Abstract
Human immunodeficiency virus-1 (HIV-1) is primarily a sexually transmitted disease. Identification of cell populations within the female reproductive tract that are initially infected, and the events involved in transmission of infection to other cells, remain to be established. In this report, we evaluated expression of HIV receptors and coreceptors on epithelial cells in the uterus and found they express several receptors critical for HIV infection including CD4, CXCR4, CCR5 and galactosylceramide (GalC). Moreover, expression of these receptors varied during the menstrual cycle. Expression of CD4 and CCR5 on uterine epithelial cells is high throughout the proliferative phase of the menstrual cycle when blood levels of oestradiol are high. In contrast, CXCR4 expression increased gradually throughout the proliferative phase. During the secretory phase of the cycle when both oestradiol and progesterone are elevated, CD4 and CCR5 expression decreased whereas CXCR4 expression remained elevated. Expression of GalC on endometrial glands is higher during the secretory phase than during the proliferative phase of the menstrual cycle. Because epithelial cells line the female reproductive tract and express HIV receptors and coreceptors, it is likely that they are one of the first cell types to become infected. The hormonal regulation of HIV receptor expression may affect a woman's susceptibility to HIV infection during her menstrual cycle. Moreover, selective coreceptor expression could account for the preferential transmission of R5-HIV-1 strains to women. In addition, these studies provide evidence that the uterus, and potentially the entire upper reproductive tract, are important sites for the initial events involved in HIV infection.
Introduction
Heterosexual transmission of human immunodeficiency virus-1 (HIV-1) accounts for 70–80% of new infections worldwide, with 80% of transmissions occurring from male to female.1 It is not well understood what promotes or contributes to the establishment of HIV infection within the female reproductive tract, or whether infection is transmitted by free virus or by virus-infected cells, both of which are present in semen.2 It is thought, however, that the amount of virus, the presence of other genital tract infections, and the effectiveness of the innate and adaptive mucosal immune systems within the female reproductive tract all contribute to the establishment of HIV-1 infection.3–6
Chemokine receptors are recognized as essential coreceptors for HIV infection and are expressed on several types of haematopoietic and non-haematopoietic cells, including epithelial cells.7–11 CCR5 was identified as a major coreceptor for macrophage-tropic (M-tropic) non-syncytium inducing strains of HIV-1 because infection is blocked by the presence of the CCR5 ligands macrophage inflammatory protein-1α (MIP-1α), MIP-1β and regulated on activation, normal T-cell expressed, and secreted (RANTES).7,12 Similarly, CXCR4 was identified as the major coreceptor for infection by T-tropic, syncytium-inducing strains of HIV-1, as infection by these strains of HIV-1 is blocked by the CXCR4 ligand stromal cell-derived factor-1 (SDF-1).7,12 These findings have led to the renaming of M-tropic strains of HIV-1 as R5 strains, T-tropic strains as X4 strains, and dual tropic strains as R5X4.7,12 Of particular interest is the finding that despite the presence of both R5- and X4-utilizing strains of HIV in infected individuals, it appears that R5 strains preferentially establish infection following transmission across mucosal surfaces.13,14 In addition to selectivity in transmission, R5 and X4 viruses elicit different pathogenic sequela,15,16 suggesting that both the tropism of the virus and the cell population that is initially infected may determine important clinical features.
Menstruation, pregnancy and immunity in the female reproductive tract are regulated by the female sex hormones oestradiol and progesterone. The onset of menstruation, typically denoted as day 1 of the menstrual cycle, is characterized by low levels of both progesterone and oestradiol. During the first 14 days of the menstrual cycle, referred to as the proliferative phase, oestradiol levels slowly rise whereas progesterone levels remain low. Immediately prior to ovulation (days 13–14), oestradiol levels rapidly peak. This results in a rise in the levels of luteinizing hormone that initiates ovulation. The second half of the menstrual cycle (days 14–28) referred to as the secretory phase, is characterized by a gradual rise in both oestradiol and progesterone. If the ovum is fertilized, progesterone levels continue to rise to promote and maintain implantation of the fetus, whereas oestradiol levels decline. If fertilization does not occur, then progesterone and oestradiol levels both decline prior to the onset of menstruation on day 28 of the cycle.
In addition to the regulation of menses, oestradiol and progesterone have been shown to influence immune cell trafficking and functional activity within the female reproductive tract. Previous findings by our group have demonstrated the existence of lymphocyte aggregates (LA) within the submucosa of the endometrium that are comprised of a core of B cells surrounded by CD8+ T cells, and encircled by macrophages.17,18 As LA are significantly smaller during the proliferative phase of the menstrual cycle than during the secretory phase, it is likely that they arise as a result of cell trafficking rather than from clonal expansion of pre-existing cells within the endometrium.18 In addition to regulating the trafficking of leucocytes into the female reproductive tract, oestradiol and progesterone also regulate immune cell function. White et al. showed that although CD8+ CD3+ cytotoxic T-cell numbers were maintained within the upper female reproductive tract throughout the menstrual cycle of premenopausal women, they were functionally active only during the proliferative phase and not during the secretory phase.19 It is hypothesized that the high levels of progesterone present during both the secretory phase and throughout pregnancy serve to inhibit T-cell cytolytic activity in order to prevent destruction of the allogeneic fetus. Thus, both progesterone and oestrogen serve important roles to regulate mucosal immunity within the female reproductive tract.
As many of the same receptors important in immunity are also involved in HIV infection, we examined the expression of HIV-1 receptors and coreceptors on uterine epithelial cells during various stages of the menstrual cycle. Although there are a few reports of CD4 expression on human epithelia, including human uterine endometrium20 there is little known about regulation of CD4 expression as a function of menstrual cycle status. In addition, CXCR4 and CCR5 expression on epithelia in the gastrointestinal tract and lung has also been described.9–11,21 Moreover, expression of chemokines including interleukin (IL)-8 and RANTES has been reported in human endometrium.22–24 It is known that HIV infection of epithelial cell lines derived from the colon is dependent on chemokine receptor expression, as infection with an X4 and galactosylceramide (GalC) binding strain of HIV-1 required interaction with CXCR4 in addition to CD4 or GalC binding.8 In these studies, virus attached to GalC-positive cells, but was not internalized if the cell did not also express CXCR4.8 We recently demonstrated infection of CXCR4+ uterine epithelial cell lines with X4, but not R5, strains of HIV25. Thus, the correlation between chemokine and chemokine receptor expression, and susceptibility to infection by HIV-1 strains of different tropism, would provide valuable information about the mechanism by which HIV-1 infects cells within the female reproductive tract and the regulation of such infection by oestradiol and progesterone.
