Abstract
The age-related increase of peripheral CD4+ CD8+ double-positive (DP) T cells in cynomolgus monkeys has been reported previously. Because the percentage of DP T cells in cynomolgus monkeys increases abruptly in parallel with the thymic involution occurring at around 11 years of age, it was suggested that thymic involution was associated with this increase. Therefore, a longitudinal study was carried out over 5 years to clarify the exact time when DP T lymphocytes start to increase in relation to the thymic involution. Twelve cynomolgus monkeys at 6 years of age were classified into three groups, based on their percentage of DP T cells, as follows: DP-High (>5% DP T cells); DP-Middle (1–5% DP T cells); and DP-Low (<1% DP T cells). In the DP-High group, the percentage of DP T cells showed an abrupt increase, of >10%, in monkeys at 7 years of age, and the prevalence of this subset correlated with a distinctive increase in the percentage of memory T cells (CD4+ CD29high, CD8+ CD28−), indicating an association with the maturation of immune function, including thymic involution. To assess the thymic function, the coding joint of T-cell receptor excision circles (cjTREC) levels in sorted T cells were analysed by polymerase chain reaction (PCR)-enzyme-linked immunosorbent assay (ELISA). The cjTREC in the T cells of the DP-High group (4362 ± 3139 copies/105 T cells) was significantly lower than that (22 722 ± 4928 copies/105 T cells) of the DP-Low group. Moreover, the mean copy number of cjTREC in naive T cells was also significantly different between the DP-High and the DP-Low group (0·457 ± 0·181 and 1·141 ± 0·107, respectively). These findings suggest that thymic involution has an influence on the age-related increase of DP T cells in cynomolgus monkeys.
Introduction
Peripheral T cells in humans are divided into major two subsets – CD4 and CD8 single positive (SP) – on the basis of mutually exclusive expression of their surface glycoproteins.1,2
During maturation in the thymus, immature CD4− CD8− double-negative (DN) T cells, which originate from the bone marrow-derived T-cell precursors, differentiate into either CD4 or CD8 SP T cells and finally emigrate into the periphery. These two subsets differ in function, as well as in their major histocompatibility complex (MHC) restrictions. The CD4 SP T cells have MHC class II restriction and function as helper/inducer T cells, whereas CD8 SP T cells are MHC class I restricted and have cytotoxic functions.1,2 In addition to these two subsets, a very low percentage [1–5% in peripheral blood leucocytes (PBL)] of T cells co-expressing both CD4 and CD8 molecules are also observed in the peripheral blood of healthy individuals.3,4 Recently, several investigators have reported a transient and/or persistent increase of peripheral DP T cells in healthy individuals – particularly in elderly people5–7– and in some individuals with neoplasia, infectious diseases or immune disorders.8,9 However, the origin and functional significance of DP T cells has remained largely unclear until now.
In contrast, a considerable number of peripheral DP T cells have been observed in chicken, swine and macaque monkeys.10–16 Our previous studies of cynomolgus monkeys have demonstrated that DP T cells:
increase in percentage in an age-dependent manner;
exhibit a CD1b− CD4+ CD8dim phenotype, typically expressing CD8αα homodimers, and are considered to be of extrathymic T-cell lineage;
have a phenotype of resting memory T cells; and
appear to have both helper and cytotoxic functions – some clones of DP T cells share the same T-cell receptor (TCR) Vβ with CD4 SP T cells, indicating that they are derived from the same origin.13–16
Interestingly, it appears that the percentage of DP T cells in cynomolgus monkeys abruptly increases in parallel with the thymic involution that occurs at around 11 years of age.14,15 This finding prompted us to investigate the relationship between age-related increase in DP T cells and the thymic involution.
Thymic function, particularly thymic output, can be assessed by quantification of recent thymic emigrants (RTE) – newly produced T cells emigrating from the thymus to the periphery. However, the lack of an appropriate phenotypic marker for these RTE has made it difficult to distinguish them from long-lived naive T cells.17–21
It has recently been reported that thymic output could be monitored by measuring the T-cell receptor excision circles (TREC).17–19 TREC are generated as episomal DNA fragments during TCR gene rearrangement in the thymus and are exported to the periphery within RTE. Because TREC are not replicated during mitosis and are diluted out with each cell division, measurement of TREC allows us to distinguish between RTE and long-lived naive T cells. Therefore, TREC have been used as biomarkers of thymic function.17–21
In this study, we attempted to identify the time-points where an increase in DP T-cell number occurs and investigated the effect of thymic involution on the age-related increase of DP T cells. To achieve this, we carried out a longitudinal study over a 5-year time-period using 12 cynomolgus monkeys (Macaca fascularis), whose thymic function was assessed by quantification of coding joint TREC (cjTREC) in the sorted T cells of monkeys with a high or a low percentage of DP T cells.
Materials and methods
Selection of monkey
In 1996, heparinized peripheral blood samples were collected from 20, apparently healthy, 6-year-old cynomolgus monkeys. After analysing the percentage and phenotype of DP T cells, 12 monkeys were selected for further longitudinal study, as discussed below.
Three monkeys were randomly selected from four with > 5% DP T cells (Fig. 1b) and were defined as the DP-High group. In view of our previous studies,13–15 the number of peripheral DP T cells in the remaining 16 monkeys were expected to increase in an age-related manner. To examine age-related dynamics of the DP T-cell level in detail, nine of the remaining 16 monkeys were subdivided into DP-Middle and DP-Low groups, as follows: the DP-Low group consisted of three monkeys with the lowest level (<1%) of DP T cells; and the DP-Middle group consisted of six monkeys, selected at random from the remaining 13 monkeys with a DP T cell level of 1–5%. Blood samples were obtained periodically over the following 5 years and analysed to determine the percentage of DP T cells and phenotype of the PBL.
