Abstract
Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis in infants under 6 months of age. Since an RSV infection does not necessarily prevent a reinfection, we asked whether RSV might subvert an effective immune response by interfering with the function of dendritic cells (DCs). Immature DCs cultured from cord blood stem cells and infected with RSV reduced the rate of interferon-γ (IFN-γ) production in co-cultured autologous naïve T cells stimulated with the superantigen TSST-1. Maturation of DCs in response to poly(IC) but not to CD40 ligand did overcome the inhibitory effect of RSV. Further experiments demonstrated that induction of apoptosis, a selective increase in CD86 expression and lack of release of pro-inflammatory cytokines were associated with inhibition of IFN-γ generation. In addition, RSV replication seemed to be essential for modulation of IFN-γ production because a virus preparation inactivated by UV irradiation had no effect. Hence, one reason for multiple reinfections by RSV might be the subversion of antiviral immune responses by interference of RSV with DC function.
Introduction
Respiratory syncytial virus (RSV) is best known for its tendency to cause bronchiolitis in infants,1,2 but it can infect all age-groups, causing upper and lower respiratory tract infections ranging in severity from subclinical infections to pneumonia and death.3–6 Repeated infections are common, and previous infection does not prevent subsequent infections, even in sequential years. Adults with previous natural RSV infection and repeatedly challenged with RSV of the same strain group developed a consistent rate of reinfection.7 Thus, RSV, like other pathogenic viruses,8,9 has most probably developed mechanisms to subvert the antiviral strategies of the host. The interaction of dendritic cells (DCs) and naïve T cells has now been recognized as playing a central role in the induction of the immune response. Antigen is engulfed and processed by immature DCs, which are found at body surfaces and in the interstitial spaces of most tissues.10 Upon contact with antigen, DCs mature and migrate from the peripheral tissues into the draining lymph nodes and acquire the capacity to trigger naïve T cells and drive polarized T helper cell type 1 responses. The capacity to trigger T-cell responses depends on the number of DCs and hence is modulated by apoptosis of DCs. Furthermore, it depends on the expression of surface molecules and cytokine secretion by DCs. Since viruses were shown to impair DC function at multiple levels11,12 we asked whether RSV can efficiently enhance apoptosis of DCs, whether RSV affects the expression of surface molecules and cytokine production of DCs, and finally whether RSV interferes with the capacity of DCs to induce the production of interferon-γ (IFN-γ) in naïve T cells.
Materials and methods
Generation of DCs from cord blood
Heparinized cord blood was obtained from newborns at the Department of Obstetrics and Gynecology (Augusta or St Elisabeth Hospital). Parents gave informed written consent before the blood was studied. The study was approved by the ethics committee of the medical faculty. Blood was drawn by puncture of the umbilical vein of the placenta after maternal blood had been wiped off. The blood for further cellular analysis was placed in a 50-ml sterile Falcon tube (Falcon, Heidelberg, Germany) containing 10 ml of Hanks' balances salt solution (HBSS) with 100 IU penicillin, 100 μg/ml streptomycin, 2·5 μg/ml amphotericin B and 50 IU/ml of heparin (Biochrom, Berlin, Germany). Only samples less than 60-min-old were processed. Mononuclear cells were isolated by Ficoll density centrifugation. Haematopoietic stem cells were isolated from mononuclear fractions through positive selection, using anti-CD34 coated microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and MiniMACS separation columns (Miltenyi Biotec). In all experiments, the isolated cells were 95–99% CD34 positive. Cells from the effluent of the MiniMACS columns contained all other mononuclear cells including naïve T cells. These cells were cryopreseved in freezing buffer [10% dimethyl sulphoxide (Sigma, Deisenhofen, Germany), 45% RPMI-1640 (Biochrom), 45% fetal calf serum (Biochrom)] at −196°. Haematopoietic progenitor cells were seeded at a density of 2 × 104 cells/ml in 24-well, flat-bottom plates (Nunc, Wiesbaden, Germany). Culture medium consisted of endotoxin-free RPMI-1640 (Biochrom, Berlin, Germany) containing 10% heat inactivated fetal bovine serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mm glutamine and 1 mm sodium pyruvate (all from Biochrom). Cultures were supplemented with recombinant human granulocyte–macrophage colony-stimulating factor (100 ng/ml, Novartis, Nürnberg, Germany) recombinant human tumour necrosis factor-α (2·5 ng/ml; Peprotech, Rocky Hill, NJ) and recombinant human stem cell factor (100 ng/ml; Peprotech). Cells were cultured at 37° in a humidified atmosphere in the presence of 5% CO2 for 12 days and split when necessary. DCs were purified using CD1a microbeads (Miltenyi Biotec) and an AutoMACS separation device (Miltenyi). These cells are referred to as immature DCs.13 Immature DCs were challenged with poly(IC) (20 mg/ml, Sigma), or with recombinant CD40 ligand (CD40L) (0·1 μg/ml) trimerized by an enhancer molecule (1 μg/ml, Alexis Biochemicals, Grünberg Germany) for 24 hr.
