Abstract
Nitric oxide (NO) produced by macrophages (Mφ) in response to interferon-γ (IFN-γ) plays a pivotal role in the control of intracellular pathogens. Current knowledge of the specific biochemical cascades involved in this IFN-γ-inducible Mφ function is still limited. In the present study, we evaluated the participation of various second messengers – Janus kinase 2 (JAK2), signal transducer and activator of transcription (STAT) 1α, MAP kinase kinase (MEK1/2), extracellular signal-regulated kinases 1 and 2 (Erk1/Erk2) and nuclear factor kappa B (NF-κB) – in the regulation of NO production by IFN-γ-stimulated J774 murine Mφ. The use of specific signalling inhibitors permitted us to establish that JAK2/STAT1α- and Erk1/Erk2-dependent pathways are the main players in IFN-γ-inducible Mφ NO generation. To determine whether the inhibitory effect was taking place at the pre- and/or post-transcriptional level, we evaluated the effect of each antagonist on inducible nitric oxide synthase (iNOS) gene and protein expression, and on the capacity of IFN-γ to induce JAK2, Erk1/Erk2 and STAT1α phosphorylation. All downregulatory effects occurred at the pretranscriptional level, except for NF-κB, which seems to exert its role in NO production through an iNOS-independent event. In addition, electrophoretic mobility shift assay (EMSA) analysis revealed that STAT1α is essential for IFN-γ-inducible iNOS expression and NO production, whereas the contribution of NF-κB to this cellular regulation seems to be minimal. Moreover, our data suggest that Erk1/Erk2 are responsible for STAT1α Ser727 residue phosphorylation in IFN-γ-stimulated Mφ, thus contributing to the full activation of STAT1α. Taken together, our results indicate that JAK2, MEK1/2, Erk1/Erk2 and STAT1α are key players in the IFN-γ-inducible generation of NO by Mφ.
Introduction
Nitric oxide (NO) is a gaseous free radical that mediates intercellular communication in most mammalian organs. It is implicated in vascular homeostasis, neurotransmission and antimicrobial defence. This simple chemical mediator is produced by nitric oxide synthase (NOS), which converts l-arginine to l-citrulline and NO.1,2 Three isoforms of the enzyme have been cloned to date. Two isoforms are Ca2+ calmodulin-dependent and defined as neuronal NOS (nNOS)3 and endothelial NOS (eNOS).4 The third isoform, inducible nitric oxide synthase (iNOS), does not need Ca2+, but necessitates calmodulin as a cofactor with which it binds tightly; moreover, iNOS is inducible in different cell types, such as macrophages (Mφ).2,5–7 NO is a major effector molecule involved in Mφ antimicrobial and cytotoxic activities against intracellular pathogens,8–10 viruses11,12 and tumours.13
NO production is tightly regulated at several levels, including transcriptional, post-transcriptional and post-translational controls.14 The induction process constitutes a first critical step of regulation, because iNOS is normally not expressed in resting Mφs. Lipopolysaccharide (LPS), the major constituent of the outer wall of Gram-negative bacteria,15 and certain cytokines such as interferon-γ (IFN-γ)16 and migration inhibitory factor (MIF),17 can directly activate Mφs to produce NO. In addition, a combination of these stimuli can have a synergistic effect on NO generation. IFN-γ has been identified as the major Mφ-priming factor when co-administered with LPS and tumour necrosis factor-α (TNF-α).18 Different transcription factors participate in the regulation of the murine iNOS promoter. While iNOS induction by LPS alone,19 or in combination with IFN-γ,20 is dependent on nuclear factor kappa B (NF-κB) heterodimers p50 (c-Rel) and p65 (Rel A), the synergistic inductive contribution of IFN-γ requires interferon gamma responsive factor (IRF)-1 binding to the IRF element21,22 and signal transducer and activator of transcription (STAT)1α binding to the gamma-interferon activated site (GAS) present in the iNOS promoter of Mφs stimulated with IFN-γ and LPS.23
Some members of the mitogen-activated protein kinase (MAPK) and Janus kinase 2 (JAK2)/STAT1α signalling pathways have also been implicated in iNOS gene expression and NO generation in response to different stimuli. Extracellular signal-regulated kinase 1 and 2 (Erk1/Erk2) and p38 MAPK cascades have been found to play a key role in the transcriptional and post-transcriptional regulation of iNOS and TNF-α in glial cells treated with LPS in the presence or absence of IFN-γ.24 Furthermore, in the same cell type, it has been suggested that JAK2 is involved in IFN-γ-dependent iNOS induction.25 In murine Mφs, p38 has been shown to be involved in LPS-mediated NF-κB activation and subsequent iNOS expression and NO release;26 a partial role has been attributed to MAPK kinase (MEK) 1/2 (the immediate upstream Erk1/Erk2 activator) in iNOS induction by LPS and IFN-γ,27 and p46 JNK/SAPK has been found to participate in iNOS regulation following ligation of TNF-α with its receptor in the presence of IFN-γ.28
In spite of the studies mentioned above, little is known about the complete mechanism underlying NO regulation in Mφs stimulated with IFN-γ per se. Further understanding of the signal transduction pathways through which this crucial microbicidal molecule is regulated in Mφs would be of paramount importance for the development of better antimicrobial treatments. This report constitutes a detailed study investigating the signalling events resulting in NO generation by Mφ in response to IFN-γ alone. Our data show that JAK2-, MEK1/2- and Erk1/Erk2-dependent signal transduction is essential to this IFN-γ-inducible function, and that STAT1α is a key transcription factor involved in the regulation of iNOS gene expression, in contrast to NF-κB.
Materials and methods
Reagents
Isotopes were obtained from ICN Pharmaceuticals Canada Ltd (Montréal, Quebéc, Canada). Recombinant murine IFN-γ (2 × 105 U/ml) was purchased from Gibco BRL (Burlington, Ont., Canada). The iNOS antibody was purchased from Cedarlane (Hornby, Ont., Canada). The Erk1/Erk2 inhibitor, apigenin, and the MEK1/2 inhibitor, PD 98059, were purchased from Calbiochem (San Diego, CA). The JAK2 inhibitor, AG-490, and the NF-κB inhibitors, CAPE and BAY 11–7082, were purchased from Biomol (Plymouth Meeting, PA). Sodium salicylate (NaS) was obtained from Sigma (St Louis, MO). Oligonucleotides specific for STAT1 (consensus sequence) and NF-κB (consensus sequence) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The STAT1α iNOS binding sequence (GAS/iNOS)23 and the non-specific Oct-2A probe were synthesized in our laboratory.
