Abstract
Constitutive expression of major histocompatibility complex class II molecules (MHC II) is restricted to dendritic cells, cells of the macrophage lineage and B lymphocytes. In all three lineages, peptide fragments of captured antigen are loaded into newly synthesized MHC II molecules. In B-lineage cells, MHC II synthesis is dramatically increased on encounter with antigen, by T-cell-derived signals and by microbial products. We have previously shown that immature B cells fail to hyperexpress MHC II after antigen receptor [B-cell receptor (BCR)] ligation, but are responsive to other stimuli. Expression of the costimulatory molecule, CD86, was similarly regulated. This suggested the existence of two pathways regulating expression of these important molecules. Here we present data supporting this hypothesis. We show that activity of the enzyme phosphatidylinositol 3-kinase is critical for MHC II hyperexpression and induction of CD86 in response to ligation of the BCR or CD38, but not for responses to other stimuli including interleukin-4, lipopolysaccharide and CD40 ligation.
Introduction
Hyperexpression of class II major histocompatibility complex molecules (MHC II) by antigen-presenting cells (APC) is a key step in ensuring the delivery of sufficient peptide-loaded MHC molecules to their surface to permit the initiation of a productive interaction with T cells. Optimal T-cell activation also requires other signals to be delivered by the APC, amongst which ligation of T-cell-expressed CD28 by CD86/CD80 is particularly important. Although dendritic cells are usually regarded as the APC most involved in presenting antigen to naïve T cells, B cells are also highly effective in this role and, for some T-cell responses, B cells appear to be obligatory. The ability of B cells to act as APCs is developmentally regulated; immature B cells from neonatal spleen or adult bone marrow are unable to process and present a protein antigen to an antigen-specific T-cell line. We have previously reported that immature B cells from neonatal spleen1 and adult bone marrow2 fail to hyperexpress MHC II after antigen receptor [B-cell receptor (BCR)] ligation, although other stimuli, including interleukin (IL)-4 and CD40 ligation were able to induce this response. BCR ligation of immature B cells also fails to induce them to express the important costimulatory molecule, CD86.3 These findings provided a molecular basis for the deficient presentation of antigen by immature B cells and also suggested that there are at least two pathways by which class II upregulation and induction of costimulatory molecules can be signalled in B cells. To explore this further, we have investigated the upregulation of MHC II and induction of CD86 in mature adult B cells by stimuli which impinge on different cell-surface receptors and examined the differential modulation of these responses.
In mature murine B cells, membrane phospholipids play a role in signal transduction, and soluble inositol trisphosphate (IP3) and membrane-bound 3-phosphoinositides are important second messengers.4 There is evidence that these pathways are developmentally regulated, as immature B cells fail to show an increase in intracellular IP3 on BCR ligation.5 Phosphatidylinositol 3-kinase (PI3K) is responsible for the production of phosphatidylinositol-(3,4,5) trisphosphate, and the B cells of mice in which PI3K has been genetically disrupted6,7 show phenotypic and functional characteristics similar to those of the immature B cells found in neonatal spleen. These observations suggested that the differences in signalling between immature and mature B cells described above might, in part, reflect differential activity of PI3K. We therefore investigated the effect of inhibiting PI3K on MHC upregulation and the expression of other activation-induced molecules. Our data show that treatment of B cells with inhibitors of PI3K prevents proliferation induced by all physiological stimuli tested. However, although this treatment inhibited hyperexpression of class II MHC in response to BCR and CD38 ligation, it did not affect the response to other stimuli. The induction of the costimulatory molecule, CD86, and the activation marker, CD69, were also differentially affected. This suggests a differential dependence on PI3K activity of at least two main pathways involved in regulating the surface expression of molecules involved in initiating and maintaining a productive interaction between B and T lymphocytes.
Materials and methods
Mice
Female BALB/c mice, bred in our animal facility, were used at 10–12 weeks of age.
