Abstract
The intraepithelial lymphocyte (IEL) network of T-cell receptor γδ+ (Vγ5+) dendritic epidermal T cells (DETC) in murine skin down-regulates cutaneous inflammation, although the mechanism is unknown. Thymosin-β4 (Tβ4), identified by serial analysis of gene expression as a predominant transcript in gut IEL, encodes both a ubiquitous actin-binding protein (UTβ4) with demonstrated capacity to inhibit neutrophilic infiltration, and a splice-variant limited to lymphoid tissue (LTβ4) with unknown bioactivity. Freshly isolated Vγ5+ DETCs expressed both forms, while only LTβ4 was preferentially up-regulated after cellular activation in vitro. To compare the anti-inflammatory properties of LTβ4 and UTβ4 in the skin in vivo, the biological activities of synthesized polypeptides were assessed using three different strategies: neutrophil infiltration by footpad λ-carrageenan injection; irritant contact dermatitis to 12-O-tetradecanoylphorbol 13-acetate; and allergic contact dermatitis to 2,4-dinitrofluorobenzene. These studies clearly showed that the anti-inflammatory activities of LTβ4 were broader and most often stronger than those of UTβ4. Thus, the activation-responsive expression of the lymph-specific form of Tβ4 may be one mechanism by which DETC, and possibly other IELs, down-regulate local inflammation.
Introduction
Situated within numerous epithelia of rodents and many other vertebrates are intraepithelial lymphocytes (IELs) composed predominantly of T cells and frequently enriched in those expressing heterodimeric γδ T-cell receptors (TCR; reviewed in ref. 1). IELs would seem ideally located to maintain epithelial integrity in the face of environmental insults, and it was recently shown that γδ cell-deficient mice are highly susceptible to chemically induced squamous cell carcinomas that in vitro can be directly targeted for cytolysis by cutaneous IELs, specifically Vγ5+ dendritic epidermal T cells (DETC).2
While DETC can kill dysregulated epithelial cells, they have also been reported to synthesize fibroblast growth factors that may promote epidermal wound healing.3 Consistent with a role for cutaneous IELs in maintaining epidermal integrity, we recently demonstrated that the skin of FVB or non-obese diabetic (NOD) mice lacking DETC becomes inflamed and functionally compromised, following αβ T-cell-mediated responses to a variety of environmental challenges, including contact allergens and irritants.4 This potential of DETC to limit internally induced disruption of epidermal integrity is consistent with earlier observations that DETC can suppress cutaneous infiltration by systemic αβ T cells reactive to auto-antigens expressed in the skin.5 Nonetheless, the mechanisms of DETC down-regulation of cutaneous inflammation are unknown.
Pro-thymosin-β4 (pTβ4) was recently identified as a predominant transcript in a serial analysis of gene expression of gut IEL.6 The pTβ4 gene encodes a ubiquitous actin-binding protein that has additionally been shown to inhibit neutrophilic infiltration (reviewed in ref. 7). Specifically, pTβ4 sulphoxide was identified as the active agent in an immunosuppressive supernatant of glucocorticoid-stimulated monocytes and macrophages, whereupon chemically synthesized pTβ4 was shown to inhibit neutrophil chemotaxis in vitro, and to inhibit λ-carrageenan-induced oedema/inflammation after injection into the mouse footpad.8 In the latter assay, oxidized pTβ4 was active whereas the native form was not. pTβ4 is synthesized as a 44 amino acid polypeptide, from which the N-terminal methionine is apparently cleaved. Upon oxidation, any residual N-terminal methionine, together with a methionine residue a further six amino acids from the N terminus, would be available to form sulphoxides. In addition to its actin-binding capacity and its anti-inflammatory potential, pTβ4 has also been reported to promote the closure of ‘scratch wounds’ in endothelial cell8,9 and keratinocyte monolayers in vitro, as well as full-thickness cutaneous wounds in vivo.10
Whereas transcripts for the ubiquitous actin-binding pTβ4 polypeptide (UTβ4) represent > 95% of pTβ4 mRNA, highly expressed across a spectrum of tissues, the pTβ4 gene additionally encodes a longer splice variant, LTβ4, reportedly limited to lymphoid tissues, such as thymus and spleen, and characterized in two preB-cell lines.11,12 LTβ4 carries an additional 98-base pair (bp) exon at the 5′ end of the gene that harbours a start codon 18 bp from its 3′ end.13 Thus, translated LTβ4 is predicted to contain six or seven additional N-terminal amino acids [(Met)-Leu-Leu-Pro-Ala-Thr-Met], depending on whether or not the initiator methionine is cleaved from LTβ4, as is the case for UTβ4.13
The bioactivity of LTβ4 is currently unresolved. Given the reported biological activities of UTβ4, we hypothesized that activated DETC might be among the lymphoid cells that express LTβ4, and that such expression might contribute to the anti-inflammatory properties of DETC in vivo. To test this, the expression of UTβ4 and LTβ4 by Vγ5+ DETCs freshly isolated from murine skin was analysed. In parallel, chemically synthesized, methionated and unmethionated UTβ4 and LTβ4 polypeptides were compared for their biological activities in three different assays of cutaneous inflammation: neutrophil infiltration by footpad λ-carrageenan injection; allergic contact dermatitis (ACD) to 2,4-dinitrofluorobenzene (DNFB); and irritant dermatitis to 12-O-tetradecanoylphorbol 13-acetate (TPA).
