Abstract
Whereas functional heavy (H)-chain antibodies devoid of light (L)- chains account for about half of the circulating immunoglobulins in Camelidae, H-chain only antibodies (HCAbs) are not produced in other healthy mammals including rodents and humans. To test the feasibility of expressing single chain antibodies in the mouse, which on account of their small size and antigen-recognition properties would have a major impact on antibody engineering strategies, we constructed a rearranged dromedary H-chain gene encoding the immunoglobulin G2a (IgG2a) isotype with specificity for hen-egg lysozyme (HEL). This IgG2a H-chain gene was introduced into mouse myeloma cells not expressing endogenous immunoglobulin H- or L-chains. Unexpectedly the mouse cells processed and expressed the introduced H-chain as naturally occurring dromedary antibody. For this the first constant (C) region exon was proficiently removed from the recombinant H-chain transcript. This resulted in specific H-chain antibodies of the correct molecular weight (2 × 50 000 MW) secreted as disulfide-linked homodimers and displayed on the mouse cell surface as glycosyl-phosphatidyl-inositol-linked B-cell receptor. The results indicate that antibody expression and maturation without immunoglobulin L-chain is feasible and paves the way for the generation of transgenic single chain antibody repertoires.
Introduction
Heteromeric antibodies consisting of multiple units of paired H- and L-chains1 emerged early in vertebrate evolution and their presence is demonstrated in all jawed vertebrates studied to date.2 In addition to these conventional antibodies, sera of camelids (sub-order Tylopoda which includes camels, dromedaries and llamas) contain a major type of antibodies composed solely of paired H-chains (heavy-chain antibodies or HCAbs).3 H-chains of homodimeric HCAbs in camelids lack the first C domain (CH1) but harbour an intact variable (V) domain (VHH) encoded by different, clearly distinguishable, V genes.4 HCAbs are absent in other mammals except in pathological cases, known as heavy chain disease, where parts of the V domain and/or CH1 exon have been removed.5 Interestingly, H-chain antibodies are present in some primitive fish, e.g. the new antigen receptor (NAR) in nurse shark and the Cos5-antibodies in ratfish.6,7 However, evolutionary analysis showed that their genes emerged and evolved independently, whereas H-chain genes in the camelids evolved from pre-existing genes used for conventional heteromeric antibodies.8 The absence of CH1 in H-chain antibodies is a common feature, most likely essential for their cellular release, and although H-chain C region genes encode the first exon it is spliced out during mRNA maturation probably due to a point mutation at the canonical splicing donor site.9,10 It is possible that H-chain-only antibodies have been selected and maintained in the camelid species for their complementary function in recognizing unusual epitopes, such as clefts on the antigen surface that are normally less antigenic for conventional antibodies.11 So it could be argued that HCAbs in the camelids are maintained because they fulfil a complementary function in their humoral immune response. In the peripheral blood of all Camelus and Lama subgroups HCAbs contribute to the immune response. They undergo antigen-mediated selection and affinity maturation, and their V domains are subjected to extensive somatic hypermutation.12,13
In the conventional murine and human antibody system, the B cells displaying immunoglobulin M (IgM) on their surface are considered to initiate the process of antibody maturation. After class switching, B cells bearing other antibody isotypes (IgG, IgA and IgE) undergo further selection and affinity maturation. The H-chain gene of the HCAbs in camelids is also obtained after DNA rearrangements and specific VHH germline genes (located within the VH gene cluster) are assembled with commonly used D and JH minigenes to code for the VHH-domain. Genomic and cDNA analyses revealed five functional dromedary γ genes, three of which (γ2a, γ2c and γ3) are always used to form the HCAb isotypes and their CH1 exon is spliced out during mRNA maturation,9,10 whereas two separate genes, γ1a and γ1b are employed for the heterotetrameric IgG isotypes.12 In the camel, serum IgM devoid of L-chains has not been found and staining of camelid B-cells for IgG H-chain-only antibodies is not yet possible because of a lack of specific antibodies. In addition, only a very low yield of H-chain-only antibody transcripts (identified by their particular V genes) spliced to Cµ (VHHDJ-Cµ) could be identified from dromedary spleen (I. Legssyer and V.K. Nguyen, manuscript in preparation and 14). Indeed using structural analysis it was concluded that it is impossible for a VHH to pair with a normal VL because the VL-interacting side of the domain is reshaped by the hydrophilic VHH hallmark amino acids and the long complementarity-determining region (CDR)3, which folds over this region.15 These observations indicate that the IgM-stage of H-chain antibodies may be transient and that the conventional IgM pathway might be circumvented.
In the investigation we focused on two essential questions: (1) can mouse B-cells produce heterologous H-chain antibodies derived from camelids; and (2) can functional H-chain-only antibodies be displayed on the cell surface to allow selection? The in vitro analysis of single chain antibody production provides crucial information for the extensive task to produce single-chain antibody repertoires in other, perhaps highly recombinogenic, cells16 and transgenic mice.17
Materials and methods
Construction of a rearranged dromedary VHH-γ2a gene
The DNA manipulations were carried out using standard polymerase chain reaction (PCR) and DNA subcloning techniques.18 In intermediate cloning steps, recombinant plasmids (pBluescript) were propagated in E. coli DH5α cells, and DNA was prepared using a Qiagen-mini® prep kit (Qiagen, Westburg, Leusden, the Netherlands). The cloning strategy was as follows: the immunoglobulin promoter region derived from the germline VHH clone cvhhp1113 was spliced by overlap PCR with the FR1 region of the VHHDJ gene encoding the lysozyme-specific antibody cAb-Lys3.19 The region from the JH5 to the CH1 exon of the Cγ2a gene (clone rg122 obtained by PCR, V.K. Nguyen, unpublished) was added as a BstEII–EcoRI fragment, and the remaining exons of the Cγ2a gene in germline configuration (clone ch51666),9 including both transmembrane (TM) exons, were added on an EcoRI–SalI fragment (Fig. 1). The 11·7 kb H-chain construct, including the TM segments on a NotI–SalI fragment, and the 7·4 kb H-chain construct, without the TM region on a NotI–KpnI fragment, were subcloned into pSV2-Neo (#459 a kind gift from M. Neuberger, LMB, Cambridge, UK) at preintroduced NotI–SalI and NotI–KpnI cloning sites, respectively. The constructs were named VHH-γ2aTM and VHH-γ2a.
