Abstract
CD59, the sole membrane regulator of the membrane attack complex of complement, is broadly and abundantly expressed in man and other mammals. In mouse, CD59 is encoded by two homologous genes. The expression patterns and functional roles of the proteins encoded by these genes, mCD59a and mCD59b, have not been well characterized. Here we describe the generation of monoclonal and polyclonal antibodies detecting specifically mCD59a and mCD59b. These reagents have been used to study function and to ascertain the cell and tissue distributions of mCD59a and mCD59b. mCD59a was broadly distributed on endothelia, erythrocytes, platelets, and on numerous other cell types in organs, a distribution pattern resembling that of CD59 in other species. In marked contrast, expression of mCD59b was restricted to germ cell elements in the testis and mature spermatozoa. Both mCD59a and CD59b inhibited human and rodent complement with similar efficiency. These findings demonstrate that the broadly distributed mCD59a is the key regulator of the terminal complement pathway in mice whereas CD59b, expressed only in testis and on sperm, probably plays other roles in vivo.
Introduction
The complement (C) system is an enzymatic cascade of proteins that forms a vital part of the innate immune system. The end product of C activation on cells is the membrane-disrupting pore, called the membrane attack complex (MAC), which is made up of single copies of C5b, C6, C7 and C8 together with multiple C9 molecules.1,2 Since C is constantly activated at low levels in serum, self cells require protection to avoid accidental lysis.3 Several membrane-bound regulators exist on human cells to inhibit the early stages of C, these include CD59, decay accelerating factor (DAF, CD55) and membrane co-factor protein (MCP, CD46).4 CD59 is the sole inhibitor of MAC formation on human cells. It is widely distributed throughout the body, present on all circulating cells, endothelia, epithelia, and in most tissues.5 Its mode of action is to block the interaction of C8 with C9, thereby preventing pore formation in cell membranes.6 It is a small (18 000–25 000 MW), globular molecule, linked to the cell membrane through a glycosyl phosphatidylinositol (GPI) anchor and comprising 77 amino acids with a single N-linked carbohydrate group at Asn-18. This carbohydrate group is large (comprising almost half the mass of CD59) and heterogeneous, human erythrocyte CD59 has at least 120 glycoforms.7
Species analogues of CD59 have been purified and/or cloned from monkeys, mice, rats, sheep and pigs.8–13 The CD59 analogues in all species are structurally similar, having apparent molecular masses in the range 18 000–25 000, a single, large N-linked carbohydrate group at or near residue 18 and all are linked to the membrane through a GPI anchor. A mouse analogue of human CD59 (mCD59) was cloned and partially characterized in this laboratory several years ago,12,14 and we have recently described the targeted deletion of the gene encoding this protein.15 The situation has been complicated by the demonstration that mice, in contrast to all other species studied to date, have two genes encoding CD59 proteins.16 The first described protein is now termed mCD59a while the product of the newly identified gene is denoted mCD59b. These two molecules were 63% identical at the amino acid level and shared all major structural features. Initial studies using Northern blot analyses detected mCD59b mRNA only in testis,16 but later work from the same authors described widespread expression both at the mRNA level and by preliminary immunostaining studies with an anti-peptide antibody.17 However, the reagents necessary for a formal investigation of the distribution of the mCD59 proteins were not available.
Here we describe the generation and characterization of novel monoclonal antibodies (mAbs) and polyclonal antisera specific for each of the mCD59 proteins. The mAbs against mCD59a were generated both in rats and in the CD59a−/− mouse and mAbs against mCD59b were generated in the CD59a−/− mouse. Purified soluble, recombinant forms of mCD59a and mCD59b were used to immunize animals and polyclonal antibodies were also generated against mCD59a in the CD59a−/− mouse and against mCD59b in rabbits. These reagents have been used to assess the distribution of mCD59a and mCD59b on cells and tissues, confirming that, while mCD59a is broadly distributed, mCD59b is essentially restricted to testis and spermatozoa.
Materials and methods
Materials and animals
All chemicals were obtained from Fisher Scientific (Loughborough, UK) or Sigma Chemical Co. (Poole, UK). Buffers were: phosphate-buffered saline (PBS; 8·1 mm Na2HPO4, 1·5 mm KH2PO4, 137 mm NaCl, pH 7·4); buffered salt solution (BSS; 137 mm NaCl, 2 mm KCl, 80 mm Na2HPO4, pH 7·3); enzyme-linked immunosorbent assay (ELISA) coating buffer (0·2 m Na2CO3/NaHCO3 pH 9·6); flow solution [FCM; PBS, 15 mm ethylenediaminetetraacetic acid (EDTA), 3 mm sodium azide, 1% bovine serum albumin (BSA), pH 7·4]; lysis buffer (5 mm Na2HPO4, 5 mm NaH2PO4, 2 mm EDTA, 0·02% sodium azide, pH 7·4); veronal-buffered saline (VBS; 2·8 mm barbituric acid, 145·5 mm NaCl, 0·8 mm MgCl2, 0·8 mm CaCl2, 0·9 mm sodium barbital, pH 7·2; Oxoid Ltd, Basingstoke, UK); GVB (VBS with 0·1% gelatin (w:v)). All tissue culture reagents were from Life Technologies (Paisley, UK). Cells were cultured in RPMI-1640, 50 U penicillin/streptomycin, 1 μg/ml amphotericin B, 2 mm glutamine, 1 mm sodium pyruvate, 10% fetal calf serum.
Horseradish peroxidase (HRPO)-conjugated goat anti-rat immunoglobulin, goat anti-mouse immunoglobulin and Extravidin were obtained from Sigma or BioRad (Poole, UK), goat anti-rabbit immunoglobulin-HRPO and fluorescein isothiocyanate (FITC) -conjugated donkey anti-rabbit immunoglobulin were obtained from Jackson Laboratories (West Grove, PA). Phycoerythrin-conjugated goat anti-mouse immunoglobulin was purchased from Dako Ltd (Ely, UK). Biotinylated mAbs anti-mCD59a and anti-mCD59b and biotin-immunoglobulin isotype control were generated in-house by incubating 1 mg of purified mAb in PBS with 2 μg of sulpho-NHS-biotin (Pierce & Warriner, Chester, UK) at room temperature for 3 hr. Free biotin was removed by gel filtration in PBS on a sephadex-G25 column (PD-10, Amersham Pharmacia, St Albans, UK). Fc Block (2.4G2), anti-B220-FITC (clone RA3-6B2) and anti-CD3e-FITC (clone 145-2C11) were obtained from Pharmingen (San Diego, CA). Anti-CD11b-FITC (Mac-1 alpha chain; clone M1/70.15) was obtained from Caltag (TCS Biologicals, Buckingham, UK). Anti-mouse Crry (clone 5D5) was a gift from Professor V. M. Holers (Denver, CO). R-phycoerythrin-conjugated streptavidin was obtained from Jackson Laboratories.