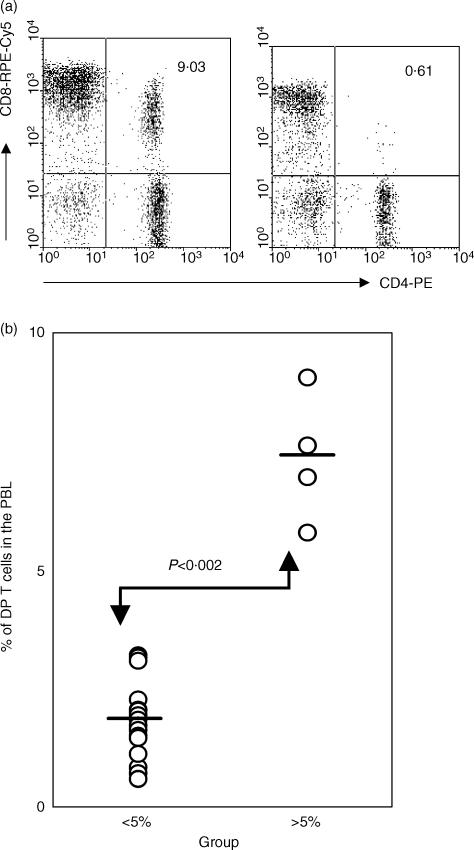
Figure 1.
Increase of peripheral double-positive (DP) T cells in cynomolgus monkeys at 6 years of age. (a) Flow cytometric analysis of CD4 and CD8 co-expression on peripheral blood lymphocytes (PBL). The results shown are representative of 20 monkeys. (b) The percentage of DP T cells in PBL of 20 monkeys at 6 years of age. Four monkeys had a significantly higher percentage (>5% in PBL) of DP T cells when compared with those of other 16 monkeys. The bars indicate the mean, and the P-value was obtained using the Student's t-test.
All the monkeys were bred and reared in Tsukuba Primate Center, National Institute of Infectious Diseases. This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institute of Infectious Diseases.22
Preparation of cells
Leucocytes were isolated, as previously described.14 Briefly, heparinized blood was treated with ACK lysis buffer (0·15 m NH4Cl, 1 mm KHCO3, 0·1 mm of Na2EDTA, pH 7·2) for 5 min at 37° to remove red blood cells, then washed with RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (FCS; Sigma, St Louis, MO), 50 IU/ml penicillin (Sigma), 50 µg/ml streptomycin (Sigma) and 2 mm l-glutamine (Sigma) (this medium was termed RPMI-10%). The peripheral blood mononuclear cells (PBMC) were isolated by density-gradient centrifugation using Ficoll–Paque (Ficoll–Hypaque; Amersham Pharmacia Biotech, Uppsala, Sweden). The leucocytes and PBMC were suspended in RPMI-10% and stored at 4° until required.
A single-cell suspension of the fetal thymus was prepared by mechanical mincing and Ficoll–Paque centrifugation. Isolated thymocytes were stored at −80° until required.
Antibodies and flow cytometry analysis
Cell-surface antigens were analysed by multicolour flow cytometry, as described previously.14 Typically, 2 × 105 cells were stained with a combination of the following monoclonal antibodies (mAbs): fluorescein isothiocyanate (FITC)-labelled anti-CD3 (FN18; Biosource, Camarillo, CA), anti-CD4 (NU-TH/1; Nichirei, Tokyo, Japan), anti-CD28 (KOLT-2; Nichirei), anti-CD29 (4B4; Coulter, Hialeah, FL) and anti-CD45RA (2H4; Coulter); phycoerythrin (PE)-labelled anti-CD4 (NU-TH/1, Nichirei), anti-CD8 (Leu-2a; Becton-Dickinson, Mountain View, CA), anti-CD28 (L293; Becton-Dickinson) and anti-CD62L (Leu8; Becton-Dickinson); and R-phycoerythrin (RPE)-Cy5-labelled anti-CD8 (DK25; DAKO, Glostrup, Denmark). The fluorescence of the stained samples was analysed using a fluorescence-activated cell sorter (FACSCalibur; Becton-Dickinson). Lymphocytes were gated on the forward- and side-scatter pattern and 10 000–20 000 events were collected. The fluorescence intensity was determined using CellQuest software (Becton-Dickinson). Automatic lymphocyte counts were performed using Sysmex K-4500 (Sysmex, Kobe, Japan). The percentage of DP T cells multiplied by the lymphocyte counts gave an absolute number of DP T cells.
For isolation of T cells, the PBMC were reacted with the following mAbs – FITC-labelled anti-CD14 (Leu-M3; Becton-Dickinson), anti-CD16 (Leu™-11a; Becton-Dickinson) and anti-CD20 (Leu™-16; Becton-Dickinson) – at 4° for 1 hr and then were washed with RPMI-10%. CD14−, CD16− and CD20− cells were sorted by Epics Elites (Coulter, Hialeah, FL). The purity of sorted T cells was always > 98%.
Preparation of the cjTREC standard DNA
Genomic DNA was isolated from fetal thymocytes using the GFM™ genomic blood DNA-purification kit (Amersham Pharmacia Biotech). Polymerase chain reaction (PCR) for the cjTREC fragment was performed in a 50-µl reaction mix containing 5 µl of 10× Ex Taq Buffer (Takara, Shiga, Japan), 4 µl of dNTPs (Takara), 0·5 µl (2·5 U) of Ex Taq DNA polymerase (Takara), 1 µl of a 10-µm concentration of each primer (forward 5′-gatacagtcttcagctgttgtaactgc-3′ and reverse 5′-ctcggaaactgactcagatgatccatg-3′) and 1 µl of genomic DNA (100 ng). Cycling conditions were 94° for 5 min, followed by 40 cycles of 30 seconds at 94°, 30 seconds at 60°, and 30 seconds at 70°. Presence of the amplified DNA product was confirmed by electrophoresis on a 2% agarose gel followed by staining with ethidium bromide.
Because of the nucleotide insertions and deletions at the recombination site of the coding joint, amplified DNA products could not be sequenced directly. Therefore, cloning was performed using a TOPO TA cloning system and the plasmids. Isolated clones were sequenced in the LI-COR sequencing apparatus (ALOKA, Tokyo, Japan).