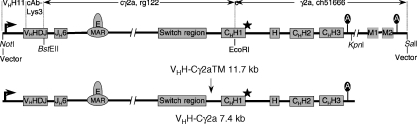
Figure 1.
Rearranged dromedary H-chain constructs, VHH-γ2a(TM), with and without TM exons. For the assembly germline fragments VHH11, rg122 and ch516669,13 were used as indicated. The V gene was replaced by a VHH, cAb-Lys3, with specificity for HEL.22 Genes and regulatory sequences are boxed. The arrow on the left denotes the immunoglobulin promoter starting with the octamer sequence. Restriction sites, NotI, BstEII, EcoRI, KpnI and SalI, are indicated and the position of the poly A sites (A) and the non-canonical splicing site of the dromedary γ2a gene at the 3′ end CH1 (⋆) are marked. MAR, matrix attachment region.
Myeloma transfection
The VHH-γ2aTM and VHH-γ2a constructs, and the pSV2-Neo cloning vector used as a control, were linearized by NotI digestion and introduced separately into NSO myeloma cells20 by two pulses using a BIORAD Gene Pulser set at 230 V and 500 µF. The transfected NSO cells were maintained at 37° and 5% CO2 in RPMI-1640 medium containing 10% fetal calf serum (FCS). After 24 hr of growth, G418 (Invitrogen, Paisley, UK) was added to a final concentration of 400 µg/ml. Several antibiotic resistant clones were chosen for each construct and grown to a density of 2–3 × 105 cells/ml for further studies.
Detection of hen-egg lysozyme (HEL)-specific antibodies by enzyme-linked immunosorbent assay (ELISA)
HEL (10 µg/ml in phosphate-buffered saline; PBS) was coated overnight at 4° onto 96-well-plates (Nunc-Maxisorb™, Life Technologies, Invitrogen, Merelbeke, Belgium). Residual protein-binding sites were blocked with PBS−1% casein for 2 hr at room temperature. Serial five-fold dilutions (100 µl) of cell-free supernatants from different clones of the transfected NSO cells were added to the wells and incubated at room temperature for 1 hr. The retention of recombinant HEL binding antibodies (anti-HEL-IgG2a) was detected with rabbit anti-camel IgG (1/1000 anti-dromedary rabbit serum R17, provided by T. Serrao, VUB, Brussels) and alkaline phosphatase-conjugated goat IgG directed against rabbit IgG (Sigma-Aldrich, Gillingham, UK) and p-nitrophenyl phosphate (pNPP) as substrate. Substrate hydrolysis was blocked after 15 min reaction with 20 µl 0·5 m ethylenediaminetetra-acetic acid and the plates were read at OD 405 nm in a microtitre plate reader (Elx808, Bio-Tek Instruments, Winooski, VT).
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blot analysis
HEL was coupled to CNBr-activated Sepharose (Amersham Pharmacia, Little Chalfont, UK; 3 mg HEL per ml resin) according to the instructions provided by the manufacturer. HEL-coupled Sepharose (50 µl wet gel) was incubated with 0·5 ml culture supernatant of the different NSO transfectants for 1 hr at room temperature to enrich the recombinant HCAbs. After repeated washes with PBS, the beads were resuspended in 100 µl SDS–sample buffer (with or without 0·5% dithiothreitol), boiled and 4 µl applied to a 10% polyacrylamide gel. Fractionated IgG1 antibodies and IgG2 HCAbs isolated from dromedary serum were applied in adjacent lanes as references. After electrophoresis, gels were stained with Coomassie Brilliant Blue to visualize the proteins. For Western blot analysis, proteins separated on SDS–PAGE were transferred onto nitrocellulose membranes (Amersham Pharmacia), using a Mini Trans-Blot Cell (Bio-Rad, Nazareth EKE, Belgium) and following standard protocols.21 The material applied on Coomassie-stained gels was in 10-fold excess compared to the material on gels analysed by Western blot. The recombinant IgG2a enriched by adsorption was detected with rabbit anti-dromedary serum as first antibody, alkaline phosphatase-conjugated goat anti-rabbit IgG as second antibody (the same reagents as in ELISA), and 5-bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium (BCIP/NBT, Sigma-Aldrich) as substrate. The molecular weight marker was the BenchMark™ prestained protein ladder (Gibco-BRL, Life Technologies, Invitrogen).