Materials and methods
DETC line
A single cell suspension of epidermal cells was prepared from normal 3-month-old C57BL/6 mice via trypsin disaggregation and subsequent Histopaque-1083 (Sigma) density gradient centrifugation as described elsewhere.14 Interface epidermal cells were cultured at 2 × 105 cells/well in a 24-well plate in 2 ml of complete RPMI media (cRPMI: RPMI-1640 supplemented with 10% fetal bovine serum, 25 mm HEPES, 20 μm l-glutamine, 10 μm sodium pyruvate, 50 μm 2-mercaptoethanol, non-essential amino acids, penicillin/streptomycin) containing 2·0 μg/ml concanavalin A (Pharmacia) and 10 U/ml murine interleukin-2 (mIL-2). The resulting cell line was expanded by serial transfer of half of the well contents to a new well and supplementing each well with 1 ml of cRPMI containing 10 U/ml mIL-2 every 3–4 days. After 10 days, the line was stained with fluorescein isothiocyanate-conjugated monoclonal antibody (mAb) F536 (anti-Vγ5; BD PharMingen) and sterile-sorted on a FACS-Vantage™ (Becton Dickinson) using cellquest™ software. The sorted Vγ5+ cells (99% F536+) were expanded for an additional 2 weeks in cRPMI containing 10 U/ml mIL-2. After washing three times in cRPMI, aliquots of 2·5 × 106 cells in cRPMI were cultured for 6 hr in either an uncoated well (‘resting’ cells) or a well previously coated with anti-CD3 mAb 2C11 (BD PharMingen) (‘activated’ cells).
Preparation of cDNA from DETC
‘Resting’ and ‘activated’ DETC were harvested and total RNA was prepared from each according to the manufacturer's directions using silica gel-based spin columns (RNEasy, Qiagen). The cDNA was reverse transcribed from 100 ng of each RNA preparation using oligo-dT (Boehringer) and Omniscript reverse transcriptase (Qiagen) according to the manufacturers' guidelines.
Cycle–course reverse transcriptase–polymerase chain reaction (RT-PCR)
For LTβ4, UTβ4 and β-actin, RT-PCR was performed in 10 μl reactions in the presence of 0·25 μm of each of the forward and reverse primers, 250 μm of each of the dNTPs (Abgene, 2·5 μm MgCl2, and 0·3 U Taq (Qiagen). Each reaction was supplemented with 0·333 μl of [32P]dCTP (10 mCi/ml, 3000 Ci/mmol, Amersham) and products were amplified for 18, 20, 22, 24, 26, or 28 cycles as follows: 94° for 30 seconds, 61° for 30 seconds, 72° for 40 seconds. Following electrophoresis of the entire reaction on a 4% polyacrylamide gel, gels were dried on drier (Model 583, BioRad) and the bands were visualized by autoradiography on X-ray film (X-Omat AR, Kodak). The primers used were as follows:
β-actin forward: 5′-TCCCTGTATGCCTCTGGTCGTACCAC-3′
β-actin reverse: 5′-CAGGATCTTCATGAGGTAGTCTGTCAG-3′
LTβ4 forward: 5′-TGCCTGTCCAGCGCAGGCACTTG-3′
UTβ4 forward: 5′-CTTCTGAGCAGATCAGACTCTCC-3′
Tβ4 (common) reverse: 5′-CTCTGCTAGCCAGACCATCAGATG-3′
Tβ4 peptide synthesis and oxidation
The four peptides [UTβ4; methionated-UTβ4 (mUTβ4), LTβ4 and methionated-LTβ4 (mlTβ4)] were synthesized by CS Bio Co. (San Diego, CA), using peptide coupling chemistry, and purified (>98%) by reverse-phase high-performance liquid chromatography. The amino acid sequences were as follows: (m)UTβ4: N-(M)SDKPDMAEIEKFDKSKLKKTETQEKNPL-PSKETIEQEKQAGES-C; and (m)LTβ4: N-(M)LLPATMSDKPDMAEIEKFDKSKLKKTE-TQEKNPLPSKETIEQEKQAGES-C. In each case, the non-methionated forms lacked the N-terminal residue shown in parentheses. The peptides, verified by matrix-assisted laser desorption ionization electro-spray (MALDI-ES) in the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale, were oxidized by the addition of an equal volume of 30% hydrogen peroxide and distilled water for 5 min at room temperature, dried under vacuum centrifugation,8 and analysed by MALDI-mass spectrometry (MALDI-MS).