Although the lower reproductive tract tissues (vagina and cervix) are generally thought of as primary sites of infection in the human female reproductive tract, a growing body of evidence suggests that the upper reproductive tract may also serve as a site for the initial infection by HIV-1. In fact, the cervix and uterus appear to have more cells expressing HIV receptors and coreceptors than the vagina (D. Anderson, personal communication). Within minutes following in vitro fertilization, spermatozoa were found in the uterus and Fallopian tubes.26 More recently, radio-opaque dye deposited into the vagina was found in the uterus and Fallopian tubes within two hours (A. Parsons, personal communication). Dye ascended into the upper reproductive tract irrespective of whether women were premenopausal, on oral contraceptives, or postmenopausal at the time of analysis. In other studies, both bacterial and viral sexually transmitted disease (STD)-causing organisms, including Clamydia trachomatis,27,28 Neisseria gonorrhoeae29,30 and HIV-131 were found to travel freely in reproductive tract secretions. By binding to sperm, these micro-organisms exploited the rapid transit of sperm from the vagina to the uterus and Fallopian tubes.32 In the case of HIV-1, virions bind to the sperm through interaction of the envelope glycoprotein gp120 with galactosyl-alkylacyl-glycerolipid (GalAAG).31,33,34 GalAAG is structurally and antigenically related to GalC which, when expressed on epithelial and neuronal cells, is a CD4 independent infectivity receptor for HIV-1, HIV-2 and simian immunodeficiency virus (SIV).35–37 Thus, both bacterial and viral STD organisms in semen are likely to reach the upper female reproductive tract tissues within minutes of deposition in the vagina. Our previous studies demonstrating that epithelial cells from the uterus and the Fallopian tubes can be infected by HIV-138 further support our hypothesis that epithelial cells in the uterus are likely to be a primary site for HIV infection.
In this study, we evaluated uterine epithelial cells for expression of CCR5, CXCR4, CD4 and GalC using immunofluorescent staining of frozen endometrial sections obtained from patients undergoing hysterectomy. These tissues were obtained from women at different stages of the menstrual cycle to correlate the level of receptor and coreceptor expression with menstrual cycle stage. The results presented here demonstrate that all four receptors are expressed on uterine epithelium and that their levels of expression vary with the stage of the menstrual cycle. These findings support the hypothesis that HIV-1 can likely infect cells in the upper reproductive tract through these receptors, and suggest that hormonal variation in HIV receptor and coreceptor expression may regulate a woman's susceptibility to infection. Moreover, the selective expression of HIV receptors and chemokine coreceptors in the uterus, and their regulation during different stages of the menstrual cycle, may in part explain the mechanism responsible for the selective transmission of CCR5-utilizing strains of HIV-1 to women.
Materials and methods
Tissues
Uterine tissue was obtained from patients undergoing hysterectomy following informed consent. Most patients included in this study were diagnosed as having leiomyomata, prolapsed uterus, or benign ovarian disease. None had a postoperative diagnosis of malignant uterine disease. Tissues to be used for immunophenotyping were dissected out from sites distal to any gross pathology and processed for preparation of frozen sections. The menstrual stage of the endometrium was determined by examining haematoxylin/eosin stained paraffin sections39 and were classified as early (four tissues), mid (three tissues), or late proliferative phase (two tissues), or early (three tissues), mid (three tissues), or late secretory phase (two tissues).
Antibodies
Mouse anti-human CXCR4 (immunoglobulin G2b (IgG2b), clone 44716.111), and IgG2b isotype control (clone 20116.11) were purchased from R & D Systems (Minneapolis, MI) and labelled with cyanine 5 (Cy5).17 Mouse anti-human CD4 (IgG2b, clone OKT4, American Type Culture Collection, Rockville, MD), mouse anti-epithelial cell antibody (IgG1, clone BerEP4, Dako, Carpinteria, CA) and a IgG1 isotype control (1711.11, R & D Systems) were labelled with cyanine 3 (Cy3).17 Mouse anti-human CCR5 (IgG2b, clone 45549.111, R & D Systems), mouse anti-human CCR5 (IgG2a, clone 2D7, Pharmingen, San Diego, CA), IgG2b isotype control (clone 20116.11, R & D Systems) and IgG2a isotype control (catalogue IC003F, R & D Systems) were purchased labelled with fluoroscein isothiocyanate (FITC). Anti-GalC (IgG3, clone mGalC, Boehringer-Mannheim, Indianapolis, IN) and IgG3 isotype control (clone FLOPC-21, Caltag, San Francisco, CA) were purchased unlabelled.
Immunofluorescent phenotyping of frozen sections
Frozen tissue sections were fixed in acetone for 15 min at room temperature and then rehydrated in a humidity chamber at 4° for 48 hr prior to staining. Combinations of three monoclonal antibodies (mAb) were applied to the sections at a concentration of 1 µg/100 µl in blocking buffer (phosphate-buffered saline (PBS)/1% bovine serum albumin (BSA)/0·1% azide containing 4 mg/ml human immunoglobulin) and incubated in humidity chambers for 2 hr at 4°. The addition of unlabelled antibodies and the fluorochrome-labelled anti-mouse IgG antibodies were performed prior to incubation of tissue sections with directly labelled antibodies. Following indirect staining, directly labelled antibodies were added in the presence of 5% normal mouse serum to prevent binding of anti-mouse antibodies to the directly conjugated mouse mAb. Completeness of blocking was controlled for as described previously.17 Unbound antibody was removed from the sections by aspiration followed by three 15-min washes in PBS/1% BSA/0·1% azide. Washed sections were fixed overnight at 4° in PBS containing 1% paraformaldehyde. Stained sections were wet-mounted in Prolong™ antifade (Molecular Probes Inc., Eugene, OR), sealed with nail varnish, and stored at 4° in the dark for up to 10 days prior to confocal imaging.