Cell culture
The murine Mφ cell line, J774, was maintained (at 37° and in an atmosphere of 5% CO2) in Dulbecco's modified Eagle's medium (Life Technologies, Inc., Rockville, MD) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), streptomycin (100 µg/ml) and 2 mm l-glutamine. J774 was obtained from the American Type Culture Collection (ATCC; Manassas, VA).
NO production
Macrophages were seeded in 24-well dishes (5 × 105 cells/well) and cultured in the presence or absence of specific inhibitors for 1 hr prior to stimulation with IFN-γ (100 U/ml). Twenty-four hours later, NO generation was evaluated by measuring the accumulation of nitrite in the culture medium, as described previously.29
Western blotting
Cells (106−107) were collected and disrupted in cold lysis buffer [20 mm Tris–HCl (pH 8·0), 0·14 m NaCl, 10% glycerol (vol/vol), 1% Nonidet P-40 (NP-40) (vol/vol), 10 µm NaF, 1 mm sodium ortho-vanadate, 100 µg/ml phenylmethylsulphonyl fluoride, and protease inhibitors (25 µg/ml aprotinin and leupeptin)]. The lysates (30 µg/lane) were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) as previously described.30 After a 1-hr blocking period in Tris-buffered saline (TBS)/Tween-20 (3% gelatin), membranes were washed and incubated with an anti-iNOS antibody. Separated and transferred proteins were also incubated with anti-phosphotyrosine-JAK2 antibody (anti-p-Y-JAK2; BioSource International, Montréal, Québec, Canada), anti-phospho-Erk1/Erk2 antibody (anti-p-Erk1/Erk2; BioSource International), anti-p-Y-STAT1 antibody and anti-p-Ser727-STAT1 antibody (kindly provided by Dr David Frank, Harvard Medical School, Boston, Massachusetts). To monitor the amount of protein loaded in each lane, membranes were stripped and reprobed with anti-JAK2 antibody (C-20 rabbit polyclonal IgG) and anti-STAT1α antibody (C-111 mouse monoclonal IgG), both purchased from Santa Cruz Biotechnology, Inc. Anti-Erk1/Erk2 (p42/p44) antibody was purchased from BioSource International. Proteins were detected with anti-mouse or anti-rabbit horseradish peroxidase (HRP)-conjugated antibodies and subsequently visualized by enhanced chemiluminescence (ECL Western blotting detection system; Amersham, Arlington Heights, IL).
Northern blot analysis
Expression of iNOS, STAT1α and IκBα genes in IFN-γ-stimulated J774 Mφs (100 U/ml, 0–8 hr), treated or not treated with specific second messenger inhibitors (1 hr prior to IFN-γ stimulation), was evaluated by Northern blot of total mRNA, as described previously.30 Briefly, after washing stimulated cells twice in PBS and extracting total RNA using TRIzol (Gibco-BRL), RNA (10 µg) was subjected to electrophoresis on 1% agarose gels, transferred onto Hybond-N filter paper and hybridized with random primer-labelled cDNA probe. Equal RNA loading was confirmed by hybridization with a glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA probe kindly provided by Dr D. Radzioch (McGill University, Montréal, Québec, Canada). All washes were performed under stringent conditions and transcripts were visualized by autoradiography. STAT1α and IκBα cDNA probes were provided by Dr D. Levy (New York University School of Medicine, New York, NY) and Dr A. Israel (Pasteur Institute, Paris, France), respectively.
Electrophoretic mobility shift assay (EMSA)
Cells grown at a density of 2 × 106 per flask were treated under different conditions. Reactions were halted by the addition of ice-cold phosphate-buffered saline (PBS). In brief, sedimented cells were resuspended in 400 µl of cold buffer A (10 mm HEPES, pH 7·9, 1·5 mm MgCl2, 10 mm KCl, 0·5 mm dithiothreitol, 0·2 mm phenylmethylsulphonyl fluoride). Then, after 15 min on ice, lysates were vortexed for 10 seconds, and 25 µl of NP-40 (10%) was added to each sample before centrifugation for 30 seconds at 12 000 g. Supernatants were discarded and cell pellets resuspended in 50 µl of cold buffer C (20 mm HEPES-KOH, pH 7·9, 25% glycerol, 420 mm NaCl, 1·5 mm MgCl2, 0·2 mm EDTA, 0·5 mm dithiothreitol, and 0·2 mm phenylmethylsulphonyl fluoride) and incubated on ice for 15 min. Cell debris was removed by centrifugation at 12 000 g for 5 min at 4°, and the supernatant stored at −70° until further use. Then, 6 µg of nuclear extract protein was subjected to electrophoresis on a 4% polyacrylamide gel. All samples were labelled with [γ-32P]dATP containing an oligonucleotide directed towards the nuclear factors of interest. After migration, the gel was dried and exposed on X-ray film (Kodak Company, Rochester, NY). The DNA oligonucleotides used were: 5′-AGTTGAGGGGACTTTCCCAGGC-3′ for NF-κB, 5′-AAGTACTTTCAGTTTCATATTACTCTA-3′ for STAT-1α (consensus sequence) and 5′-CTTTTCCCCTAACAC-3′ for STAT-1α (GAS/iNOS sequence).23 Supershift assays were performed by preincubation of nuclear extracts with 2 µg of the polyclonal antibody STAT1α (Santa Cruz Biotechnology), in the presence of all components of the binding reaction described above, for 30 min at 4°. The non-specific probe Oct-2A (5′-GGAGTATCCAGCTCCGTAGCATGCAAATCCTCTGG-3′) was used to confirm the specificity of the DNA/nuclear protein reaction.
Statistical analyses
Statistically significant differences were determined using the analysis of variance (anova) module of SAS software (version 6·07, SAS Institute, Cary, NC) and the Fisher least significant difference test. P-values of < 0·05 were deemed statistically significant. All data are presented as mean ± standard error of the mean (SEM).
Results
IFN-γ-induced NO production and iNOS expression in murine J774 Mφs
Exposure to IFN-γ resulted in nitrite production and iNOS mRNA expression in dose- and time-dependent manners (Fig. 1). Maximal nitrite generation was obtained 24 hr following the addition of 100–500 U/ml of IFN-γ (Fig. 1a). Therefore, in the following experiments, cells were treated with 100 U/ml of IFN-γ for 24 hr. Maximal iNOS gene expression was achieved when cells were stimulated with 100 U/ml of IFN-γ during an 8-hr time-period. However, increased iNOS mRNA levels were already detectable at low doses of IFN-γ (10 U/ml) and were considerable from 4–24 hr poststimulation (Fig. 1b). Based on these observations, further experiments evaluating iNOS mRNA expression were conducted following cell stimulated with 100 U/ml of IFN-γ for 8 hr.