Preparation of RSV and infection with RSV
A viral stock (RSV Long strain) was prepared by infection of HEp2 cells with a low input multiplicity of infection (MOI) as described previously.14 When infection was advanced, cell supernatants were harvested and then disrupted by ultrasonication. The debris was pelleted by low-speed centrifugation. Supernatants containing viruses were frozen at − 80°. Infectivity titres of stock viruses were determined by inoculation of serial dilutions into HEp-2 cells. Virus growth was detected by observation of typical cytopathic effects followed by immunocytochemical staining of infected cell monolayers. Cell lines and virus preparations were tested for mycoplasma by polymerase chain reaction with a mycoplasma detection kit as described in the manufacturer's manual (American Type Culture Collection, Manassas, VA). To inactivate the virus an aliquot of the suspension was irradiated with ultraviolet light (254 nm, 265 μW/cm2) for 15 min.
Fluorescence staining
Cultured cells were washed twice, the concentration was adjusted to 2 × 105 cells/ml in HBSS and the cells were then incubated with the appropriately diluted antibodies against surface proteins for 20 min at 4°. Expression of co-stimulatory molecules was detected by appropriate fluorescein isothiocycanate (FITC)-labelled antibodies specific for HLA-DR, CD86, CD83, respectively (all from Pharmingen, San Diego, CA).
To determine the rate of RSV infection permeabilization of the cell membrane was achieved by resuspension in 100 μl HBSS containing 0·1% saponin and 0·01 m HEPES buffer (saponin buffer). The permeabilized cells were incubated with biotinylated goat-anti-RSV (Biodesign, Saco, ME) for 20 min at 4°, washed with saponin buffer and subsequently incubated with FITC-labelled streptavidin for 20 min at 4° in the dark. After washing with saponin buffer the cells were resuspended in 200 μl HBSS for flow cytometric analysis.
Viability of the cells after RSV infection was evaluated by staining cells with FITC-labelled annexin V (Pharmingen) and propidium iodide (PI). The samples were analysed on a FACScan® using cell quest software (Becton Dickinson).
Stimulation of naïve T cells
Naïve autologous cord blood CD4+ CD45RA+ T cells were obtained from the effluent of the stem cell separations by negative sorting with anti-HLA-DR, anti-glycophorin, anti-CD8, anti-CD56, anti-CD45RO magnetic beads (Miltenyi) using an AutoMACS separation device (Miltenyi). For the superantigen stimulation DCs were further incubated for 1 hr at 37° with mitomycin C (50 μg/ml, Sigma) washed and cultured at different numbers in 96-well plates (Nunc, Wiesbaden, Germany) with 10 ng/ml TSST-1 (Toxin Technology, Sarasota, FL) and 5 × 104 CD4+ CD45RA+ T cells/well. Plates were tested for [3H]thymidine incorporation on day 5.
In parallel cultures 5 × 103 DCs and 5 × 104 naïve T cells/well were analysed for cytokine production by intracellular staining. After 3 days of incubation, cytokine secretion was inhibited by brefeldin 1 μm (Sigma Deisenhofen, Germany) for 16 hr. The cytokines were measured as described before.15 Cells were washed in HBSS (Biochrom, Berlin, Germany) and then fixed in ice-cold HBSS containing 4% paraformaldehyde (Riedel-de Haën, Seelze, Germany) for 10 min. Cells were washed twice. Permeabilization of the cell membrane was achieved by resuspension in 100 μl HBSS containing 0·1% saponin (Sigma Deisenhofen, Germany) and 0·01 m HEPES buffer (saponin buffer). The permeabilized cells were incubated with FITC-labelled antibodies against T-cell receptor (TCR) -Vβ2 chain (Beckman Coulter, Unterschleissheim, Germany), biotinylated anti-IFN-γ and phycoerythrin-labelled anti-interleukin-4 (IL-4) (Pharmingen, Heidelberg, Germany) for 20 min at 4°, washed and then incubated with streptavidin-Tricolor (Medac, Hamburg, Germany) for 20 min at 4°. After washing with saponin buffer the cells were resuspended in 200 μl HBSS for flow cytometric analysis.