Confocal scanning laser microscopy
Antibody-stained sections were imaged using a Bio-Rad MR1000 Confocal Scanning Laser Microscope system equipped with a krypton/argon laser and a 40× oil immersion objective (Hercules, CA). Laser power, photomultiplier tube gain and enhancement factors were determined for the FITC, Cy3 and Cy5 channels using the single fluorochrome-stained sections to ensure effective cross-channel compensation.
Image analysis and fluorescence quantitation
Images were analysed on an IBM PC compatible computer using Adobe® Photoshop® version 5.5 (Adobe Systems Inc., San Jose, CA) equipped with plugins from the Image Analysis Tool Kit version 3.0 (Reindeer Games Inc., Raleigh, NC). To estimate fluorescence intensity for individual glands, the path tools of Photoshop were used to segment out each gland using morphology to define glandular margins. The average fluorescence/µm2 of epithelial cells in each gland was then determined for each of the three fluorescent channels.
Statistical analysis
Differences in the average fluorescence intensity on glands were evaluated by Mann–Whitney tests using GraphPad Prism® software.40 Co-expression of receptors on glands was evaluated by Spearman rank correlation using the same software.
Results
Identification of epithelial cells within endometrial sections
The mAb BerEP4 was used as a marker to identify epithelial cells within uterine sections. The intensity of staining of the mAb BerEP4 varied between individual uterine glands within the same section (Fig. 1a and Fig. 2), and between samples from patients who were at different menstrual cycle stages (Fig. 3). BerEP4 reactivity was generally higher in the stratum basalis (lower one-third of the uterus, closest to the myometrium) than in the stratum functionalis (upper two-thirds of the uterus, closest to the lumen; Fig. 2). The median fluorescence intensity of BerEP4 staining on glandular tissue increased gradually during the proliferative phase of the menstrual cycle, and dropped during the secretory phase to levels equal to or below that present in the early proliferative phase (data not shown). Because the level of expression of BerEP4 varied considerably in different regions of the same gland and between glands from patients in different menstrual cycle stages, the fluorescence intensity of BerEP4 staining could not be used to normalize the fluorescence intensity of other cell surface markers. Rather, BerEP4 staining together with morphology were used to identify individual glands within the uterine tissue.
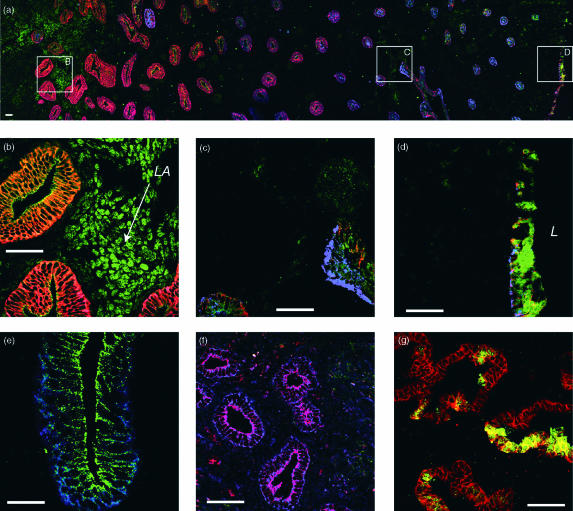
Figure 1.
Expression of BerEP4, CD4, GalC, CCR5 and CXCR4 on uterine epithelial cells. Scale is indicated on each panel by a bar representing 50 µm. (a–d) Distribution of BerEP4 (epithelial cell), CCR5 and CXCR4 expression in a late proliferative phase endometrium. Frozen sections were directly stained with three fluorescently tagged monoclonal antibodies: an epithelial cell specific antibody (Cy3-labelled clone BerEP4, red colour in (a–d)), anti-CCR5 (FITC-labelled clone 2D7, Green colour in (a–d)) and anti-CXCR4 (Cy5-labelled 12G5, blue colour in (a–d). (a) A montage of overlapping images extending from the myometrial interface (far left) to the luminal epithelium (right-hand side). (b–d) Higher magnification fields from the same region (as indicated by the boxes in (a)). BerEP4 reactivity decreases progressively from the myometrial interface towards the luminal epithelium (red colour in (a)). The luminal epithelium, in contrast to the adjacent glands, retains a relatively intense BerEP4 expression (red colour in (a) and (d)). CCR5 expression is prominent on the lymphoid aggregates located in the stratum basalis immediately adjacent to the myometrium (‘LA’ in (b)). Epithelial expression of CCR5 is low in the stratum basalis and increases in the proximal third of the stratum functionalis, thereafter decreasing towards the lumen (a) and Fig. 2). Again, the luminal epithelium is the exception to this pattern where CCR5 shows intense reactivity (green colour in (d). With the exception of the occasional gland, CXCR4 expression is low in the stratum basalis and proximal third of the stratum functionalis but increases in the outer two thirds of the stratum functionalis (a) and Fig. 2) and is maintained on the luminal epithelium (d), L indicates the position of the uterine lumen). (e) CCR5 is localized on the lateral and apical aspects of the epithelial cells and expression is low or absent on the basolateral aspects (green) whereas CXCR4 expression is more uniformly distributed on all aspects of the epithelial cells (blue). (f) CD4 expression on glandular epithelial cells. The CD4 expression is primarily localized to the apical surface of the epithelial cells (red), CXCR4 staining is evident on all aspects of the epithelial cells in this late proliferative tissue (blue). Apical staining appears purple indicating colocalization of CD4 and CXCR4. (g) GalC expression on glandular epithelial cells. GalC expression is present on patches of epithelial cells on some individual endometrial glands, in secretory phase endometria (green, GalC, red, BerEP4).