Antibodies and reagents
The following rat antibodies to murine molecules were used in this study: anti-I-Ad (NIM-R4); anti-CD86 (2D10, a gift from Dr D Faherty; Hoffmann-La Roche, Nutley, NJ); anti-CD38; anti-CD40 (FGK65, a gift from Dr J. Anderson; Basel Institute, Basel, Switzerland) and biotinylated anti-B220 (RA3-6B2; Pharmingen, San Diego, CA). Fluorescein-coupled hamster anti-mouse CD69 (HI.2F3) was purchased from Serotec (Oxford, UK). Non-commercial antibodies were used as tissue-culture supernatants or were partially purified by ammonium-sulphate precipitation and/or chromatography on protein G or diethylaminoethyl (DEAE) sepharose and, where appropriate, were coupled with biotin or fluorescein using standard methods. Biotinylated antibodies were revealed using phycoerythrin (PE)-conjugated streptavidin (Serotec). Recombinant murine IL-4 was obtained from Genzyme (Cambridge, MD); lipopolysaccharide (LPS) (Escherichia coli 055B5), wortmannin, ionomycin and phorbol 12-myristate 13-acetate (PMA) from Sigma Aldrich (Poole, UK), LY294002 from Calbiochem (Nottingham, UK) and F(ab′)2 goat anti-mouse µ from Jackson Laboratories (West Grove, PA).
Cell preparation and culture
B cells were prepared from spleen-cell suspensions by treatment with rat anti-mouse Thy-1 and guinea-pig complement, as described previously.1 For proliferation experiments, cells were cultured for 72 hr in 96-well plates at 2 × 105 cells/well in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 2 mm glutamine, 1 mm sodium pyruvate and 5 × 10−5m 2-mercaptoethanol (complete medium). Cultures were pulsed with 0·5 µCi [3H]thymidine (Amersham, Bucks., UK) for the final 4 hr, before being harvested and uptake measured by liquid-scintillation spectroscopy. For marker-upregulation experiments, cells (2 × 106 cells/ml) were cultured in 24-well plates for 20–48 hr with the indicated stimuli and changes in the expression of MHC II, CD86 and CD69 were analysed by flow cytometry. The timing of analysis was based on the kinetics of increased expression of these molecules, which is first observed at 12–16 hr poststimulation, peaks by 24 hr and decreases after 60–72 hr. Inhibitors were added to the cells 30 min before the relevant stimuli.
Cell staining and flow cytometry
Cells (106) were stained as described previously.1 Flow cytometry was performed using a FACScan flow cytometer (Becton-Dickinson, Oxford, UK). Dead cells were excluded on the basis of their forward- and side-scatter characteristics; an electronic gate was set to include cells with the characteristics of lymphocytes. Analysis was based on the collection of 5000–20 000 events.
Results
Effect of PI 3K inhibition on B-cell proliferation
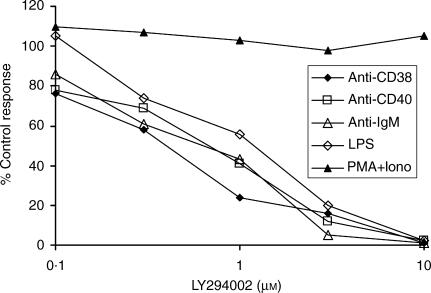
To assess the role of PI3K in regulating B-cell proliferation, spleen B cells were pretreated with the inhibitor LY294002 for 30 min prior to culture with various growth-promoting stimuli (Fig. 1). Proliferative responses induced by ligation of surface immunoglobulin M (sIgM), CD38 and CD40 were all highly sensitive, with 50% inhibitory concentrations (IC50s) ranging from 0·4 µm to 0·8 µm, and no consistent difference between their sensitivities observed in three experiments. LPS-induced proliferation was also inhibited in a dose-dependent manner with an IC50 of 1–2 µm. Proliferation induced by a combination of PMA and ionomycin was, in contrast, completely resistant to treatment. The inhibitory concentrations of LY294002 in this assay correspond closely with those found for the isolated enzyme and, taken together with the lack of response to PMA + ionomycin (a stimulus which bypasses membrane-signalling events) suggests the effects are a result of the specific requirement of PI3K in the affected signal-transduction pathways. The dependency of proliferation induced by anti-µ, anti-CD40 and LPS on PI3K is in agreement with the findings of Fruman et al.7 but this is the first report that signalling via CD38 in mature murine B cells requires PI3K. Venkataraman et al.8 also reported the dependence on PI3K of LPS-induced proliferation and IL-6 secretion by mouse B cells, with proliferation being 50% inhibited by approximately 2–3 µm LY294002, a value very similar to the IC50 reported here.