Data acquisition and analysis
A FACScan® flow cytometer (Becton Dickinson, Mountain View, CA) equipped with filter settings for FITC (530 nm) (FL-1), for phycoerythrin (585 nm) (FL-2) and for Tricolor emitting in deep red (< 650 nm) (FL-3) was used. Between 10 000 and 50 000 events were acquired in list mode and analyzed using lysis II® software.
Enzyme-lined immunosorbent assay (ELISA)
Cytokines were measured with ELISA kits (R & D, Wiesbaden, Germany) on cell-free supernatants. Data are expressed as picograms per ml ± SD of triplicate cultures. The IL-12 ELISA detects the bioactive IL-12p70 heterodimer only.
Statistical analysis
Results with and without RSV were compared by paired t-test as indicated.
Results
Infection of immature and mature DCs with RSV
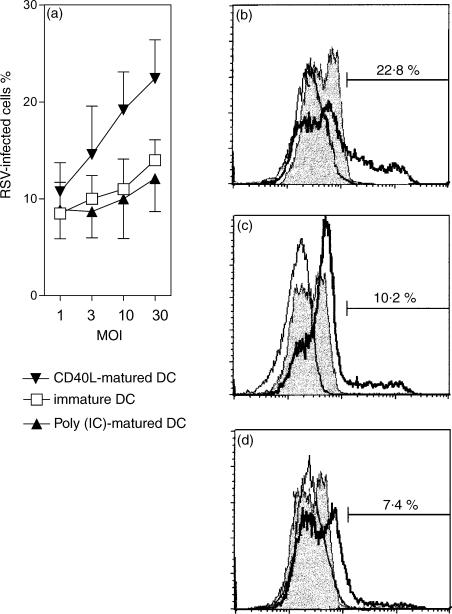
Initial studies were designed to determine the infectivity of RSV in immature and mature DCs. Immature DCs were allowed to differentiate in culture for 12 days. Maturation was stimulated by addition of either trimerized CD40L or poly(IC) for 24 hr. The cells were infected with increasing MOI (Fig. 1). The percentages of infected cells were measured 24 hr after infection by staining with a RSV-specific polyclonal antiserum. A clear difference was observed in the percentage of infected cells, which was two-fold higher in CD40L stimulated DCs compared with poly(IC) matured DCs and immature DCs.
Figure 1.
Infection of DCs with RSV. Human cord-blood-derived DCs were infected with increasing MOI of RSV. After 24 hr of infection at 37°, cells were fixed, permeabilized, and stained for RSV antigen. The percentage of infected cells was determined by flow cytometry. (a) The rate of infection depending on multiplicity of infection (MOI) and pretreatment of the DCs. The results are expressed as mean ± SD of four independent experiments. ▾, CD40L-matured DCs; □, immature DCs; ▴, poly(IC)-matured DCs. (b–d) Histograms obtained with (b) CD40L-matured DCs, (c) immature DCs and (d) poly(IC)-matured DCs at a MOI of 10. The solid lines delineate controls with FITC-streptavidin, the shaded histograms controls with FITC-streptavidin and an irrelevant biotinylated antibody and the bold lines delineate measurements with anti-RSV antibody.
DCs infected with RSV die by apoptosis
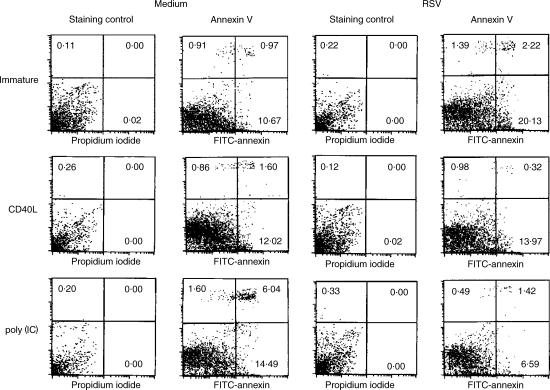
RSV is highly cytopathic in several cell types.16 To determine whether immature DCs infected with RSV were dying via apoptosis, we stained immature and mature DCs infected with RSV at a MOI of 10 with FITC–annexin V, which binds to phosphatidylserine on the surface of cells undergoing early apoptosis.17 After 24 hr of infection a significant number of apoptotic cells (20% Annexin V+, PI−) and cells undergoing secondary necrosis (2% Annexin V+ and PI+) were apparent in the immature DC population (Fig. 2). DCs matured by trimerized CD40L did not show enhanced apoptosis after RSV infection. In contrast, DCs matured by poly(IC) were resistant to the induction of apoptosis by RSV (Fig. 2, lower panel) and showed even lower rates of apoptosis then DCs treated with poly(IC) alone. The data indicate that RSV has substantial cytopathic effects as a result of apoptosis in immature DCs.
