Abstract
There is great potential for novel vaccines based on recombinant proteins and synthetic peptides. Unfortunately these antigens often lack the immunogenicity of whole, killed pathogens used in traditional vaccines. Thus there is strong interest in the identification of immunological adjuvants with low reactogenicity, but high potency, to enhance immune responses and realize the potential of these new vaccine strategies. CD40 antibodies have been shown to have adjuvant effects when administered at very high doses. These large doses are impractical and induce a cascade of cytokine release giving rise to septic shock-like symptoms, as well as splenomegaly and polyclonal antibody production. We show here that a very small amount of CD40 antibody can exhibit potent adjuvant effects when attached to soluble antigen. The lack of detectable systemic effects indicates that this method may be a powerful and practical means of enhancing the efficacy of recombinant vaccines.
Introduction
Recent advances in molecular biology have led to a rapid increase in the development of potential new vaccines. However, most recombinant proteins and synthetic peptides are poorly immunogenic and the only adjuvants currently available for human use are relatively weak. Thus, to take best advantage of these advances it is essential that new adjuvants are developed.
There are a number of novel adjuvants under development, many of which contain bacterial cell wall derivatives such as muramyl-dipeptides; or surface active agents such as saponins.1 A major aim with most adjuvants under development is to keep reactogenicity as low as possible with adjuvanticity as high as possible. As the properties of the compounds giving rise to these two effects are often identical, this can prove difficult.2 Most adjuvants, including those currently in development, have been designed empirically without any initial understanding of their mode of action. Understanding of immunology has contributed to great advances in the rational design of vaccines; and we are now in a position also to apply this knowledge to a rational design of adjuvants.
The major signal in T-cell help to B cells, which drives or costimulates B-cell activation, proliferation, differentiation and antibody production, is mediated through expression of the antigen CD154 on activated T cells. This binds to CD40, which is constitutively expressed on B cells, dendritic cells, macrophages and other cell types (for reviews see 3–5). We have previously shown that very large doses of anti-CD40 can mimic T-cell help in vivo in responses against T-independent antigens, such as capsular polysaccharides.6–8 T-dependent protein antigens by definition induce T-cell help, which is mediated through CD40 ligation. Thus the background response is higher, nevertheless we have shown that administration of a 0·5-mg dose of the anti-CD40 antibody 1C109,10 together with T-dependent antigens can lead to a significantly enhanced specific antibody response (our unpublished observations). Others have shown powerful effects of large doses of anti-CD40 on T helper and cytotoxic T lymphocyte responses.11–14 The doses of antibody (up to 1 mg/mouse) needed to obtain these enhancing effects also induce highly undesirable side effects including polyclonal stimulation of B cells leading to splenomegaly,6,8,15 increased total serum immunoglobulin levels,6 pro-inflammatory cytokine release (11,16 and our unpublished observations), and septic shock like symptoms, which can lead to death.17–19 Doses of antibody of this magnitude, besides being impractical, would clearly not be suitable for use in vaccination due to the side effects.
The work described here illustrates a means of reducing CD40 antibody doses, while enhancing adjuvant effects and removing antibody-associated toxicity.
Materials and methods
Determining effects of anti-CD40 monoclonal antibody (mAb) dose on toxicity and adjuvanticity
Five doses of anti-CD40 mAb, 1C10 or isotype matched control GL117 were injected into groups of six female BALB/c mice, along with a fixed dose (10 µg/mouse) of chicken egg ovalbumin (OVA). Five days after immunization, three animals were killed and spleens were removed and weighed. Ten days after immunization the remaining three mice were bled via the dorsal tail vein and serum levels of anti-OVA, anti-rat immunoglobulin and polyclonal immunoglobulin determined by enzyme-linked immunosorbent assay (ELISA).
Comparison of adjuvanticity of anti-CD40 and alum
Groups of five female BALB/c mice were immunized, via the intraperitoneal route, with 10 µg of 1C10 or GL117 isotype control, the latter being in either soluble form or precipitated with alum by standard techniques.20 Ten days later mice were bled via the dorsal tail vein and serum levels of anti-rat immunoglobulin determined by standard ELISA techniques using plates coated with rat immunoglobulin G2a (IgG2a). Titres were calculated as log10 of the highest reciprocal dilution at which the optical density (OD) from the test serum was higher than the OD from normal mouse serum. Student's t-test was used for statistical analysis.