Figure 1.
Effects of LY294002 on B-cell proliferation driven by various stimuli. BALB/c B cells (2 × 105) were preincubated with different concentrations of LY294002 for 30 min before the addition of lipopolysaccharide (LPS) (10 µg/ml), F(ab′)2 anti-µ (10 µg/ml), anti-CD40 (10 µg/ml), anti-CD38 (1 : 200 dilution of s/n) or phorbol 12-myristate 13-acetate (PMA) (5 ng/ml) + ionomycin (1 µm). After 68 hr, the cultures were pulsed with [3H]thymidine and harvested 4 h later. Data shown are derived from the means of triplicate cultures and represent the inhibition produced by each concentration of inhibitor compared with control cultures lacking LY294002. Uptakes ranged from 7500 counts per minute (c.p.m.) (control stimulated with anti-CD38) to 85 775 c.p.m. (LPS controls). The data shown are representative of three similar experiments.
Selective effect of LY294002 on MHC class II hyperexpression
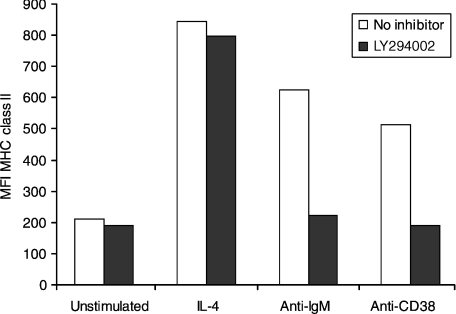
The effect of PI3K inhibition on class II upregulation by murine B cells was assessed after stimulating class II hyperexpression with IL-4, anti-µ or anti-CD38. As seen in Fig. 2, all three stimuli induced marked MHC II upregulation, but differed in their sensitivity to LY294002. Hyperexpression induced by BCR (sIgM) and CD38 ligation was almost completely abrogated in the presence of 10 µm inhibitor, whilst that induced by IL-4 was essentially unaffected.
Figure 2.
Differential effects of inhibition of phosphoinositide 3-kinase (PI3K) on major histocompatibility complex (MHC) II hyperexpression induced by interleukin-4 (IL-4), anti-µ and anti-CD38. BALB/c B cells preincubated with or without the inhibitor LY294002 (10 µm), as described in the legend to Fig. 1, were further cultured with the indicated stimuli [F(ab′)2 anti-µ, 5 µg/ml; anti-CD38, 1 : 200 dilution of s/n; or IL-4, 50 U/ml] for 24 hr before being harvested and stained for surface expression of MHC class II. The data shown represent the mean fluorescence intensity (MFI) of 5000 events, and are representative of three experiments. The MFI of cells with and without inhibitor was compared for each stimulus by using the Student's t-test. For unstimulated and IL-4-treated cells, the difference was not significant (P = 0·113 and 0·051, respectively). For anti-immunoglobulin M (anti-IgM)- and anti-CD38-treated cells the differences were significant (P < 0·0001).