DNA isolation and PCR–enzyme-linked immunosorbent assay (ELISA)
Genomic DNA was prepared from sorted T cells using 10 mm Tris (pH 8·0) containing 50 µg/ml proteinase K (Boehringer Mannheim, Indianapolis, IN). The cell/proteinase K solution (105 cells/5 µl) was incubated at 56° for 1–2 hr, and then inactivated at 95° for 15 min. The 5 µl of lysate was considered to be equivalent to 1 × 105 cells owing to no additional purification steps.
Quantification of cjTREC was performed using a PCR–ELISA system, according to the manufacturer's instructions (Boehringer Manheim) and a previously described method.17 For Dig-labelling of the PCR product, 2 µl of genomic DNA (equivalent to 4 × 104 T cells) was used as template and the PCR reaction was performed in a final 50-µl reaction volume, consisting of 2 µm primers (forward 5′-ctaataaaaagatcctcaagggtccagactg-3′ and reverse 5′-cctatttgttaaggcacattaaaatctcccactg-3′), 1× PCR buffer, 2·5 mm MgCl2, 20 µm Dig-labelling mix and 0·02 U/µl Taq polymerase (Boehringer Mannheim). Cycling conditions were as follows: 95° for 5 min; 25 cycles of 30 seconds each at 90, 60 and 72°; then a final extension step at 72° for 7 min. Standard cjTREC, at different copies (50 000, 5000, 500, 50, and 0), were co-amplified with each PCR reaction.
ELISA for Dig detection of cjTREC was performed using a Dig-Detection kit (Boehringer Manheim), as previously described.17 Briefly, 10 µl of each PCR product was denatured at room temperature for 10 min, using 20 µl of denaturation solution, and then 220 µl of a hybridization solution containing 7·5 pmol/ml of a biotin-labelled internal probe (5′-tctgtgtctagctcccagcc-3′) was added. After mixing well, 200 µl of the mixture was transferred into the streptavidin-coated plate and incubated at 55° for 3 hr. After three washes, 200 µl of anti-Dig peroxidase solution (10 mU/ml) was added to each well. The plate was incubated at 37° for 30 min and washed, as previously described.17 As chromogen, 200 µl of ABTS substrate solution was added and incubated at 37° for 30 min. The plate was gently shaken at every step of the incubation.
The absorbance was measured at a wavelength of 405 nm and a reference of 490 nm on an automatic ELISA reader (Thermo max, Molecular Devices Corp., Sunnyvale, CA). The copy number of an unknown sample was calculated using the standard curve obtained from the cjTREC standard DNA.
Statistics
Statistical analysis was conducted using statistica (Statsoft Inc., Tulsa, OK). The Student's t-test and the Duncan test were applied to compare the relationships between variables. Significant differences were considered to occur at a P-value of < 0·05.
Results
Monkeys with a higher percentage of DP T cells tend to have a high percentage of CD4 SP and CD8 SP T cells with memory phenotype
Among the 20 monkeys tested at 6 years of age, DP T cells comprised 0·6–9% of the PBL (Fig. 1a). Four monkeys had significantly higher percentages (7·37 ± 1·36%) of DP T cells than the other monkeys (1·82 ± 0·82%) of the group (Fig. 1b). Consistent with a previously published report,15 a proportion of increased DP T cells expressed a resting memory phenotype (CD28− CD29high), and the percentage of DP T cells with the CD28+ CD29low phenotype was similar in the two groups (data not shown).
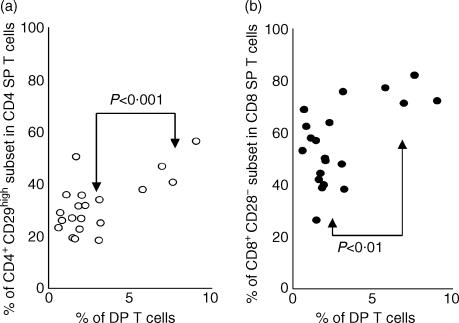
We first wished to investigate whether an increase in the percentage of DP T cells is related to phenotypic change in the PBL, particularly proportional changes in the CD4 SP or CD8 SP T cells with a memory phenotype. To achieve this, the cell-surface expression of CD28 and CD29, which are markers associated with resting memory T cells in cynomolgus monkeys,14 was assessed. Figure 2 shows that the percentages of CD4+ CD29high (45·44 ± 8·23%) and CD8+ CD28− (75·77 ± 5·04%) subsets were significantly higher in the four monkeys with a higher percentage of DP T cells than in the others of the same group (28·47 ± 8·19% and 51·09 ± 12·91%, respectively), at 6 years of age.
Figure 2.
Percentages of CD4 single-positive (SP) and CD8 SP T cells with memory phenotype in cynomolgus monkeys at 6 years of age. To evaluate the percentage of T cells with memory phenotype, the leucocytes isolated from 20 monkeys were stained with CD28-labelled fluorescein isothiocyanate (FITC) or CD29-FITC, CD4-labelled phycoerythrin (PE), and CD8-labelled R-phycoerythrin (RPE)-Cy5. In four monkeys with a higher percentage of double-positive (DP) T cells, the levels of memory CD4 SP (a) and CD8 SP (b) T cells were significantly higher than those of other monkeys. The P-value was obtained using the Student's t-test.
Age-related dynamics of an increase in DP T cells were different among each group
To identify the time-point(s) where the increase in DP T cells occurs, we selected 12 monkeys from the initial group of 20, according to the criteria described in the Materials and Methods, and divided them into three groups: DP-High (>5% DP T cells in PBL; n = 3); DP-Middle (1–5% DP T cells in PBL; n = 6); and DP-Low (<1% DP T cells in PBL; n = 3).