Immunization with anti-CD40–avidin conjugates
Groups of five BALB/c mice were immunized i.p with 10 µg of avidin (Sigma, Poole, UK), avidin mixed with 10 µg biotinylated 1C10 or GL117 isotype control antibody, biotinylated 1C10 with the biotin sites preblocked by preincubation for 30 min on ice with 10 µg streptavidin, or non-biotinylated 1C10. All antigens were diluted in phosphate-buffered saline (PBS) and administered in a volume of 0·2 ml. Mice were bled 14 days later and the end-point titres assayed by ELISA against avidin or rat IgG2a using plates directly coated with antigen at 10 µg/ml in PBS, overnight at 4°.
All titres expressed as log10 of the highest reciprocal dilution at which the OD from the test serum was higher than the OD from normal mouse serum and statistical significance was calculated from the logarithmic titres by Student's t-test, undetectable titres were assigned a titre of 1, the lowest detectable titre (expressed as log10). Figures in parentheses are standard deviations. Significance was determined against the results for the top group of each cluster in the table.
N-Succinimidyl S-acetylthioacetate (SATA)-mediated conjugation of mAbs to recombinant OVA and herpes virus glycoprotein D
SATA, obtained from Sigma was used to conjugate antibodies to the two antigens used in this study (OVA and herpes simplex virus glycoprotein D (HSVgD)). 1C10 and control mAb GL117 were dialysed overnight against conjugation buffer (50 mm phosphate, 1 mm ethylenediaminetetra-acetic acid (EDTA)) and then concentrated to 5 mg/ml using a 30 000 MW cut-off centrifugal filter. Immediately prior to use, 6·5 mg of SATA was dissolved in 500 µl dimethylsulphoxide. One ml of the concentrated antibody solution was then incubated at room temperature (RT) for 30 min with 10 µl of the SATA solution. The reacted antibody solution was then washed three times over a 30 000 MW cut-off centrifugal filter. Sulphhydryl groups introduced into the antibodies were then de-protected by incubating each mAb with 100 µl of 0·5 m hydroxylamine (in 50 mm phosphate, 25 mm EDTA, pH 7·5) per ml of antibody solution. This reaction was allowed to proceed for 2 hr at RT. Meanwhile, for HSVgD conjugation, maleimide activation of the recombinant HSVgD (Viral Therapeutics Inc. Ithica, NY) was performed using sulpho-succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sulpho-SMCC) obtained from Sigma. HSVgD was concentrated to 8 mg/ml in PBS and 1 mg of sulpho-SMCC added to 500 µl of the HSVgD solution. Following 60 min incubation at RT, the maleimide activated HSVgD was washed extensively with conjugation buffer, over a 30 000 MW cut-off centrifugal filter. Commercially available imject maleimide-activated OVA (Pierce, Rockford, IL) was used as the antigen in OVA mAb conjugates. Maleimide-activated antigens were then reacted with SATA-treated mAb at antigen to antibody ratios of 1·5 : 1 and 2 : 1 for OVA and HSVgD, respectively. These reactions were allowed to proceed for 1·5 hr at RT and were stopped by the addition of 2-mercaptoethanol to a final concentration of 10 mm. The protein conjugates were then extensively dialysed against PBS, quantified by the Bradford assay, filter sterilized and stored at 4° until used.
Analysis of mAb-antigen conjugates
mAb–OVA and mAb–HSVgD conjugates were analysed by flow cytometric analysis on CD40-transfected fibroblasts in order to determine functional activity of CD40 mAb and presence of coupled herpes antigen. Control (L929) cells and CD40-transfected fibroblasts were incubated with conjugates at 10 µg/ml (in PBS, 0·1% bovine serum albumin, 0·01% NaN3) for 20 min on ice. Following three washes, detection of bound herpes glycoprotein D was confirmed using a mouse anti-HSV-1 antibody supplied by Dako (Cambridgeshire, UK) (20 min, on ice). Detection of OVA binding was confirmed using mouse anti-OVA serum (available in-house). Staining with fluoroscein isothiocyanate-labelled anti-mouse immunoglobulin enabled visualization of binding using a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA).