Preparation of mRNA and reverse transcriptase–(RT)–PCR
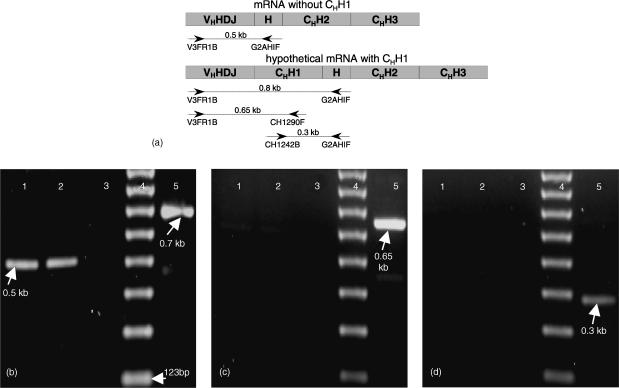
The QuickPrep micro mRNA purification kit (Amersham Pharmacia) was used for the preparation of mRNA from 107 transfected NSO cells and first strand cDNA was synthesized using the ‘Ready-to-Go’ kit (Amersham Pharmacia). PCR conditions using 0·5 µl cDNA were; 30 cycles of 45 s at 94°, 30 s at 52° and 45 s at 72°. Three combinations of specific oligonucleotides allowed the analysis of the CH1 splicing junctions (Fig. 4a): (1) V3FR1B (5′-GAGGTGCAGCTGGTGGCGTCTGGAGGAGG-3′), derived from the sequence of the VHH/VH-FR1 region and G2AHIF (5′-GGGACACGTGCATTCTGGTTCA-3′), a sequence annealing at the long hinge region of dromedary Cγ2a; (2) V3FR1B and CH1290F (5′-CTCTTGTCGACCTTGGTGCTGCTG-3′), representing a conserved sequence of the first constant exon of all camelid Cγ genes; and (3) CH1242B (5′-GCATCTAGACCGGMAAGACCTTCAYCT-3′), a consensus sequence of the first constant exon of all camelid Cγs, and the long hinge-specific G2AHIF primer. A lack of the CH1 exon sequence from the mRNA will result in a ∼0·5 kb PCR fragment employing oligonucleotides (1) whilst no amplification products are expected in PCR reactions using oligonucleotide combinations (2) and (3).
Figure 4.
Identification of H-chain transcription products. (a) Hypothetically, two mRNA products, with and without CH1, could be obtained from the introduced H-chain gene construct. Possible RT-PCR amplification products are indicated with the expected sizes in kb. RT-PCR products using (b) VH (V3FR1B) and hinge (G2AHIF) (c) VH (V3FR1B) and CHH1 (CH1290F) and (d) CHH1 (CH1242B) and hinge (G2AHIF) oligonucleotides. RNA was derived from NSO cells transfected with the following constructs: VHH-γ2a-TM (lane 1), VHH-γ2a (lane 2) and the parental pSV2 vector (lane 3). The 123 bp size ladder (the position of the 123 bp monomer is indicated in b) is shown in lane 4. Lane 5 displays the RT–PCR products of the cloned dromedary immunoglobulin-γ1 cDNA possessing the CH1 exon. This results in the expected CH1 containing fragments of 695 bp (0·7 kb), 646 bp (0·65 kb) and 334 bp (0·3 kb) using the oligonucleotides to perform the experiments shown in b, c and d, respectively.
Flow cytometry analysis
HEL and affinity-purified rabbit anti-camel IgG were biotinylated using biotin-X-sulpho-NHS (Calbiochem, Nottingham, UK) as described.11 Clones from the NSO transfectants (106 cells) were resuspended in 200 µl RPMI medium and incubated with 5 µg biotinylated HEL at 4° for 45 min. Unbound antigen was removed by two washings with PBS containing 0·002% Triton-X-100 and 0·01% sodium azide, and FITC-labelled streptavidin (PharMingen, San Diego, CA) was added to detect cells that captured HEL. After 30 min incubation the cells were washed and resuspended in 2 ml PBS. The cells were analysed with a FACSvantage flow cytometer (Becton Dickinson, Mountain View, CA). To analyse the H-chain anchorage in the membrane, the cells were incubated with 10 U/ml phosphatidyl-inositol-specific phospholipase C (PI-PLC; ICN, Cedarwood, Basingstoke, UK) prior to staining with biotinylated rabbit anti-camel IgG and fluorescein isothiocyanate (FITC)-labelled streptavidin. Untreated cells were used as control.
Results
The dromedary VHH-γ2a(TM) H-chain gene construct
A rearranged H-chain gene was constructed from VHH and Cγ2a gene segments used in a dromedary to express HCAbs. The promoter region including the octamer was obtained from a VHH gene in germline configuration (VHH11).13 The V-exon was replaced by a rearranged VHHDJH, an RT–PCR fragment (cAb-Lys3) obtained from a HEL-specific dromedary HCAb22 (Fig. 1). This intermediate construct was extended 3′ of JH by a BstEII–EcoRI fragment from JH5 to the naturally occurring EcoRI site in the CH1 exon of dromedary Cγ2a. The fragment contained the JH6 segment and sequences homologous to the human Eµ intron enhancer and matrix attachment region (denoted E and MAR (matrix attachment region) in Fig. 1). The Cγ2a gene was completed by addition of a genomic fragment from the EcoRI site in CH1 downstream to, and including, the putative TM exons and the poly A sites.9 The VHH-γ2aTM gene construct on a 11·7 kb NotI–SalI fragment was subcloned into the pSV2-Neo vector and the VHH-γ2a gene without the TM exons was inserted on a 7·4 kb NotI–KpnI fragment. The assembled VHH-γ2a(TM) H-chain constructs represent a rearranged dromedary H-chain gene in genomic configuration with appropriate 5′-and 3′ regions to allow expression.