Induction of cutaneous inflammation
Experimental groups of eight to 10 female BALB/c and FVB mice, 8–10 weeks of age, were housed in temperature-controlled rooms and given food and water ad libitum. Observations and measurements were made by an investigator blinded to the experimental group.
λ-Carrageenan injection
After measuring the baseline thickness of the hind footpads with a spring-loaded engineer's micrometer, mice were injected subcutaneously in both hind paws with 340 μg of λ-carrageenan in 40 μl. Six hours, 24 hr and 48 hr after the injection of λ-carrageenan, footpads were re-measured and swelling was calculated by subtracting the baseline from the experimental measurements. For each mouse, increases in right and left footpad thickness were averaged. Thymosin peptides were injected as previously described:8 (i) intraperitoneally with 100 μl of 3·50 × 10−5 m peptide solution, 30 min before footpad injection; (ii) intradermally into the footpad with 40 μl of 8·75 × 10−5 m peptide solution and λ-carrageenan at time 0, and (iii) intraperitoneally with 100 μl of 3·50 × 10−5 m peptide solution, 6 hr after footpad injection. For each group, the mean increase in footpad thickness was calculated, and compared to other groups using a one-tailed Student's t-test. The experiment was repeated four times
Allergic contact dermatitis (ACD)
Mice were sensitized on day 0 by epicutaneous application to razor-shaved abdominal skin of 25 μl of 0·5% DNFB in a mixture of acetone : olive oil (4 : 1). Before the challenge with DNFB, mice were injected (i) intraperitoneally with 100 μl of 3·50 × 10−5 m thymosin peptide solution (30 min before DNFB) and (ii) intradermally with 40 μl of 8·75 × 10−5 m peptide solution in both ears (1 min prior to DNFB challenge). On day 5, after measuring baseline ear thickness with an engineer's micrometer, mice were challenged by applying 10 μl of 0·2% DNFB in acetone : olive oil to each side of each ear. Ears were re-measured 6 hr, 24 hr and 48 hr after challenge, and data were expressed as the response above baseline (i.e. ear thickness 24 hr after challenge minus ear thickness immediately prior to challenge) ± 1 standard error of the mean (SEM). For each mouse, increases in right and left ear thickness were averaged. The experiment was repeated twice.
Irritant contact dermatitis
After measuring the baseline ear thickness, naïve mice were injected intraperitoneally (i.p.) with either 100 μl of phosphate-buffered saline (PBS), or 100 μl of 3·50 × 10−5 m peptide solution [mTβ4-so, or mlTβ4-so (n = 10 per group)]. After 30 min, mice were injected intradermally with 40 μl of either PBS or 8·75 × 10−5 m solution of the appropriate peptide in both ears. One minute later, 40 nmol TPA (in 10 μl acetone) was applied to the anterior side of each ear. Six and 24 hr after the topical application of TPA, the ears were re-measured, and the increases in ear thickness between baseline and 6 hr and between baseline and 24 hr were calculated. For each mouse, increases in right and left ear thickness were averaged.
Statistics
Groups were compared for differences in the means using a one-tailed Student's t-test; P ≤ 0·05 was considered statistically significant.