Immunization with mAb–antigen conjugates
Four groups of five BALB/c mice were immunized via the intraperitoneal (i.p.) route with 10 µg of mAb–OVA conjugate (anti-CD40 or control mAb), 10 µg of OVA/1C10 mix (4 µg OVA/6 µg 1C10) or with 10 µg of OVA alone. For the HSV immunogen, four groups of five BALB/c mice were immunized via the i.p. route with 10 µg of mAb–HSVgD conjugate (anti-CD40 or control mAb), 10 µg of HSVgD/1C10 mix (4 µg HSVgD/6 µg 1C10) or with 10 µg of HSVgD alone. Ten days after immunization, mice were bled via the dorsal tail vein and serum separated following overnight incubation of the blood at 4°. Serum anti-OVA and anti-HSV titres were determined by standard ELISA techniques on enzyme immunoassay plates coated with the corresponding antigen (OVA or HSVgD at10 µg/ml in PBS) overnight at 4°.
Results
Toxic effects of CD40 mAbs
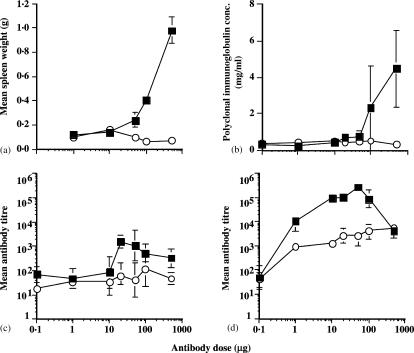
Decreasing doses of anti-CD40 or isotype control antibodies were administered to mice. As expected, the highest dose of anti-CD40 (0·5 mg) induced significant splenomegaly and polyclonal antibody production (Fig. 1a, b, respectively). Neither spleen size, nor total immunoglobulin production were reduced to normal levels until the dose of antibody was lowered to 10 µg/mouse.
Figure 1.
Effect of anti-CD40 mAb dose on toxicity and adjuvanticity. The figure illustrates the dose–response effect of 1C10 on induction of splenomegaly, polyclonal immunoglobulin production and adjuvanticity, respectively. Seven doses of 1C10 (filled squares) or GL117 (open circles) were administered to groups of 4 female BALB/c mice.(a) Effect of mAb dose on spleen weight (g) was determined at 5 days post immunization. Filled squares represent animals immunized with 1C10 and open circles represent GL117 immunized mice. (b) Effect of mAb dose on polyclonal immunoglobulin levels (mg/ml). Filled squares represent 1C10 and open circles GL117 immunized mice. Antigen specific antibody responses to OVA (c) and rat immunoglobulin (d) were determined by ELISA on day 10. As above, filled squares represent 1C10 and open circles, GL117. For all figures, error bars indicate standard error of the mean.
Adjuvant effects of CD40 mAb correlate with toxicity
In order to determine the relationship between these side-effects, and the adjuvant effect of CD40 antibody mixed with antigen, mice were immunized with a mixture of the anti-CD40 antibody 1C10, or isotype control antibody at various doses, and OVA at a fixed dose of 10 µg/mouse. As for the toxic effects described above, the adjuvant effect on the antibody response against coadministered OVA also declined to background by the time the antibody dose had fallen to 10 µg (Fig. 1c). Clearly, if CD40 antibodies are to have any role as adjuvants for prophylactic vaccines, a means of removing the side effects resulting from widespread stimulation of B cells, macrophages and other antigen-presenting cells must be found.
A stronger adjuvant effect at non-reactogenic doses of CD40 mAb.