Table 1 shows individual changes in the percentages and absolute numbers of DP T cells over the 5-year time-period of the study. In the monkeys of the DP-High group, the percentage of DP T cells showed a rapid elevation to approximately 10% at 7 years of age (P < 0·002), representing a significant increase in the absolute number of DP T cells (430 ± 185 cells/µl blood) when compared with those (81 ± 49 and 31 ± 49/µl, respectively) in the DP-Middle and DP-Low groups. Over the 5-year period of the study, the absolute numbers of DP T cells in the DP-High group were maintained at > 270 cells/µl, except for that in monkey no. 9 at 8·8 years of age. Among monkeys in the DP-Middle group, the percentage of DP T cells in three (in monkeys 4, 7 and 8) gradually increased to around 5% at 9 years of age and basically similar results were obtained in the kinetics of absolute numbers. In particular, the absolute number of DP T cells in monkeys 4 and 8 was maintained at > 250 cells/µl after 8·8 years of age and this value is close to the minimum level in the DP-High group. On the other hand, the percentages and absolute numbers of DP T cells in the DP-Low group were maintained (in monkeys 5 and 6) at around 1%, or were slightly increased (see monkey no. 3) over 5 years, compared to levels at 6 years of age.
Table 1.
Age-related individual changes in percentage and absolute number of DP T cell subset over 5 years
| Age of monkeys | 6.5 | 7.3*† | 7.7*† | 8.8*† | 9.4 | 10.0*†‡ | 11.0*†‡ | 11.6* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | No | %A | Abs.B | % | Abs. | % | Abs. | % | Abs. | % | Abs. | % | Abs. | % | Abs. | % | Abs. |
| 2 | 5.80 | NT | 11.41 | 342/3000 | 9.08 | 418/4600 | 8.21 | 378/4600 | 22.21 | NT | 10.82 | 552/5100 | 16.82 | 454/2700 | 17.23 | NT | |
| DP-High | 9 | 6.97 | NT | 11.32 | 306/2700 | NT | NT | 8.89 | 196/2200 | 12.79 | NT | 9.48 | 474/5000 | 11.43 | 274/2400 | 14.10 | NT |
| 12 | 7.63 | NT | 10.89 | 643/5900 | 10.46 | 785/7500 | 10.74 | 859/8000 | 10.99 | NT | 9.55 | 678/7100 | 8.82 | 459/5200 | 10.47 | NT | |
| 1 | 1.85 | NT | 1.72 | 71/4100 | 2.85 | 77/2700 | 3.53 | 74/2100 | 2.23 | NT | 2.42 | 80/3300 | 2.00 | 50/2500 | NT | NT | |
| DP-Middle | 4 | 2.03 | NT | 2.22 | 104/4700 | 3.52 | 197/5600 | 5.04 | 373/7400 | 5.55 | NT | 5.25 | 341/6500 | 5.48 | 384/7000 | NT | NT |
| 7 | 2.05 | NT | 2.24 | 47/2100 | 2.75 | 72/2600 | 4.79 | 187/3900 | 6.50 | NT | 4.31 | 134/3100 | 6.08 | 97/1600 | NT | NT | |
| 8 | 1.72 | NT | 2.50 | 168/6700 | 3.06 | 147/4800 | 4.54 | 250/5500 | 5.39 | NT | 4.52 | 303/6700 | 4.66 | 256/5500 | NT | NT | |
| 10 | 1.13 | NT | 2.08 | 31/1500 | 2.48 | 112/4500 | 3.51 | 112/3200 | 4.40 | NT | 2.47 | 141/5700 | 3.85 | 116/3000 | NT | NT | |
| 11 | 1.45 | NT | 1.93 | 64/3300 | 1.60 | 37/4200 | 1.99 | 111/5600 | 2.61 | NT | 2.58 | 126/4900 | 2.57 | 77/3000 | NT | NT | |
| 3 | 0.72 | NT | 0.86 | 38/4400 | 0.90 | 71/7900 | 1.28 | 97/7600 | 2.68 | NT | 2.03 | 175/8600 | 2.78 | 120/4300 | 3.10 | NT | |
| DP-Low | 5 | 0.83 | NT | 3.75 | 38/1000 | 0.86 | 11/1300 | 0.97 | 23/2400 | 0.80 | NT | 0.90 | 40/4400 | 0.60 | 17/2800 | 0.70 | NT |
| 6 | 0.61 | NT | 0.99 | 17/1700 | 0.94 | 20/2100 | 0.92 | 24/2600 | 0.74 | NT | 1.11 | 48/4300 | 1.07 | 32/3000 | 1.22 | NT | |
indicates the percentages of DP T cells in the PBL.
indicates the absolute numbers of DP T cells and lymphocyte subset per μl blood. (DP T cell count/lymphocyte count).
All absolute numbers were compared by Duncan test.
Indicate significant differences (P < 0.05) within DP-High versus DP-Low respectively.
Indicate significant differences (P < 0.05) within DP-High versus DP-Middle respectively.
Indicate significant differences (P < 0.05) within DP-Middle versus DP-Low, respectively.
NT, not tested
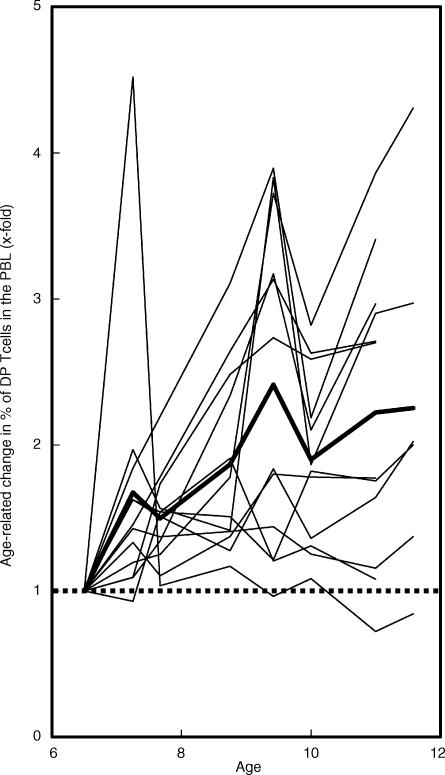
As shown above, individual differences were observed in the dynamics of age-related increase in DP T cells. However, the percentages of this subset showed a tendency to generally increase over the 5 years of the study as compared to those obtained when the monkeys were 6 years of age (Fig. 3), and this age-related increase is consistent with data of our cross-sectional studies.14,15
Figure 3.