Results
Expression of pTβ4 splice-variants by Vγ5+ DETC
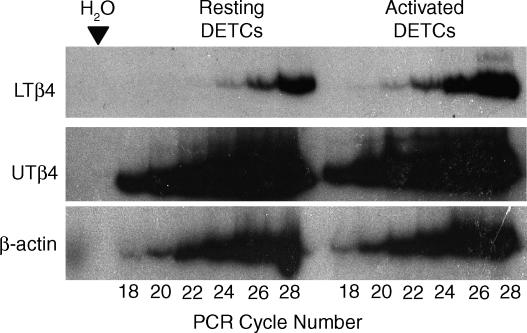
Our recent analysis of the gene expression profiles of intestinal TCRαβ+ and γδ+ IELs using serial analysis of gene expression revealed that Tβ4 was expressed at very high levels in both IEL subsets.6 Therefore, we wished to determine whether Tβ4 is also expressed by cutaneous γδ+ IELs (specifically by Vγ5+ DETC that comprise the vast majority of skin IELs in the mouse), and to determine whether TCR-mediated activation of DETC results in up-regulated expression of either form of Tβ4. Since no mAbs directed against murine Tβ4 are readily available, we examined the relative levels of Tβ4 splice variants by quantitative PCR reactions, carried out on cDNAs prepared from a short-term line of Vγ5+ DETC both before (‘resting’ DETC) and 6 hr after stimulation with anti-CD3 mAb (‘activated’ DETC). In the method employed, each cDNA is amplified in the linear range for 18, 20, 22, 24, 26 and 28 cycles in the presence of 32P-dCTP and primers for either UTβ4, LTβ4, or β-actin; Fig. 1 shows the reaction products visualized by autoradiography, and confirms that between cycles 18 and 26, the signal is increasing in a linear fashion relative to cycle number.
Figure 1.
Differential expression of LTβ4 in activated DETCs. Autoradiogram of gel electrophoresed showing 32P-labelled products, sampled at 18, 20, 22, 24, 26 and 28 cycles, covering a linear range pre-established by pilot experiments. The products were generated using primers specific for LTβ4, UTβ4 and β-actin, as indicated. As determined by densitometry, LTβ4 is expressed at higher levels in activated DETCs than in resting DETCs, while the expression of UTβ4 is similar in resting and activated DETCs.
The data show that both splice variants are expressed by resting and activated DETC. While the overall levels of UTβ4 expression clearly exceed the levels of LTβ4 in both resting and activated cells, UTβ4 expression levels are largely unaffected by TCR-mediated activation. Conversely, LTβ4 expression is clearly up-regulated, with a signal becoming clearly apparent at 22 cycles (with a very faint signal at 20 cycles) that was not apparent prior to activation. The LTβ4 signal from activated DETC is likewise stronger at all cycles thereafter (Fig. 1). Densitometry indicated that LTβ4 RNA expression was up-regulated more than four-fold by TCR-mediated activation.
Synthesis of thymosins for bioassay
Available evidence indicates that the major fraction of UTβ4 undergoes N-terminal methionine processing,8,12 but the known rules for aminopeptidase activity15 do not permit one to make the same assumption for LTβ4. Therefore, in our experiments to determine the biological activities of UTβ4 and LTβ4, we synthesized both N-terminal methionated and unmethionated forms of each polypeptide. The average molecular weights and mass errors, as determined by MALDI-ES, of the synthesized Tβ4 peptides were as follows: UTβ4: 4962·4 MW, 0·009%; mUTβ4: 5093·13 MW, 0·032%; LTβ4: 5592·52 MW, 0·026%; mlTβ4: 5722·92 MW, 0·012%. Because of previous findings demonstrating the anti-inflammatory effects of oxidized UTβ4, all peptides were oxidized prior to use, as described.8 Thymosins have no oxidizable amino acids (e.g. cysteine residues) other than methionine. To assess whether the extent of oxidation is consistent de facto with oxidation only of methionines, the polypeptides were exposed for 5 min to hydrogen peroxide. As seen in Table 1, the change in molecular weight in each case was consistent with oxidation of each of its methionine residues to methionine sulphoxide.
Table 1.
Oxidations of thymosin peptides
| Met resid. | Original MW | Oxidized MW | Diff. | oxidations | |
|---|---|---|---|---|---|
| UTβ4 | 1 | 4962·40 | 4980·48 | 18·08 | 1·13 |
| mUTβ4 | 2 | 5093·13 | 5124·92 | 31·79 | 1·99 |
| LTβ4 | 2 | 5592·52 | 5621·96 | 29·44 | 1·84 |
| mlTβ4 | 3 | 5717·68 | 5766·62 | 48·94 | 3·06 |
resid., residues; MW, molecular weight; Diff., difference.
Number of oxidations was calculated as the difference in MW divided by the MW of oxygen (15·9994).