Mouse blood was obtained fresh from the animal facility of the University of Wales College of Medicine (UWCM), by cardiac puncture under terminal anaesthesia and cells were separated as described below. Myeloma cell line SP2/0-Ag14, Chinese hamster ovary (CHO) and EL4 cells were obtained from ECACC (Porton Down, UK).
Both mCD59a−/− and wild-type control mice (C57Bl6/129Sv) were generated as described elsewhere.15 BALB/c mice were obtained from the animal facility at UWCM. Dark Agouti (DA) rats were obtained from Harlan (Oxford, UK).
Production of Fc-fusion proteins and antibodies
To create a mCD59a-immunoglobulin G (IgG1)Fc fusion protein, sequence encoding the signal peptide and a truncated form of mCD59a (C-terminal residue Lys72) was cloned into the expression vector pIgplus (R & D systems, Abingdon, UK). To obtain high expression levels, the DNA encoding the whole mCD59a-Fc construct was subsequently cloned into pDR2ΔEF1α as previously described.18 A fusion protein comprising mCD59b attached to the Fc portion of human IgG4 was generated by cloning sequence encoding the signal peptide and a truncated form of mCD59b (C-terminal residue Ser 85) directly into pDR2ΔEF1α upstream of and in frame with DNA encoding the hinge and Fc domains of human IgG4. CHO cells were transfected and stable lines were generated by hygromycin B selection.19 Both mCD59a-Fc and mCD59b-Fc were purified from tissue culture supernatant on glass-immobilized protein A (Prosep A; Bioprocessing Ltd, Consett, UK).
Rat mAbs to mCD59a were made by immunization of DA rats with mCD59a-Fc using standard schedules. Spleens were removed from immune rats, cells were harvested and fused with the SP2/0-Ag14 myeloma. Hybridomas were selected and screened for anti-mCD59a mAb as described below, and positive wells were subcloned by limiting dilution to monoclonality. Rat mAbs were isotyped by ELISA using biotin-conjugated antibodies specific for each rat antibody isotype (Pharmingen, Becton Dickinson, Oxford, UK).
Mouse mAbs to mCD59a were generated by immunization of mCD59a−/− mice with mCD59a-Fc using standard schedules and fusion as described above.
Mouse mAbs to mCD59b were generated from CD59a−/− mice immunized with EL4 cells expressing mCD59b (see below). These immunizations were performed before the mCD59b-Fc fusion protein was available. Tail bleeds were screened by flow cytometry for reactivity with CHO cells expressing mCD59b; cells transfected with ‘empty’ vector were used as a negative control as described.19 The mAbs were produced as described above and screened using flow cytometry on expressing and control cells. Mouse mAbs were isotyped using the IsoStrip mouse mAb isotyping kit (Boehringer Mannheim, Indianapolis, IN).
Sera from mCD59a-Fc-immunized rats or mice and supernatants from individual fusion wells were screened by ELISA. Alternate rows of 96-well plates were coated with 100 μl of either 1 μg/ml of human IgG (Sigma), mCD59a-Fc, or as a control, a mouse DAF-Fc fusion protein.18 After blocking, serum (diluted 1 : 10 in PBS) or fusion well supernatant was added to one of each coated well type and incubated. Plates were washed and the appropriate secondary antibody (HRPO-conjugated goat anti-rat immunoglobulin or goat anti-mouse immunoglobulin) was added, and incubated for 1 hr at 37°. Plates were washed and developed by adding 100 μl of OPD substrate (Dako) to each well. Absorbance at 490 nm was read in a BioRad plate reader.
The mAbs of appropriate isotypes were purified from tissue culture supernatant on Prosep A or Prosep G. IgM mAbs were purified on Thiosorb-M (Bioprocessing). Protein concentration was determined using the Pierce Protein Assay Reagent (Pierce & Warriner) with BSA as standard.
Polyclonal anti-mCD59a was generated using standard protocols in CD59−/− mice by immunization with mCD59a-Fc; animals were exsanguinated and antiserum harvested. Polyclonal anti-mCD59b was similarly generated in rabbits by immunization with either the mCD59b-Fc fusion protein or a mCD59b-specific peptide (residues 70–84; KNLDGLEEPNNAETS), synthesized in-house as an octameric multiple array peptide on a lysine core (Applied Biosystems). Antiserum was harvested from coagulated blood and tested for reactivity against fusion protein or peptide.
Expression of mCD59a and mCD59b in CHO and EL4 cells
Adherent CHO cells were transfected with expression vector using Lipofectamine (Life Technologies) as described previously.19 A mouse T-cell line (EL4; known to be negative for mCD59a expression) was transfected by electroporation. Briefly, EL4 cells were washed into ice-cold RPMI-1640 and resuspended at 107/ml. Cells (450 μl) were mixed with 10 μg DNA in a 0·4-cm cuvette, incubated for 15 min on ice and electroporated at 274 V, 950 μF. Cells were left on ice for 5 min and then taken into culture medium. Stable lines were generated by selection with 600 μg/ml Hygromycin B (Life Technologies). The mCD59a-expressing cell line was heterogeneous as judged by flow cytometry using the mAb against mCD59a-Fc. To select for cells expressing high amounts of mCD59a, cells were preincubated under sterile conditions with a blocking mAb anti-Crry, washed into GVB and incubated at 0·5 × 106/ml for 45 min at 37° with 25% rat serum, a dose chosen in preliminary experiments to cause approximately 50% lysis of the mCD59a-expressing line. Surviving cells were returned to culture and expression of mCD59a was assessed 3 days later. Although no mAb against CD59b was then available, EL4 cells expressing mCD59b were treated similarly to select for C resistance. Again, the amount of rat serum used was chosen following titration to cause approximately 50% lysis of expressing cells.
Lysates of transfected cells were prepared by incubating cells for 30 min at room temperature at 2 × 107/ml in PBS, 2% nonidet-P40, 1 mm phenylmethylsulphonyl fluoride, 10 mm EDTA, 10 μg/ml leupeptin (Sigma) and 10 μg/ml pepstatin A (Sigma). Lysates were centrifuged for 10 min at 15 000 g, an aliquot of the supernatant was added to an equal volume of non-reducing sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) loading buffer.
Mouse erythrocyte ghosts were made by suspending erythrocytes in cold lysis buffer; ghosts were washed by centrifugation and resuspension. Washed ghosts were mixed with an equal volume of SDS–PAGE loading buffer.