Transfected NSO cells secrete HEL-specific polypeptides
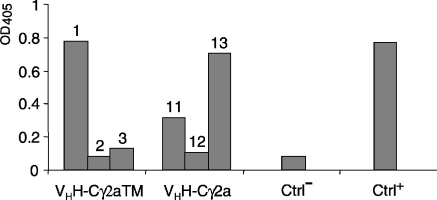
To investigate whether mouse B cells can process rearranged dromedary HCAb genes, NSO myeloma cells were transfected with the VHH-γ2aTM and the VHH-γ2a constructs and, as a control, the pSV2-Neo vector. To examine antibody production several different cell clones for each transfection were grown in Petri dishes to a density of 2–3 × 105 cells/ml. The culture supernatant was then analysed by ELISA on HEL-coated plates and antibody binding was identified using anti-antibody serum from rabbits immunized with camel H-chain-only IgG. A 1/5000 dilution of dromedary antiserum (D54) raised against HEL was used as positive control.11 Illustrated in Fig. 2 are the results for several supernatants from transfections with the VHH-γ2aTM and the VHH-γ2a construct, which show that clones 1 and 13 exhibited binding similar to that of the positive control serum. These clones were used for further characterization and it could be shown that in ELISA the binding of the antibodies produced by the mouse cells could be inhibited when free HEL at ≥ 0·5 µg/ml was added (data not shown). This showed that murine NSO cells transfected with the VHH-γ2aTM and VHH-γ2a constructs are capable of recognizing and utilizing the dromedary H-chain gene to secrete HEL-specific antibody products.
Figure 2.
In vitro production of HEL-specific dromedary immunoglobulin. Antibodies produced by NSO cells transfected with VHH-γ2a-TM (clones 1, 2 and 3), VHH-γ2 (clones 11, 12 and 13) or the parental vector (Ctrl−) were tested in ELISA using a 1/50 dilution of the culture supernatant from cultures from randomly selected individual colonies. For the assay plates were coated with HEL and bound antibody was detected with rabbit anticamel immunoglobulin. Dromedary anti-HEL immunoglobulin (diluted 1/5000) served as a positive control (Ctrl+).
Dromedary HCAbs are produced in mouse B-cells
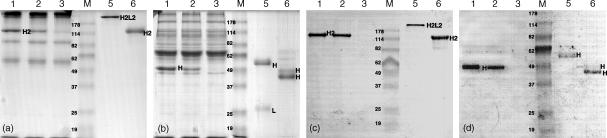
HEL-binding polypeptides secreted by the NSO cells were further characterized by SDS–PAGE and Western blot. For this culture supernatant from the transfectants was incubated with HEL-coupled to Sepharose which allowed the capture of specific proteins and the removal of interfering serum components before separation by SDS–PAGE (Fig. 3). In Coomassie stainings, despite extensive background, additional bands of ∼120 000 MW could be identified under non-reducing conditions (Fig. 3a, lanes 1 and 2) and of ∼50 000 MW under reducing conditions (Fig. 3b, lanes 1 and 2) which were not present in control lanes using supernatant from cells transfected with the parental pSV2-neo vector (Figs 3a, b, lane 3). This was confirmed in Western blots where unequivocal results showed only one band of ∼120 000 MW under non-reducing conditions (Fig. 3c, lanes 1 and 2) and one band of ∼50 000 MW under reducing conditions (Fig. 3d, lanes 1 and 2), with the controls in lane 3 showing no bands or background at all. From the apparent MW it was concluded that the 120 000 MW band corresponds to disulphide-linked H2 homodimers and the 50 000 MW band corresponds to single H-chain. However, HCAbs in H2 form secreted by the NSO cells appear to migrate slightly slower than the 114 000 MW protein marker and thus two H-chains do not fully reconstitute the MW of a homodimer. At present we have no explanation for the discrepancy in MW, which could indicate secondary, non-covalent, attachments. However, this seems to be a common feature also found in camel HCAbs as shown in lane 6.3
Figure 3.
MW analysis of recombinant dromedary immunoglobulin produced by NSO cells. SDS–PAGE of dromedary antibodies from culture supernatant adsorbed with HEL coupled to sepharose and either stained with Coomassie Brilliant Blue (a and b) or revealed by Western blot (c and d). Culture supernatant was applied from NSO cells transfected with VHH-γ2aTM (lane 1), VHH-γ2a (lane 2) and vector only (lane 3). As controls, isotypes representing conventional antibodies, IgG1 in H2L2 configuration (lane 5), and HCAbs, IgG2 in H2 form (lane 6), fractionated from dromedary serum were loaded in adjacent lanes. Proteins were separated under non-reducing (a and c) and under reducing conditions (b and d). The sizes of the marker bands (M) are indicated.
The appearance of a single band in Western blots (Fig. 3c, d, lanes 1 and 2) indicates that the secreted antigen-binding H-chains are homogeneous and that putative, differentially processed, H-chains are not released from the mouse cells. Furthermore, the apparent MW of the recombinant H-chain polypeptide should be indicative of the presence of the various domains. It is evident for the reduced samples (Fig. 3b, d) that the H-chains from the NSO-derived HCAbs (lanes 1 and 2) have a distinctly smaller MW than the H-chain of conventional antibodies isolated from dromedary serum (lane 5). From cDNA sequences it is known that the dromedary H-chain of conventional antibodies contains a VH, CH1, CH2 and CH3 domain (∼15 000 MW per domain). The difference in MW between the H-chain of the conventional antibodies and the H-chain of the recombinant HCAb suggests that one domain is missing as in the H-chain of naturally occurring HCAbs in camelids where the CH1 is absent. However at first sight, the apparent MW of the recombinant antibody secreted by the NSO transfectants is slightly larger than that of IgG2 HCAbs, fractionated from dromedary serum (in Fig. 3b, d compare lanes 1 and 2 with lane 6). A reason for this is that a VHH gene from a HEL-specific HCAb was used for the construct,22 which encodes a long CDR3 loop of 24 amino acids and is 7–8 amino acids longer than the average CDR3. This compares well with the IgG2 fraction isolated from dromedary serum which is heterogeneous with two discernible H-chain bands (Fig. 3b, d, lane 6). It is likely that the lower and most intense band corresponds to an abundant HCAb isotype with a hinge region of ∼15 amino acids (IgG2c) and the less intense band of lower electrophoretic mobility corresponds to the dromedary IgG2a isotype with a 35 amino acid long hinge,12 which is equivalent to the recombinant H-chain gene construct.