Figure 2.
Immature DCs infected with RSV die by apoptosis. Immature and mature DCs were uninfected or infected with RSV (MOI 10). The extent of apoptosis and necrosis was determined by staining the cells with FITC–annexin V and propidium iodide (PI) after 24 hr. Results shown are representative of four experiments.
RSV up-regulates membrane expression of CD86
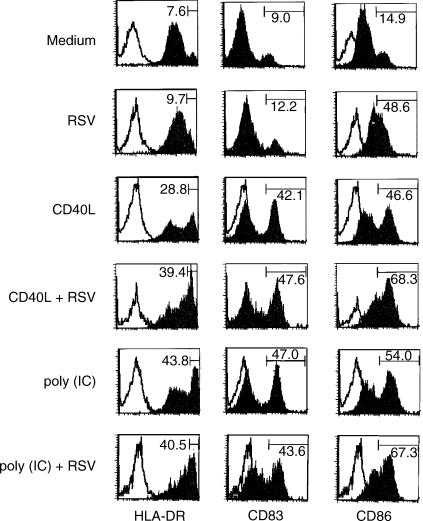
To investigate whether RSV induces maturation of human DCs, cord-blood-derived DCs were cultured with RSV (MOI 10) for 24 hr and then analysed for surface expression of major histocompatibility complex and co-stimulatory molecules. Figure 3 shows that RSV up-regulates predominantly expression of CD86 in untreated immature DC as well as in DC matured under the influence of CD40L or poly(IC). In contrast, RSV induces only a modest increase in the expression of HLA-DR and CD83, which are maturation markers for DC.
Figure 3.
Surface phenotype of DCs exposed to RSV. Immature DCs were left untreated or were stimulated for 24 hr with CD40L or poly(IC) and subsequently treated with RSV (MOI 10) for further 24 hr. The histograms show fluorescence values on gated large cells. The open histograms represent the controls of DCs stained with isotype-matched irrelevant mAb. The figures indicate the percentage of highly positive (HLA-DR, CD86) or positive (CD83) cells for each sample. The data are from one representative experiment out of six performed.
RSV induces cytokine production by DCs
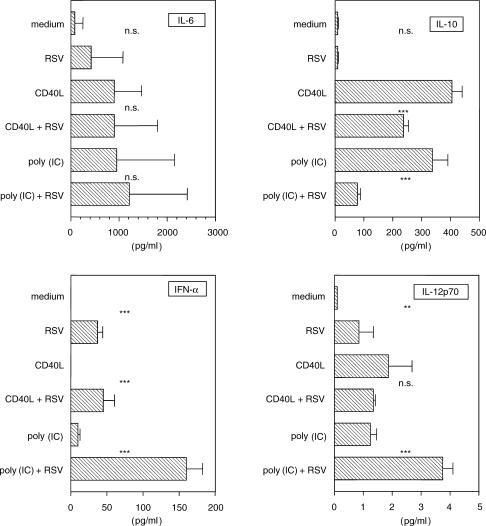
The stimuli that induce DC maturation generally stimulate secretion of cytokines by these cells. We therefore asked whether RSV induced production of these mediators by DCs. Figure 4 shows that while CD40L and poly(IC) stimulated the secretion of different amounts of IL-6, IL-10, IL-12p70 and IFN-α by DCs, RSV (MOI 10) induced the production of only low concentrations of these cytokines. To address the question whether RSV affects mature DCs we evaluated cytokine generation in DCs matured upon simulation with CD40L or poly(IC). While IL-6 generation was only marginally affected, RSV inhibited the generation of IL-10 and increased the generation of IL-12p70 and IFN-α in particular in poly(IC) matured DCs.
Figure 4.
Modulation of cytokine production by RSV. DCs were treated with RSV (MOI 10) and supernatants were collected after 24 hr and analysed with specific ELISA. The results represent the means ± SD of six independent experiments. Measurements with and without RSV were compared by paired t-test. n.s., denotes not significant, *P < 0·05, **P < 0·01 and ***P < 0·001.