As the anti-mouse CD40 antibodies used above are rat immunoglobulins, they are themselves immunogenic to mice. We therefore assessed the anti-rat IgG2a antibody response induced by administration of progressively decreasing doses of anti-CD40 or control antibody, and found a strikingly different picture to that found for anti-CD40/antigen mixtures. Anti-rat IgG titres reach a very high level in response to much smaller doses of anti-CD40 antibody, with enhancements in the region of 100-fold (as compared with response to control antibody) seen at doses down to 10 µg/mouse, and a significant adjuvant effect still evident at 1 µg/mouse (Fig. 1d).
Comparison of adjuvanticity versus alum
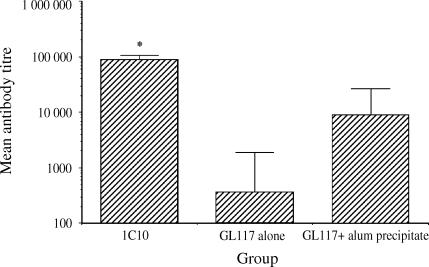
We sought to assess the efficacy of the anti-CD40 mAb as an adjuvant compared with a current, widely used formulation, alum. This was achieved by immunizing mice with soluble anti-CD40 or its isotype control, either in soluble form or precipitated with alum and measuring the antibody response against the rat immunoglobulins. Low anti-rat titres were detected against the soluble rat immunoglobulin and this response was increased using alum as an adjuvant. In comparison the anti-rat response against the anti-CD40 mAb was substantially and significantly higher than isotype control, when either in soluble form or when precipitated with alum (see Fig. 2). The efficacy of the anti-CD40 mAb as an adjuvant is illustrated by the superior response against antigen compared with alum, in which the response was some 10-fold higher.
Figure 2.
Comparison of adjuvanticity of anti-CD40 and alum. Significantly enhanced antibody responses against the anti-CD40 mAb 1C10 were observed in comparison with the isotype-matched control mAb GL117 precipitated with the commonly used adjuvant alum, as determined by ELISA. In each case error bars represent standard error of the mean and the asterisk statistical significance (P < 0·05) compared with all groups as determined by Student's t-test.
Attachment of antigen to antibody induces a potent adjuvant effect at low doses of conjugate
The major difference between the induction of anti-OVA responses (Fig. 1c) and anti-rat IgG2a (Fig. 1d) responses is that in the latter case the immunogen (rat IgG2a) is physically attached to the CD40 binding moiety (rat immunoglobulin antigen-binding domains). It appeared possible therefore that very low doses of anti-CD40 antibodies might become highly effective, non-reactogenic adjuvants when physically attached to antigen. To further address this question we used biotinylated rat (IgG2a) anti-mouse CD40 antibody 1C10, and produced conjugates of antigen and anti-CD40 by using avidin as an antigen, as it binds to biotin with extremely high affinity. Biotinylated anti-CD40 or isotype control antibodies were mixed with avidin prior to injection and anti-avidin responses monitored. The data in Table 1 show that a mixture of 10 µg biotin anti-CD40 mAb and 10 µg avidin induced a very strongly enhanced primary antibody response to avidin when compared with avidin alone, or avidin plus a biotinylated isotype control antibody. As expected, this powerful adjuvant effect was totally dependent upon physical connection between the antigen and anti-CD40. Biotinylated, but not unbiotinylated, 1C10 enhanced responses against avidin, with this response being abrogated by preincubation of the antibody with streptavidin to block the biotinylated sites (Table 1). Abrogation of the anti-avidin response in the above cases had no effect on the potent anti-rat IgG2a responses induced by the CD40 antibodies, indicating that attachment of the antigen to the CD40 binding moieties is the important factor, even when another antigen is mixed with the conjugate.
Table 1.
Co-attachment of antigen and anti-CD40 is essential for the adjuvant effect
| Titre determined against | |||
|---|---|---|---|
| Immunization | Avidin | Rat IgG2a | |
| Avidin | 1·23 (0·39) | 1 (0) | |
| Avidin + biot 1C10 | 4·66 (0·13) | P < 10−6 | 4·51 (0·44) P < 10−6 |
| Avidin + biot GL117 | 2·2 (0·59) | NS | 2·1 (0·26) NS |
| Avidin + streptavidin + biot 1C10 | 1·45 (0·89) | NS | 4·53 (0·24) P < 10−6 |
| Avidin + unbiotinylated 1C10 | 1·59 (1·34) | NS | 4·53 (0·24) P < 10−6 |
Antibody responses of mice against avidin and rat IgG2a 14 days after a single immunization with anti-CD40 and avidin in the combinations shown. Immune responses where antigen is attached to a CD40 binding moiety are shown in bold. Significantly enhanced responses is indicated by P < 10−6.