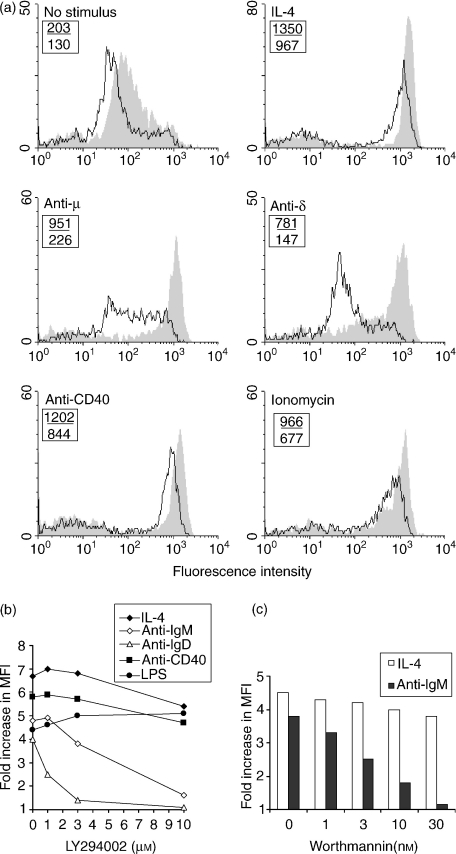
These findings were confirmed and extended by the data shown in Fig. 3. Class II upregulation induced by BCR ligation with anti-µ and anti-δ was clearly inhibited in the presence of LY294002. Interestingly, the response to ligation of immunoglobulin D (IgD) was distinctly more sensitive than that induced by BCR ligation with anti-µ. In contrast, cells stimulated with IL-4, anti-CD40, LPS or ionomycin remained able to hyperexpress class II, even at an inhibitor concentration of 10 µm. The lack of effect of inhibition of PI3K on class II upregulation induced by CD40 ligation is particularly important because CD40 ligation has been shown to lead to activation of PI3K in B cells9 and proliferation induced by this treatment was clearly sensitive to the inhibitor. These data also suggest that the essential role for PI3K in upregulation of class II expression is probably in receptor-proximal signalling events, rather than in those processes involved in routing the newly synthesized molecules to the cell surface, because cells treated with 10 µm LY294002 could upregulate MHC II in response to some stimuli.
Figure 3.
Effects of LY294002 and wortmannin on the ability of various stimuli to induce major histocompatibility complex (MHC) class II upregulation. (a) BALB/c B cells, treated as described in the legend to Fig. 2, were cultured with the indicated stimuli [anti-immunoglobulin D (anti-IgD), 20 µg/ml; ionomycin, 0·3 µm; anti-CD40, 5 µg/ml; concentrations of the other stimuli were as stated in the legend to Fig. 2] with (–) or without (shaded) LY294002 (10 µm). The data shown represent the fluorescence on 10 000 cells per sample. The numbers shown in the upper left of each histogram represent the mean fluorescence intensity (MFI) of MHC class II fluorescence in the presence (lower value) or absence (upper value) of inhibitor. Similar results were obtained in two other, similar, experiments. (b) BALB/c B cells pretreated with the indicated concentrations of LY294002 were cultured at 2 × 106 cells/ml for 24 hr with the indicated stimuli [lipopolysaccharide (LPS), 10 µg/ml; concentrations of the other stimuli were as stated in the legend to Fig. 2] before being harvested for staining and fluorescence-activated cell sorter (FACS) analysis. The data represent the fold increases in the MFI of MHC class II staining compared with those seen for control cells cultured without stimulation, and are representative of two similar experiments. (c) BALB/c B cells, precultured with the indicated concentrations of wortmannin, were further cultured for 24 hr in the presence of F(ab′)2 anti-µ (5 µg/ml) or interleukin-4 (IL-4) (50 U/ml) before expression of MHC class II was examined by staining and FACS analysis. The data shown represent the fold increase in MFI of MHC class II staining over that seen for cells cultured without stimulation. Similar results were obtained in two other experiments. The Student's t-test was used to compare the MFIs of untreated cells stimulated with IL-4 or anti-IgM with those of cells treated with the indicated concentrations of inhibitor. For IL-4 stimulation, the MFI of cells treated with 1, 3 or 10 nm wortmannin was not significantly different from that of control cells, at 30 nm the difference was just significant (P = 0·04). For anti-IgM stimulation, the differences were significant at all inhibitor concentrations (P < 0·0002).