Changes in percentage of the double-positive (DP) T-cell subset in 20 cynomolgus monkeys over the 5-year study period. Percentages of the DP T-cell subset are shown as x-fold change, compared with those obtained from the monkeys when 6 years of age. The bold line indicates the mean value among the 20 monkeys tested. The dashed horizontal line indicates no change, compared with those (1x) at 6 years of age.
As previously reported,13 the proportion of DP T cells showing an increase during this study typically expressed the CD4+ CD8dim phenotype (data not shown), indicating that they exhibit the CD8αα homodimer7,8,13 and also the memory phenotype (CD28− CD29high). The percentage of DP T cells with the CD28+ CD29low phenotype rarely increased in any individual over the 5 years of the study (data not shown).
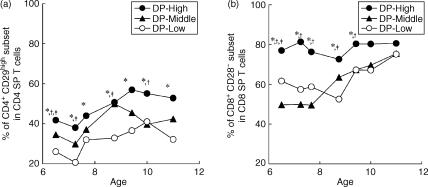
Figure 4 shows age-related changes, among each group, in the percentage of CD4 SP and CD8 SP T cells with memory phenotype. In the DP-High group, a high percentage of the CD8+ CD28− subset in CD8 SP T cells was observed in monkeys at 6 years of age and this subset was maintained at around 80% for the 5 years of the study. In contrast, in the DP-Middle and DP-Low groups, the CD8+ CD28− subset did not show an increase until the monkeys were 8 and 9 years of age, respectively, and reached a comparable level with that of the DP-High group when the monkeys reached 11 years of age (Fig. 4b). In the case of CD29 expression, although the mean percentage of the CD4+ CD29hi subset in CD4 SP T cells was different among the three groups, the age-related dynamics of this subset was similar in each group over the 5 years of the study (Fig. 4a).
Figure 4.
Age-related changes in percentage of the CD4 single-positive (SP) (a) and CD8 SP (b) T cells with memory phenotype over the 5-year study period. All values were compared by using the Duncan test. *, † and ‡ indicate significant differences (P < 0·05) within double-positive (DP)-High versus DP-Low, DP-High versus DP-Middle, and DP-Middle versus DP-Low, respectively.
The percentage sum of CD4 SP and DP T cells showed minimal changes in each group during the 5-year study period
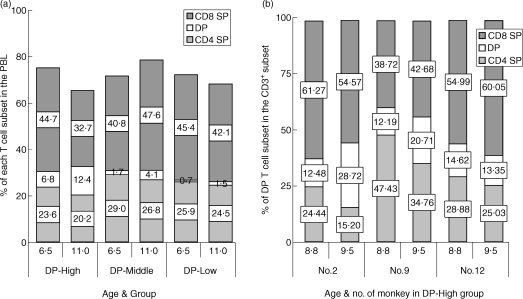
We next investigated proportional changes in each T-cell subset – CD4 SP, CD8 SP and DP T cells – among the three groups of monkeys during the 5-year study period. As shown in Fig. 5(a), the increase of DP T cells was accompanied by a decrease of CD4 SP T cells in the PBL, and the percentage sum of CD4 SP and DP T cells remained essentially unchanged in each group during the 5-year study period, despite changes in percentages of the CD8 SP T-cell subset. Because there was no data on the absolute number of DP T cells at 6 years of age, we were unable to show changes in absolute number of cells in the two subsets. However, based on the data shown in Table 1, changes in absolute numbers may be expected to be similar to those shown as percentages in Fig. 5(a).
Figure 5.
Age-related changes in each T cell subset – CD4 single positive (SP), CD8 SP and double-positive (DP) T cells – over the 5-year study period. (a) Although the percentage of each T-cell subset was variable, the percentage sum in the CD4 SP and DP T cells was essentially unchanged in each group during the 5-year study period. (b) In two monkeys of the DP-High group, a rapid increase was observed in the proportion of DP T cells over an 8-month time-period. Data shown represent the proportional changes of T-cell subsets (CD4 SP, CD8 SP and DP T cells), in total CD3+ cells, over 8 months. Values shown in the boxes represent the percentage of each subset.
To further confirm this observation, we also analysed the proportional change of CD4 SP and CD8 SP T cells in the CD3+ subsets, when DP T-cell subset showed the most marked increase during each period of time. Over 8 months, the percentage of DP T cells in two monkeys of the DP-High group increased about twofold and CD4 SP T cells showed a rapid decrease during the same time-period (Fig. 5b). Consequently, the sum of percentages in the CD4 SP and DP T cells in the CD3+ subsets rarely changed in these two monkeys.
Monkeys with a higher percentage of DP T cells have fewer copies of cjTREC in sorted T cells
In previous reports14,15 it was suggested that the age-related increase of DP T cells in cynomolgus monkeys might be associated with thymic involution. To test this hypothesis, we measured cjTREC levels, which are used as a biomarker of thymic output, in sorted T cells of monkeys in the DP-High and DP-Low group.
To adapt the PCR–ELISA method to quantification of cjTREC in cynomolgus monkeys, we first cloned and sequenced cjTREC isolated from fetal thymocyte of the cynomolgus monkey. The cjTREC sequence of the cynomolgus monkey showed 94·68% homology to that of human cjTREC (data not shown). This clone was then used as the cjTREC standard DNA in the following PCR–ELISA. Based on the standard curve created by plotting different copy numbers of this clone (data not shown), the standards ranged from 5 × 104 to 5 × 102 copies, with a distilled water control having 0 copies.