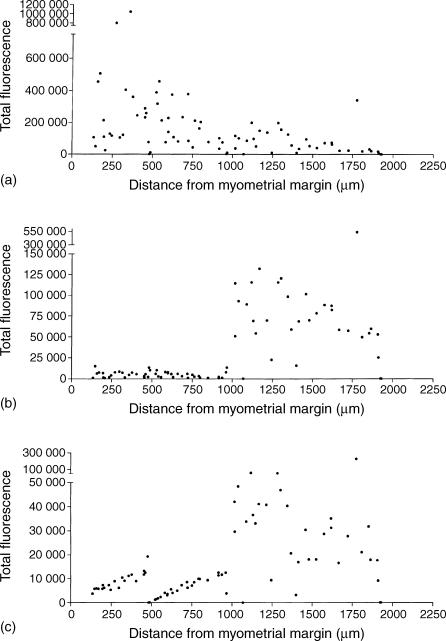
Figure 2.
Epithelial cell BerEP4 and chemokine receptor expression varies between glands and by location. Image analysis of the composite figure shown in Fig. 1a shows the differential expression of BerEP4 (a), CCR5 (b) and CXCR4 (c) across the endometrium. Total pixel intensity for each fluorochrome for individual glands is plotted against the distance from the myometrial–endometrial interface in the direction of the lumen. These results confirm the subjective impression obtained from visual examination of Fig. 1: BerEP4 is high in the stratum basalis and decreases in the functionalis, whereas CCR5 and CXCR4 are low in the stratum basalis and increase in the stratum functionalis.
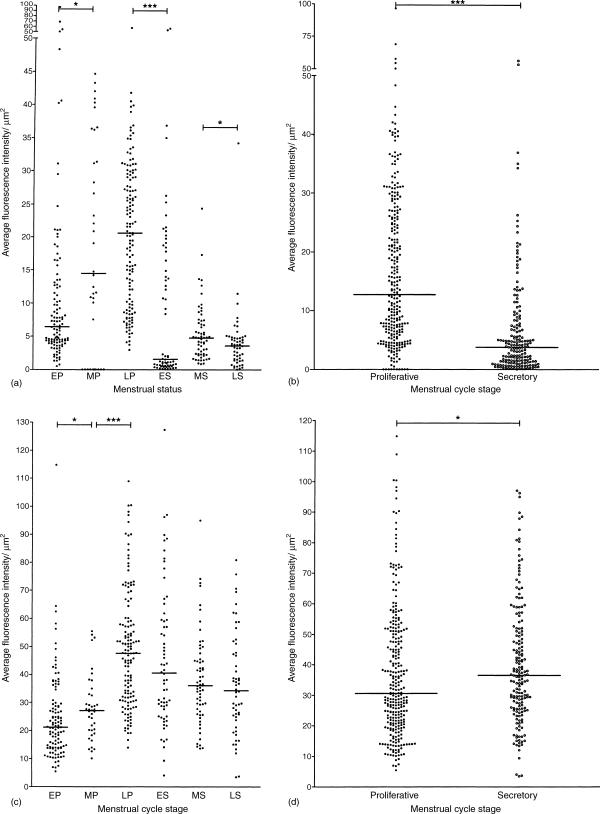
Figure 3.
CCR5 and CCR4 expression on epithelial cells varies with the stage of the menstrual cycle. Each dot represents the average fluorescence intensity per µm2 epithelium on an individual gland. (a) CCR5 expression during the early, middle and late stages of the proliferative phase and the early middle and late stages of the secretory phase. The median bars indicate that the level of expression of CCR5 increases with progress through the proliferative phase but is lower during the early, mid and late secretory phase; (b) this is reflected in a higher overall CCR5 expression in the proliferative phase. (c) CXCR4 expression during the early, middle and late stages of the proliferative phase and the early, middle and late stages of the secretory phase. The median bars indicate that the CXCR4 expression also increases during the early, middle and late stages of the proliferative phase but does not decline significantly during the secretory phase; (d) this is reflected in an overall slightly higher expression during the secretory phase. EP, early proliferative; MP, mid proliferative; LP, late proliferative; ES, early secretory; MS, mid secretory; LS, late secretory. Bracketed pairs indicate that the differences in median intensities between those adjacent pairs were statistically significant (Mann–Whitney test, * = P < 0·05, **P < 0·005, ***P < 0·0005).
Epithelial cell expression of chemokine receptors
Using confocal microscopy, we determined that glandular and luminal epithelium from the uterus express both CCR5 and CXCR4 (Fig. 1). Expression was found to be independent of the disease diagnosis (data not shown).
The intensity of epithelial staining of CCR5 and CXCR4 varied among individual glands within each uterus (Fig. 1a–d). Generally, expression of these chemokine receptors was lower in the glandular epithelium of the stratum basalis and increased progressively through the stratum functionalis towards the lumen (Fig. 2bc). The luminal epithelium generally expressed high amounts of both CCR5 and CXCR4 (Fig. 1a and d). As an internal control for mAb binding, we found that CCR5 was also expressed on T cell aggregates in the stratum basalis (Fig. 1a and b), but was largely absent on macrophages, stromal T cells and intraepithelial lymphocytes (IEL). In contrast, CXCR4 expression was absent from T-cell aggregates as well as stromal macrophages and T cells, but was present on some IEL (data not shown).
To determine whether chemokine receptor expression varied during the menstrual cycle, multiple individual glands from patients at different menstrual stages were compared for CCR5 and CXCR4 expression. CCR5 expression on epithelial cells increased during the proliferative phase and then decreased during the secretory phase (Fig. 3). In contrast, CXCR4 staining rose during the proliferative phase and remained elevated throughout the secretory phase of the menstrual cycle (Fig. 3). CCR5 reactivity was most prominent on the apical and lateral aspects of the epithelial cells (Fig. 1e), whereas CXCR4 staining was more evenly distributed on the cell surfaces. However, CXCR4 expression was more apically distributed during the secretory phase of the menstrual cycle (Fig. 1f).
Epithelial CD4 expression
We examined uterine epithelial cells for CD4 expression, and compared expression among tissues from various menstrual cycle stages. CD4 was primarily localized on the apical side of epithelial cells and staining was punctate in nature (Fig. 1f). CD4 staining intensity was significantly higher in proliferative phase compared to the secretory phase of the menstrual cycle (Fig. 4a).