Cell lysates and ghosts were boiled for 2 min prior to loading on 15% SDS–PAGE in the Hoeffer Mighty Small Gel system (Hoeffer Scientific Instruments, Newcastle-under-Lyme, UK). Gels were electroblotted onto nitrocellulose and probed with the relevant antibodies using standard protocols. Blots were developed using a chemiluminescent substrate (SuperSignal, Pierce & Warriner) and captured on Kodak X-ray film.
Tissue expression and function of mCD59a and mCD59b
The mCD59a−/− and wild-type mice were killed, and tissue samples were collected immediately, mounted on cork boards with optimum cutling temperature compound (OCT) (EMS Laboratories Ltd, Ashford, UK) and snap frozen in isopentane. Frozen sections were cut on a CME cryostat (Shandon, Life Sciences International Europe, Runcorn, UK) and placed onto Snowcoat X-tra glass slides (Surgipath, Peterborough, UK). Sections were fixed in acetone for 10 min. For staining with mAbs, sections were treated with H2O2 to eliminate endogenous peroxidase activity and biotin and avidin binding sites were blocked using a commercial blocking kit (Vector Labs, Peterborough, UK). Biotinylated anti-mCD59a and anti-mCD59b mAbs (mCD59a.1; mCD59b.1, respectively) were first titrated on kidney or testis tissue sections to identify an appropriate dilution which was subsequently used on all remaining tissue types. Sections were incubated with mAbs for 1 hr at room temperature, washed in PBS then incubated with HRPO-Extravidin (1 : 100 dilution in PBS/1% BSA) for 1 hr at room temperature. After washing, sections were stained for 5 min with 0·05% 4,4-diaminobenzidine (Sigma) in PBS/0·02% H2O2, washed in water and counter-stained for 20 seconds with haematoxylin. Sections were dehydrated by immersing in 100% ethanol, washed in Xylene and mounted in a neutral mounting medium (XAM, BDH, Dorset, UK). For staining with polyclonal antisera, rabbit anti-mCD59b antiserum was first incubated overnight at 4° with mCD59a-Fc (1 mg/ml) to adsorb any reactivity towards Fc and mCD59a. The antiserum was centrifuged to remove the immunoprecipitate prior to application to sections essentially as described above. FITC-labelled donkey anti-rabbit IgG was used to detect bound antibody. Slides were viewed using a Leica microscope and representative images were captured using a digital camera.
Expression of mCD59a and mCD59b on blood cells was assessed by flow cytometry. Erythrocytes and platelets were obtained by centrifugation of EDTA-anticoagulated mouse blood at 2000 g, washing the pelleted cells in flow solution and resuspending to 0·1% erythrocytes, in flow solution. Leucocytes were made from heparin anticoagulated mouse blood by dilution (1 : 10) in erythrocyte lysis buffer (0·83% NH4Cl, 0·1% KCO3, 0·1 mm EDTA), centrifugation at 1300 g to pellet, followed by washing the pelleted leucocytes in flow solution. Leucocytes were standardized to a cell density of 106/ml for staining. Forward and side scatter in conjunction with two-colour flow cytometry was used to distinguish the various cell types. B220+ staining was used to identify B cells, CD3e was used to identify T cells and CD11b+ (Mac-1) was used to identify granulocytes. Each cell preparation was incubated with saturating amounts (determined by titration on erythrocytes or transfected cells) of the appropriate mAb, either unlabelled or biotinylated, for 25 min at 4°. Leucocytes were first incubated with 10 μg/ml 2.4G2 (Fc Block; Pharmingen) for 15 min, then with 10 μg/ml of the appropriate mAb or isotype control diluted in flow solution. Cells were washed three times in flow solution, then incubated for 25 min at 4° with the appropriate secondary reagent, a 1 : 100 dilution of streptavidin-conjugated R-phycoerythrin for biotinylated mAb or the appropriate FITC-labelled secondary antibody for unlabelled mAb. Cells were washed three times, and analysed on a Becton Dickinson FACScalibur (Oxford, UK). For each cell type, 5000 events were collected and all samples were run in triplicate.
Cells transfected with mCD59a or mCD59b were similarly analysed by flow cytometry following incubation either with a 1 : 200 dilution of antiserum (prefusion screen of mice immunised with mCD59b-expressing cells), a 1 : 2 dilution of culture supernatant (screening of anti-mCD59b hybridoma clones) or 10 µg/ml pure IgG/IgM (anti-Crry, anti-mCD59a, anti-mCD59b).
Functional assay for mCD59 on erythrocytes and transfected cells
Blood was collected from CD59a−/− mice and wild-type littermates into 10 mm EDTA. Erythrocytes were isolated by centrifugation for 5 min at 1300 g, and washed twice in PBS. A 2% suspension of erythrocytes was made from packed, washed cells in PBS. Erythrocytes were incubated on ice for 15 min with a 1 : 100 dilution of a rabbit antiserum against mouse erythrocytes that had been depleted of anti-mCD59a reactivity.15 Sensitized erythrocytes (EA) were washed twice in VBS (5 min, 1300 × g), then incubated for 20 min at 37° with a 1 : 10 dilution in VBS of C8-depleted human serum.20 The EAC5b-7 cells so formed were washed into PBS/10 mm EDTA. To complete the lytic pathway of C, rat serum diluted in PBS/10 mm EDTA was titrated to identify a serum dose at which lysis of the EAC5b-7 cells following a 15-min incubation at 37° was approximately 35% (1 : 3000 for wild-type erythrocytes and 1 : 10000 for mCD59a−/− erythrocytes). EAC5b-7 cells from each source were then incubated with anti-mCD59a mAb (10 μg/ml; 5 min on ice), washed once and then incubated with rat serum under the conditions and dilutions defined above.
To assess the function of mCD59a and mCD59b expressed on EL4, transfected and control cells were washed into GVB and resuspended at 106/ml. Cells (100 μl) were incubated for 45 min at 37° with 100 μl of rat or human serum diluted in GVB. Tubes containing cells were transferred onto ice and 100 μl of 6 μg/ml propidium iodide (PI) in flow buffer was added. Cell death was analysed by assessing PI permeability by flow cytometry. In the case of lysis by rat serum, EL4 cells were preincubated with 10 μg/ml blocking anti-Crry, and washed twice in GVB, prior to the lysis step. All assays were performed in triplicate.