As summarized in Table 2, the cjTREC level in the sorted T cells of the DP-High group was 4362 ± 3139 copies/105 T cells and this number was about five times less than that (22 722 ± 4928 copies/105 T cells) of the DP-Low group. However, the cjTREC concentration is prone to becoming diluted-out by cell division or clonal expansion, even if the thymic output is constant.20,21 It is therefore important that cjTREC levels are compared with those of naive T cells which are thought to be a subset with a very rare dividing rate in vivo. We next evaluated, in the sorted T cells, the percentage contributed by the ‘truly naive’ CD45RA+ CD62L+ subset and compared it with the cjTREC levels (Table 2).
Table 2.
cjTREC levels in the sorted T cells of DP-High and DP-Low group
| cjTRECs (copies/105 T cells) | ||||||
|---|---|---|---|---|---|---|
| Group | No. | % of DP T cells in the PBL | % of naive subset in the sorted T cellsA | Number of copiesB | Mean ±SD | The mean copy number of cjTRECs in each naive T cells† |
| DP-High | 2 | 17.23 | 6.33 | 2 778 | 4 362 ± 3 139* | 0.439 |
| 9 | 14.10 | 8.15 | 2 330 | 0.286 | ||
| 12 | 10.10 | 12.33 | 7 978 | 0.647 | ||
| DP-Low | 3 | 3.10 | 16.38 | 17 098 | 22 722 ± 4 928 | 1.044 |
| 5 | 0.70 | 23.06 | 26 283 | 1.140 | ||
| 6 | 1.22 | 19.70 | 24 785 | 1.258 | ||
The T cells were sorted out by deletion of CD14+, CD16+, and CD20+ lymphocytes from the PBMC. Sorted T cells (1×106) were used for preparation of genomic DNA and a part of them was stained with CD45RA-FITC and CD62L-PE. The naive T cell subset was defined by CD45RA+CD62L+ phenotype.
Indicates significant difference (P < 0.006) in TREC levels between DP-High and DP-Low group (Student's t test).
Firstly, the number of naive subset in the 105 T cells was calculated by the percentageA of naive subset in the sorted T cells and then this number was compared with cjTREC levelsB in the 105 T cells measured in this study. If all naive T cells in the periphery are RTE, this value can be maximally calculated at approximately 1.26 [20].
TCR gene rearrangements, by which signal joint (sj)TREC and cjTREC are generated, occur at identical sites in 70% of TCRαβ T cells and consequently identical TREC are generated.19,20 Although these rearrangement events occur in both alleles, it was found that all TCRαβ T cells have their TREC generated on at least one allele and about 80% of TCRαβ T cells have their TREC generated on both alleles.20 Therefore, if all naive T cells in the periphery are RTE, the mean copy number of cjTREC in each naive T cell can be maximally calculated at approximately 1·26 as follows:
 |
The mean copy number of cjTREC in each naive T cell was calculated by using the percentage of naive subsets in the sorted T cell and cjTREC levels measured in this study (Table 2). In the DP-High group, this mean copy number (0·457 ± 0·181) was significantly lower than that (1·141 ± 0·107) of the DP-Low group (P < 0·005).
Discussion
In previous cross-sectional studies on cynomolgus monkeys14,15 we have reported on the age-related increase in the percentage of DP T cells in the periphery. This subset was characterized as showing a marked increase in monkeys at around 11 years of age and this finding suggested that an age-related increase in DP T cells might be influenced by thymic involution.
Our findings initially showed that a higher percentage of DP T cells (>5% in the PBL) first appeared in monkeys at 6 years of age. These four monkeys with a higher percentage of DP T cells at this age had significantly higher percentages of memory subsets CD4+ CD29high (45·44 ± 8·23% in the CD4 SP T cells) and CD8+ CD28− (75·77 ± 5·04% in the CD8 SP T cells) than the other monkeys with a lower percentage of DP T cells (Fig. 2). These percentages of memory subsets were comparable to those of 15-year-old monkeys in which it is accepted that thymic involution, as well as age-related remodelling of peripheral DP T cells, is complete.14,15,23 This observation suggests that the prevalence of DP T cells in peripheral blood may reflect the maturation of immune function.
In a further longitudinal study, age-related dynamics of DP T cells were clearly different among each group (Table 1). The question arises as to why these individual differences occur in monkeys of the same age. One possible explanation may be that there are individual differences in the extent to which thymic involution progresses. At this point, it is important to note that impairment of thymic function by mediastinal irradiation results in the expansion of CD4CD8αα+ or CD8αα+ T cells in humans.24
The thymus is a central lymphoid organ that provides a microenvironment for T-cell development and is characterized by age-related atrophy.25–28 Thymic involution is one of the most dramatic age-related changes in the immune system. Therefore, it is well known that thymic output has an influence on various immunological events, such as differentiation, proliferation and composition of T-cell subsets.28 Nevertheless, only limited studies on the effect of thymic involution in the immune system have been reported as a result of difficulty in directly sampling the thymus. Several groups recently developed a novel assay that can assess thymic function by quantification of sjTREC or cjTREC levels.
Our results show that cjTREC levels (4362 ± 3139 copies/105 T cells) and percentage of the naive subset (8·94 ± 3·08%), in the sorted T cells of the DP-High group, were significantly lower than those (22 722 ± 4928 copies/105 T cells and 19·71 ± 3·34%, respectively) of the DP-Low group (Table 2). This observation suggests that the age-related increase in DP T cells is associated with thymic involution. However, this difference of cjTREC levels between the two groups can be attributed to an increase in memory T cells as well as a decrease in RTE by diminished thymic output in the DP-High group (Figs 2 and 4), because these cjTREC concentrations are diluted out as a result of peripheral T-cell division or clonal expansion, even if thymic output is constant. Therefore, we compared cjTREC levels in naive T cells which are considered to be a subset with a very rare dividing rate in vivo, of the DP-High group and the DP-Low group.