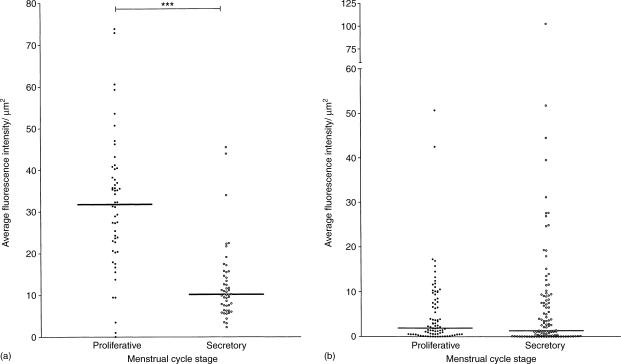
Figure 4.
CD4 and GalC expression on epithelial cells in proliferative and secretory endometria. Each dot represents the average fluorescence intensity per µm2 epithelium on an individual gland. (a) CD4 expression during the proliferative phase and the secretory phase. The median bars indicate that the level of expression of CD4 was higher in the proliferative phase than in the secretory phase. No significant differences in expression were observed between the early, middle and late stages of the proliferative phase or between the early, middle and late stages of the secretory phase. (b) GalC expression during the proliferative and secretory phases. Although the median levels of expression of GalC were similar in both phases, several glands in secretory phase tissues expressed high amounts of GalC. Bracketed pairs indicate that the differences in median intensities between menstrual phases were statistically significant (Mann–Whitney test, * = P < 0·05, **P < 0·005, ***P < 0·0005).
Epithelial GalC expression
GalC, an alternative infectivity receptor, was also expressed in uterine epithelium. Expression was found on relatively few endometrial glands, and was usually restricted to the luminal aspects of the cells (Fig. 1g). A small number of individual glands stained more intensely in some secretory endometria than that seen in tissues from the proliferative stage of the menstrual cycle (Fig. 4b).
Coexpression of chemokine receptors and CD4 on epithelial cells
The coexpression of the HIV receptor CD4 and coreceptors CXCR4 and CCR5 at different stages of the menstrual cycle was determined by Spearman rank correlation analysis. The relative fluorescence intensity of CXCR4 and CCR5 staining on individual glands was compared to that of CD4. When the coexpression of CXCR4 and CD4 was evaluated, there was a significant positive correlation between these markers during the late proliferative and late secretory phases (Table 1). In contrast, CCR5 and CD4 coexpression was only correlative in the early secretory phase. CXCR4 and CCR5 were coexpressed on individual glands only in the mid-secretory phase of the menstrual cycle, suggesting that the expression of these coreceptors on epithelial cells is relatively independent.
Table 1.
Co-expression of chemokine receptors and CD4 on uterine epithelial cells
| Proliferative | Secretory | |||||
|---|---|---|---|---|---|---|
| Early | Mid | Late | Early | Mid | Late | |
| CXCR4 versus CD4 | NS | NS | 0·6154(P = 0·0252) | NS | NS | 0·6103(P = 0·0093) |
| CCR5 versus CD4 | NS | NS | NS | 0·7275(P = 0·0032) | NS | NS |
| CCR5 versus CXCR4 | NS | NS | NS | NS | 0·676(P = 0·0021) | NS |
Discussion
The vast majority of new HIV-1 infections in women are the result of heterosexual transmission.1 Understanding the mechanism by which HIV-1 infects cells within the female reproductive tract and the conditions that regulate infection would be helpful in the design of intervention strategies to reduce the rate of new infections. It is possible that HIV-1 could infect cells within the female reproductive tract by a different mechanism than that used in the peripheral blood. This would include the possibility that cells other than T lymphocytes and macrophages are the initial targets of infection. Moreover, the expression of cellular receptors and coreceptors used by HIV-1 for infection may be differentially regulated on target cells in the mucosae than in the peripheral blood. Because R5 strains of HIV-1 are selectively transmitted across mucosal surfaces during the initial infection13 it is important to determine whether this selection takes place at the level of the target cell, the virus, or both.
Our studies focused on defining the expression of HIV-1 receptors and coreceptors on epithelial cells that line the lumen of the uterus, as a first step in defining potential cell targets within the female reproductive tract. We reasoned that epithelial cells would be one of the first cell types to come in contact with HIV-1, and might play a role in the replication of virus and in transmission of the infection to leucocytes present in the submucosa. Our findings demonstrate that key HIV receptors and coreceptors are present on these cells, and that their expression varies as a function of the menstrual cycle. The hormonal regulation of receptor and coreceptor expression may be an important mechanism that controls susceptibility to HIV-1 infection within the female reproductive tract.
The expression of CXCR4 and CCR5 on haematopoietic cells has been shown to be essential for HIV-1 infectivity.7,41,42 Recognizing that 70–80% of all new cases of HIV infection occur as a result of sexual transmission prompted us to determine the extent to which CD4, CXCR4, CCR5 and GalC are expressed on epithelial cells within the female reproductive tract, and whether their expression is dependent on the stage of the menstrual cycle. The likelihood that HIV-1 can establish an initial primary infection in the uterus is high based on the potential for this virus to enter the uterine cavity within minutes after deposition in the vagina. We found that the glandular and luminal epithelium of the human uterus express both the major HIV-1 receptor CD4 and the epithelial cell associated receptor GalC, as well as the major HIV coreceptors CCR5 and CXCR4.
The role of the sex hormones progesterone and oestradiol in regulating chemokine receptor expression at mucosal sites is not well known. Our studies indicate that cell surface expression of these receptors is likely to be under hormonal control because expression varies with the stage of the menstrual cycle. In support of this hypothesis, are findings that progesterone inhibits IL-2-induced up-regulation of CCR5 and CXCR4 expression on activated T cells from the peripheral blood.43 Moreover, this regulation of coreceptor expression correlated with a reduced susceptibility to infection by both R5 and X4 tropic strains of HIV-1. In addition to regulating expression of chemokine receptor expression, sex hormones can also affect HIV-1 transmission by their effects on the recruitment of lymphocytes and monocytes to mucosal tissues. Our previous studies17,18 as well as studies by others44–47 demonstrated that the distribution of leucocyte populations within reproductive tract tissues varied with the stage of the menstrual cycle, during pregnancy, and after menopause.