Results
Monoclonal and polyclonal antibody production
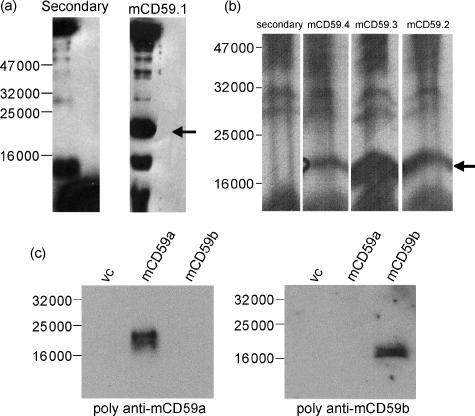
From the rat fusion, a single well positive against mCD59a-Fc but negative against the control proteins was detected from the original screen and brought through two rounds of recloning. This mAb, named mCD59a.1, was isotyped by ELISA and found to be IgG1. From the CD59a−/− mouse fusion for mCD59a, many wells were positive and five clones were taken through two rounds of recloning. These mAbs, termed mCD59a.2 to mCD59a.6 were isotyped using a commercial kit and were, respectively, IgM, IgG1, IgG2a, IgG1 and IgG1. The specificity of the various mAbs for mCD59a was confirmed by Western blotting of a nonreducing 15% SDS–PAGE of mouse erythrocyte ghosts (Fig. 1a, b). All the mAbs specifically recognized a broad band of 18 000–20 000 MW in the erythrocyte ghosts, the expected molecular mass of mCD59a. Other bands present in blots were also present when probed only with secondary antibody and represent non-specific reactivity in the polyclonal anti-immunoglobulin reagents. The rat mAb (mCD59a.1) and appropriate secondary antibody gave fewer non-specific bands and a sharper staining for mCD59a. All mAbs strongly stained mouse erythrocytes when analysed by flow cytometry (Fig. 2a). No staining of erythrocytes from the mCD59a−/− mouse was obtained (not shown). The mAbs were also reactive with mCD59a-transfected cells but not with vector control or CD59b-transfected cells when tested by Western analysis (not shown) or flow cytometry (Fig. 2b). Lysates of cells transfected with mCD59a or mCD59b were also stained with polyclonal antisera raised against mCD59a-Fc and mCD59b-Fc fusion proteins (Fig. 1c). These gave strong and specific staining in the expressing lines and showed no cross-reactivity between mCD59a and mCD59b. On Western analyses, mCD59a ran with a mobility of about 20 000 as expected, whereas mCD59b had a slightly faster mobility (approximately 18 000) (Fig. 1c).
Figure 1.
Western blotting with anti-mCD59 antibodies. (a, b) Western blots of mouse erythrocyte ghosts run under non-reducing conditions on a 15% SDS–PAGE, stained with mCD59.1 rat anti-mCD59a (a) or three of the six mouse mAbs anti-mCD59a (b). In each case, secondary antibody alone shows various bands indicating that these reagents cross-react with mouse proteins, but the specific mouse CD59a protein is clearly detected as a broad band at 18 000–20 000 MW (arrowed). (c) Western blots of EL4 cells transfected with mCD59a or mCD59b (or empty vector) probed with mouse polyclonal antiserum against mCD59a or rabbit polyclonal antiserum against mCD59b.
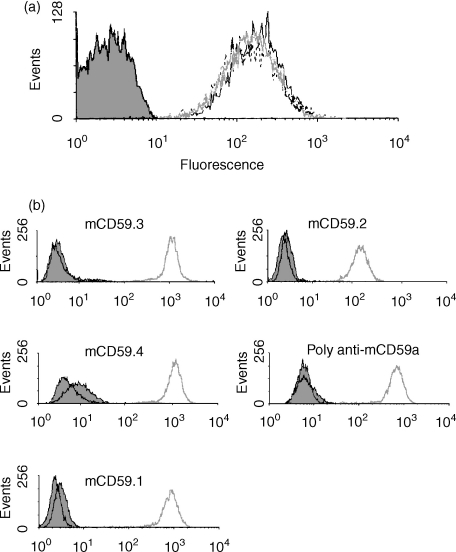
Figure 2.
Flow cytometry analysis using mAb anti-mCD59a. (a) Mouse erythrocyte staining with mAb mCD59.2 (grey line), mCD59.3 (solid black line) or mCD59.4 (dashed line). Control erythrocytes stained with second antibody alone are shown in the filled profile. (b) EL4 cells transfected with mCD59a (grey line), mCD59b (black line) or empty vector (filled profile) were stained with the various mAbs against mCD59a or with the mouse polyclonal antiserum against mCD59a and analysed by flow cytometry.
From the mCD59a−/− mouse fusion for mCD59b, despite a strong immune response in the immunized mice, only a single mAb was obtained, termed mCD59b.1. The mAb was strongly reactive with mCD59b-transfected CHO and EL4 cells but not with vector control or mCD59a-transfected cells in flow cytometry (Fig. 3), but did not detect mCD59b in Western analyses of transfected cell lysates. The antibody was isotyped and shown to be IgM.
Figure 3.
Flow cytometry analysis of expression of mCD59a and mCD59b. EL4 cells transfected with mCD59a, mCD59b, or with empty vector were stained with mAb mCD59.3 anti-mCD59a (solid black line), mCD59b.1 anti-CD59b (dashed line), 5D5 anti-Crry (grey line) or second antibody alone (filled profile) and analysed by flow cytometry.
Polyclonal antisera against the respective fusion proteins and the mCD59b peptide, when tested in ELISA against the immunizing antigen all had titres > 1 : 1024 compared to non-immune control sera.
Tissue distribution of mCD59a and mCD59b
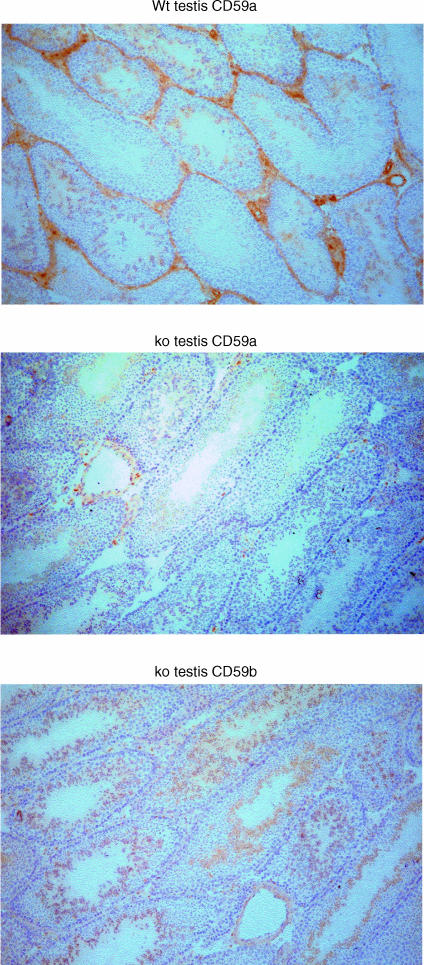
Acetone-fixed frozen sections of the prinicipal organs were stained as described in methods using mAb against mCD59a and mCD59b. A summary of the results is presented in Table 1. mCD59a was broadly distributed, expressed on endothelia throughout the animal and on numerous other cell types in the various organs, confirming and extending our earlier findings.15 In the kidney, the glomeruli, distal tubules and collecting ducts stained strongly for mCD59a, while proximal tubules were negative. In the heart, cardiac myocytes stained for mCD59a, although the strongest staining was seen on endothelium lining the chambers and major vessels. Myocytes in striated muscle also stained uniformly for mCD59a. In the liver, hepatocytes stained uniformly for mCD59a, with occasional cells, likely to be Kupffer cells, staining more strongly. In the skin, the epidermis and dermis stained weakly, while supporting striated muscle stained strongly. In the spleen, weak staining was observed in the red pulp areas, while the white pulp was negative. Endothelium of central arterioles of the white pulp stained strongly. In the brain, cerebral cortex showed weak, diffuse staining whereas in cerebellum, mCD59a was localized to white matter and the molecular layer while Purkinje cells and the granular layer were negative. Staining for mCD59a in the testis was typical of CD59 expression patterns in other species in that the Leydig cells and connective tissue surrounding the seminiferous tubules were strongly stained while immature spermatogonia and mature spermatozoa at the centre of the seminiferous tubules stained weakly (Fig. 4). These same tissues from the mCD59a−/− mouse were also tested and were uniformly negative.