Chemical conjugation of CD40 mAb to antigens
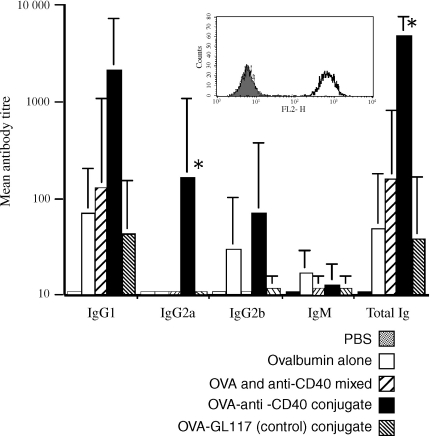
The avidin–biotin system would, of course, not be suitable for the production of real conjugate vaccines. We therefore assessed a number of other possible means of direct conjugation using OVA as a model antigen. The most successful method involved the cross-linking of maleimated OVA to the antibody using the cross linker SATA.21 Conjugates of OVA and 1C10 were shown to retain CD40 binding activity as assessed by flow cytometry on CD40 expressing cells (Fig. 3, insert) and induced a significantly enhanced antibody response against OVA, compared with OVA alone, the isotype control conjugate and the anti-CD40 mAb/OVA mixture (Fig. 3). This significant enhancement was observed in both the OVA specific total immunoglobulin and in the IgG2a subclass.
Figure 3.
In vitro binding and in vivo immune responses to anti-CD40–OVA conjugates. Enhanced antibody responses to 1C10–OVA conjugates as determined by ELISA. Histogram shows mean antibody titres for each isotype in mice immunized with; PBS, OVA alone OVA and anti-CD40 mixed, OVA–anti-CD40 conjugate, and OVA–GL117 (control) conjugate. Error bars indicate standard error and asterisks indicate statistical significance (P < 0·05) as determined by Student's t-test. Functional activity of CD40 mAb and presence of coupled OVA was determined by flow cytometric analysis on CD40 transfected fibroblasts (see insert). The filled histogram represents background levels of binding, the broken line represents binding by the isotype matched control antibody GL117-OVA conjugate and the thick solid line binding of the anti-CD40-OVA conjugate.
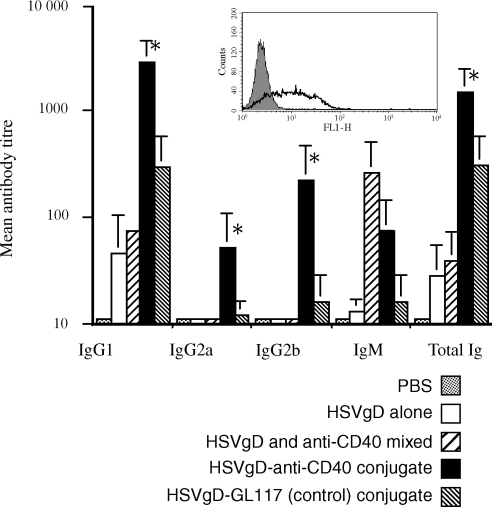
Of course none of the above antigens are of real relevance to vaccination. Finally therefore, we produced conjugates of 1C10 with recombinant glycoprotein D (gD) from herpes simplex virus, which is a prime candidate for non-living vaccines against HSV.22 Conjugates were produced as for OVA, by maleimating gD and using the hetero-bifunctional cross-linker, SATA. Conjugates were tested for presence of gD and retention of CD40 binding by flow cytometric analysis on CD40 expressing L929 cells (Fig. 4, insert). Conjugates using a 2 : 1 (molar : molar) ratio of gD to 1C10 gave the best binding and were used in immunization studies. A single i.p. immunization with 10 µg 1C10-gD conjugate induced strongly enhanced antibody responses against gD in comparison with the control GL117 conjugate, a mixture of 1C10 and gD, or gD alone (Fig. 4). As with the earlier responses to OVA, enhanced responses were apparent in the HSVgD specific total immunoglobulin and IgG2a. In addition, significant enhancement of the IgG1 and IgG2b subclasses were observed in responses to the HSVgD conjugate.