This report is the first to examine CD4 expression on human uterine epithelial cells during the menstrual cycle, and extends the previously reported expression of CD4 on human uterine epithelium and endometrial-derived cell lines.20 In the present study, CD4 was most highly expressed on epithelial cells from uterus during the proliferative phase of the menstrual cycle, and expression was reduced on these cells during the secretory phase. CCR5 expression on glandular epithelium was also highest during the proliferative phase of the menstrual cycle, and expression was also found to be lower during the secretory phase. In contrast, CXCR4 expression increased during the proliferative phase and remained high throughout the secretory phase. Taken together, these results suggest that during the proliferative phase of the menstrual cycle, epithelial cells could bind and become infected preferentially with R5 and dual tropic HIV strains. In contrast, the high expression of CXCR4 during the secretory phase in the absence of CCR5 suggests that epithelial cells may be more susceptible to X4 or dual tropic HIV strains during the second half of the menstrual cycle. If, however, epithelial cells require the coexpression of CD4 and one or the other of the chemokine receptors for infection, we would speculate that infection by X4 strains of HIV would occur most likely during late proliferative phase, and by R5 strains during the early secretory phase of the menstrual cycle.
During the secretory phase of the menstrual cycle, however, CD4 levels are relatively low and may not be expressed at sufficient levels for HIV to infect epithelial cells through this receptor. That infection can occur during the secretory phase is suggested by our finding that the alternative HIV receptor, GalC is expressed on uterine epithelial cells. GalC expression was evident on the luminal aspects of some individual glands within secretory phase tissues and was low or absent in proliferative phase endometria. This difference in expression may be the result of changes in the expression of the enzymes involved in GalC synthesis, or in the degree to which GalC is capped with sialic acid. Expression levels of galactosyltransferase and sialyltransferase, enzymes that are involved in the synthesis of GalC are hormonally controlled and their expression in the uterus varies during the menstrual cycle.48 Similar to CD4, GalC was largely restricted in its expression to the apical surface of the epithelial cells, suggesting that it would be accessible in vivo for HIV binding. HIV can bind both to GalC and to its sulphated derivative sulphatide.34 Sulphatide expression is increased in the human endometrium during the secretory phase, although the cell types and subcellular localization of this molecule have not been determined.49 Further studies would be required to determine whether HIV is able to infect uterine epithelial cells through GalC and its sulphated derivative.
Evidence that progesterone increases HIV infectivity in mucosal tissues comes from both epidemiological studies and from SIV infectivity studies in primates, where administration of depo-provera and progesterone containing oral contraceptives increased the risk of infection, whereas oestrogen had a protective effect.5,50–53 Our findings that CD4, GalC, CXCR4 and CCR5 are expressed on uterine epithelial cells at different levels during the menstrual cycle, provides further evidence that susceptibility to HIV infection may be influenced by the female sex hormones through regulation of these critical receptors. Alternatively, oestradiol and/or progesterone may influence HIV susceptibility by increasing epithelial cell proliferation, thus providing additional target cells for infection. Studies are currently in progress to further elucidate the effects of sex hormones on HIV-1 infection of epithelial cells from the female reproductive tract.
Another mechanism that is likely to control HIV infection within the female reproductive tract, and that appears to be under hormonal control, is secretory leucocyte protease inhibitor (SLPI) production by epithelial cells and macrophages. SLPI has been shown to have potent anti-HIV-1 activity.54 We previously reported SLPI production and secretion by primary cultures of uterine epithelial cells from premenopausal women but not from epithelial cells from post-menopausal women.55 SLPI produced by epithelial cell cultures inhibited colony formation from both Gram-negative and Gram-positive bacteria, and this activity was completely neutralized with an anti-SLPI antisera. Thus, the hormonal regulation of SLPI production together with its anti-HIV activity are likely to be important factors in the susceptibility of women to HIV infection. For example, fluctuations in SLPI levels within the female reproductive tract may partly explain why the risk of infection (1 : 1000–1 : 10 000 per act of sexual intercourse) remains low under conditions of repeated viral exposure. Studies are needed to define the role of SLPI and other innate virucidal agents in inhibiting the spread of HIV-1 infection.
In summary, these findings suggest that the upper female reproductive tract is likely to be a site susceptible to HIV infection. Further, they indicate that differing hormonal conditions, as seen during the menstrual cycle, may alter susceptibility to infection. Studies are underway to evaluate the effect of altering hormone levels on HIV-1 infection, chemokine receptor expression, and proliferation of uterine epithelial cells.
Acknowledgments
This work was supported by a National Institutes of Health grants AI34478, AI/NS51877 (CRW) and AI43837, and by a Merit Review from the Department of Veterans Affairs. Confocal scanning laser microscopy was performed in the Herbert C. Englert Cell Analysis Laboratory, which was established with a grant from the Fannie E. Rippel Foundation and is supported in part by the core grant of the Norris Cotton Cancer Center (CA-23108). The authors would like to thank Vincent A. Memoli, MD, Section Chief of Anatomic Pathology, Department of Pathology, for facilitating procurement of tissues; Peter Seery and Maryalice Achbach, Medical Technologists, Section of Anatomic Pathology, for inspecting and dissecting tissue specimens; Laura Wolfe for interfacing with respect to patient surgical schedules; surgeons of the Department of Surgery: John Currie, Stephen Andrews, Jackson Beecham, Joan Barthold, John Ketterer, Eileen Kirk, Benjamin Mahlab, Paul Manganiello, Eric Sailer, Barry Smith and William Young; OR nurses: Jeanette Sawyer, Tracy Stokes, Fran Reinfrank, and Jaclyn Logren; clinical support: Joanne Lavin, Chris Ramsey, Nancy Leonard, and Tamara Krivit; confocal consultants: Alice Givan and Kenneth Orndorff.