Table 1.
Tissue distribution of mCD59a and mCD59b shown by immunostaining
| Tissue | Cell type | CD59a | CD59b |
|---|---|---|---|
| Blood (flow cytometry) | platelets | + + | – |
| erythrocytes | + + | – | |
| leucocytes | – | – | |
| Cerebral cortex | white matter | + – | – |
| grey matter | + – | – | |
| Cerebellum | molecular layer | + + | – |
| granular layer | – | – | |
| white matter | + | – | |
| Purkinje cells | – | – | |
| Heart | cardiac muscle | + + | – |
| endothelium | + + + | – | |
| Kidney | proximal tubules | – | – |
| distal tubules | + + | – | |
| glomerulus | + + | – | |
| collecting duct | + + | – | |
| endothelium | + + + | – | |
| Liver | hepatocytes | + | – |
| Striated muscle | myocytes | + | – |
| big cells | + + + | – | |
| Skin | epidermis | + + | – |
| dermis | + – | – | |
| basal hair follicle cells | – | – | |
| Testis | mature sperm | + + | + + |
| spermatogonia | + | + + | |
| Leydig cells | + + | – | |
| Spleen | white pulp | – | – |
| red pulp | + – | – | |
| arteriole (endothelium) | + + | – | |
| Lung | bronchial epithelium | + + | – |
| alveoli | + + | – |
Figure 4.
Immunohistochemical analysis of mCD59a and mCD59b expression in testis. Testes from wild-type (wt) and CD59a−/− (ko) mice were stained for mCD59a and mCD59b using appropriate mAb. Staining for mCD59a is abundant in wild-type but completely absent in CD59a−/− mice. Expression of mCD59b was identical in wild-type and CD59a−/− mice and only the latter is shown. Slides were viewed using a Leica microscope and representative images were captured using a digital camera. Original magnifications all ×200.
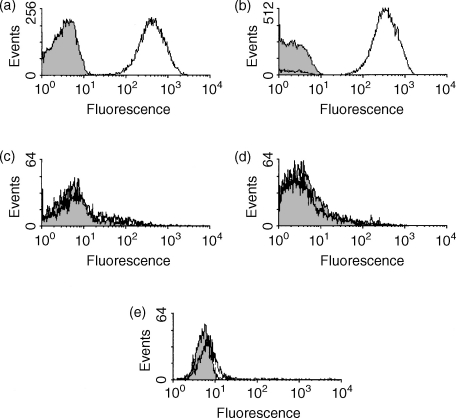
By flow cytometry, erythrocytes (Fig. 5a) and platelets (Fig. 5b) expressed mCD59a at a high and uniform level. B cells (B220+), T cells (CD3e+) and granulocytes were consistently negative for mCD59a (Fig. 5c, d and e, respectively). Blood cells from the mCD59a−/− mouse were all negative for mCD59a. These results were confirmed using other anti-CD59a mAbs from the panel.
Figure 5.
Expression of mCD59a on blood cells assessed by flow cytometry. Blood from BALB/c mice was collected in heparin. Isolated cells were stained for flow cytometry with a biotin-isotype control antibody (shown in grey), or biotinylated mCD59.1 (shown in black); (a) Erythrocytes and (b) platelets. Leucocytes were determined by forward and side scatter and then subdivided using two-colour flow cytometry. mCD59.1 staining is shown on: (c) B cells (determined by B220+ events), (d) T cells (CD3e+ events) and (e) granulocytes (CD11b+ events). At least 5000 events were collected for each cell type.
In contrast to the broad distribution of mCD59a revealed by immunostaining with specific mAbs, staining of the same panel of cells and tissues with the mAb mCD59b.1, reactive with mCD59b, revealed a positive signal only in testis (Fig. 4). Staining for mCD59b was restricted to the core of the seminiferous tubules where spermatids and spermatozoa were strongly positive, a pattern distinct from that of mCD59a in testis, described above. Tissues from the mCD59a−/− mouse were also stained and gave an identical staining pattern in the only positive tissue, the testis. Endothelium was negative for mCD59b and no staining of blood cells within vessels was observed. All blood cell types were uniformly negative for mCD59b as assessed by flow cytometry following staining with mCD59b.1 mAb (not shown). This mAb strongly stained cells transfected to express mCD59b (Fig. 3).
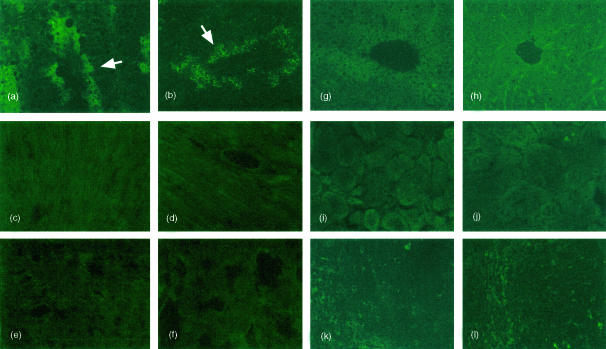
To confirm the observed distribution pattern of mCD59b, a panel of tissues was also stained with a polyclonal antiserum raised against the mCD59b-Fc fusion protein. The antiserum specifically detected mCD59b in Western blot analyses (Fig. 1c) and in flow cytometry on transfected cells (not shown). Sections from wild-type and mCD59a−/− mice were stained with the antiserum (adsorbed against mCD59a-Fc) and detected using fluorescent secondary antibodies to maximize sensitivity. Strong staining was detected in testis (Fig. 6a, b). The pattern of staining was identical to that observed with the mAb against mCD59b and was the same in wild-type (Fig. 6a) and mCD59a−/− (Fig. 6b) mice. All other tissues examined were essentially negative, although a weak and inconsistent signal was occasionally detected in liver (Fig. 6g, h) and in the red pulp of spleen (Fig. 6k, l).
