. 1986;261:3414. [PubMed] [Google Scholar]
- 51.Bird TA, Schooley K, Dower SK, Hagen H, Virca GD. Activation of nuclear transcription factor NF-kappaB by interleukin-1 is accompanied by casein kinase II-mediated phosphorylation of the p65 subunit. J Biol Chem. 1997;272:32606. doi: 10.1074/jbc.272.51.32606. [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Westerheide SD, Hanson JL, Baldwin AS. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 53.Naumann M, Scheidereit C. Activation of NF-kappa B in vivo is regulated by multiple phosphorylations. EMBO J. 1994;13:4597. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474. [PubMed] [Google Scholar]
- 55.Wang SW, Pawlowski J, Wathen ST, Kinney SD, Lichenstein HS, Manthey CL. Cytokine mRNA decay is accelerated by an inhibitor of p38-mitogen-activated protein kinase. Inflamm Res. 1999;48:533. doi: 10.1007/s000110050499. [DOI] [PubMed] [Google Scholar]
- 56.Wu GD, Lai EJ, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J Biol Chem. 1997;272:2396. [PubMed] [Google Scholar]
- 57.Hobbie S, Chen LM, Davis RJ, Galan JE. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550. [PubMed] [Google Scholar]
- 58.Wesselborg S, Bauer MK, Vogt M, Schmitz ML, Schulze-Osthoff K. Activation of transcription factor NF-kappaB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J Biol Chem. 1997;272:12422. doi: 10.1074/jbc.272.19.12422. [DOI] [PubMed] [Google Scholar]
- 59.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 60.Bergmann M, Hart L, Lindsay M, Barnes PJ, Newton R. IkappaBalpha degradation and nuclear factor-kappaB DNA binding are insufficient for interleukin-1beta and tumor necrosis factor-alpha-induced kappaB-dependent transcription. Requirement for an additional activation pathway. J Biol Chem. 1998;273:6607. doi: 10.1074/jbc.273.12.6607. [DOI] [PubMed] [Google Scholar]
- 61.Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, Glembotski CC. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J Biol Chem. 2000;275:23814. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]
- 62.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin AG, Fresno M. Tumor necrosis factor-alpha activation of NF-kappa B requires the phosphorylation of Ser-471 in the transactivation domain of c-Rel. J Biol Chem. 2000;275:24383. doi: 10.1074/jbc.M909396199. [DOI] [PubMed] [Google Scholar]
- 64.Norris JL, Baldwin AS. Oncogenic Ras enhances NF-kappaB transcriptional activity through Raf-dependent and Raf-independent mitogen-activated protein kinase signaling pathways. J Biol Chem. 1999;274:13841. doi: 10.1074/jbc.274.20.13841. [DOI] [PubMed] [Google Scholar]
- 65.Anrather J, Csizmadia V, Soares MP, Winkler H. Regulation of NF-kappaB RelA phosphorylation and transcriptional activity by p21 (ras) and protein kinase Czeta in primary endothelial cells. J Biol Chem. 1999;274:13594. doi: 10.1074/jbc.274.19.13594. [DOI] [PubMed] [Google Scholar]
- 66.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 67.Mayo MW, Madrid LV, Westerheide SD, Jones DR, Yuan XJ, Baldwin AS, Whang YE. PTEN blocks tumor necrosis factor-induced NF-kappa B-dependent transcription by inhibiting the transactivation potential of the p65 subunit. J Biol Chem. 2002;277:11116. doi: 10.1074/jbc.M108670200. [DOI] [PubMed] [Google Scholar]
- 68.Chen CY, Shyu AB. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 69.Wilson GM, Brewer G. The search for trans-acting factors controlling messenger RNA decay. Prog Nucl Acid Res Mol Biol. 1999;62:257. doi: 10.1016/s0079-6603(08)60510-3. [DOI] [PubMed] [Google Scholar]
- 70.Winzen R, Kracht M, Ritter B, et al. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poon M, Liu B, Taubman MB. Identification of a novel dexamethasone-sensitive RNA-destabilizing region on rat monocyte chemoattractant protein 1 mRNA. Mol Cell Biol. 1999;19:6471. doi: 10.1128/mcb.19.10.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 73.Rovin BH, Wilmer WA, Danne M, Dickerson JA, Dixon CL, Lu L. The mitogen-activated protein kinase p38 is necessary for interleukin 1beta-induced monocyte chemoattractant protein 1 expression by human mesangial cells. Cytokine. 1999;11:118. doi: 10.1006/cyto.1998.0409. [DOI] [PubMed] [Google Scholar]
- 74.Goebeler M, Kilian K, Gillitzer R, Kunz M, Yoshimura T, Brocker EB, Rapp UR, Ludwig S. The MKK6/p38 stress kinase cascade is critical for tumor necrosis factor-alpha-induced expression of monocyte-chemoattractant protein-1 in endothelial cells. Blood. 1999;93:857. [PubMed] [Google Scholar]
- 75.Mainiero F, Soriani A, Strippoli R, Jacobelli J, Gismondi A, Piccoli M, Frati L, Santoni A. RAC1/P38 MAPK signaling pathway controls beta1 integrin-induced interleukin-8 production in human natural killer cells. Immunity. 2000;12:7. doi: 10.1016/s1074-7613(00)80154-5. [DOI] [PubMed] [Google Scholar]
- 76.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inan MS, Tolmacheva V, Wang QS, Rosenberg DW, Giardina C. Transcription factor NF-kappaB participates in regulation of epithelial cell turnover in the colon. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1282. doi: 10.1152/ajpgi.2000.279.6.G1282. [DOI] [PubMed] [Google Scholar]
- 78.Hommes D, van den Blink B, Plasse T, et al. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate-to-severe Crohn's disease. Gastroenterology. 2002;122:7. doi: 10.1053/gast.2002.30770. [DOI] [PubMed] [Google Scholar]
- 79.Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 Mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]