Figure 1.
Interferon-γ (IFN-γ)-induced macrophage nitric oxide (NO) generation and inducible nitric oxide synthase (iNOS) mRNA expression. (a) J774 Mφs were seeded in 24-well dishes (5 × 105 cells/well) and stimulated with increasing doses of IFN-γ (1–500 U/ml) for 24 hr. Then, supernatants were collected and submitted to the colorimetric Greiss reaction to evaluate nitrite production. (b) Cells were stimulated with different concentrations of IFN-γ (1–500 U/ml) for 8 hr or with 100 U/ml of IFN-γ for different time-periods (2–24 hr). Next, total mRNA was extracted and subjected to Northern blot analysis to evaluate iNOS gene expression. A glyceraldehyde 3-phosphate dehydrogenase (GAPDH) probe was used to confirm equal RNA loading. These results are representative of one of three independent experiments.
Effect of JAK2, Erk1/Erk2 and MEK1/2 inhibitors on IFN-γ-induced iNOS expression and NO generation
Previous studies have reported some evidence regarding the involvement of the JAK2/STAT1α signalling pathway in murine Mφ iNOS induction by IFN-γ alone or in combination with LPS.21,23 In view of these observations obtained independently, we were interested in identifying the exact role played by these second messengers as well as that of the Erk1/Erk2 MAPK cascade in the regulation of iNOS expression and NO release following IFN-γ-stimulation per se. Initial identification of potentially involved signalling pathways was performed by using specific inhibitors recognized to selectively block JAK2, Erk1/Erk2 and MEK1/2 (Fig. 2). As JAK2 is rapidly activated following IFN-γ ligation to its receptor,31 the effect of AG-490, a JAK2 inhibitor, on NO generation was therefore tested. As shown in Fig. 2(a), this compound down-regulated NO release in a dose-dependent manner, leading to a 92·7% reduction when added at maximal concentrations (75 µm), thus indicating the pivotal role played by JAK2-dependent signalling events in IFN-γ-inducible NO production. Similarly, we found that the Erk1/Erk2 inhibitor, apigenin, was also capable of completely blocking (100% inhibition at 50 µm) IFN-γ-inducible Mφ NO generation in a dose-dependent manner (Fig. 2b). Moreover, knowing that MEK1/2 has been reported to activate Erk1/Erk2 by phosphorylation of their tyrosine and threonine residues in non-phagocytic cells,32 it was of interest to test whether MEK1/2 could be implicated in IFN-γ-mediated Mφ NO generation. In Fig. 2(c) we show that following treatment with increasing doses of PD 98059, a specific MEK1/2 inhibitor, the capacity of Mφ to produce NO was reduced by 90% at subcytotoxic doses recognized to completely block MEK1/2 activity. This high level of inhibition suggests that MEK1/2-dependent events are involved in Mφ IFN-γ-induced Erk1/Erk2 activation, which is, in turn, necessary for NO induction.
Figure 2.
Effect of specific inhibitors of Janus kinase 2 (JAK2), extracellular signal-regulated kinase 1 and 2 (Erk1/Erk2) and MAPK kinase 1 and 2 (MEK1/2) on interferon-γ (IFN-γ)-induced macrophage nitric oxide (NO) generation, and inducible nitric oxide synthase (iNOS) gene and protein expression. Cells were treated with increasing doses of AG-490 (JAK2 inhibitor) (a), apigenin (Erk1/Erk2 inhibitor) (b) or PD 98059 (MEK1/2 inhibitor) (c) for 1 hr prior to stimulation with IFN-γ (100 U/ml). After 24 hr of incubation, supernatants were collected and submitted to the Greiss reaction to evaluate nitrite generation (mean ± SEM, n = 3). (d) to (f) iNOS regulation has been monitored in cells pretreated, as described above, with specific inhibitors prior to stimulation with IFN-γ for 8 hr (iNOS gene expression) or 24 hr (iNOS protein expression). After incubation, either total RNA or total proteins were extracted, and used for Northern and Western blots, respectively. These results are representative of one of three experiments performed independently. GAPDH, glyceraldehyde 3-phosphate dehydrogenase probe used to confirm equal RNA loading.
To evaluate whether the antagonist-mediated NO inhibition reported in Fig. 2(a)–2(c) occurred at the pre- or post-transcriptional level, the impact of each inhibitor on iNOS gene and protein expression was monitored. As shown in Fig. 2(d), inhibition of JAK2 reduced IFN-γ-mediated iNOS expression by more than 90%, indicating that abrogation of NO production by blocking JAK2-dependent signals is as a result of pretranscriptional downregulation of iNOS. Similarly, iNOS induction in cells treated with apigenin was blocked at the highest concentration tested (50 µm) (Fig. 2e). As expected, MEK1/2 inhibition completely abrogated iNOS expression (Fig. 2f), a total reduction that correlated with the high level of downregulation observed for the NO production (Fig. 2c). These results suggest that JAK2, Erk1/Erk2 and MEK1/2 kinases play a pivotal role in the regulation of iNOS expression at the pretranscriptional level, probably by leading to transcription factor activation.
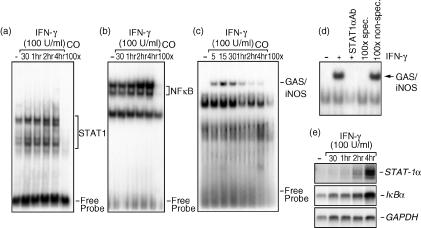
Mφ STAT1α and NF-κB nuclear translocation in response to IFN-γ stimulation
The promoter region of iNOS contains an array of putative transcription factor recognition boxes, including three GAS elements, two NF-κB sites, two IFN-stimulated response elements (ISREs), two recognition sites for activator protein 1 (AP-1), two TNF response elements (TNF-RE), and one X-box.33 We observed that blockage of second messengers, JAK2 and Erk1/Erk2, recognized to be partly involved in the signalling events resulting in STAT1α activation,34–36 led to a complete inhibition of IFN-γ-induced iNOS expression. Based on these data, we were interested in establishing the kinetic profiles of STAT1α and NF-κB transcription factors in response to IFN-γ to further correlate their induction with iNOS expression. We found that nuclear translocation of both STAT1α (Fig. 3a) and NF-κB (Fig. 3b), as well as STAT1α binding to the GAS/iNOS sequence (Fig. 3c), occurred over a 4-hr time-period following stimulation with IFN-γ. Of interest, STAT1α nuclear translocation (Fig. 3a), and its binding to the specific portion of the murine iNOS promoter (Fig. 3c), were detected significantly earlier (5–30 min poststimulation) compared with NF-κB nuclear translocation (Fig. 3b), which was observable only 2–4 hr later. In addition, supershift assays allowed us to define the nature of the IFN-γ-induced iNOS binding complex. As illustrated in Fig. 3(d), when nuclear extracts from IFN-γ-treated cells (1 hr) were incubated with a specific antibody against STAT1α, the complex binding was completely abrogated. The specificity of this complex was demonstrated by the fact that unlabelled GAS/iNOS oligonucleotide could compete effectively for binding, while an unrelated Oct-2A probe could not. This set of results indicates that STAT1α activation is a rapid and early process in the biochemical cascade leading to iNOS expression, whereas the late activation of NF-κB suggests a lesser participation for this factor and that its translocation could be the result of a second stimulation involving secreted molecules, such as TNF-α.