The effects on class II hyperexpresssion of another relatively specific PI3K inhibitor, the fungal metabolite wortmannin, were also examined. The data (Fig. 3c) show that upregulation of MHC class II induced by BCR ligation was inhibited in the presence of 3 nm wortmannin and almost completely abrogated at 30 nm. In contrast, hyperexpression induced by IL-4 was unaffected by wortmannin, even at a concentration of 30 nm.
PI 3K activity is required for the induction of CD86 and CD69 expression
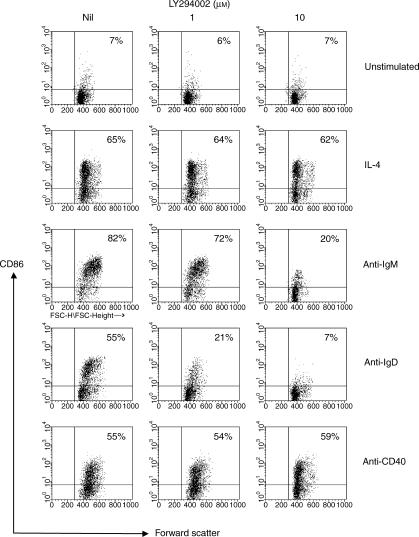
Resting B cells do not express B7·2 (CD86) or CD69, but the expression of these molecules is induced when B cells are activated by various stimuli. Induction of CD86 is important because its interaction with CD28 on T cells provides a powerful costimulatory signal. The effect of inhibiting PI3K with LY294002 on the induction of these molecules was therefore investigated. Induction of CD86 by cross-linking of the BCR with anti-µ was almost completely inhibited in the presence of 10 µm LY294002, and induction by anti-δ was even more sensitive, being significantly inhibited at 1 µm (Fig. 4). Upregulation of CD86 by IL-4 and by ligation of CD40 were, however, unaffected.
Figure 4.
Inhibition of phosphoinositide 3-kinase (PI3K) selectively affects the induction of CD86 by B-cell receptor (BCR) ligation. B cells pretreated with LY294002, as shown, were cultured with the indicated stimuli (the concentrations of which are as stated in the legend to Fig. 3a) for 24 hr before being stained for the expression of CD86. The data shown represent the fluorescence on 5000 cells analysed, the numbers in the upper right quadrant correspond to the percentage of CD86+ cells in each sample. Essentially identical data were obtained in a second, independent experiment.
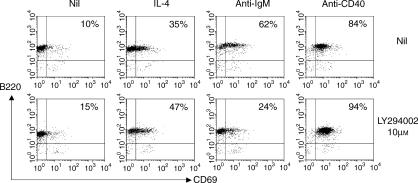
A similar pattern was observed when inducible expression of CD69 was examined (Fig. 5). In the presence of 10 µm LY294002, CD69 induction by anti-µ was markedly inhibited, but that induced by IL-4 and anti-CD40 was not affected. As expression of both CD86 and CD69 is induced de novo on stimulation, these data support the idea that PI3K activation is probably critical for signalling events that influence the level of gene transcription.
Figure 5.
The induced expression of CD69 is inhibited by inactivation of phosphoinositide 3-kinase (PI3K). BALB/c B cells, preincubated as described in the legend to Fig. 2, were stimulated as indicated for 18 hr (concentrations of the different stimuli were as stated in the legend to Fig. 2) and CD69 expression on B cells was analysed by flow cytometry. The numbers shown in the upper right quadrant indicate the percentage of cells that were B220+ CD69+ and are derived from 10 000 events analysed. Two other experiments gave similar data.