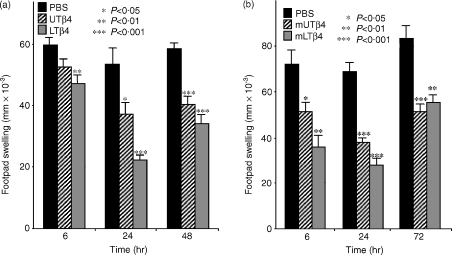
Activities of UTβ4 and LTβ4 in λ-carrageenan-induced inflammation
Oxidised UTβ4 and LTβ4 were compared to PBS vehicle alone for their capacity to suppress inflammation induced by intradermal injection with λ-carrageenan into the footpad. λ-Carrageenan injection results in an oedematous, neutrophil-rich inflammatory response that can be quantified as footpad swelling over baseline. As seen in Fig. 2(a), this study confirmed the reported anti-inflammatory activity of UTβ4;8 at 6 hr, 24 hr and 48 hr, UTβ4 suppressed footpad inflammation by 12% (P = 0·048), 30% (P = 0·012), and 31% (P < 0·0001), respectively, relative to PBS. Additionally, this study demonstrated that LTβ4 also has substantial anti-inflammatory activity. Suppression relative to PBS at 6 hr, 24 hr and 48 hr, respectively, was 21% (P = 0·0053), 58% (P < 0·0001), and 42% (P < 0·00001) (Fig. 2). Relative to UTβ4, LTβ4 was more effective at suppressing inflammation at every time-point, with the difference in footpad thickness at 24 hr being statistically significant (0·22 ± 0·03 vs. 0·37 ± 0·03, P = 0·0002).
Figure 2.
λ-Carrageenan induced inflammation, representative of neutrophil-mediated inflammation. Micrometer-measured increases in footpad swelling in mice injected with λ-carrageenan intradermally into the footpad, and treated with PBS; UTβ4 or its methionated derivative; LTβ4 or its methionated derivative. (a) At 24 hr, mice that received LTβ4 had a significantly reduced footpad swelling than mice receiving either PBS or UTβ4. (b) At both 6 hr and 24 hr mice that received mlTβ4 had a significantly reduced footpad swelling than mice receiving either PBS or mUTβ4.
Figure 2(b) shows that methionated UTβ4 also has anti-inflammatory activity; footpad thickness was suppressed by 29% (P = 0·011), 45% (P < 0·00001), and 38% (P = 0·00053) at 6 hr, 24 hr, and, 48 hr, respectively. Likewise, mlTβ4 was anti-inflammatory as footpad thickness was suppressed by 50% (P = 0·0011), 59% (P < 0·0001), and 34% (P = 0·0053) at 6 hr, 24 hr and 48 hr. At the 6 hr and 24 hr time-points, the anti-inflammatory activities of mlTβ4 were significantly greater than those of mUTβ4 (P = 0·035 and 0·017, respectively).
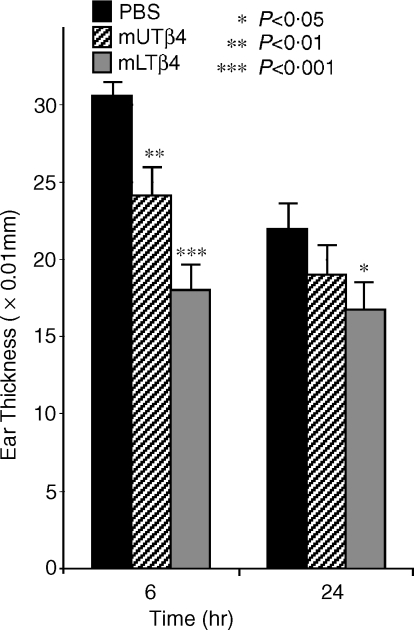
Activities of UTβ4 and LTβ4 in irritant contact dermatitis (ICD)
The methionated forms of Tβ4 were next compared for their ability to suppress ICD induced by topical application of TPA to ear skin. We have previously shown that TPA-induced ICD is exaggerated in TCRδ−/− mice.4 At 6 hr post-application of TPA, inflammation in mice treated with mUTβ4 was suppressed by 21% (P = 0·0027) relative to PBS-treated controls (Fig. 3). By 24 hr, suppression was 13% (although this was not statistically significant). By contrast, there was a statistically significant suppression of inflammation by mlTβ4 at both of these time-points: 41% (P < 0·000001) at 6 hr, and 24% (P = 0·023) at 24 hr, respectively. At 6 hr, the suppression of ear swelling in mlTβ4-treated mice was significantly greater than that in mUTβ4-treated mice (18·00 ± 1·58 vs. 24·10 ± 1·83, P = 0·011).