T-cell priming and inhibition of IFN-γ generation
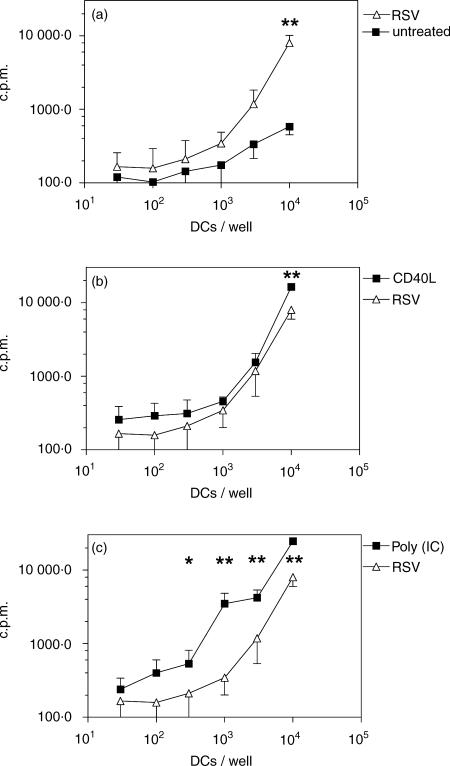
RSV is known to modulate T-cell responses.14 Thus, we asked whether RSV-treated DCs were able to prime naïve T lymphocytes and whether T-cell polarization is perturbed. To this end, DCs were first stimulated with RSV, poly(IC), CD40L, or left untreated and then incubated with TSST-1, a superantigen that activates preferentially T cells bearing the TCR-Vβ2 chain. Purified CD4+ CD45RA+ T cells were stimulated in the presence of autologous DCs. DCs that had been stimulated with RSV (MOI 10), poly(IC), or CD40L all induced a high proliferative response in naïve T cells as compared to untreated DC (Fig. 5).
Figure 5.
Stimulation of naive CD4+ CD45RA+ T cells. Immature DCs were left untreated or were stimulated for 24 hr with the indicated stimuli. An mixed lymphocyte reaction assay was then set up where mitomycin-C-treated DC were cultured at different cell numbers with 5 × 104 purified autologous CD4+ CD45RA+ T cells and incubated with the superantigen TSST-1. The proliferative response was measured after 5 days induced by RSV-infected DCs (MOI 10) is compared with (a) immature DCs; (b) CD40L-matured DCs and (c) poly(IC)-matured DCs;. The data from six experiments are presented as mean ± SD. Measurements with and without RSV were compared by paired t-test. *P < 0·05; **P < 0·01.
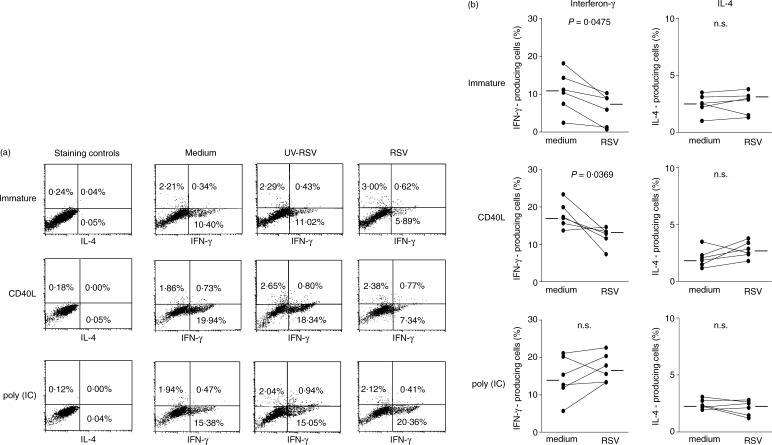
We then analysed the pattern of IL-4 and IFN-γ production by TSST-1-activated, TCR-Vβ2-bearing T lymphocytes (Fig. 6). The T cells polarized in the presence of RSV produced less IFN-γ in cultures with immature DCs or with CD40L-matured DCs. In contrast, in the presence of DCs that had been stimulated simultaneously with poly(IC) and RSV the proportion of T cells producing IFN-γ was increased compared to cultures treated with poly(IC) alone. Inhibition of IFN-γ generation was abrogated by UV irradiation of the virus suspension. Under the chosen culture conditions RSV had no effect on IL-4 generation.
Figure 6.