Figure 6.
Immunofluorescence analysis of mCD59b expression in mCD59a−/− and wild-type mice. The various tissues were stained with mCD59a-Fc-adsorbed antiserum against mCD59b and developed as described in the Materials and methods. (a,c,e,g,i,k) wild-type mouse; (b,d,f,h,j,l) mCD59a−/− mouse. (a,b) testis; (c,d) heart; (e,f) lung; (g,h) liver; (i,j) kidney; (k,l) spleen. Arrows in (a,b) indicate strongly stained cells close to the lumen of the testicular follicles. Original magnification ×400 for testis, heart, liver and spleen, ×200 for lung and kidney.
Assessment of functional of mCD59a and mCD59b
The C inhibitory activity of mCD59a was first assessed on mouse erythrocytes by measuring the ability of the anti-mCD59a mAbs to functionally block the protein. Wild-type and mCD59a−/− erythrocytes bearing C5b-7 sites were incubated with or without 10 μg/ml of the various mAbs prior to completion of the C cascade by addition of rat serum as a source of C8 and C9. The rat anti-mCD59a mAb mCD59.1 and three of the five mouse anti-mCD59a mAb (mCD59.2, mCD59.3 and mCD59.4) caused increased lysis of wild-type erythrocytes, an approximate doubling of lysis compared with wild-type erythrocytes without mAb, indicating that mCD59a was protecting erythrocytes from lysis and that the majority of the mAbs were function blockers. The remaining two mAbs did not significantly enhance lysis. None of the mAbs enhanced lysis of mCD59a−/− erythrocytes, confirming that the effect was mCD59a-dependent.
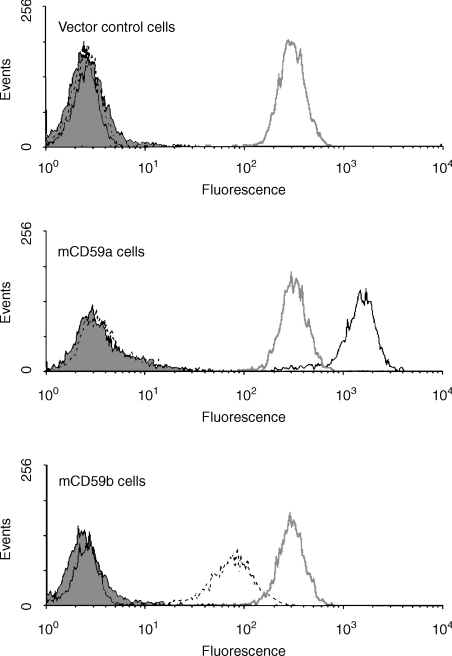
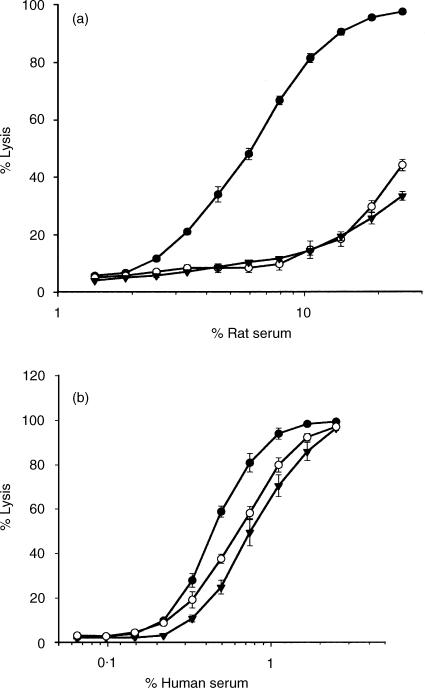
To test the ability of mCD59b to inhibit C, and compare with mCD59a, EL4 cells were transfected with mCD59a, with mCD59b, or with ‘empty’ vector. Initial analysis of the mCD59a-expressing line demonstrated that expression was heterogeneous, with clear high-expressing and low-expressing populations (not shown). A single treatment of cells with rat serum at a concentration predetermined to cause partial lysis efficiently removed the non-/low-expressing cells to yield a homogeneous and stable level of expression of mCD59a (Fig. 3). Cells expressing mCD59b were treated in the same way with rat serum to eliminate any negative/low-expressing cells and a homogeneous population expressing mCD59b was obtained (Fig. 3). As different mAb were used for the detection of mCD59a and mCD59b, it was not possible directly to compare the expression levels in the two lines. Nevertheless, as the same expression vector and method of selection was used for each line, it was assumed that the expression levels were similar. Expression of Crry, an endogenously expressed internal control, was identical in the two cell lines. mCD59a and mCD59b-expressing cells and control cells were incubated with either rat or human serum and lysis was assessed by measuring propidium iodide permeability. Expression of either mCD59a or mCD59b efficiently protected expressing cells from lysis by rat C and the degree of protection was similar (Fig. 7). In each case, the protective effect was less marked, but reproducible, against human serum (Fig. 7). Mouse serum was not tested in this system as EL4 were resistant to lysis by mouse serum even after mAb blockade of Crry.
Figure 7.
Functional analysis of mCD59a and mCD59b on transfected cells. EL4 transfected with mCD59a (○), mCD59b (▾) or empty vector (•) were incubated with various dilutions of rat (a) or human (b) serum as a source of complement and lysis measured as described in methods. Each point is the mean ± SD of triplicate determinations.
Discussion
We here describe the generation of antibodies against mCD59a and mCD59b and their application to analysis of the distribution and function of these proteins. These reagents have been used to demonstrate that mCD59a, like CD59 analogues in other species, is broadly expressed on cells and tissues, whereas expression of mCD59b is restricted to testis, present on germ cells and mature spermatozoa. The majority of the mAb against mCD59a were derived by immunization of mCD59a−/− mice, demonstrating the utility of gene-deleted mice for the generation of mAb against mouse proteins. Of the numerous clones obtained from a single fusion, five (mCD59a.2 to mCD59a.6) were further characterized. All identified native mCD59a as a broad band of 18 000–20 000 MW on Western blots of erythrocyte membranes under non-reducing conditions and stained transfected cells and erythrocytes in flow cytometry. Four of the six mAb generated against mCD59a inhibited the function of the protein as illustrated by increased C lytic susceptibility in a terminal pathway assay following antibody treatment of normal mouse erythrocytes but not of mCD59a−/− erythrocytes. This finding suggests that the majority of the mAb obtained targeted similar regions around the ‘active site’ of CD59.21 In an attempt to obtain mouse mAb against mCD59b, mCD59a−/− mice were immunized with mCD59b-expressing cells. Immunization generated a strong, specific antibody response, a result that suggested that the immunized mice either did not express mCD59b or expressed the protein only at immunologically isolated sites. Despite the strong immune response, only a single mAb specific for mCD59b was obtained from the fusion. This mAb did not detect mCD59a and none of the mAb raised against mCD59a detected mCD59b on cells transfected with the respective cDNA. Western blot analyses of lysates of transfected cells with the respective mAb showed that mCD59a had a larger apparent Mr (20 000 versus 18 000; Fig. 1c) despite the fact that mCD59b contained an additional 10 amino acids. The presence of a second potential site for N-glycosylation in mCD59a (N69), absent in mCD59b, might provide an explanation for the higher Mr of mCD59a.