Figure 3.
TPA irritant contact dermatitis assay. Micrometer-measured increases in ear thickness in naive mice in which ICD was elicited by application of TPA, and that were treated with PBS; methionated UTβ4; or methionated LTβ4. The mlTβ4 suppressed TPA-induced ear swelling significantly more than PBS at 6 hr and 24 hr, and significantly more than mUTβ4 at 6 hr.
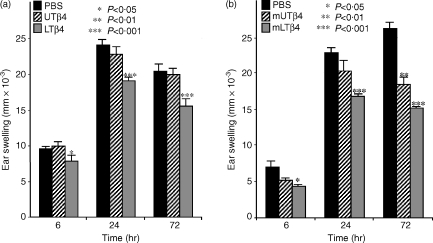
Activities of UTβ4 and LTβ4 in allergic contact dermatitis (ACD)
UTβ4 and LTβ4 were next compared to PBS for their ability to inhibit ACD inflammation induced by epicutaneous sensitization of abdominal skin with 25 μl 0·5% DNFB followed by epicutaneous challenge to ear skin with 20 μl 0·2% DNFB. As with ICD, ACD is highly exaggerated in TCRδ−/− mice, and can be restored to normal levels by selective reconstitution with DETC.4 At no time-point in this assay did UTβ4 show any significant anti-inflammatory activity: − 4% (NS), 5% (NS), and 2% (NS) at 6 hr, 24 hr, and, 72 hr, respectively (Fig. 4a). By contrast, LTβ4 suppressed the ACD ear swelling response by 17% (P = 0·030), 21% (P = 0·00031), and 24% (P = 0·00052) at 6 hr, 24 hr, and, 72 hr, respectively. The effects were more pronounced when the methionated forms were compared for their ability to suppress ACD (Fig. 4b). Relative to PBS, mLTβ4 suppressed ear swelling by: 37% (P = 0·042), 27% (P < 0·0001), and 42% (P < 0·00000001) at 6 hr, 24 hr and 72 hr, respectively, whereas mUTβ4 showed a significant effect only at 72 hr (29% lower than PBS, P = 0·0040). The suppression of ACD observed with mLTβ4 was significantly greater than that observed with mUTβ4 at 24 hr and 72 hr (P = 0·026 and 0·010, respectively). In summary, assessment of ACD-induced inflammation reveals that LTβ4 has significantly greater anti-inflammatory properties than does UTβ4, irrespective of whether or not the N-terminal methionine is retained.
Figure 4.
DNFB allergic contact dermatitis assay. ACD was elicited in mice previously sensitized on abdominal skin with DNFB by epicutaneous challenge of the ear skin with low-dose DNFB. Micrometer-measured increases in ear thickness mice treated with PBS; UTβ4 or its methionated derivative; or LTβ4 or its methionated derivative. (a) At 6 hr, 24 hr, and 72 hr after challenge, mice that received LTβ4 demonstrated a significant reduction in ear swelling response when compared to mice receiving PBS or UTβ4. There was no significant suppression of ACD by UTβ4. (b) mlTβ4 showed a significant reduction in ear swelling response at 24 hr and 72 hr relative to mice that received PBS or mUTβ4.
Discussion
The data presented in this paper confirm that oxidized UTβ4 has anti-inflammatory properties. However, the anti-inflammatory activities were selective for particular assays as shown by the lack of activity in response to ACD. By contrast, oxidized LTβ4 demonstrated significant anti-inflammatory activity in all three assays employed. Thus, LTβ4 has the potential to be a potent immunological effector produced by lymphocytes. The hitherto unrecognized and potent activity of LTβ4 in ACD is particularly provocative because this is a common, clinically relevant condition, primarily regulated by the lymphoid compartment.