The signal in Western blots obtained for the HCAb transfectants is located in a single band which allows an estimation of the expression levels. The captured HCAbs obtained from 20 µl culture supernatant yield a signal about twice as strong as what has been obtained from 0·1 µg purified control HCAbs applied in lane 6 (Fig. 3d). This suggests that 1 ml supernatant from NSO cells transfected with the VHH-γ2aTM or the VHH-γ2a construct can produce ∼10 µg/ml HEL-specific HCAbs. Such secretion levels are similar to the levels obtained from endogenous antibodies expressed in myeloma and hybridoma cells.
Dromedary H-chain transcripts are correctly spliced in NSO cells
From PAGE and Western analysis it can be concluded that HEL-captured antibodies secreted by the NSO cells are in homogeneous form with a MW corresponding to that of naturally occurring IgG2a dromedary HCAbs. To investigate heterologous splice products of the VHH-γ2a H-chain in the mouse cells RT–PCR was employed using forward oligonucleotides for the V-gene or CH1 exon in combination with reverse oligos for CH1 or the hinge exon (Fig. 4). The results using mRNA from the transfectants showed a single 0·5 kb fragment, identifying the transgenic mRNA product without CH1 exon (Fig. 4b, lanes 1 and 2). No band of a predicted 0·8 kb was obtained which would have identified mRNA with retained CH1 exon sequence. The absence of the CH1 exon in the VHH-γ2a spliced products was further confirmed by RT–PCR using CH1-specific oligonucleotides, which failed to amplify any products (Fig. 4c, d, lanes 1 and 2). However, control amplification using mRNA from a rearranged conventional dromedary H-chain gene resulted in the expected bands (Fig. 4, lane 5). The results confirm that the removal of the CH1 exon from the primary VHH-γ2a(TM) transcript is proficiently carried out in the mouse cells.
HEL-specific HCAbs are displayed on mouse cells
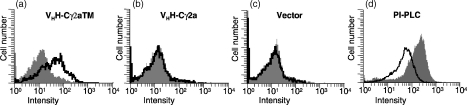
To investigate whether HCAbs can be presented on the surface of NSO cells transfected with the VHH-γ2aTM or the VHH-γ2a construct, cells were incubated with biotinylated HEL and, separately, biotinylated rabbit anti-camel IgG followed by incubation with FITC-labelled streptavidin (Fig. 5). Analysis of the staining by flow cytometry identified HEL-specific HCAbs on the surface of cells expressing the VHH-γ2aTM construct (Fig. 5a) but not on cells with introduced VHH-γ2a construct or control cells (Fig. 5b, c, respectively). The signal intensity implies that heterologous HCAbs are efficiently transported and anchored in the mouse cell membrane with adequate use of the transmembrane exons.
Figure 5.
Flow cytometry analysis of NSO transfectants. Cells transfected with VHH-γ2aTM (a), VHH-γ2a (b) and vector only (c) were incubated with biotinylated HEL and stained with FITC-labelled streptavidin (open profiles). The background signal obtained when biotinylated HEL was omitted is superimposed (shaded histograms). (d) Cells transfected with the VHH-γ2aTM construct were incubated with (open profile) or without (shaded histogram) PI-PLC prior to staining with biotinylated rabbit anti-dromedary immunoglobulin followed by FITC-labelled streptavidin. Each histogram represents ∼104 cells.
To identify the cell surface anchoring type of the HCAbs – which could be accomplished by association with the Igα/Igβ coreceptor or, alternatively, by glycosyl-phosphatidyl-inositol (GPI) anchoring23 or in the ‘naked’ form24– we incubated the surface immunoglobulin+ transfectants with phosphatidyl-inositol-specific phospholipase C (PI-PLC) which specifically releases GPI-linked proteins from the cell surface but has no effect on naked or Igα/Igβ-associated surface immunoglobulin.23 The results (Fig. 5d) show a net reduction in fluorescence intensity upon PI-PLC treatment of the cells, reflecting the removal of GPI-linked surface immunoglobulin. This demonstrates that dromedary HCAbs devoid of L-chain can be transported to and expressed on the cell surface of NSO mouse B cells as a GPI-linked receptor.