The mean copy number of cjTREC per one naive T cell was significantly different from that of cells of both the DP-High group and the DP-Low group (0·457 ± 0·181 and 1·147 ± 0·107, respectively). This observation suggests that low cjTREC levels in monkeys of the DP-High group are caused by undersupply of RTE rather than by diminution of naive T cells in the periphery, emphasizing a decline of thymic output by thymic involution. However, we cannot completely discount another possibility – that naive T cells of the DP-High group divide at a higher frequency than those of the DP-Low group – because it has been recently reported that naive T cells could be activated by stimulatory cytokines, independent of antigen, without phenotypic change.21 Nevertheless, it should be noted that naive cell proliferation without phenotypic change was generally observed in the absence of thymic output or in state of diminished thymic output (such as in adult thymectomized mice) and following transfer of T cells to irradiated mice.29,30 Diminished thymic output in cynomolgus monkeys with higher percentage of DP T cells therefore may result in an increase in proliferation of this naive cell type, without phenotypic change, in the context of T homeostatic control.
On the other hand, it should be noted that the age-related increase of DP T cells could not be explained only by thymic involution. The variation in percentage of DP T cells in cynomolgus monkeys was considered to be the result of remarkable individual differences, as about 25% of monkeys over 11 years of age still have a low percentage (<5%) of DP T cells.14,15 In our recent familial study on cynomolgus monkeys, it was suggested that the percentage of DP T cells was genetically controlled, with a high heritability (h2: 0·55) that was estimated from the regression of offspring on mid-parent. Additionally, the offspring from parents with a low percentage (<5%) of DP T cells also had a low percentage of this subset when they were over 11 years old (W. W. Lee et al., manuscript in preparation). It is possible that this phenomenon may be linked to certain MHC types, associated with resistance and susceptibility to infectious agents, which may become recall antigen to DP T cells. Interestingly, the appearance of DP T cells in chickens is inherited in a dominant Mendelian manner.11
Several lines of evidence indicate that DP T cells are derived from CD4 SP T cells. Studies on human and swine suggest that after activation, CD4 SP memory T cells acquire the ability to express the CD8α chain and permanently maintain the CD4+CD8αα+ phenotype.8,10,12,31 Our previous single-strand conformation polymorphism (SSCP) data on TCR clonotype in cynomolgus monkeys indicated that some clones of DP T cells share the same TCR Vβ with CD4 SP T cells.16 From these findings, DP T cells in cynomolgus monkeys are probably derived from CD4 SP T cells via phenotypic change. Our present data support this hypothesis. During the 5 years of the present study, the overall percentage sum of the CD4 SP and DP T cells remained essentially unchanged in each group, regardless of any proportional change in each T-cell subset (Fig. 5). Because it seems that the proportion of mature T cells is regulated by homeostatic mechanisms that maintain the size of each subset and total size of the T-cell pool at a near-constant level,32,33 it is conceivable that the sum of the CD4 SP and DP T cells, as observed in this study, is regulated by these mechanisms. Recently, our preliminary data showed that highly purified CD4 SP T cells were induced to express CD8αα homodimer molecule on the their surface in response to anti-CD3/CD28 polyclonal activation (W. W. Lee et al., unpublished observations).
Taken together, the present results suggest that the prevalence of peripheral DP T cells in cynomolgus monkeys is associated with the maturation of immune function, and age-related increase in this subset is influenced by thymic involution.
Acknowledgments
We thank the staff of The Corporation for Production and Research of Laboratory Primates for animal care and blood collection, and Mr Hiroaki Shibata for technical advice and reading of the manuscript. This study was supported by funding for Comprehensive Research on Ageing and Health from the Japan Foundation for Ageing and Health.
References
- 1.Miceli MC, Parnes JR. Role of CD4 and CD8 in T cell activation and differentiation. Adv Immunol. 1993;53:59–122. doi: 10.1016/s0065-2776(08)60498-8. [DOI] [PubMed] [Google Scholar]
- 2.Zamoyska R. CD4 and CD8: modulator of T-cell receptor recognition of antigen and of immune responses? Curr Opin Immunol. 1998;10:82–7. doi: 10.1016/s0952-7915(98)80036-8. [DOI] [PubMed] [Google Scholar]
- 3.Blue ML, Daley JF, Levine H, Schlossman SF. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985;134:2281–6. [PubMed] [Google Scholar]
- 4.Lanier LL, Ruitenberg JJ, Phillips JH. Human CD3+ T lymphocytes that express neither CD4 nor CD8 antigens. J Exp Med. 1986;164:339–44. doi: 10.1084/jem.164.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laux I, Khoshnan A, Tindell C, Bae D, Zhu X, June CH, Effros RB, Nel A. Response differences between human CD4+ and CD8+ T cells during CD28 costimulation: implications for immune cell-based therapies and studies related to the expansion of double-positive T-cells during ageing. Clin Immunol. 2000;96:187–97. doi: 10.1006/clim.2000.4902. [DOI] [PubMed] [Google Scholar]
- 6.Colombatti A, Doliana R, Schiappacassi M, Argentini C, Tonutti E, Feruglio C, Sala P. Age-related persistent clonal expansions of CD28− cells: phenotypic and molecular TCR analysis reveals both CD4+ and CD4+ CD8+ cells with identical CDR3 sequences. Clin Immunol Immunopathol. 1998;89:61–70. doi: 10.1006/clin.1998.4580. [DOI] [PubMed] [Google Scholar]