In their original studies, Young and colleagues showed a dose-dependent anti-inflammatory effect of oxidized UTβ4 following λ-carrageenan injection into mouse footpads. Twenty micrograms of oxidized UTβ4 produced equivalent suppression to that induced by 0·5 mg/kg of dexamethasone.8 It was suggested that the anti-inflammatory activity of oxidized UTβ4 (UTβ4-so) serves as a safety-feedback signal, its oxidation reflecting an oxidizing environment that often correlates with host cell damage. Since oxidative damage may be reversed by methionine sulphoxide reductase, the oxidation and reduction of the methionine residue on UTβ4 might well act as a sensitive regulatory sentinel.8 Indeed, the feedback mechanism provoked by oxidation of methionine residue six (M6) in UTβ4 may itself promote methionine sulphoxide reductase and the repair of oxidative damage. In this regard, LTβ4, with an additional oxidizable methionine residue (Table 1), has a potentially greater capacity to reduce oxidative stress. Such a mechanism may account for the greater anti-inflammatory activity seen in our comparative analyses of LTβ4 versus UTβ4. Additional studies will be necessary to compare the unmethionated with the methionated forms of LTβ4 directly, for their relative levels of expression in vivo, for their biological efficacy ‘head-to-head’, and for the effects of selective single versus multiple methionine oxidations.
Over the past 5 years, several experimental systems have identified an anti-inflammatory role for IELs, whether or not they express TCRγδ or TCRαβ4,5,16,17 The work of Girardi et al.4 showing that the skin of DETC-deficient mice becomes spontaneously inflamed, indicates that the anti-inflammatory activities of DETC are a feature of normal physiology. While the mechanisms underlying this biological activity remain uncertain, the findings that Tβ4 is heavily expressed by TCRαβ+ and TCRγδ+ IELs in the gut,6 and that LTβ4 is substantially up-regulated in DETC following activation via the TCR (Fig. 1) identify LTβ4 as a candidate contributor to the local regulation of inflammation by IELs. Future studies should test this hypothesis, for example by assessing the biological activity of TCRγδ+ DETC that lack LTβ4.
UTβ4, originally isolated from a bovine thymus preparation called thymosin fraction 5, was thought to be a thymic hormone that initiated the early stages of T-cell differentiation.11 It showed significant activity in the induction of terminal deoxynucleotidyltransferase in murine thymocytes,18 and in the inhibition of macrophage migration.19 Since these original studies, UTβ4 was found to be broadly expressed and to have a highly conserved structure across human, rat, bovine and mouse species.12,20 This provoked the hypothesis that UTβ4 has a more general function than originally proposed, and it has since been shown to function intracellularly in the regulation of the equilibrium between globular and filamentous actin.21 While this activity might seem far removed from an extracellular anti-inflammatory activity, one might consider that a cell's response to stress might involve both a re-organization of its cytoskeleton, to permit cell migration, and a feedback regulation of oxidative damage and wound healing. Therefore, one wonders whether LTβ4 is a specialized form of thymosin that has evolved specifically through its capacity to mediate the latter activities. Interestingly, optimal binding of UTβ4 to actin requires the N-terminal residues.22 Since the N terminus is the region of the protein that differs in LTβ4, it is conceivable that LTβ4 does not bind actin as well. Clearly, the differential biological activities of either form of Tβ4 will require the selective mutation of UTβ4 and LTβ4.
Finally, we remain ignorant of how either form of Tβ4 mediates extracellular activities, because there is no documented mechanism for how UTβ4 or LTβ4 may be released from cells. Possibilities include their exocytosis in a complex with a second protein that harbours a conventional hydrophobic signal sequence, or release from cells dying as a result of oxidative damage. Alternatively, either form may share with proteins such as HIV TAT and Drosophila antennapedia, the intrinsic ability to shuttle across membranes.23,24
Acknowledgments
This work was supported by N.I.H. (R.E.T., M.G.); the Dermatology Foundation (M.G.); and the Wellcome Trust (A.C.H.); and utilized core laboratory facilities of the Yale Skin Diseases Research Core Center. All mice used in this study were bred and maintained in an AALAC-accredited facility and handled according to approved protocols. We thank Stephanie Donaldson for assistance with mouse breeding, and laboratory colleagues for discussions.