Analysis of intracellular cytokine production by T-cells. Cytokine production by TCR-Vβ2+ T cells stimulated in the presence of autologous DCs pretreated with the indicated stimuli and incubated with the superantigen TSST-1. The T-cells expressing TCR-Vβ2 chain, which are activated by TSST-1, were 10% of the purified CD4+ CD45RA+ T cells. (a) The data are from one representative experiment. (b) shows the comparison of all six cultures treated with medium alone or with RSV. Horizontal bars represent the mean. The results were compared by paired t-test.
Discussion
We have shown that immature DCs infected with RSV reduced the rate of IFN-γ production in co-cultured naïve T cells. Reduction of IFN-γ generation by RSV is most probably a mechanism whereby the virus evades the immune response of the host. It has been shown before that IFN-γ18 rather than IFN-α19 is essential for limiting RSV infections. In mice, the resolution of RSV infection coincides with IFN-γ production.20 Prophylactic intranasal IFN-γ gene transfer decreases RSV replication and infection.21 In human epithelial cells the IFN-γ-mediated inhibition of RSV infection involves the 2′-5′ oligoadenylate synthetase/RNase L pathway. T-cell responses like IFN-γ generation are modulated by DCs and viruses have evolved several means to interfere with DC function.11
Interestingly, RSV-mediated reduction of IFN-γ generation was found in CD40L-matured but not poly(IC)-matured DCs. To elucidate the mechanisms involved we asked whether the reduction of IFN-γ production could be attributed to modulation of survival, expression of surface markers, or cytokine production by DCs in response to viral infection.
In immature DCs RSV induced an increase in apoptosis. It is conceivable that early apoptosis of RSV-infected DCs could prevent efficient T-cell activation and could account for suppression of cell-mediated immunity. Following lymphocytic choriomeningitis virus infection in mice immune system-mediated destruction of DCs results in generalized immune suppression.22
CD40L did not inhibit apoptosis nor did it enhance induction of IFN-γ, although it is a potent stimulus for DC maturation. This might be because the CD40L-matured DC showed a marked increase in the rate of RSV infection. This is in line with a previous report showing that, CD40L does not inhibit the cytopathic effect of influenza virus.23 Indeed CD40L-mediated stimulation of DCs infected with measles virus has been shown to enhance viral replication.24
In contrast to CD40L, poly(IC) alone induced an increased frequency of apoptotic DC and in parallel an augmented proliferation and a slight increase of IFN-γ generation in DC–T-cell co-cultures. These observations are consistent with an increased degree of activation induced by poly(IC). Poly(IC) is a synthetic source of dsRNA and activation of NF-κB through dsRNA-activated protein kinase increases apoptosis25 as well as co-stimulatory properties of DCs.
In contrast, the combination of poly(IC) and RSV infection induced a clear decrease in apoptosis, a decreased infection rate and an increase in IFN-γ induction in DC–T-cell co-cultures. Interestingly, RSV has been demonstrated to inhibit NF-κB-mediated apoptosis,26 an observation consistent with the results of our experiments. In addition, latent 2′-5′ oligoadenylate synthetase which is constitutively expressed by DCs27 is activated by dsRNA to increase synthesis of 2′-5′ oligoadenylate that is required to activate RNase L. Activated RNase L non-specifically degrades single-stranded RNAs and thus limits virus production.28 This mechanism has been demonstrated to be crucial for the inhibition of RSV replication.18 RSV-mediated inhibition of IFN-γ synthesis seems to be confined to replicating virus because UV-irradiated virus preparations had virtually no effect.
DC co-stimulatory activity depends largely on the expression of surface molecules.29 In our experiments, immature DCs were only partially activated upon infection with RSV. DCs infected by RSV displayed a significant increase in expression of CD86 but only a minor increase in CD83 and HLA-DR expression. An impaired expression of maturation markers has been demonstrated for DCs infected with herpes simplex virus,30 hepatitis C virus31 and vaccinia virus.32 In addition, it has been show that these viruses inhibit maturation of DCs induced by other stimuli.
No inhibition of activation markers was demonstrated in DCs matured with CD40L and poly(IC), indicating that RSV infection does not interfere with maturation but selectively induces CD86 expression. CD86 is recognized as the ligand for CD28 and as a consequence activates an important co-stimulatory pathway in the activation of naïve T cells. Indeed, CD28 engagement increases the expression of the down-modulatory molecule CTLA-4, induces the differentiation of T helper type 2 (Th2) cells, and has an obligatory role in the homeostasis of regulatory T cells.33 Thus, the selective increase of CD86 expression may be one of the reasons why IFN-γ is down-regulated in our experiments. However, additional mechanisms have to be involved because CD40L-matured DCs induced decreased IFN-γ production after RSV infection but expressed high levels of the DC maturation markers CD83 and HLA-DR and conversely, poly(IC)-matured DCs, which exhibited nearly the same expression pattern of surface markers induced in increased production of IFN-γ.