The mAb were used to compare the distribution of mCD59a and mCD59b proteins. The expression pattern for mCD59a resembled that of CD59 in other species in that it was widely and abundantly expressed, with the highest expression observed on endothelium. While, in most respects, the distribution of mCD59a was very similar to that of human CD59, there were interesting differences. mCD59a was expressed, albeit weakly, in the white matter of the brain and cerebellum, whereas in humans, rats and pigs, white matter was CD59-negative.22–24 Purkinje cells in the mouse cerebellum were negative for mCD59a, as reported in the pig.24 Expression of mCD59a in kidney was similar to that in human, rat and pig; glomeruli, distal tubules and collecting ducts all expressed high levels of mCD59a.22–24 In the testis, strong staining was obtained for support cells between the seminiferous tubules and weaker staining at the centre of the tubules, where spermatids and maturing spermatozoa are located. In mouse spleen, weak staining was present in red pulp but other areas were negative. This was in contrast to the expression of CD59 seen in the pig and the rat where the majority of cells were positive with clear areas of negative cells which corresponded to the B- and T-cell zones, respectively.24,25 The expression pattern of CD59 in human spleen has not been reported. In peripheral blood, erythrocytes and platelets expressed high levels of mCD59a, whereas T and B lymphocytes and granulocytes were all mCD59a-negative. CD59 is expressed on erythrocytes in all species examined but platelets in rat, sheep and pig have been reported to be negative.9,24,26 In man, all leucocyte populations were positive for CD59, whereas in rats, T cells were CD59-negative and in pig, an uncharacterized subpopulation of leucocytes were CD59-negative. The distribution pattern for mCD59b was markedly different. Positive staining was obtained only in testis and only for germ cells and maturing spermatozoa in the centre of the seminiferous tubules. Mature spermatozoa obtained from mouse semen stained for mCD59b in head, neck and tail with no staining of midpiece (not shown). No specific staining was detected in any other tissue and all blood cell types were negative for mCD59b. The distribution pattern obtained with mAb anti-CD59b was corroborated using specific polyclonal antisera raised against a mCD59b-Fc fusion protein and a peptide derived from the unique C-terminal extension in mCD59b (residues 70–84).
The first description of mCD59b reported the presence of mCD59b mRNA only in mouse testis.16 However, a recent study using an anti-peptide (residues 37–61) polyclonal reagent to examine the distribution pattern of mCD59b protein claimed broad distribution of mCD59b in mice.17 Both mCD59a and mCD59b were found in kidney, brain, lung, spleen, testis and in all blood vessels; blood cell expression was not reported. In contrast, we found, using a specific mAb against mCD59b and two different polyclonal antisera, expression only in testis. We contend that our analyses, using agents of proven specificity, reveal the true distribution of mCD59b. This contention is strengthened by analyses in the mCD59a−/− mouse where all staining for mCD59a is lost, whereas the testis-specific staining for mCD59b is retained. The fact that mCD59a−/− mice immunized with mCD59b mounted a strong antibody response against the protein and yielded a specific mAb reactive with mCD59b also militates against the protein being broadly distributed. Expression restricted to the testis, an immunologically privileged site, would not restrict an immune response against mCD59b, whereas broad expression would. Of note, Qin et al. reported that mCD59a was expressed only in spermatids whereas mCD59b was expressed only in mature spermatozoa.17 Our data demonstrate that mature spermatozoa express both mCD59a and mCD59b, albeit with different patterns of distribution.
Our findings confirm that both mCD59a and mCD59b are effective regulators of C, acting to inhibit lysis by rodent and human serum. Qin et al. expressed mCD59a and mCD59b on CHO cells at similar copy number and reported that the latter was six times more active at inhibiting rat C.17 On the mCD59a-negative mouse cell line EL4, expression of either mCD59a or mCD59b caused similar degrees of resistance against human and rat C, suggesting that function was conserved but not enhanced in mCD59b. The observed conservation of functional activity may give additional clues to the residues essential for interaction with the forming MAC. Identification of residues in CD59 conserved between species have previously been used to map this putative active site and identify key residues, including W40, R53 and E56.10,12,21 Comparisons across these key residues in mCD59a and mCD59b reveal conservation of W40 in both, R53 in mCD59b (Q in mCD59a) and E56 in mCD59a (V in mCD59b). The presence of the additional 10 residues at the C terminus of CD59b and a second potential site for N-glycosylation in mCD59a (N69) might also have consequences for function. Further functional analyses and swapping of regions and single residues between these two molecules will be informative.
Why do mice alone of all species examined possess two forms of CD59? Whereas it is clear from the knockout mouse that mCD59a is an important C regulator, acting to protect from damage cells exposed to C,15 the highly restricted expression of mCD59b, present only on developing and mature spermatozoa, suggests different roles. CD59 in other species has been implicated as an adhesion molecule,27 and it is possible that mCD59b plays a role in sperm–egg interaction. The specific mAb against mCD59b described here provide tools to investigate this possibility. Creation of a mCD59b−/− mouse and the mCD59a/mCD59b double knockout would also provide clues to the roles of these proteins in the male reproductive system.
Acknowledgments
This work was funded by The Wellcome Trust (ref. 16668) through the award of a Senior Clinical Fellowship to B.P.M. The authors wish to thank Richard Mead, Jayne Chamberlain and Miriam Vigar for invaluable assistance.
Abbreviations
- C
complement
- E
erythrocyte
- MAC
membrane attack complex
- GPI
glycosyl phosphatidylinositol
- GVB
gelatin veronal buffer
- VBS
veronal-buffered saline
Note Added in Proof
Whilst in press, a paper appeared describing deletion of the CD59b gene (Qin X, Krumrei N, Grubissich L, Dobarro M, Aktas H, Perez G, Halperin JA. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity 2003; 18:217–27).