Abbreviations
- ACD
allergic contact dermatitis
- AMU
atomic mass units
- DETC
dendritic epidermal T cells
- F-MOC
9-fluorenylmethoxycarbonyl
- IEL
intraepithelial lymphocytes
- LTβ4
lymphoid spliced variant of thymosin β4
- MALDI-MS
matrix-assisted laser desorption ionization mass spectrometry
- MALDI-ES
matrix-assisted laser desorption ionization electrospray
- Tβ4
thymosin-β4
- TPA
12-O-tetradecanoylphorbol 13-acetate
- UTβ4
ubiquitous thymosin β4
References
- 1.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Girardi M, Oppenheim D, Lewis J, Filler R, Tigelaar RE, Hayday AC. The regulation of squamous cell carcinoma development by γδ T cells. Science. 2001;294:605–9. [Google Scholar]
- 3.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–9. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 4.Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, Tigelaar RE. Resident skin-specific gamma delta T cells provide local, non-redundant regulation of cutaneous inflammation. J Exp Med. 2002;195:855–67. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiohara T, Moriya N, Hayakawa J, Itohara S, Ishikawa H. Resistance to cutaneous graft-vs.-host disease is not induced in T cell receptor δ gene-mutant mice. J Exp Med. 1996;183:1483–9. doi: 10.1084/jem.183.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shires J, Theodoridis E, Hayday AC. Biological insights into murine TCR γδ(+) and TCR αβ(+) intrepithelial lymphocytes provided by the serial analysis of gene expression. Immunity. 2001;15:419–34. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 7.Huff T, Muller CS, Otto AM, Netzker R, Hannappel E. β Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–20. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 8.Young JD, Lawrence AJ, MacLean AG, Leung BP, McInnes IB, Canas B, Pappin DJC, Stevenson RD. Thymosin β4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nature Med. 1999;5:1424–7. doi: 10.1038/71002. [DOI] [PubMed] [Google Scholar]
- 9.Malinda KM, Goldstein AL, Kleinman HK. Thymosin beta4 stimulates directional migration of human umbilical vein endothelial cells. FASEB J. 1997;11:474–81. doi: 10.1096/fasebj.11.6.9194528. [DOI] [PubMed] [Google Scholar]
- 10.Malinda KM, Sidhu GS, Mani H, Banaudha K, Maheshwari RK, Goldstein AL, Kleinman HK. Thymosin beta4 accelerates wound healing. J Invest Dermatol. 1999;113:364–8. doi: 10.1046/j.1523-1747.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Marquez J, Dosil M, Segade F, Bustelo XR, Pichel JG, Dominguez F, Freire M. Thymosin-beta 4 gene. Preliminary characterization and expression in tissues, thymic cells, and lymphocytes. J Immunol. 1989;143:2740–4. [PubMed] [Google Scholar]
- 12.Rudin CM, Engler P, Storb U. Differential splicing of thymosin beta 4 mRNA. J Immunol. 1990;144:4857–62. [PubMed] [Google Scholar]
- 13.Li X, Zimmerman A, Copeland NG, Gilbert DJ, Jenkins NA, Yin HL. The mouse thymosin beta 4 gene: structure, promoter identification, and chromosome localization. Genomics. 1996;32:388–94. doi: 10.1006/geno.1996.0133. [DOI] [PubMed] [Google Scholar]
- 14.Nixon-Fulton JL, Bergstresser PR, Tigelaar RE. Thy-1+ epidermal cells proliferate in response to concanavalin A and interleukin 2. J Immunol. 1986;136:2776–86. [PubMed] [Google Scholar]
- 15.Black DL. Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell. 2000;103:367–70. doi: 10.1016/s0092-8674(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 16.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–9. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195:1491–7. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pazmino NH, Ihle JN, McEwan RN, Goldstein AL. Control of differentiation of thymocyte precursors in the bone marrow by thymic hormones. Cancer Treat Rep. 1978;62:1749. [PubMed] [Google Scholar]
- 19.Thurman GB, Low TLK, Rossio JL, Goldstein AL. Specific and nonspecific macrophage migration inhibition. In: Goldstein AL, Chirigos M, editors. Lymphokines and Thymic Factors Their Potential in Cancer Therapeutics. New York: Raven Press; 1981. p. 145. [Google Scholar]
- 20.Weber A, Nachmias VT, Pennise CR, Pring M, Safer D. Interaction of thymosin beta 4 with muscle and platelet actin: implications for actin sequestration in resting platelets. Biochemistry. 1992;31:6179–85. doi: 10.1021/bi00142a002. [DOI] [PubMed] [Google Scholar]
- 21.De La Cruz EM, Pollard TD. Structural biology: actin′up. Science. 2001;293:616–18. doi: 10.1126/science.1063558. [DOI] [PubMed] [Google Scholar]
- 22.Vancompernolle K, Goethals M, Huet C, Louvard D, Vandekerckhove J. G- to F-actin modulation by a single amino acid substitution in the actin binding site of actobindin and thymosin beta 4. EMBO J. 1992;11:4739–46. doi: 10.1002/j.1460-2075.1992.tb05579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the antennapedia homeodomain translocates thruogh biological membranes. J Biol Chem. 1994;269:10444–50. [PubMed] [Google Scholar]
- 24.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–17. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]