Figure 3.
Nuclear translocation of signal transducer and activator of transcription 1α (STAT1α) and nuclear factor-κB (NF-κB) transcription factors in interferon-γ (IFN-γ)-stimulated J774 macrophages. Nuclear extracts from cells either left untreated or stimulated with IFN-γ (100 U/ml) for different time-periods (0–4 hr) were incubated with a [γ-32P]-labelled STAT1α (consensus sequence) (a), NF-κB (b), or STAT1α [IFN-γ-activated site (GAS)/inducible nitric oxide synthase (iNOS) sequence] (c) probe and were subjected to electrophoretic mobility shift assay (EMSA) analysis. The last lane in each panel represents nuclear extracts from IFN-γ-treated cells incubated with an excess of unlabelled oligonucleotide in order to evaluate band specificity. (d) For supershift assays, nuclear extracts from cells stimulated with IFN-γ (1 hr) were or were not incubated with a specific antibody against STAT1α for 1 hr before EMSA. Binding specificity was tested by adding, to nuclear extracts from 1-hr IFN-γ-treated cells, a 100-fold molar excess of either a cold GAS/iNOS oligonucleotide or a non-specific Oct-2A probe. (e) Macrophages were stimulated with IFN-γ (100 U/ml) for different time-periods (0–4 hr). Following RNA extraction, STAT1α and IκBα gene expression were determined using Northern blot analysis. Equal RNA loading was confirmed by hybridization with a glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA probe. These results are representative of one of three separate experiments.
The expression level of IκBα is a good indicator of NF-κB activation. Following the ubiquitin/proteasome-mediated degradation of IκBα, NF-κB is free to translocate to the nucleus and IκBα protein needs to be generated to terminate NF-κB activity.37 On the other hand, STAT1α expression is only a consequence of its autoregulation and, to date, it has not been correlated with any negative regulatory mechanism controlling its transcriptional activity.34 Therefore, our EMSA observations, indicating STAT1α and NF-κB activation, were further confirmed by monitoring STAT-1α and IκBα gene expression in Mφs subjected to IFN-γ over the same time-period. As shown in Fig. 3(e), both STAT1α (upper panel) and IκBα (lower panel) mRNA levels increased in a time-dependent manner, reaching maximal values at 4 hr poststimulation.
Selective attenuation of STAT1α nuclear translocation by JAK2, Erk1/Erk2 and MEK1/2 inhibitors
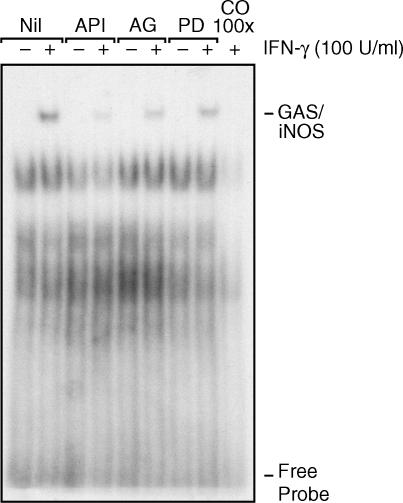
In comparison to NF-κB, we have shown that STAT1α activation is rapidly induced in response to IFN-γ. As we further observed that STAT1α binding to the specific iNOS sequence (GAS/iNOS) was also rapidly induced, we were interested in specifically evaluating the role of the second messengers JAK2, Erk1/Erk2 and MEK1/2 in this regulatory event. As illustrated in Fig. 4, a considerable reduction of STAT1α binding to its GAS/iNOS site was apparent in cells incubated with maximal doses of specific JAK2, Erk1/Erk2 and MEK1/2 inhibitors prior to treatment with IFN-γ. This result is in agreement with our observations reported above and further supports the pivotal roles played by these second messengers and by STAT1α in the signalling events resulting in iNOS expression and NO generation in response to IFN-γ.
Figure 4.
Effect of specific second-messenger inhibitors on interferon-γ (IFN-γ)-induced signal transducer and activator of transcription 1α (STAT1α) binding to the murine inducible nitric oxide synthase (iNOS) promoter. J774 macrophages were treated with specific inhibitors against extracellular signal-regulated kinase 1 and 2 (Erk1/Erk2) (50 µm of apigenin, lanes 3 and 4), Janus kinase 2 (JAK2) (75 µm of AG-490, lanes 5 and 6) and MEK1/2 (50 µm of PD 98059, lanes 7 and 8) for 1 hr prior to IFN-γ stimulation for 15 min. The last lane represents nuclear extracts preincubated with unlabelled oligonucleotide (100 ×) for band specificity evaluation. This result is representative of one of three different experiments.
Impact of second messenger inhibitors on IFN-γ-induced JAK2, Erk1/Erk2 and STAT1α phosphorylation
To further delineate and confirm the signalling process involving JAK2, Erk1/Erk2 and STAT1α in the regulation of NO production, we evaluated the induction of their phosphorylation in response to IFN-γ by performing Western blot analyses. As shown in Fig. 5, phosphorylation of the tyrosyl residues of JAK2 (Fig. 5a) and STAT1α (Fig. 5b) was detectable within 5 min poststimulation and sustained for all selected time-points over a 60-min period. On the other hand, phosphorylation of the Ser727 residue of STAT1α seemed slightly shifted in time, as it was clearly apparent only after 15 min (Fig. 5c) and peaked at 60 min. Moreover, Erk1/Erk2 phosphorylation was clearly observed at 15 min up to 60 min poststimulation (Fig. 5d). Protein phosphorylation on specific residues of these three signalling molecules is a good indicator of their state of activation in response to IFN-γ. Furthermore, our observation that the STAT1α Ser727 residue is phosphorylated around the same time as Erk1/Erk2 suggests their possible involvement in this activation process. The present result is in agreement with the concept that maximal STAT1α activation requires both tyrosine and serine phosphorylation.38
Figure 5.