- 7.Tonutti E, Sala P, Feruglio C, Yin Z, Colombatti A. Phenotypic heterogeneity of persistent expansions of CD4+ CD8+ T cells. Clin Immunol Immunopathol. 1994;73:312–20. doi: 10.1006/clin.1994.1204. [DOI] [PubMed] [Google Scholar]
- 8.Suni MA, Ghanekar SA, Houck DW, Maecker HT, Wormsley SB, Picker LJ, Moss RB, Maino VC. CD4+ CD8dim T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Eur J Immunol. 2001;31:2512–20. doi: 10.1002/1521-4141(200108)31:8<2512::aid-immu2512>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan YB, Landay AL, Zack JA, Kitchen SG, Al-Harthi L. Upregulation of CD4 on CD8+ T cells: CD4dim CD8bright T cells constitute an activated phenotype of CD8+ T cells. Immunology. 2001;103:270–80. doi: 10.1046/j.1365-2567.2001.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuckermann FA. Extrathymic CD4/CD8 double positive T cells. Vet Immunol Immunopathol. 1999;72:55–66. doi: 10.1016/s0165-2427(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 11.Luhtala M, Lassila O, Toivanen P, Vainio O. A novel peripheral CD4+ CD8+ T cell population; inheritance of CD8alpha expression on CD4+ T cells. Eur J Immunol. 1997;27:189–93. doi: 10.1002/eji.1830270128. [DOI] [PubMed] [Google Scholar]
- 12.Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996;87:500–12. [PMC free article] [PubMed] [Google Scholar]
- 13.Akari H, Terao K, Murayama Y, Nam KH, Yoshikawa Y. Peripheral blood CD4+ CD8+ lymphocytes in cynomolgus monkeys are of resting memory T lineage. Int Immunol. 1997;9:591–7. doi: 10.1093/intimm/9.4.591. [DOI] [PubMed] [Google Scholar]
- 14.Nam KH, Akari H, Terao K, Itagaki S, Yoshikawa Y. Age-related changes in major lymphocyte subsets in cynomolgus monkeys. Exp Anim. 1998;47:159–66. doi: 10.1538/expanim.47.159. [DOI] [PubMed] [Google Scholar]
- 15.Nam KH, Akari H, Terao K, Ohto H, Itagaki S, Yoshikawa Y. Age-dependent remodeling of peripheral blood CD4+ CD8+ T lymphocytes in cynomolgus monkeys. Dev Comp Immunol. 1998;22:239–48. doi: 10.1016/s0145-305x(97)00058-x. [DOI] [PubMed] [Google Scholar]
- 16.Nam KH, Akari H, Terao K, Shibata H, Kawamura S, Yoshikawa Y. Peripheral blood extrathymic CD4+ CD8+ T cells with high cytotoxic activity are from the same lineage as CD4+ CD8− T cells in cynomolgus monkeys. Int Immunol. 2000;12:1095–103. doi: 10.1093/intimm/12.7.1095. [DOI] [PubMed] [Google Scholar]
- 17.Al-Harthi L, Marchetti G, Steffens CM, Poulin J, Sekaly R, Landay A. Detection of T cell receptor circles (TRECs) as biomarkers for de novo T cell synthesis using a quantitative polymerase chain reaction-enzyme linked immunoabsorbent assay (PCR-ELISA) J Immunol Methods. 2000;237:187–97. doi: 10.1016/s0022-1759(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 18.Sodora DL, Douek DC, Silvestri G, Montgomery L, Rosenzweg M, Igarashi T, Bernacky B, Johnson RP, et al. Quantification of thymic function by measuring T cell receptor excision circles within peripheral blood and lymphoid tissues in monkeys. Eur J Immunol. 2000;30:1145–53. doi: 10.1002/(SICI)1521-4141(200004)30:4<1145::AID-IMMU1145>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Potis MA, Haase AT, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 20.Hazenberg MD, Verschuren MC, Hamann D, Miedema F, van Dongen JJ. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med. 2001;79:631–40. doi: 10.1007/s001090100271. [DOI] [PubMed] [Google Scholar]
- 21.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, Coutinho RA, Lange JM, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036–42. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 22.Honjo S. The Japanese Tsukuba Primate Center for Medical Science (TPC): an outline. J Med Primatol. 1985;14:75–89. [PubMed] [Google Scholar]
- 23.Yoshida T. Comparison between non-human primates and humans: characterization of growth patterns. Human Sci. 1994;6:52–77. [in Japanese] [Google Scholar]
- 24.Watanabe N, De Rosa SC, Cmelak A, Hoppe R, Herzenberg LA, Roederer M. Long-term depletion of naive T cells in patients treated for Hodgkin's disease. Blood. 1997;90:3662–72. [PubMed] [Google Scholar]
- 25.Haynes BF, Hale LP. The human thymus. A chimeric organ comprised of central and peripheral lymphoid components. Immunol Res. 1998;18:175–92. doi: 10.1007/BF02788778. [DOI] [PubMed] [Google Scholar]
- 26.Aspinall R, Andrew D. Thymic involution in ageing. J Clin Immunol. 2000;20:250–6. doi: 10.1023/a:1006611518223. [DOI] [PubMed] [Google Scholar]
- 27.Bertho JM, Demarquay C, Moulian N, Van Der Meeren A, Berrih-Aknin S, Gourmelon P. Phenotypic and immunohistological analyses of the human adult thymus: evidence for an active thymus during adult life. Cell Immunol. 1997;179:30–40. doi: 10.1006/cimm.1997.1148. [DOI] [PubMed] [Google Scholar]
- 28.Hirokawa K, Utsuyama M, Kasai M, Kurashima C, Ishijima S, Zeng YX. Understanding the mechanism of the age-change of thymic function to promote T cell differentiation. Immunol Lett. 1994;40:269–77. doi: 10.1016/0165-2478(94)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–90. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–35. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Donovan MR, Jones DRE, Robins RA, Li KF, Shim HK, Zheng Z, Arlett CF, Capulas E, et al. Co-cultivation of CD4+ and CD8+ human T-cells leads to the appearance of CD4+ cells expressing CD8 through de novo synthesis of the CD8 α-subunit. Hum Immunol. 1999;60:1018–27. doi: 10.1016/s0198-8859(99)00098-1. [DOI] [PubMed] [Google Scholar]
- 32.Marrack P, Bender J, Hildeman D, Jordan M, Mitchell T, Murakami M, Sakamoto A, Schaefer BC, et al. Homeostasis of αβTCR+ T cells. Nat Immunol. 2000;1:107–11. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 33.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9–14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]