Discussion
For most immune responses, cognate interactions between antigen-specific T and B cells are essential. An effective T : B dialogue depends on the ligation of a variety of membrane molecules on both partners. These elicit a complex set of signalling cascades which regulate the biological responses of the participants. Although PI3K has been firmly established as a critical signalling intermediate in cellular responses to a variety of stimuli, its precise role in regulating B-cell development and differentiation is not completely resolved.
Previous reports have examined the requirement for PI3K in B-cell proliferation, differentiation and survival.6–8,10 We have extended these studies by investigating the role of PI3K in mediating events which are critical for the establishment and effective development of T : B collaboration. PI3K activity was rapidly induced on stimulation of human B cells with CD40L and the polyclonal activator, Staphylococcus aureus.10 Proliferative responses to both stimuli were inhibited by prior treatment with wortmannin, and induction of immunoglobulin secretion was also sensitive to inhibitors of PI3K. Interestingly, wortmannin had a much more profound inhibitory effect on immunoglobulin secretion than on proliferation, leading the authors to speculate that distinct signal-transduction pathways are involved in proliferation and differentiation. Mice with a targeted disruption of the gene encoding the p85α subunit of PI3K not only showed reduced proliferative responses to stimulation with LPS, and CD40 and BCR ligation, but also displayed reduced responses to TI antigens and defects in B-cell development.6,7 One of these reports7 also demonstrated that the proliferation of normal murine B cells in response to the same stimuli was inhibited by prior exposure to 10 µm LY294002. Our data (Fig. 1) are in agreement with these observations. However, we have also shown that B-cell proliferation, induced by ligation of cell-surface CD38, is PI3K dependent and that proliferation induced by LPS appears to be slightly less sensitive to the inhibitor than that induced by the other stimuli. The signals involved in B-cell proliferation in response to ligation of CD38 are not well understood and the involvement of PI3K has not been previously reported. There is, however, evidence that CD38 signalling ‘hijacks’ the signalling pathways used by the BCR11 and our data are consistent with this.
The effects of inhibiting PI3K on stimulus-induced upregulation of MHC II expression were different from those on proliferation induced by the same stimuli. In particular, MHC II hyperexpression following CD40 ligation was essentially unaffected, although the proliferative response to this stimulus was highly sensitive. MHC II upregulation by IL-4 also occurred in the presence of both wortmannin and LY294002. Thus, PI3K appears to be required for signalling MHC upregulation after stimulation via the BCR or CD38 ligation, but dispensable for induction of the same response by IL-4 and CD40 ligation. It is also interesting that only those stimuli which do not require PI3K to induce MHC II upregulation by mature, adult B cells are capable of inducing this response in immature B cells.1,2 Those stimuli that do require PI3K do not upregulate MHC II in immature B cells. Investigation of the activation of PI3K following BCR ligation in immature B cells is currently under investigation. Despite the PI3K-independent nature of MHC II upregulation by IL-4 or CD40 ligation, both of these stimuli have been shown to induce activation of PI3K in B-lineage cells9,12 and CD40-driven proliferation is PI3K-dependent (see ref. 7 and this report), as is IL-4-induced B-cell survival.7 Fruman et al. also found a differential requirement for PI3K in B-cell responses driven by CD40 ligation, as LY294002 was shown to inhibit proliferation but to have no effect on survival induced by this stimulus.