Figure 4.
In vitro binding and in vivo immune responses to anti-CD40–HSVgD conjugates. Enhanced antibody responses to 1C10–HSVgD conjugates as determined by ELISA. Histogram shows mean antibody titres for each isotype in mice immunized with; PBS, HSVgD alone, HSVgD and anti-CD40 mixed, HSVgD-anti-CD40 conjugate, and HSVgD-GL117 (control) conjugate. Error bars indicate standard error and asterisks indicate statistical significance (P < 0·05) as determined by Student's t-test. Functional activity of CD40 mAb and presence of coupled glycoprotein D was determined by flow cytometric analysis on CD40 transfected fibroblasts (see insert). The filled histogram represents background levels of binding, the broken line represents binding by the isotype matched control antibody GL117–HSVgD conjugate and the thick solid line binding of the anti-CD40–HSVgD conjugate.
Discussion
There is currently much interest in the identification of novel, potent immunological adjuvants. Much of this work has been performed empirically. A complex material or mixture of compounds is discovered to have adjuvant activity, and this is then further processed, purified or modified in an attempt to obtain a pure material which retains a high level of adjuvanticity while having as few reactogenic effects as possible. In most cases this has proven a difficult, if not impossible balance to achieve, and the mode of action of the adjuvant may then remain unknown or may be determined in retrospect. We and others have been attempting to take a rational approach to the design of immunological adjuvants; using knowledge of the workings of the immune system to tailor adjuvants with very specific properties and predicted modes of action which should produce low or no side effects. Such rationally designed adjuvants have included recombinant cytokines23,24 and fusions with complement component C3d.25
We have shown that CD40 antibodies in large, and thus impractical doses can mimic T-cell help for T-independent antigens when given in vivo with antigen.5,6 Others have shown similar effects of large doses of CD40 antibodies on enhancing responses to T dependent, protein and peptide antigens.11–14,16,26,27 The doses required for an adjuvant effect of CD40 mAb when mixed with antigen are highly reactogenic, inducing splenomegaly and polyclonal antibody production. We have shown here that this relatively weak adjuvant effect correlates very well with unacceptable side effects in dose–response analyses. In contrast, antibody responses against the CD40 mAb (rat IgG2a) itself were considerably more strongly enhanced than responses against a coadministered antigen (OVA) and this enhancement remained very strong at doses of CD40 mAb at which no polyclonal stimulation was seen. This adjuvant effect was found to be substantially more potent than the commonly used alum formulation. The enhanced response against rat IgG2a was not peculiar to this antigen, as a similar potent adjuvant effect of CD40 was seen on anti-avidin responses when biotinylated CD40 mAb was mixed with avidin prior to immunization. The results indicated a very strong enhancement of primary antibody responses against avidin of between 100- and 1000-fold. In addition there was a potent enhancement of anamnestic responses against a second injection of avidin alone, indicating a strong boosting of memory, which is a requirement for many vaccines (not shown). Removal of the link between CD40 mAb and avidin by preincubation of mAb with streptavidin; or by the use of non-biotinylated mAb; completely abrogated the adjuvant effect on avidin, but not on the anti-rat response. This indicates that the important factor is the linkage of CD40 binding domain with antigenic domains, and not the size of complexes produced, or the form of the antigen.
Chemical conjugation of CD40 mAb with another model antigen, OVA, and with the candidate HSV vaccine antigen, glycoprotein D, enhanced immunogenicity of these antigens to a similar extent, indicating that this system may be applied to a range of antigens, including real vaccine candidates.