Time-dependent activation of Janus kinase 2 (JAK2), signal transducer and activator of transcription 1α (STAT1α) and extracellular signal-regulated kinase 1 and 2 (Erk1/Erk2) by interferon-γ (IFN-γ) in J774 macrophages: effect of signalling inhibitors on their phosphorylation states. Cells were treated with IFN-γ (100 U/ml) for different time-periods (0–60 min) and cell lysates were subjected to immunoblot analysis by using antibodies specific for the phosphorylated forms of Janus kinase 2 (JAK2) (a); STAT1α (b) and (c); and Erk1/Erk2 (d). (e) To monitor IFN-γ-dependent Erk1/Erk2, JAK2 and STAT1α activation in the presence of apigenin, AG-490 or PD 98059, cells were treated with maximal doses of these specific inhibitors for 1 hr prior to stimulation with IFN-γ (for 15 min). Cell lysates were then subjected to Western blotting and incubated with the same antibodies mentioned above (for panels a–d). Equal protein loading was verified by using anti-JAK2, anti-STAT1α and anti-Erk1/Erk2 specific antibodies. Results are representative of three experiments performed independently.
As reported above, IFN-γ can rapidly induce JAK2, Erk1/Erk2 and STAT1α phosphorylation, corroborating previous observations.39,40 To accurately determine the specificity of the signalling inhibitors used to block iNOS expression and NO generation in J774 Mφs, we evaluated their capacity to selectively block JAK2, Erk1/Erk2 and STAT1α phosphorylation. As depicted in Fig. 5(e), cells were treated with maximal doses of Erk1/Erk2 (apigenin; lanes 3 and 4), JAK2 (AG-490; lanes 5 and 6) and MEK1/2 (PD 98059; lanes 7 and 8) inhibitors for 1 hr prior to stimulation with IFN-γ (100 U/ml, 30 min). Our data confirm that Erk1/Erk2 phosphorylation is inhibited by apigenin and PD 98059, reflecting a direct implication of MEK1/2 in Erk1/Erk2 tyrosine and threonine residue phosphorylation. Moreover, JAK2 phosphorylation is selectively blocked by AG-490, which was also reflected by a significant, but partial, reduction of STAT1α tyrosyl residue phosphorylation. This observation suggests that kinase(s) other than JAK2 could phosphorylate STAT1α on its tyrosyl residue. The use of apigenin clearly indicates, for the first time in Mφs, that Erk1/Erk2 MAPK are involved in STAT1α Ser727 phosphorylation in response to IFN-γ, while the tyrosyl phosphorylation of JAK2 and STAT1α was not significantly affected in the presence of this compound. Moreover, the inhibition of MEK1/2 by PD 98059 led to a significant reduction of IFN-γ-induced STAT1α seryl residue phosphorylation compared with a slight reduction on its tyrosyl residue. Collectively, this series of experiments strongly suggests that JAK2, Erk1/Erk2 and MEK1/2 play a pivotal role in the regulation of STAT1α activation and iNOS expression, and that Erk1/Erk2 MAPK are responsible for Mφ STAT1α Ser727 phosphorylation in response to IFN-γ.
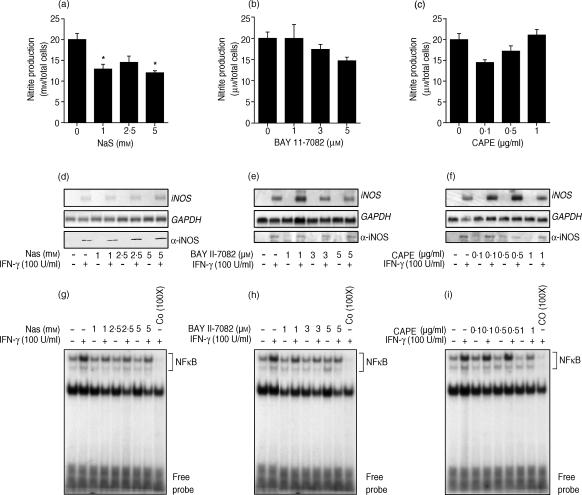
Effect of NF-κB inhibitors on IFN-γ-induced iNOS expression and NO generation
According to our results, JAK2, Erk1/Erk2 and MEK1/2 are important players in the regulation of STAT1α activity and the subsequent IFN-γ-mediated NO release. However, as the iNOS regulatory element contains two NF-κB sites,33 it was of importance to evaluate the extent to which NF-κB-regulated events are involved in this cellular process. As depicted in Fig. 6(a), addition of the NF-κB inhibitor, NaS, resulted in only a partial inhibition of NO release at the highest subcytotoxic concentration (5 mm), which was shown to totally inhibit NF-κB nuclear translocation (Fig. 6g). Moreover, as illustrated in Fig. 6(b),6(c), the use of increasing concentrations of the IκB inhibitor, BAY 11-7082 (1–5 µm), and the NF-κB inhibitor, CAPE (0·1–1 µg/ml), did not lead to a significant reduction of IFN-γ-mediated NO production. These results suggest that NF-κB is not a major player in IFN-γ-induced Mφ NO generation compared with STAT1α, which seems to be the main transcription factor involved in the activation of this Mφ function.
Figure 6.
Effect of nuclear factor-κB (NF-κB) antagonists on interferon-γ (IFN-γ)-inducible macrophage nitric oxide (NO) generation. Cells were treated with increasing doses of sodium salicylate (NaS) (a), BAY 11-7082 (b) or CAPE (c) for 1 hr prior to stimulation with IFN-γ (24 hr). Then, NO generation was measured by the Greiss reaction. Panels (g), (h) and (i) show the effect of NF-κB inhibitors on inducible nitric oxide synthase (iNOS) gene and protein expression. Cells were treated, as described above, prior to stimulation with IFN-γ for either 8 hr (iNOS gene) or 24 hr (iNOS protein). Panels (g), (h) and (i) illustrate IFN-γ-induced NF-κB nuclear translocation in the presence of the different NF-κB antagonists. The last lane of each panel represents nuclear extracts preincubated with an excess (100 ×) of NF-κB unlabelled oligonucleotide for band-specificity determination. These results are representative of one of three experiments performed independently.