References
- 1.Podack ER, Tschopp J. Membrane attack by complement. Mol Immunol. 1984;21:589–603. doi: 10.1016/0161-5890(84)90044-0. [DOI] [PubMed] [Google Scholar]
- 2.Morgan BP. Mechanisms of tissue damage by the membrane attack complex of complement. Complement Inflamm. 1989;6:104–11. doi: 10.1159/000463082. [DOI] [PubMed] [Google Scholar]
- 3.Lachmann PJ, Hughes-Jones NC. Initiation of complement activation. Springer Semin Immunopathol. 1984;7:143–62. doi: 10.1007/BF01893018. [DOI] [PubMed] [Google Scholar]
- 4.Morgan BP, Harris CL. Complement Regulatory Proteins. London: Academic Press; 1999. [Google Scholar]
- 5.Davies A, Lachmann PJ. Membrane defence against complement lysis: the structure and biological properties of CD59. Immunol Res. 1993;12:258–75. doi: 10.1007/BF02918257. [DOI] [PubMed] [Google Scholar]
- 6.Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, Lachmann PJ. Human protectin (CD59), an 18, 0–20, 000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71:1–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Rudd PM, Morgan BP, Wormald MR, Harvey DJ, van den Berg CW, Davis SJ, Ferguson MA, Dwek RA. The glycosylation of the complement regulatory protein, human erythrocyte CD59. J Biol Chem. 1997;272:7229–44. doi: 10.1074/jbc.272.11.7229. [DOI] [PubMed] [Google Scholar]
- 8.Hughes TR, Meri S, Davies M, Williams JD, Morgan BP. Immunolocalization and characterization of the rat analogue of human CD59 in kidney and glomerular cells. Immunology. 1993;80:439–44. [PMC free article] [PubMed] [Google Scholar]
- 9.van den Berg CW, Harrison RA, Morgan BP. The sheep analogue of human CD59: purification and characterization of its complement inhibitory activity. Immunology. 1993;78:349–57. [PMC free article] [PubMed] [Google Scholar]
- 10.Rushmere NK, Harrison RA, van den Berg CW, Morgan BP. Molecular cloning of the rat analogue of human CD59: structural comparison with human CD59 and identification of a putative active site. Biochem J. 1994;304:595–607. doi: 10.1042/bj3040595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fodor WL, Rollins SA, Bianco-Caron S, Burton WV, Guilmette ER, Rother RP, Zavoico GB, Squinto SP. Primate terminal complement inhibitor homologues of human CD59. Immunogenetics. 1995;41:51. doi: 10.1007/BF00188435. [DOI] [PubMed] [Google Scholar]
- 12.Powell MB, Marchbank KJ, Rushmere NK, van den Berg CW, Morgan BP. Molecular cloning, chromosomal localization, expression, and functional characterization of the mouse analogue of human CD59. J Immunol. 1997;158:1692–702. [PubMed] [Google Scholar]
- 13.Hinchliffe SJ, Rushmere NK, Hanna SM, Morgan BP. Molecular cloning and functional characterization of the pig analogue of CD59: relevance to xenotransplantation. J Immunol. 1998;160:3924–32. [PubMed] [Google Scholar]
- 14.Holt DS, Powell MB, Rushmere NK, Morgan BP. Genomic structure and chromosome location of the gene encoding mouse CD59. Cytogenet Cell Genet. 2000;89:264–7. doi: 10.1159/000015630. [DOI] [PubMed] [Google Scholar]
- 15.Holt DS, Botto M, Bygrave AE, Hanna SM, Walport MJ, Morgan BP. Targeted deletion of the CD59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood. 2001;98:442–49. doi: 10.1182/blood.v98.2.442. [DOI] [PubMed] [Google Scholar]
- 16.Qian YM, Qin X, Miwa T, Sun X, Halperin JA, Song WC. Identification and functional characterization of a new gene encoding the mouse terminal complement inhibitor CD59. J Immunol. 2000;165:2528–34. doi: 10.4049/jimmunol.165.5.2528. [DOI] [PubMed] [Google Scholar]
- 17.Qin X, Miwa T, Aktas H, et al. Genomic structure, functional comparison, and tissue distribution of mouse Cd59a and Cd59b. Mamm Genome. 2001;12:582–9. doi: 10.1007/s00335-001-2060-8. [DOI] [PubMed] [Google Scholar]
- 18.Harris CL, Spiller OB, Morgan BP. Human and rodent decay-accelerating factors (CD55) are not species restricted in their complement-inhibiting activities. Immunology. 2000;100:462–70. doi: 10.1046/j.1365-2567.2000.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiller OB, Harris CL, Morgan BP. Efficient generation of monoclonal antibodies against surface-expressed proteins by hyperexpression in rodent cells. J Immunol Met. 1999;224:51–60. doi: 10.1016/s0022-1759(99)00008-3. [DOI] [PubMed] [Google Scholar]
- 20.Abraha A, Morgan BP, Luzio JP. The preparation and characterization of monoclonal antibodies to human complement component C8 and their use in purification of C8 and C8 subunits. Biochem J. 1988;251:285–92. doi: 10.1042/bj2510285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodian DL, Davis SJ, Morgan BP, Rushmere NK. Mutational analysis of the active site and antibody epitopes of the complement-inhibitory glycoprotein, CD59. J Exp Med. 1997;185:507–16. doi: 10.1084/jem.185.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meri S, Waldmann H, Lachmann PJ. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991;65:532–7. [PubMed] [Google Scholar]
- 23.Funabashi K, Okada N, Matsuo S, Yamamoto T, Morgan BP, Okada H. Tissue distribution of complement regulatory membrane proteins in rats. Immunology. 1994;81:444–57. [PMC free article] [PubMed] [Google Scholar]
- 24.Hanna SM, Williams GT, Van Den Berg CW, Morgan BP. Characterization in vitro and in vivo of the pig analogue of human CD59 using new monoclonal antibodies. Immunology. 1998;95:450–9. doi: 10.1046/j.1365-2567.1998.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiller OB, Hanna SM, Morgan BP. Tissue distribution of the rat analogue of decay-accelerating factor. Immunology. 1999;97:374–84. doi: 10.1046/j.1365-2567.1999.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes TR, Piddlesden SJ, Williams JD, Harrison RA, Morgan BP. Isolation and characterization of a membrane protein from rat erythrocytes which inhibits lysis by the membrane attack complex of rat complement. Biochem J. 1992;284:169–76. doi: 10.1042/bj2840169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menu E, Tsai BC, Bothwell AL, Sims PJ, Bierer BE. CD59 costimulation of T cell activation. CD58 dependence and requirement for glycosylation. J Immunol. 1994;153:2444–56. [PubMed] [Google Scholar]