Discussion
The formation of HCAbs in camelids is decided by rearrangement of a VHH gene to commonly used D and JH segments25 and (switch?) recombination to a Cγ gene that permits the removal of CH1.9,12 The VHH genes are distinct from conventional VH genes; they accommodate changes in key residues normally in contact with the VL domain in the antigen-binding site of conventional antibodies.15 Nevertheless, the genomic organization of the VHH genes (i.e. promoter, leader signal, intron, V-exon, recombination signal sequence) is otherwise remarkably similar to that of the conventional VH counterparts.25 It has been reasoned that VHH genes have recently evolved from conventional VH genes after the emergence of the Tylopoda (>50 million years ago) which makes it likely that both types are accommodated in the V gene cluster of the H-chain locus.25 This is supported by the observation that both the VH and VHH gene segments appear to rearrange to the same D and JH gene segments to form either a conventional antibody or a HCAb.13 Furthermore, the emergence of HCAbs in camelids appears to depend on yet another gene adaptation, not found in other jawed vertebrates, occurring in a subset of their Cγ genes.9,10 It was proposed that in these genes a point mutation at the canonical splice signal sequence might cause the excision of the first C-region domain;9 a removal which may permit assembly and secretion of homodimeric H-chains.8 However, the exact processing and the extent of the CH1 removal from the camelids VHHDJH-γ primary transcript remains presently obscure. Nevertheless, the precise removal of the CH1-containing sequences from the RNA transcript of HCAb genes appears to be performed with equal efficiency in dromedary and mouse cells. Although this has not yet been tested directly in dromedary B cells, we have never identified any dromedary cDNA clone derived from the genomic HCAb-specific γ genes, which retained the CH1 exon. The removal of the CH1 exon may be essential to allow HCAb secretion. Previously, it was observed that hybridoma or myeloma cell lines harbouring immunoglobulin genes with deleted CH1 exon retain the ability to secrete homodimeric H-chains without associated L-chains.26,27 It has been established that the CH1 domain participates actively in the regulation of the assembly and secretion of conventional H2L2 antibodies. The nascent translated H-chain polypeptide associates non-covalently with the H-chain binding protein (BiP or grp78) via BiP association sites in CH1.28 The BiP/H-chain complex is retained in the endoplasmic reticulum by virtue of the KDEL sequence at the carboxy terminus of BiP29 and the H-chain is not secreted unless BiP is displaced by the L-chain.28,30,31 BiP is expressed constitutively in many cell lines29 and is also present in the NSO plasmacytoma line used in this study.26 Thus, the lack of CH1 is likely to permit unhindered transit of the H-chain polypeptide through the endoplasmic reticulum to allow secretion and appropriate surface deposition. Furthermore, the loss of BiP association may also prevent degradation of the H-chain. H-chains with the long hydrophobic transmembrane region anchor in the lipid bilayer, whilst the short hydrophilic C-terminal region of the secretory form H-chains ensures their release from the cell in the absence of associated BiP. However, exclusive H-chain-only antibody production in camelids may also involve interaction with species-specific cellular factors important for expression of HCAb genes, processing of transcripts, and the assembly of the translation products into functional antigen-binding entities. For these reasons, the utilization of a heterologous system to produce bona fide HCAbs from the rearranged dromedary immunoglobulin H-chain genes is far from obvious.
The rearranged H-chain expressed in NSO cells, which produced HEL-specific HCAbs in the dromedary, was constructed with no alteration that would favourably bias expression in mouse B-cells. Thus, secretion and surface expression of HEL-specific HCAbs in a heterologous system established that RNA processing, H-chain assembly and cellular transport utilize commonly recognized signals provided by the dromedary VHH-γ2a(TM) construct. Apparently, neither the VHH hallmark amino acids, nor the presence of a long CDR3 loop of 24 amino acids, caused folding problems. Furthermore, the non-canonical antigen binding loop structures tethered by an interloop disulphide bond were truly formed as in cAb-Lys3 HCAbs.19 Indeed the quite respectable protein expression levels suggest that the intrinsic alterations of the dromedary HCAbs are well recognized and dealt with by the mouse cells, and that dromedary-specific factors are either not essential or can be by-passed by the mouse transcription, translation and secretion machinery.
The expression of surface immunoglobulin on NSO cells came as a surprise because plasmacytomas are at a B-cell differentiation stage when intracellular immunoglobulin H-chain polypeptides with attached transmembrane regions are retained and degraded, and hence they normally express little or no membrane-anchored immunoglobulin.32,33 Nevertheless, membrane and secretory polypeptides can be produced34 and one introduced H-chain construct provides both, the secretory and transmembrane form, whilst the other, shorter construct, provides only the secretory form. As no membrane association is found in transfections with the construct encoding the secretory form only this implies that surface expression relies on using the transmembrane exons. The display of dromedary HCAbs on the surface of NSO cells suggests that heterologous H-chain polypeptides with TM regions can persist. Reasons for this could be that the modification of the H-chain, in particular the removal of CH1, provides an altered signal for H-chain recognition, transport through the cytoplasm and association with accessory molecules. For cells producing conventional antibodies, membrane-bound immunoglobulin (associated H- and L-chain) is cotransported onto the cell surface in a complex with the Igα/Igβ dimer35–37 or alternatively with a GPI linker independent of the presence or absence of Igα/Igβ23,38 or in a ‘naked’ form.24,39 The extensive reduction in fluorescence intensity of transgenic NSO cells stained with anti-camel immunoglobulin after PI-PLC treatment (Fig. 5d) demonstrates that antibodies lacking CH1 can be displayed on the cell surface via conventional GPI linkage.40,41 Thus, the dromedary HCAbs are functionally displayed on the NSO cell surface like a GPI-linked IgG receptor. The Igα chain, as indeed many late stage B-cell markers, is generally not expressed in immunoglobulin-secreting plasma cells38 and this also holds true for NSO cells which nevertheless express Igβ (M. Neuberger, personal communication). However, it is unclear if the lack of Igα initiates surface expression via GPI or if the dromedary VHH-γ2aTM polypeptide can associate with the coreceptor heterodimer as a membrane bound BCR. This has major implications on B-cell developmental processes as it raises the question whether conventional BCR expression of a dromedary HCAb without L-chain is possible. In this context it is interesting to note that staining of camel lymphocytes for immunoglobulin H- and L-chain on the cell surface has been attempted but did not unambiguously demonstrate surface IgG H-chain-only expression. A reason for this may be that the staining reagents raised against ruminant immunoglobulin fail if there is broad epitope diversity.42 Unfortunately there is no information about pre B-cell development in camelids or whether a µ H-chain without CH1 can associate with a surrogate L-chain to form the pre-BCR necessary to progress B-cell development. However, from gene targeting studies in the mouse it is clear that B-cell development without surrogate L-chain can progress,43 whilst B-cell development without L-chain is blocked after H-chain expression and maturation up to the immature B-cell stage.44
Presence of the BCR is essential to govern B-cell survival and differentiation.24 Thus, HCAb deposition on the cell surface is of key importance for the formation of the HCAb repertoire.45,46 Expression of the membrane form strongly suggests the presence of memory B cells for HCAbs in camelids. Such cells would undergo an antibody-maturation process leading to HCAbs with improved affinities for the antigen.13 The finding of extensive diversification of HCAbs13 but the failure to detect an IgM isotype without l-chains in camelids (12 and I. Legssyer, personal communication), has unexpected implications for HCAb ontogeny as it questions the involvement of µ+ B cells bearing conventional IgM as precursors of HCAb producing cells. For this reason it becomes important to re-assess the development of B cells expressing HCAbs. The successful expression of HCAbs by mouse cells, transfected with a dromedary VHH-γ2a construct, offers the possibility in future research to investigate the developmental regulation of camelid HCAbs in a transgenic mouse model and to perform in vivo and in vitro HCAb maturation experiments.