To further support these observations, we also evaluated the effect of the various NF-κB antagonists on iNOS gene and protein expression. As expected, cells treated with the NF-κB inhibitors, NaS (Fig. 6d), BAY 11-7082 (Fig. 6e) and CAPE (Fig. 6f), did not show a significant reduction of iNOS expression, correlating with the data obtained for the production of nitrite (Fig. 6a, 6b, 6c). Collectively, these experiments provide evidence suggesting that NF-κB does not play a key role in the regulation of IFN-γ-induced iNOS expression at the pre- and post-transcriptional levels.
As control experiments, EMSA were performed to demonstrate that NaS, BAY 11-7082 and CAPE inhibit IFN-γ-induced NF-κB nuclear translocation. As reported in Fig. 6(g), 6(h) and 6(i), administration of these compounds at subcytotoxic concentrations completely blocked NF-κB nuclear translocation induced by IFN-γ. These observations confirm that antagonist-mediated inhibition of NF-κB nuclear translocation can only slightly affect NO release in response to IFN-γ, and further suggest a minimal role for NF-κB and a major role for JAK2/STAT1α and Erk1/Erk2 MAPK in the IFN-γ-dependent regulation of NO.
Discussion
Several studies have been performed in order to elucidate the signalling mechanisms through which iNOS expression is regulated in response to a combination of various proinflammatory agents, such as LPS and cytokines.20,23,24 In contrast, very little is known regarding the transductional events involved in the regulation of iNOS expression and NO release in Mφs following IFN-γ stimulation per se. To further increase our understanding about the IFN-γ-induced signals resulting in this important Mφ function, the present study was designed to establish the role played by JAK2/STAT1α, Erk1/Erk2- and NF-κB-dependent pathways in IFN-γ-induced NO generation. Our results strongly suggest that JAK2, MEK1/2 and Erk1/Erk2 play pivotal roles in the activation of STAT1α, which, in turn, appears to be essential for full IFN-γ-inducible production of NO. However, in contrast, a minimal role for the transcription factor, NF-κB, is suggested in this IFN-γ-mediated regulatory process.
In view of these findings, further experiments were conducted to determine whether this cellular regulation was taking place at the pre- and/or post-transcriptional level(s). We found that JAK2, as well as Erk1/Erk2 and MEK1/2 inhibitors, exerted their down-regulatory effect on iNOS expression at the pretranscriptional level. Our observations are in agreement with those of Kitamura and colleagues,25 who reported the role played by JAK2 in IFN-γ-mediated iNOS expression in glial cells. Moreover, our data, indicating a key role for Erk1/Erk2 in IFN-γ-inducible Mφ iNOS regulation, are perfectly in agreement with previous studies which have associated Erk1/Erk2 activation with iNOS induction by a combination of proinflammatory stimuli.41–43
In contrast to our observations for the JAK2/STAT1α and Erk1/Erk2 antagonists, the NF-κB inhibitor, NaS, did not affect iNOS mRNA and protein expression; however, it was able to reduce NO generation by ∼20%. This observation could be, at least in part, explained by the non-specific effect of NaS on nuclear factors and signalling molecules others than NF-κB. The use of newly developed NF-κB antagonists, which act by blocking either NF-κB translocation (CAPE) or IκBα degradation (BAY 11-7082), provided us with more direct evidence to support the notion that NF-κB plays a minimal role in IFN-γ-induced iNOS expression and NO generation. In fact, no significant iNOS or NO inhibition was detectable in cells treated with either of these two compounds, despite the fact that both BAY 11-7082 and CAPE were found to block IFN-γ-induced NF-κB translocation in a dose-dependent manner. In contrast to our results, partial NF-κB implication in IFN-γ-induced Mφ NO generation and iNOS mRNA expression was previously demonstrated by using the non-specific NF-κB inhibitor, NaS.44 This discrepancy might result from the presence of endotoxin, which would have, in turn, favoured NF-κB-dependent iNOS expression. Alternatively, these differences could also be related to the state of differentiation of the cell lines used in each study.
Different studies have reported the involvement of STAT1α and NF-κB transcription factors in the regulation of NO generation, in the context of costimulation.20,23 Extending these previous findings, our results allow us to propose that STAT1α is the main transcription factor involved in IFN-γ-induced NO generation by Mφ. Whereas specific inhibition of IFN-γ-mediated NF-κB nuclear translocation was not paralleled by downregulation of NO generation, both iNOS expression and STAT1α binding to the iNOS promoter were completely abrogated by JAK2, MEK1/2 and Erk1/Erk2 antagonists, strongly suggesting that full STAT1α activation by these second messengers is required for NO induction of Mφ in response to IFN-γ. According to the data collected so far, it is conceivable that the requirement for STAT1α in Mφ NO induction might be a result of both its direct and indirect effects on the iNOS gene. On one hand, we and others23 have shown that STAT1α binds to the GAS sites present in the murine iNOS promoter to initiate transcription. On the other hand, STAT1α has also been found to contribute indirectly to increase iNOS activity by inducing gene expression of IRF-1,45 another transcription factor required for full responsiveness of the iNOS promoter to IFN-γ.22 Further studies will be needed to fully understand the involvement of STAT1α in IFN-γ-mediated Mφ NO regulation.
To more directly investigate the putative contribution of the various second messengers – JAK2, STAT1α, MEK1/2 and Erk1/Erk2 – to the IFN-γ-dependent iNOS regulation, we examined the capacity of IFN-γ to induce their phosphorylation, thus reflecting their activation. As reported for cells of the monocyte/Mφ lineage,39,46,47 we showed that the presence of IFN-γ resulted in phosphorylation of JAK2 and STAT1α. We also confirmed the specificity of the JAK2 inhibitor, AG-490, by showing that it can block IFN-γ-induced JAK2 tyrosyl residue phosphorylation, whereas none of the other antagonists used affected its activation. It is well established that JAK2 is responsible for STAT1α phosphorylation on its tyrosyl residue34 and we further corroborated this by showing that JAK2-mediated STAT1α tyrosyl residue phosphorylation is downregulated by the JAK2-specific antagonist, AG-490. However, the incomplete inhibition of STAT1α tyrosyl phosphorylation in the presence of this compound suggests that other kinase(s) could be implicated in this regulatory event. Consistent with this hypothesis, it has been demonstrated that the protein tyrosine kinase, Pyk2, mediates JAK-dependent activation of STAT1α in IFN-γ-stimulated fibroblasts.35 Based on these observations, it is plausible that Pyk2 could also participate in Mφ STAT1α tyrosyl phosphorylation in response to IFN-γ.