In B lymphocytes, PI3K activation appears to be an early event following BCR ligation and functions as a signal initiator for downstream pathways through recruitment of molecules containing PH domains, including the phosphotyrosine kinases (PTKs) Btk and Tec, the serine/threonine kinase, Akt, and the adapter proteins, Bam32 and Gab-1. These molecules are recruited to the membrane by binding to phosphatidylinositol-3, 4,5-trisphosphate (PIP3), generated by PI3K activity. Given the importance of PI3K in BCR signalling, it was not surprising that BCR-induced MHC II upregulation was sensitive to inhibition of PI3K by both LY294002 and wortmannin. It is interesting to note, however, that inhibition of MHC II upregulation required higher concentrations of inhibitor than were needed to affect proliferative responses.
MHC II hyperexpression, induced by ligation of either the IgM or IgD components of the BCR, were sensitive to inhibition of PI3K. Surprisingly, MHC II upregulation (and induction of CD86) following ligation of IgD was noticeably affected at inhibitor concentrations much lower than those needed to inhibit anti-IgM-induced upregulation (Figs 3 and 5). Although some differences have been observed in signalling following ligation of the two isotypes,13 the B cells of mice transgenic for either sIgD anti-hen egg lysozyme (HEL) or sIgM anti-HEL have been shown to be equally capable of activation, deletion or being rendered anergic when encountering antigen in the appropriate context at the appropriate developmental stage.14 The apparently greater dependence of sIgD-mediated class II hyperexpression on PI3K suggests a tighter coupling of this isotype to PI3K pathways. This is interesting in the light of other evidence for differential reliance of IgD signalling on PI3K activity.15 The observation that signalling via IgD results in a weaker and shorter activation of the PI3K pathways than signalling via IgM in human lymphoma cells, which are resistant to IgD-mediated apoptosis,16 suggests that the greater sensitivity of IgD-mediated MHC upregulation to the PI3K inhibitor might occur because this stimulus induces lower PI3K activity, which is easier to inhibit than a more robust activation induced by IgM ligation. To assess this we are currently examining PI3K activation by sIgM and sIgD in normal, mature murine B cells.
The molecules downstream of PI3K have differing affinities for PIP3, suggesting that some may be recruited at lower levels of PI3K activity than others.4 This may explain the different sensitivities of different responses to inhibition of PI3K. PI3K pathways have been clearly implicated at multiple stages of B-cell differentiation, and the outcome of signalling is developmentally regulated. Levels of the adapter protein, Bam32, have been shown to vary during B-cell maturation and to be differentially modulated following ligation of CD40 or the BCR,17 and this may account for the differing reliance of these responses on PI3K activity. Similar variations in the expression of this or other adapter proteins could also influence the PI3K dependence of other signalling pathways.
In summary, our data provide clear evidence for two distinct pathways leading to stimulus-induced MHC II hyperexpression in murine B lymphocytes. One pathway, initiated by BCR ligation (or stimuli which utilize overlapping second messengers) involves PI3K; the other pathway(s), initiated through toll-like receptors, IL-4R and CD40, proceed independently of PI3K activation. Further dissection of these pathways is clearly important.