Abbreviations
- GalC
galactosylceramide
- GalAAG
galactosyl-alkylacyl-glycerolipid
- Cy3
cyanine 3
- Cy5
cyanine 5
- IEL
intraepithelial lymphocyte
References
- 1.Flaskerud JH, Ungvarski PJ. Overview and update of HIV disease. In: Ungvarski PJ, Flaskerud JH, editors. HIV/AIDSA Guide to Primary Care Management. Philadelphia: WB Saunders Co; 1999. p. 1. [Google Scholar]
- 2.Pearce-Pratt R, Phillips DM. Studies of adhesion of lymphocytic cells: Implications for sexual transmission of human immunodeficiency virus. Biol Reprod. 1993;48:431. doi: 10.1095/biolreprod48.3.431. [DOI] [PubMed] [Google Scholar]
- 3.Pedraza MA, del Romero J, Roldan F, Garcia S, Ayerbe MC, Noriega AR, Alcami J. Heterosexual transmission of HIV-1 is associated with high plasma viral load levels and a positive viral isolation in the infected partner. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;21:120. [PubMed] [Google Scholar]
- 4.Cohen CR, Plummer FA, Mugo N, et al. Increased interleukin-10 in the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS. 1999;13:327. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 5.Plummer FA. Heterosexual transmission of human immunodeficiency virus type 1 (HIV). interactions of conventional sexually transmitted diseases, hormonal contraception and HIV-1. AIDS Res Human Retroviruses. 1998;14(Suppl. 1):S5. [PubMed] [Google Scholar]
- 6.Plummer FA, Ball TB, Kimani J, Fowke KR. Resistance to HIV-1 infection among highly exposed sex workers in Nairobi: what mediates protection and why does it develop? Immunol Lett. 1999;66:27. doi: 10.1016/s0165-2478(98)00182-5. [DOI] [PubMed] [Google Scholar]
- 7.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors. roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 8.Delezay O, Koch N, Yahi N, Hammache D, Tourres C, Tamalet C, Fantini J. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. AIDS. 1997;11:1311. doi: 10.1097/00002030-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117:359. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 10.Jordan NJ, Kolios G, Abbot SE, Sinai MA, Thompson DA, Petraki K, Westwick J. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest. 1999;104:1061. doi: 10.1172/JCI6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murdoch C, Monk PN, Finn A. Functional expression of chemokine receptor CXCR4 on human epithelial cells. Immunology. 1999;98:36. doi: 10.1046/j.1365-2567.1999.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger EA. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11:S3. [PubMed] [Google Scholar]
- 13.Meng G, Wei X, Wu X, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nature Med. 2002;8:114. doi: 10.1038/nm0202-150. [DOI] [PubMed] [Google Scholar]
- 14.Van't Wout A, Kootstra N, Mulder-Kampinga G, et al. Macrophage-tropic variants initiate human immunodeficiency virus type-1 infection after sexual, parenteral and vertical transmission. J Clin Invest. 1994;94:2060. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkowitz R, Alexander S, McCune J. Causal relationships between HIV-1 coreceptor utilization, tropism, and pathogenesis in human thymus. AIDS Res Hum Retroviruses. 2000;16:1039. doi: 10.1089/08892220050075291. [DOI] [PubMed] [Google Scholar]
- 16.Harouse J, Gettie A, Tan R, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 17.Yeaman GR, Guyre PM, Fanger MW, et al. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukocyte Biol. 1997;61:427. [PubMed] [Google Scholar]
- 18.Yeaman G, Collins J, Fanger M, Wira C, Lydyard P. CD8+ T cells in human uterine endometrial lymphoid aggregates: evidence for accumulation of cells by trafficking. Immunology. 2001;102:434. doi: 10.1046/j.1365-2567.2001.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White HD, Crassi KM, Givan AL, Stern JE, Green WR, Wira CR. CTL activity within the human female reproductive tract: Influence of stage of the menstrual cycle and menopause. J Immunol. 1997. p. 37. [PubMed]
- 20.Weisz-Carrington P, Bardawil W, Cintron J, Duarte B, Farraj M, Buschmann R. Presence of CD4 on normal endometrial epithelium and endometrial line HEC-1-B. FASEB J. 1991;5:A1265. [Google Scholar]
- 21.Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, Ringler DJ. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol. 1997;151:1341. [PMC free article] [PubMed] [Google Scholar]
- 22.Jones RL, Kelly RW, Critchley HO. Chemokine and cyclooxygenase-2 expression in human endometrium coincides with leukocyte accumulation. Hum Reprod. 1997;12:1300. doi: 10.1093/humrep/12.6.1300. [DOI] [PubMed] [Google Scholar]
- 23.Arici A, MacDonald P, Casey M. Progestin regulation of interleukin-8 mRNA levels and protein synthesis in human endometrial stromal cells. J Steroid Biochem Mol Biol. 1996;58:71. doi: 10.1016/0960-0760(96)00016-7. [DOI] [PubMed] [Google Scholar]
- 24.Kelly RW, Illingworth P, Baldie G, Leask R, Brouwer S, Calder AA. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum Reprod. 1994;9:253. doi: 10.1093/oxfordjournals.humrep.a138491. [DOI] [PubMed] [Google Scholar]
- 25.Asin S, Wildt-Perinic D, Mason S, Howell A, Wira C, Fanger M. HIV-1 infection of human uterine epithelial cells: viral shedding and cell contact mediated infectivity. J Infect Dis. 2003. in press. [DOI] [PubMed]
- 26.Settlage DS, Motoshima M, Tredway DR. Sperm transport from the external cervical os to the fallopian tubes in women: a time and quantitation study. Fertil Steril. 1973;24:655. doi: 10.1016/s0015-0282(16)39908-3. [DOI] [PubMed] [Google Scholar]
- 27.Wolner-Hanssen P, Mardh PA. In vitro tests of the adherence of Chlamydia trachomatis to human spermatozoa. Fertil Steril. 1984;42:102. doi: 10.1016/s0015-0282(16)47966-5. [DOI] [PubMed] [Google Scholar]
- 28.Friberg J, Confino E, Suarez M, Gleicher N. Chlamydia trachomatis attached to spermatozoa recovered from the peritoneal cavity of patients with salpingitis. J Reprod Med. 1987;32:120. [PubMed] [Google Scholar]