In addition to tyrosine phosphorylation, maximal STAT1α activation has been found to require phosphorylation on a single serine residue, Ser727.38,48,49 Extending previous studies reporting MAPK involvement in STAT1α Ser phosphorylation in IFN-γ-treated fibroblasts,35,36 our data clearly indicate a role for Erk1/Erk2 in IFN-γ-dependent STAT1α Ser phosphorylation in Mφs. Even though Erk1/Erk2 activation has been shown to be impaired by Jak2 inactivation,50–52 we did not detect any downregulation of Erk1/Erk2 phosphorylation by blocking JAK2. A possible explanation of this contradictory result may lie in the fact that other tyrosine kinases, such as Pyk2, might activate Erk1/Erk2 via alternative pathways. In fact, Pyk2 has been identified as one of the signalling mediators critical for the G-protein-coupled receptor to the MAPK pathway53 and it has been shown to activate MAPK through a Ras-dependent mechanism.54
As we found that blockage of the Erk1/Erk2 pathway abrogated both STAT1α phosphorylation and binding to the iNOS promoter, and significantly reduced iNOS and NO induction by IFN-γ, our results allow us to propose a mechanism of IFN-γ-mediated Mφ NO regulation, which seems to require Erk1/Erk2-dependent signals for optimal STAT1α activity and subsequent iNOS gene expression. Alternatively, different lines of evidence seem to support the possibility that Erk1/Erk2 might be potentiating iNOS induction by favouring the activation of transcription factors others than STAT1α. In this regard, both IRF-1 activation and iNOS expression by LPS and/or IFN-γ were found to be downregulated by selective blockage of the Erk1/Erk2 pathway.55 In addition, Erk1/Erk2 were recently shown to contribute to AP-1-dependent murine iNOS promoter activation.56 A more detailed understanding of the specific roles of the various activated kinases and transcription factors will contribute to fully characterize the implication of Erk1/Erk2 on Mφ NO regulation in response to IFN-γ.
Collectively, the findings reported in the present study suggest that JAK2-, MEK1/2- and Erk1/Erk2-dependent signalling events play pivotal roles in IFN-γ-inducible Mφ NO generation, and that STAT1α is a key transcription factor involved in the regulation of iNOS gene expression. Overall, a better knowledge of the transductional mechanisms by which IFN-γ triggers Mφ microbicidal functions, such as production of NO, could permit the development of biological response modifiers useful for the modulation of various leucocyte functions in order to control infectious agents and immune responses.
Acknowledgments
We would like to thank Drs Levy and Israel for the donation of STAT1α and IκBα cDNA probes, respectively. This work was supported by grants from the Canadian Institute of Health Research (CIHR) to M.O. M.O. is a member of a CIHR Group in Host–Pathogen Interactions. M.O. holds a CIHR Investigator Award and is a Burroughs Wellcome Fund Awardee in Molecular Parasitology. J.B. is the recipient of a CIHR PhD studentship. M.J. is the recipient of a Ministère de l'Éducation du Québec PhD studentship.
References
- 1.Lowenstein CJ, Snyder SH. Nitric oxide, a novel biologic messenger. Cell. 1992;70:705–7. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- 3.Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P450 reductase. Nature. 1991;351:714–8. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 4.Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci USA. 1992;89:6348–52. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992;176:599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–8. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 8.Green SJ, Nacy CA, Meltzer MS. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol. 1991;50:93–103. doi: 10.1002/jlb.50.1.93. [DOI] [PubMed] [Google Scholar]
- 9.Liew FY. The role of nitric oxide in parasitic diseases. Ann Trop Med Parasitol. 1993;87:637–42. doi: 10.1080/00034983.1993.11812822. [DOI] [PubMed] [Google Scholar]
- 10.Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994;120:227–37. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–8. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 12.Croen KD. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993;91:2446–52. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 14.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 15.Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–42. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 17.Cunha FQ, Weiser WY, David JR, Moss DW, Moncada S, Liew FY. Recombinant migration inhibitory factor induces nitric oxide synthase in murine macrophages. J Immunol. 1993;150:1908–12. [PubMed] [Google Scholar]
- 18.Amber IJ, Hibbs JB, Jr, Parker CJ, Johnson BB, Taintor RR, Vavrin Z. Activated macrophage conditioned medium: identification of the soluble factors inducing cytotoxicity and the l-arginine dependent effector mechanism. J Leukoc Biol. 1991;49:610–20. doi: 10.1002/jlb.49.6.610. [DOI] [PubMed] [Google Scholar]
- 19.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–8. [PubMed] [Google Scholar]
- 20.Kim YM, Lee BS, Yi KY, Paik SG. Upstream NF-kappaB site is required for the maximal expression of mouse inducible nitric oxide synthase gene in interferon-gamma plus lipopolysaccharide-induced RAW 264.7 macrophages. Biochem Biophys Res Commun. 1997;236:655–60. doi: 10.1006/bbrc.1997.7031. [DOI] [PubMed] [Google Scholar]
- 21.Martin E, Nathan C, Xie QW. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med. 1994;180:977–84. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamijo R, Harada H, Matsuyama T, et al. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–5. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Morrison DC, Parmely TJ, Russell SW, Murphy WJ. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J Biol Chem. 1997;272:1226–30. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 24.Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–41. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura Y, Takahashi H, Nomura Y, Taniguchi T. Possible involvement of Janus kinase Jak2 in interferon-gamma induction of nitric oxide synthase in rat glial cells. Eur J Pharmacol. 1996;306:297–306. doi: 10.1016/0014-2999(96)00212-9. [DOI] [PubMed] [Google Scholar]
- 26.Chen CC, Wang JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol Pharmacol. 1999;55:481–8. [PubMed] [Google Scholar]
- 27.Caivano M. Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW264 macrophages. FEBS Lett. 1998;429:249–53. doi: 10.1016/s0014-5793(98)00578-x. [DOI] [PubMed] [Google Scholar]