The mode of action by which CD40 conjugates enhance immunogenecity of candidate antigens is unclear and this is currently under investigation. CD40 has diverse expression patterns and the relative importance of CD40 stimulation on various cells remains to be elucidated. Clear candidates for mediating these effects are dendritic cells and B cells. We know that the adjuvant effect is not mediated by simple targeting of B lymphocytes as similar conjugates prepared using the pan-B-cell marker CD45R (B220) do not display the adjuvant effect (data not shown). Similarly, the effect cannot be attributed to simple targeting of Fc receptor positive cells as the isotype-matched control antibody conjugate does not lead to enhanced immunogenecity (see Results). However, CD40 expression patterns are not the same as those of FcR and CD45R. Previous work by Carayanniotis et al. has shown that antibodies against major histocompatibility complex class II can be used to enhance antibody responses to antigen in a similar system.28 We are currently investigating the relative roles of dendritic cells and B cells in responses to CD40 mAb/antigen conjugates and preliminary data indicates the effect is, at least in part, mediated by direct action on B cells.
It is noteworthy that all of the strong responses shown here were induced by a single immunization, without any boost. Effective immunization with single doses is a major aim of the World Health Organization and other organizations. There are considerable social advantages and cost-savings to be made by restricting immunizations to one dose of each vaccine.29
There is a pressing need for well-defined, potent immunological adjuvants which CD40 ligation has the potential to answer.
Acknowledgments
T.B. was supported by Adjuvantix Ltd. A.M. was supported by a bursary awarded by the Department of Medical Microbiology, University of Sheffield, J.C. was supported by the Wellcome Trust (Project No. 061268).
References
- 1.Edelman R. Adjuvants for the future. In: Levine MM, Woodrow GC, Kaper JB, Cobon GS, editors. New Generation Vaccines. 2. New York: Marcel Dekker; 1997. [Google Scholar]
- 2.Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben Efraim S, Gupta CK. Adjuvants – a balance between toxicity and adjuvanticity. Vaccine. 1993;11:293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 3.Gordon J, Pound JD. Fortifying B cells with CD154: an engaging tale of many hues. Immunology. 2000;100:269–80. doi: 10.1046/j.1365-2567.2000.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol. 1997;9:330–7. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 6.Dullforce P, Sutton DC, Heath AW. Enhancement of T-cell independent immune responses in vivo by CD40 antibodies. Nat Med. 1998;4:88–91. doi: 10.1038/nm0198-088. [DOI] [PubMed] [Google Scholar]
- 7.Barr TA, Heath AW. Enhanced in vivo immune responses to bacterial lipopolysaccharide by exogenous CD40 stimulation. Infect Immun. 1999;67:3637–40. doi: 10.1128/iai.67.7.3637-3640.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vinuesa CG, MacLennan ICM, Holman M, Klaus GGB. Anti-CD40 antibody enhances responses to polysaccharide without mimicking T cell help. Eur J Immunol. 1999;29:3216–24. doi: 10.1002/(SICI)1521-4141(199910)29:10<3216::AID-IMMU3216>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Heath AW, Wu WW, Howard MC. Monoclonal antibodies to murine CD40 define two distinct functional epitopes. Eur J Immunol. 1994;24:1828–34. doi: 10.1002/eji.1830240816. [DOI] [PubMed] [Google Scholar]