Cytokines released by antigen-presenting cells are a further potential mechanism, which might influence T-cell polarization.34 Whereas the generation of IL-6 is significantly influenced neither by RSV nor by CD40L nor by poly(IC), the generation of IL-10 is diminished by RSV in CD40L- as well as poly(IC)-matured DCs. This finding was unexpected because IL-10 generation in response to RSV has been described before35 and is known to decrease IFN-γ production.36 However, previous work has shown that RSV stimulates IL-10 generation in macrophages rather than DCs.34
RSV alone and in combination with CD40L induced low but detectable levels of IL-12 generation but at the same time suppressed the differentiation of IFN-γ-producing cells. This finding is also unexpected because IL-12 is a known stimulator of Th1 differentiation.37,38 Recently, however, it has been shown that IL-12 induces Th1 polarization in concert with IL-27, IL-23 and IL-18.39 In this regard it is interesting that high IL-12 levels and presumably other Th1 polarizing cytokines were produced in response to RSV infection in poly(IC)-matured DCs and that this combination led to a significant increase in IFN-γ-generating T cells.
The highly significant increase in IFN-α, another known inducer of Th1 differentiation,40 in response to poly(IC) and RSV supports this theory. The synergistic effect of poly(IC) and RSV on IFN-γ generation and thus on the Th1-polarizing capacity of DCs might be explained by known mechanisms. The transcription of type I IFN is shown to depend on the dsRNA-activated protein kinase41 and IFN-α generation is further enhanced by the virus-dependent phosphorylation of interferon regulatory factor (IRF)-3 and)-7.42
In conclusion, we have shown that RSV infection of DC suppressed IFN-γ generation in co-cultivated naïve T lymphocytes and that this effect was related to the induction of apoptosis, a selective induction of CD86, and the decrease of Th1 polarizing cytokines. It remains to be shown whether reduction of IFN-γ production is related to clinical severity in RSV bronchiolitis.
Acknowledgments
This work was supported by grants from the Bundesministerium für Bildung und Forschung (01GC9801). The expert technical assistance of Veronika Baumeister and Angelika Michel is acknowledged.
References
- 1.Henderson F, Collier A, Clyde W, Denny F. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–4. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 3.Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358:1410–6. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315:1060–4. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall C, Geiman J, Biggar R, Kotok D, Hogan P, Douglas G. Respiratory syncytial virus infections within families. N Engl J Med. 1976;294:414–9. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–8. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 8.Klagge I, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol. 1999;80:823–33. doi: 10.1099/0022-1317-80-4-823. [DOI] [PubMed] [Google Scholar]
- 9.Wiertz EJ, Mukherjee S, Ploegh HL. Viruses use stealth technology to escape from the host immune system. Mol Med Today. 1997;3:116–23. doi: 10.1016/S1357-4310(96)10059-9. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 11.Palucka K, Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol. 2002;14:420–31. doi: 10.1016/s0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 12.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 13.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–61. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 14.Thurau AM, Streckert HJ, Rieger CH, Schauer U. Increased number of T cells committed to IL-5 production after respiratory syncytial virus (RSV) infection of human mononuclear cells in vitro. Clin Exp Immunol. 1998;113:450–5. doi: 10.1046/j.1365-2249.1998.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–7. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Arens MQ, Swierkosz EM, Schmidt RR, Armstrong T, Rivetna KA. Enhanced isolation of respiratory syncytial virus in cell culture. J Clin Microbiol. 1986;23:800–2. doi: 10.1128/jcm.23.4.800-802.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 18.Behera AK, Kumar M, Lockey RF, Mohapatra SS. 2′-5′ Oligoadenylate synthetase plays a critical role in interferon-gamma inhibition of respiratory syncytial virus infection of human epithelial cells. J Biol Chem. 2002;277:25601–8. doi: 10.1074/jbc.M200211200. [DOI] [PubMed] [Google Scholar]
- 19.Atreya PL, Kulkarni S. Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type I interferons and human MxA. Virology. 1999;261:227–41. doi: 10.1006/viro.1999.9835. [DOI] [PubMed] [Google Scholar]