References
- 1.Tasker L, Marshall-Clarke S. Immature B cells from neonatal mice show a selective inability to up-regulate MHC class II expression in response to antigen receptor ligation. Int Immunol. 1997;9:475–84. doi: 10.1093/intimm/9.4.475. [DOI] [PubMed] [Google Scholar]
- 2.Marshall-Clarke S, Tasker L, Parkhouse RM. Immature B lymphocytes from adult bone marrow exhibit a selective defect in induced hyperexpression of major histocompatibility complex class II and fail to show B7.2 induction. Immunology. 2000;100:141–51. doi: 10.1046/j.1365-2567.2000.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall-Clarke S, Reen D, Tasker L, Hassan J. Neonatal immunity: how well has it grown up? Immunol Today. 2000;21:35–41. doi: 10.1016/s0167-5699(99)01548-0. [DOI] [PubMed] [Google Scholar]
- 4.Marshall AJ, Niiro H, Yun TJ, Clark EA. Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cgamma pathway. Immunol Rev. 2000;176:30–46. doi: 10.1034/j.1600-065x.2000.00611.x. [DOI] [PubMed] [Google Scholar]
- 5.Yellen AJ, Glenn W, Sukhatme VP, Cao XM, Monroe JG. Signaling through surface IgM in tolerance-susceptible immature murine B lymphocytes. Developmentally regulated differences in transmembrane signaling in splenic B cells from adult and neonatal mice. J Immunol. 1991;146:1446–54. [PubMed] [Google Scholar]
- 6.Suzuki H, Terauchi Y, Fujiwara M, Aizawa S, Yazaki Y, Kadowaki T, Koyasu S. Xid-like immunodeficiency in mice with disruption of the p85alpha subunit of phosphoinositide 3-kinase. Science. 1999;283:390–2. doi: 10.1126/science.283.5400.390. [DOI] [PubMed] [Google Scholar]
- 7.Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, Cantley LC. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–7. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 8.Venkataraman C, Shankar G, Sen G, Bondada S. Bacterial lipopolysaccharide induced B cell activation is mediated via a phosphatidylinositol 3-kinase dependent signaling pathway. Immunol Lett. 1999;69:233–8. doi: 10.1016/s0165-2478(99)00068-1. [DOI] [PubMed] [Google Scholar]
- 9.Ren CL, Morio T, Fu SM, Geha RS. Signal transduction via CD40 involves activation of lyn kinase and phosphatidylinositol-3-kinase, and phosphorylation of phospholipase C gamma 2. J Exp Med. 1994;179:673–80. doi: 10.1084/jem.179.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aagaard-Tillery KM, Jelinek DF. Phosphatidylinositol 3-kinase activation in normal human B lymphocytes. J Immunol. 1996;156:4543–54. [PubMed] [Google Scholar]
- 11.Lund FE, Yu N, Kim KM, Reth M, Howard MC. Signaling through CD38 augments B cell antigen receptor (BCR) responses and is dependent on BCR expression. J Immunol. 1996;157:1455–67. [PubMed] [Google Scholar]
- 12.Izuhara K, Harada N. Interleukin-4 activates two distinct pathways of phosphatidylinositol-3 kinase in the same cells. Biochem Biophys Res Commun. 1996;229:624–9. doi: 10.1006/bbrc.1996.1854. [DOI] [PubMed] [Google Scholar]
- 13.Kim KM, Reth M. Function of B-cell antigen receptor of different classes. Immunol Lett. 1995;44:81–5. doi: 10.1016/0165-2478(94)00219-h. [DOI] [PubMed] [Google Scholar]
- 14.Brink R, Goodnow CC, Crosbie J, Adams E, Eris J, Mason DY, Hartley SB, Basten A. Immunoglobulin M and D antigen receptors are both capable of mediating B lymphocyte activation, deletion, or anergy after interaction with specific antigen. J Exp Med. 1992;176:991–1005. doi: 10.1084/jem.176.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey GB, Scott DW. Role of phosphatidylinositol 3-kinase in anti-IgM- and anti-IgD-induced apoptosis in B cell lymphomas. J Immunol. 2001;166:1618–26. doi: 10.4049/jimmunol.166.3.1618. [DOI] [PubMed] [Google Scholar]
- 16.Jiang A, Clark EA. Involvement of Bik, a proapoptotic member of the Bcl-2 family, in surface IgM-mediated B cell apoptosis. J Immunol. 2001;166:6025–33. doi: 10.4049/jimmunol.166.10.6025. [DOI] [PubMed] [Google Scholar]
- 17.Marshall AJ, Niiro H, Lerner CG, Yun TJ, Thomas S, Disteche CM, Clark EA. A novel B lymphocyte-associated adaptor protein, Bam32, regulates antigen receptor signaling downstream of phosphatidylinositol 3-kinase. J Exp Med. 2000;191:1319–32. doi: 10.1084/jem.191.8.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]