- 10.Barr T, Heath A. Functional activity of anti-CD40 mAbs correlates to the position of binding relative to CD154. Immunology. 2001;102:39–43. doi: 10.1046/j.1365-2567.2001.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French RR, Chan HC, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nature Med. 1999;5:548–53. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 12.Sotomayor EM, Borrello I, Tubb E, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nature Med. 1999;5:780–7. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 13.Diehl L, Den Boer A, Schoenberger SP, et al. CD40 activation in vivo overcomes peptide-induced peripheral T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 14.Ninomiya A, Ogasawara K, Kajiro K, Takada A, Kida H. Intranasal administration of a synthetic peptide vaccine encapsulated in liposome together with an anti-CD40 antibody induces protective immunity against influenza A virus in mice. Vaccine. 2002;20:3123–9. doi: 10.1016/s0264-410x(02)00261-x. [DOI] [PubMed] [Google Scholar]
- 15.Erickson LD, Vogel LA, Cascalho M, Wong J, Wabl M, Durell BG, Noelle RJ. B cell immunopoiesis. visualizing the impact of CD40 engagement on the course of T cell-independent immune responses in an Ig transgenic system. Eur J Immunol. 2000;30:3121–31. doi: 10.1002/1521-4141(200011)30:11<3121::AID-IMMU3121>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferlin WG, von der Weid T, Cottrez F, Ferrick DA, Coffman RL, Howard MC. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. Eur J Immunol. 1998;28:525–31. doi: 10.1002/(SICI)1521-4141(199802)28:02<525::AID-IMMU525>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Hixon JA, Anver MR, Blazar BR, Panoskaltsis-Mortari A, Wiltrout RH, Murphy WJ. Administration of either anti-CD40 or interleukin-12 following lethal total body irradiation induces acute lethal toxicity affecting the gut. Biol Blood Marrow Transpl. 2002;8:316–25. [PubMed] [Google Scholar]
- 18.Hixon JA, Blazar BR, Anver MR, Wiltrout RH, Murphy WJ. Antibodies to CD40 induce a lethal cytokine cascade after syngeneic bone marrow transplantation. Biol Blood Marrow Transpl. 2001;7:136–43. doi: 10.1053/bbmt.2001.v7.pm11302547. [DOI] [PubMed] [Google Scholar]
- 19.Hixon J, Blazar BR, Murphy WJ. Acute toxicity of CD40 stimulation following syngeneic bone marrow transplantation. Role Interferon-Gamma Exp Hematol. 2000;28(Suppl. 1):75. [Google Scholar]
- 20.Hudson L, Hay FC. Practical Immunology. 3. Oxford, UK: Blackwell Scientific Publications; 1989. [Google Scholar]
- 21.Baiu DC, Prechi J, Tchorbanov A, et al. Modulation of the humoral immune response by antibody-mediated antigen targeting to complement receptors and Fc receptors. J Immunol. 1999;162:3125–30. [PubMed] [Google Scholar]
- 22.Corey L, Langenberg AG, Ashley R, et al. The Chiron HSV Vaccine Study Group. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: Two randomized controlled trials. JAMA. 1999;282:331–40. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 23.Heath AW. Cytokines as immunological adjuvants. In: Powell MF, Newman MJ, editors. Vaccine Design, the Subunit and Adjuvant Approach. New York: Plenum Press; 1995. pp. 645–58. [Google Scholar]
- 24.Dong P, Brunn C, Ho RJ. Cytokines as vaccine adjuvants. In: Powell MF, Newman MJ, editors. Vaccine Design, the Subunit and Adjuvant Approach. New York: Plenum Press; 1995. pp. 625–43. [Google Scholar]
- 25.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 26.Ito D, Ogasawara K, Iwabuchi K, Inuyama Y, Onoe K. Induction of CTL responses by simultaneous administration of liposomal peptide vaccine with anti-CD40 and anti-CTLA-4 mAb. J Immunol. 2000;164:1230–5. doi: 10.4049/jimmunol.164.3.1230. [DOI] [PubMed] [Google Scholar]
- 27.Ito D, Ogasawara K, Matsushita T, et al. Effective priming of cytotoxic T lymphocyte precursors by subcutaneous administration of peptide antigens in liposomes accompanied by anti-CD40 and anti-CTLA-4 antibodies. Immunobiology. 2000;201:527. doi: 10.1016/S0171-2985(00)80072-8. [DOI] [PubMed] [Google Scholar]
- 28.Carayanniotis G, Barber GH. Adjuvant-free IgG responses induced with antigen coupled to antibodies against class II MHC. Nature. 1987;327:59–61. doi: 10.1038/327059a0. [DOI] [PubMed] [Google Scholar]
- 29.Gerber MA. The Jordan Report 2000. Accelerated Development of Vaccines Division of Microbiology and Infectious Diseases. Bethesda, MD: National Institute of Allergy and Infectious Diseases National Institutes of Health; 2000. [Google Scholar]