Acknowledgments
We thank R. Hamers and M. Neuberger for their valuable discussions. This work was supported by the Babraham Institute and by a Research Grant for Developmental Projects (UK partner) and by FWO-krediet aan navorsers, VLIR and VIB grants (Belgian partner).
Abbreviations
- BCR
B-cell receptor
- C
constant (region)
- CDR
complementarity determining region
- D
diversity (segment)
- E
enhancer
- FR
framework
- H
heavy (chain)
- HCAb
heavy chain antibody
- HEL
hen-egg lysozyme
- J
joining (segment)
- L
light (chain)
- NAR
new antigen receptor
- PI-PLC
phosphatidyl-inositol-specific phospholipase C
- pNPP
p-nitrophenyl phosphate
- TM
transmembrane
- V
variable (region)
References
- 1.Padlan EA. Anatomy of the antibody molecule. Mol Immunol. 1994;31:169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 2.Litman GW, Anderson MK, Rast JP. Evolution of antigen binding receptors. Annu Rev Immunol. 1999;17:109–47. doi: 10.1146/annurev.immunol.17.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–8. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 4.Muyldermans S, Atarhouch T, Saldanha J, Barbosa JA, Hamers R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994;7:1129–35. doi: 10.1093/protein/7.9.1129. [DOI] [PubMed] [Google Scholar]
- 5.Alexander A, Steinmetz M, Barritault D, Frangione B, Franklin EC, Hood L, Buxbaum JN. γ Heavy chain disease in man: cDNA sequence supports partial gene deletion model. Proc Natl Acad Sci U S A. 1982;79:3260–4. doi: 10.1073/pnas.79.10.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg AS, Avila D, Hughes M, Hughes A, McKinney EC, Flajnik MF. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374:168–73. doi: 10.1038/374168a0. [DOI] [PubMed] [Google Scholar]
- 7.Rast JP, Amemiya CT, Litman RT, Strong SJ, Litman GW. Distinct patterns of IgH structure and organization in a divergent lineage of chrondrichthyan fishes. Immunogenetics. 1998;47:234–45. doi: 10.1007/s002510050353. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen VK, Su C, Muyldermans S, van der Loo W. Heavy-chain antibodies in Camelidae; a case of evolutionary innovation. Immunogenetics. 2002;54:39–47. doi: 10.1007/s00251-002-0433-0. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen VK, Hamers R, Wyns L, Muyldermans S. Loss of splice consensus signal is responsible for the removal of the entire C (H) 1 domain of the functional camel IGG2A heavy-chain antibodies. Mol Immunol. 1999;36:515–24. doi: 10.1016/s0161-5890(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 10.Woolven BP, Frenken LG, van der Logt P, Nicholls PJ. The structure of the llama heavy chain constant genes reveals a mechanism for heavy-chain antibody formation. Immunogenetics. 1999;50:98–101. doi: 10.1007/s002510050694. [DOI] [PubMed] [Google Scholar]
- 11.Lauwereys M, Ghahroudi MA, Desmyter A, Kinne J, Holzer W, De Genst E, Wyns L, Muyldermans S. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998;17:3512–20. doi: 10.1093/emboj/17.13.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen VK, Desmyter A, Muyldermans S. Functional Heavy-chain Antibodies in Camelidae. Adv Immunol. 2001;79:261–96. doi: 10.1016/s0065-2776(01)79006-2. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen VK, Hamers R, Wyns L, Muyldermans S. Camel heavy-chain antibodies: diverse germline V (H) H and specific mechanisms enlarge the antigen-binding repertoire. EMBO J. 2000;19:921–30. doi: 10.1093/emboj/19.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen VK. Generation of heavy chain antibodies in Camelids PhD Thesis. Belgium: Free University of Brussels; 2002. [Google Scholar]
- 15.Muyldermans S, Cambillau C, Wyns L. Recognition of antigens by single-domain antibody fragments: the superfluous luxury of paired domains. Trends Biochem Sci. 2001;26:230–5. doi: 10.1016/s0968-0004(01)01790-x. [DOI] [PubMed] [Google Scholar]
- 16.Sonoda E, Morrison C, Yamashita YM, Takata M, Takeda S. Reverse genetic studies of homologous DNA recombination using the chicken B-lymphocyte line, DT40. Philos Trans R Soc Lond B Biol Sci. 2001;356:111–7. doi: 10.1098/rstb.2000.0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson IC, Zou X, Popov AV, et al. Antibody repertoires of four and five feature translocus mice carrying human immunoglobulin heavy chain and kappa and lambda light chain YACs. J Immunol. 1999;163:6898–906. [PubMed] [Google Scholar]
- 18.Sambrook J, Russell DW. Molecular Cloning. 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 19.Desmyter A, Transue TR, Ghahroudi MA, Thi MH, Poortmans F, Hamers R, Muyldermans S, Wyns L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat Struct Biol. 1996;3:803–11. doi: 10.1038/nsb0996-803. [DOI] [PubMed] [Google Scholar]
- 20.Galfre G, Milstein C. Chemical typing of human kappa light chain subgroups expressed by human hybrid myelomas. Immunology. 1982;45:125–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Burnette WN. ‘Western blotting’. electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 22.Ghahroudi MA, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997;414:521–6. doi: 10.1016/s0014-5793(97)01062-4. [DOI] [PubMed] [Google Scholar]