- 20.Matsuse H, Behera AK, Kumar M, Rabb H, Lockey RF, Mohapatra SS. Recurrent respiratory syncytial virus infections in allergen-sensitized mice lead to persistent airway inflammation and hyperresponsiveness. J Immunol. 2000;164:6583–92. doi: 10.4049/jimmunol.164.12.6583. [DOI] [PubMed] [Google Scholar]
- 21.Kumar M, Behera AK, Matsuse H, Lockey RF, Mohapatra SS. Intranasal IFN-gamma gene transfer protects BALB/c mice against respiratory syncytial virus infection. Vaccine. 1999;18:558–67. doi: 10.1016/s0264-410x(99)00185-1. [DOI] [PubMed] [Google Scholar]
- 22.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–70. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–9. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Servet-Delprat C, Vidalain PO, Azocar O, Le Deist F, Fischer A, Rabourdin-Combe C. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J Virol. 2000;74:4387–93. doi: 10.1128/jvi.74.9.4387-4393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SB, Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology. 1994;199:491–6. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- 26.Thomas KW, Monick MM, Staber JM, Yarovinsky T, Carter AB, Hunninghake GW. Respiratory syncytial virus inhibits apoptosis and induces NF-kappa B activity through a phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem. 2002;277:492–501. doi: 10.1074/jbc.M108107200. [DOI] [PubMed] [Google Scholar]
- 27.Tiefenthaler M, Marksteiner R, Neyer S, et al. M1204, a novel 2′,5′ oligoadenylate synthetase with a ubiquitin-like extension, is induced during maturation of murine dendritic cells. J Immunol. 1999;163:760–5. [PubMed] [Google Scholar]
- 28.Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu Rev Biochem. 1987;56:727–77. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 29.Steinman RM, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol. 1999;60:562–7. doi: 10.1016/s0198-8859(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 30.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–53. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 31.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–6. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 32.Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox WI, Steinman RM, Bhardwaj N. Vaccinia Virus Inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163:6762–8. [PubMed] [Google Scholar]
- 33.Bour-Jordan H, Blueston JA. CD28 function: a balance of costimulatory and regulatory signals. J Clin Immunol. 2002;22:1–7. doi: 10.1023/a:1014256417651. [DOI] [PubMed] [Google Scholar]
- 34.Bartz H, Buning-Pfaue F, Turkel O, Schauer U. Respiratory syncytial virus induces prostaglandin E2, IL-10 and IL-11 generation in antigen presenting cells. Clin Exp Immunol. 2002;129:438–45. doi: 10.1046/j.1365-2249.2002.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konig B, Streckert HJ, Krusat T, Konig W. Respiratory syncytial virus G-protein modulates cytokine release from human peripheral blood mononuclear cells. J Leukoc Biol. 1996;59:403–6. doi: 10.1002/jlb.59.3.403. [DOI] [PubMed] [Google Scholar]
- 36.Palma JP, Yauch RL, Kang HK, Lee HG, Kim BS. Preferential induction of IL-10 in APC correlates with a switch from Th1 to Th2 response following infection with a low pathogenic variant of Theiler's virus. J Immunol. 2002;168:4221–30. doi: 10.4049/jimmunol.168.8.4221. [DOI] [PubMed] [Google Scholar]
- 37.Parronchi P, Mohapatra S, Sampognaro S, et al. Effects of interferon-alpha on cytokine profile, T cell receptor repertoire and peptide reactivity of human allergen-specific T cells. Eur J Immunol. 1996;26:697–703. doi: 10.1002/eji.1830260328. [DOI] [PubMed] [Google Scholar]
- 38.Sinigaglia F, D'Ambrosio D, Panina-Bordignon P, Rogge L. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol Rev. 1999;170:65–72. doi: 10.1111/j.1600-065x.1999.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 39.Robinson DS, O'Garra A. Further checkpoints in Th1 development. Immunity. 2002;16:755–8. doi: 10.1016/s1074-7613(02)00331-x. [DOI] [PubMed] [Google Scholar]
- 40.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–8. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 41.Der SD, Lau AS. Involvement of the double-stranded-RNA-dependent kinase PKR in interferon expression and interferon-mediated antiviral activity. Proc Natl Acad Sci USA. 1995;92:8841–5. doi: 10.1073/pnas.92.19.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy DE, Marie I, Smith E, Prakash A. Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J Interferon Cytokine Res. 2002;22:87–93. doi: 10.1089/107999002753452692. [DOI] [PubMed] [Google Scholar]