- 29.James AN, Knox JM, Williams RP. Attachment of gonococci to sperm. Influence of physical and chemical factors. Br J Vener Dis. 1976;52:128. doi: 10.1136/sti.52.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James-Holmquest AN, Swanson J, Buchanan TM, Wende RD, Williams RP. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect Immun. 1974;9:897. doi: 10.1128/iai.9.5.897-902.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brogi A, Presentini R, Solazzo D, Piomboni P, Costantino-Ceccarini E. Interaction of human immunodeficiency virus type 1 envelope glycoprotein gp120 with a galactoglycerolipid associated with human sperm. AIDS Res Human Retroviruses. 1996;12:483. doi: 10.1089/aid.1996.12.483. [DOI] [PubMed] [Google Scholar]
- 32.Howard TL. Bacterial hitch-hikers. J Urol. 1971;106:94. doi: 10.1016/s0022-5347(17)61232-1. [DOI] [PubMed] [Google Scholar]
- 33.Brogi A, Presentini R, Moretti E, Strazza M, Piomboni P, Costantino-Ceccarini E. New insights into the interaction between the gp120 and the HIV receptor in human sperm (human.sperm/gp120/galactoglycerolipid/antigalactosylceramide/seminolipid/spermatogonia) J Reprod Immunol. 1998;41:213. doi: 10.1016/s0165-0378(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 34.Gadella BM, Hammache D, Pieroni G, Colenbrander B, van Golde LM, Fantini J. Glycolipids as potential binding sites for HIV. topology in the sperm plasma membrane in relation to the regulation of membrane fusion. J Reprod Immunol. 1998;41:233. doi: 10.1016/s0165-0378(98)00061-8. [DOI] [PubMed] [Google Scholar]
- 35.Yahi N, Baghdiguian S, Moreau H, Fantini J. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J Virol. 1992;66:4848. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yahi N, Sabatier JM, Baghdiguian S, Gonzalez-Scarano F, Fantini J. Synthetic multimeric peptides derived from the principal neutralization domain (V3 loop) of human immunodeficiency virus type 1 (HIV-1) gp120 bind to galactosylceramide and block HIV-1 infection in a human CD4-negative mucosal epithelial cell line. J Virol. 1995;69:320. doi: 10.1128/jvi.69.1.320-325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantini J, Cook DG, Nathanson N, Spitalnik SL, Gonzalez-Scarano F. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc Natl Acad Sci USA. 1993;90:2700. doi: 10.1073/pnas.90.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howell AL, Edkins RD, Rier SE, Yeaman GR, Stern JE, Fanger MW, Wira CR. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J Virol. 1997;71:3498. doi: 10.1128/jvi.71.5.3498-3506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noyes RW, Hertig AT. Dating the endometrial biopsy. Fertil Steril. 1955;1:3. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 40.Motulsky HJ. Analyzing Data with Graphpad Prism. San Diego, CA: GraphPad Software Inc.; 1999. [Google Scholar]
- 41.Feng Y, Broder C, Kennedy P, Berger E. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 42.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 43.Vassiliadou N, Tucker L, Anderson DJ. Progesterone-induced inhibition of chemokine receptor expression on peripheral blood mononuclear cells correlates with reduced HIV-1 infectability in vitro. J Immunol. 1999;162:7510. [PubMed] [Google Scholar]
- 44.Bonatz G, Hansmann M, Buchholz F, Mettler L, Radzun H, Semm K. Macrophage- and lymphocyte-subtypes in the endometrium during different phases of the ovarian cycle. Int J Gynaecol Obstet. 1992;37:29. doi: 10.1016/0020-7292(92)90974-n. [DOI] [PubMed] [Google Scholar]
- 45.Kamat B, Isaacson P. The immunocytochemical distribution of leukocytic subpopulations in human endometrium. Am J Pathol. 1987;127:66. [PMC free article] [PubMed] [Google Scholar]
- 46.Bulmer J, Lunny D, Hagin S. Immunohistochemical characterization of stromal leukocytes in nonpregnant human endometrium. Am J Reprod Immunol Microbiol. 1988;17:83. doi: 10.1111/j.1600-0897.1988.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 47.Givan AL, White HD, Stern JE, Colby E, Guyre P, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix and vagina. Am J Reprod Immunol. 1997;38:350–9. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 48.Nelson JD, Jato-Rodriguez JJ, Labrie F, Mookerjea S. 1977 Glycosyltransferase and UDP-galactose pyrophosphatase activities in the endometrium during oestrous cycle of the rat. J Endocrinol. 1997;73:53. doi: 10.1677/joe.0.0730053. [DOI] [PubMed] [Google Scholar]
- 49.Kubushiro K, Kojima K, Mikami M, Nozawa S, Iizuka R, Iwamori M, Nagai Y. Menstrual cycle-associated alteration of sulfogalactosylceramide in human uterine endometrium: possible induction of glycolipid sulfation by sex steroid hormones. Arch Biochem Biophysics. 1989;268:129. doi: 10.1016/0003-9861(89)90573-0. [DOI] [PubMed] [Google Scholar]
- 50.Sodora D, Gettie A, Miller C, Marx P. Vaginal transmission of SIV. assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl. 1):S119–23. [PubMed] [Google Scholar]
- 51.Guimaraes MD, Munoz A, Boschi-Pinto C, Castilho EA. HIV infection among female partners of seropositive men in Brazil. Rio de Janeiro Heterosexual Study Group. Am J Epidemiol. 1995;142:538. doi: 10.1093/oxfordjournals.aje.a117672. [DOI] [PubMed] [Google Scholar]
- 52.Marx P, Spira A, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nature Med. 1996;2:1084. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 53.Smith S, Baskin G, Marx P. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis. 2000;182:708. doi: 10.1086/315776. [DOI] [PubMed] [Google Scholar]
- 54.Hocini H, Becquart P, Bouhlal H, Adle-Biassette H, Kazatchkine M, Belec L. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin Diagn Lab Immunol. 2000;7:515. doi: 10.1128/cdli.7.3.515-518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fahey J, Wira C. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]