- 23.Wienands J, Reth M. Glycosyl-phosphatidylinositol linkage as a mechanism for cell-surface expression of immunoglobulin D. Nature. 1992;356:246–8. doi: 10.1038/356246a0. [DOI] [PubMed] [Google Scholar]
- 24.Williams GT, Dariavach P, Venkitaraman AR, Gilmore DJ, Neuberger MS. Membrane immunoglobulin without sheath or anchor. Mol Immunol. 1993;30:1427–32. doi: 10.1016/0161-5890(93)90104-j. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen VK, Muyldermans S, Hamers R. The specific variable domain of camel heavy-chain antibodies is encoded in the germline. J Mol Biol. 1998;275:413–8. doi: 10.1006/jmbi.1997.1477. [DOI] [PubMed] [Google Scholar]
- 26.Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, Williams G, Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990;60:781–90. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- 27.Morrison SL. Murine heavy chain disease. Eur J Immunol. 1978;8:194–9. doi: 10.1002/eji.1830080311. [DOI] [PubMed] [Google Scholar]
- 28.Haas IG, Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983;306:387–9. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- 29.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 30.Hendershot L, Bole D, Köhler G, Kearney JF. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987;104:761–7. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendershot LM. Immunoglobulin heavy chain and binding protein complexes are dissociated in vivo by light chain addition. J Cell Biol. 1990;111:829–37. doi: 10.1083/jcb.111.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sitia R, Alberini C, Biassoni R, Rubartelli A, DeAmbrosis S, Vismara D. The control of membrane and secreted heavy chain biosynthesis varies in different immunoglobulin isotypes produced by a monoclonal B cell lymphoma. Mol Immunol. 1988;25:189–97. doi: 10.1016/0161-5890(88)90067-3. [DOI] [PubMed] [Google Scholar]
- 33.Hombach J, Sablitzky F, Rajewsky K, Reth M. Transfected plasmacytoma cells do not transport the membrane form of IgM to the cell surface. J Exp Med. 1988;167:652–7. doi: 10.1084/jem.167.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitia R, Neuberger MS, Milstein C. Regulation of membrane IgM expression in secretory B cells: translational and post-translational events. EMBO J. 1987;6:3969–77. doi: 10.1002/j.1460-2075.1987.tb02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hombach J, Lottspeich F, Reth M. Identification of the genes encoding the IgM-alpha and Ig-beta components of the IgM antigen receptor complex by amino-terminal sequencing. Eur J Immunol. 1990;20:2795–9. doi: 10.1002/eji.1830201239. [DOI] [PubMed] [Google Scholar]
- 36.Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990;343:760–2. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- 37.Schamel WW, Reth M. Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity. 2000;13:5–14. doi: 10.1016/s1074-7613(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 38.Sakaguchi N, Kashiwamura S, Kimoto M, Thalmann P, Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988;7:3457–64. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuberger MS, Patel KJ, Dariavach P, Nelms K, Peaker CJ, Williams GT. The mouse B-cell antigen receptor: definition and assembly of the core receptor of the five immunoglobulin isotypes. Immunol Rev. 1993;132:147–61. doi: 10.1111/j.1600-065x.1993.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 40.Iglesias A, Nichogiannopoulou A, Williams GS, Flaswinkel H, Köhler G. Early B cell development requires µ signaling. Eur J Immunol. 1993;23:2622–30. doi: 10.1002/eji.1830231036. [DOI] [PubMed] [Google Scholar]
- 41.Cherayil BJ, MacDonald K, Waneck GL, Pillai S. Surface transport and internalization of the membrane IgM H chain in the absence of the Mb-1 and B29 proteins. J Immunol. 1993;151:11–9. [PubMed] [Google Scholar]
- 42.Ungar-Waron H, Yagil R, Brenner J, Paz R, Partosh N, Van Creveld C, Lubashevsky E, Trainin Z. Reactions of peripheral blood mononuclear cells (PBMC) of camels with monoclonal antibodies against ruminant leukocytes. Comp Immunol Microbiol Infect Dis. 2003;26:137–43. doi: 10.1016/s0147-9571(02)00037-1. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu T, Mundt C, Licence S, Melchers F, Martensson IL. VpreB1/VpreB2/lambda 5 triple-deficient mice show impaired B cell development but functional allelic exclusion of the IgH locus. J Immunol. 2002;168:6286–93. doi: 10.4049/jimmunol.168.12.6286. [DOI] [PubMed] [Google Scholar]
- 44.Zou X, Piper TA, Smith JA, Allen ND, Xian J, Brüggemann M. Block in development at the pre-B-II to immature B cell stage in mice without Igκ and Igλ light chain. J Immunol. 2003;170:1354–61. doi: 10.4049/jimmunol.170.3.1354. [DOI] [PubMed] [Google Scholar]
- 45.Torres RM, Flaswinkel H, Reth M, Rajewsky K. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science. 1996;272:1804–8. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- 46.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–83. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]