- 28.Chan ED, Winston BW, Uh ST, Wynes MW, Rose DM, Riches DW. Evaluation of the role of mitogen-activated protein kinases in the expression of inducible nitric oxide synthase by IFN-gamma and TNF-alpha in mouse macrophages. J Immunol. 1999;162:415–22. [PubMed] [Google Scholar]
- 29.Evans TG, Thai L, Granger DL, Hibbs JB., Jr Effect of in vivo inhibition of nitric oxide production in murine leishmaniasis. J Immunol. 1993;151:907–15. [PubMed] [Google Scholar]
- 30.Olivier M, Romero-Gallo BJ, Matte C, Blanchette J, Posner BI, Tremblay MJ, Faure R. Modulation of interferon-gamma-induced macrophage activation by phosphotyrosine phosphatases inhibition. Effect on murine Leishmaniasis progression. J Biol Chem. 1998;273:13944–9. doi: 10.1074/jbc.273.22.13944. [DOI] [PubMed] [Google Scholar]
- 31.Greenlund AC, Farrar MA, Viviano BL, Schreiber RD. Ligand-induced IFN gamma receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91) EMBO J. 1994;13:1591–600. doi: 10.1002/j.1460-2075.1994.tb06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–9. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 33.Xie QW, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon-gamma and bacterial lipopolysaccharide. Trans Assoc Am Physicians. 1993;106:1–12. [PubMed] [Google Scholar]
- 34.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 35.Takaoka A, Tanaka N, Mitani Y, et al. Protein tyrosine kinase Pyk2 mediates the Jak-dependent activation of MAPK and Stat1 in IFN-gamma, but not IFN-alpha, signaling. EMBO J. 1999;18:2480–8. doi: 10.1093/emboj/18.9.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song JH, So EY, Lee CE. Increased serine phosphorylation and activation of STAT1 by oncogenic Ras transfection. Mol Cells. 2002;13:322–6. [PubMed] [Google Scholar]
- 37.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 38.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 39.Lucas DM, Lokuta MA, McDowell MA, Doan JE, Paulnock DM. Analysis of the IFN-gamma-signaling pathway in macrophages at different stages of maturation. J Immunol. 1998;160:4337–42. [PubMed] [Google Scholar]
- 40.Hu J, Roy SK, Shapiro PS, Rodig SR, Reddy SP, Platanias LC, Schreiber RD, Kalvakolanu DV. ERK1 and ERK2 activate CCAAAT/enhancer-binding protein-beta-dependent gene transcription in response to interferon-gamma. J Biol Chem. 2001;276:287–97. doi: 10.1074/jbc.M004885200. [DOI] [PubMed] [Google Scholar]
- 41.Ajizian SJ, English BK, Meals EA. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999;179:939–44. doi: 10.1086/314659. [DOI] [PubMed] [Google Scholar]
- 42.Kan H, Xie Z, Finkel MS. TNF-alpha enhances cardiac myocyte NO production through MAP kinase-mediated NF-kappaB activation. Am J Physiol. 1999;277:H1641–6. doi: 10.1152/ajpheart.1999.277.4.H1641. [DOI] [PubMed] [Google Scholar]
- 43.Kristof AS, Marks-Konczalik J, Moss J. Mitogen-activated protein kinases mediate activator protein-1-dependent human inducible nitric-oxide synthase promoter activation. J Biol Chem. 2001;276:8445–52. doi: 10.1074/jbc.M009563200. [DOI] [PubMed] [Google Scholar]
- 44.Kepka-Lenhart D, Chen LC, Morris SM., Jr Novel actions of aspirin and sodium salicylate: discordant effects on nitric oxide synthesis and induction of nitric oxide synthase mRNA in a murine macrophage cell line. J Leukoc Biol. 1996;59:840–6. doi: 10.1002/jlb.59.6.840. [DOI] [PubMed] [Google Scholar]
- 45.Pine R, Canova A, Schindler C. Tyrosine phosphorylated p91 binds to a single element in the ISGF2/IRF-1 promoter to mediate induction by IFN alpha and IFN gamma, and is likely to autoregulate the p91 gene. EMBO J. 1994;13:158–67. doi: 10.1002/j.1460-2075.1994.tb06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovarik P, Stoiber D, Novy M, Decker T. Stat1 combines signals derived from IFN-gamma and LPS receptors during macrophage activation. EMBO J. 1998;17:3660–8. doi: 10.1093/emboj/17.13.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eilers A, Georgellis D, Klose B, Schindler C, Ziemiecki A, Harpur AG, Wilks AF, Decker T. Differentiation-regulated serine phosphorylation of STAT1 promotes GAF activation in macrophages. Mol Cell Biol. 1995;15:3579–86. doi: 10.1128/mcb.15.7.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David M, Petricoin E, III, Benjamin C, Pine R, Weber MJ, Larner AC. Requirement for MAP kinase (ERK2) activity in interferon alpha- and interferon beta-stimulated gene expression through STAT proteins. Science. 1995;269:1721–3. doi: 10.1126/science.7569900. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S. Requirement of serine phosphorylation for formation of STAT-promoter complexes. Science. 1995;267:1990–4. doi: 10.1126/science.7701321. [DOI] [PubMed] [Google Scholar]
- 50.Hiraguri M, Miike S, Sano H, Kurasawa K, Saito Y, Iwamoto I. Granulocyte–macrophage colony-stimulating factor and IL-5 activate mitogen-activated protein kinase through Jak2 kinase and phosphatidylinositol 3-kinase in human eosinophils. J Allergy Clin Immunol. 1997;100:S45–51. doi: 10.1016/s0091-6749(97)70004-6. [DOI] [PubMed] [Google Scholar]
- 51.Matsumiya T, Imaizumi T, Itaya H, Shibata T, Yoshida H, Sakaki H, Kimura H, Satoh K. Production of growth related oncogene protein-alpha in human umbilical vein endothelial cells stimulated with soluble interleukin-6 receptor-alpha: role of signal transducers, janus kinase 2 and mitogen-activated kinase kinase. Life Sci. 2002;70:3179–90. doi: 10.1016/s0024-3205(02)01560-6. [DOI] [PubMed] [Google Scholar]
- 52.Madamanchi NR, Li S, Patterson C, Runge MS. Thrombin regulates vascular smooth muscle cell growth and heat shock proteins via the JAK-STAT pathway. J Biol Chem. 2001;276:18915–24. doi: 10.1074/jbc.M008802200. [DOI] [PubMed] [Google Scholar]
- 53.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–50. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 54.Lev S, Moreno H, Martinez R, et al. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–45. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 55.Faure V, Hecquet C, Courtois Y, Goureau O. Role of interferon regulatory factor-1 and mitogen-activated protein kinase pathways in the induction of nitric oxide synthase-2 in retinal pigmented epithelial cells. J Biol Chem. 1999;274:4794–800. doi: 10.1074/jbc.274.8.4794. [DOI] [PubMed] [Google Scholar]
- 56.Cho MK, Suh SH, Kim SG. JunB/AP-1 and NF-kappa B-mediated induction of nitric oxide synthase by bovine type I collagen in serum-stimulated murine macrophages. Nitric Oxide. 2002;6:319–32. doi: 10.1006/niox.2001.0415. [DOI] [PubMed] [Google Scholar]