Abstract
Sub-unit vaccines utilizing purified mycobacterial proteins or DNA vaccines induce partial protection against mycobacterial infections. For example, immunization with DNA vaccines expressing the gene for the immunodominant 35 000 MW protein, common to Mycobacterium avium and Mycobacterium leprae but absent from the Mycobacterium tuberculosis complex, conferred significant protection against infection with either virulent M. avium or M. leprae in mice. However, the level of protection was equivalent to that obtained with the viable, attenuated vaccine, Mycobacterium bovis, bacille Calmette–Guèrin (BCG). The cytokine, interleukin (IL)-12, is essential for priming naïve CD4+ T lymphocytes to differentiate into interferon-γ (IFN-γ)-secreting T cells. We have used a novel self-splicing vector expressing both chains of murine IL-12 to determine if plasmid IL-12 would increase the efficacy of a vaccine expressing the M. avium 35 000 MW protein (DNA-Av35). Co-immunization with p2AIL-12 and DNA-Av35 led to a significant increase in the number of antigen-specific IFN-γ secreting cells and total amount of IFN-γ released, but a concomitant fall in the antibody response to the 35 000 MW protein. This pattern of response was associated with enhanced clearance of M. avium from the liver and spleen of coimmunized mice, and was significantly more effective than BCG or DNA-Av35. alone. Following M. avium challenge there was significant increase in the expansion of the 35 000 MW antigen-reactive T cells in the coimmunized mice. Therefore, plasmid-delivered IL-12 acts as an effective adjuvant to increase the protective efficacy of a single DNA vaccine against M. avium infection above that achieved by BCG, and this strategy may improve the efficacy of subunit vaccines against M. leprae and M. tuberculosis.
Introduction
Mycobacterial infections remain major causes of mortality and morbidity worldwide. Tuberculosis causes 2–3 million deaths and 9 million new cases per annum,1 and there is a continuing high case detection rate of leprosy in endemic countries despite the implementation of antimicrobial control programs. Infection with members of the Mycobacterium avium complex are responsible for significant morbidity in patients with human immunodeficiency virus/acquired immune deficiency syndrome and other immunodeficiency disorders,2 and are increasingly recognized as a cause of pulmonary disease in immunocompetent individuals.3 Immunization with the vaccine strain M. bovis bacille Calmette–Guèrin (BCG) provides partial, but variable, protection against tuberculosis and leprosy4 and new approaches to immunization against mycobacterial infections are urgently required. Recently a number of protein and DNA vaccines expressing single mycobacterial antigens have been found to induce partial protection against experimental infection with M. tuberculosis,5–8M. leprae9 or M. avium,10,11 suggesting that non-replicating subunit vaccines may be effective in humans. However, of the 18 different anti-tuberculosis DNA vaccines reported thus far, none were more effective than BCG (reviewed in 12). In the case of M. avium and M. leprae infection, DNA vaccines expressing the shared immunodominant 35 000 MW protein induced protective immunity equivalent to, but not greater than, that afforded by BCG against either infection.9,10 Therefore, the protective immunity provided by DNA and other subunit vaccines must be increased if they are to be considered as practical replacements for BCG.
The cytokine, interleukin (IL)-12, plays a major role in the induction of interferon-γ (IFN-γ) T helper 1 (Th1)-like cell responses, which are essential for protective immunity against mycobacterial infections.13 It promotes Th1-like responses through the secretion of IL-2 and IFN-γ, while inhibiting Th2-like responses.14 Neutralization of IL-1213,15 or genetic deletion of the p40 chain of IL-1216 results in progressive infection with M. avium in mice, and mutations in the IL-12 signalling pathway are associated with progressive M. avium infection in humans.17 Dendritic cells (DCs) rather than macrophages are the major source of IL-12 during M. avium infection,18 and the levels of IL-12 secreted are related to the degree of virulence of the M. avium strain.19 The addition of recombinant IL-12 protein as an adjuvant increases efficacy of vaccines against protozoal and viral pathogens, such as Leishmania and herpes simplex virus (HSV),20,21 and recently was found to enhance the protective efficacy of culture filtrate proteins against M. avium.19 Furthermore, vector-encoded IL-12 enhanced the immune response to both DNA and protein vaccines.22 Therefore we have investigated whether codelivery of a plasmid encoding both chains of murine IL-12 with a DNA vaccine expressing the M. avium 35 000 MW antigen is more effective than BCG at inducing protective immunity against systemic infection with a virulent strain of M. avium.
Materials and methods
Plasmid vaccines and recombinant protein
The DNA vaccine expressing M. avium 35 000 MW protein (DNA-Av35) was prepared by cloning the gene for the M. avium 35 000 MW antigen into the vector pJW4303 between the cytomegalovirus early/intermediate promoter and the bovine growth hormone polyadenylation sequence, as previously described.10 The parental plasmid, pJW4303, was used as the control vector (DNA-Neg). The plasmid expressing murine IL-12 (p2AIL12) was prepared by cloning the genes for the p40 and p35 chains of Il-12 on either side of the self-cleaving peptide termed 2A, of the foot-and-mouth-disease virus (FMDV).23,24 This permits the conjugate expression of both chains in the same cell. Transient transduction of HEK293 cells with p2AIL12 confirmed the release of biologically active IL-12·23 The gene for M. avium 35 000 MW protein was expressed in M. smegmatis and the recombinant 35 000 MW protein purified by monoclonal antibody affinity chromatography.25
Animals and immunization schedules
Female C57BL/6 mice were obtained as specific pathogen-free mice from ARC (Perth, Australia) and maintained in specific pathogen-free conditions. Groups of mice (n = 5) were immunized between 8 and 12 weeks of age with 50 µg of each plasmid in volume of 50 µl by intramuscular injection (i.m.) into the tibialis anterior muscle of each hindleg. Mice were immunized three times, at three weekly intervals, with DNA-Av35 (100 µg) and p2AIL12 or the control plasmid (100 µg each) or DNA-Neg and p2AIL12 or the control plasmid (100 µg each) to provide equivalent amounts of total DNA. Mice immunized with BCG were administered 5 × 104 colony-forming units (c.f.u.) subcutaneously (s.c.) 12 weeks prior to challenge. Mice coimmunized with BCG and p2AIL12 received p2AIL12 (300 µg i.m.i) at the same time as the BCG. These experiments were approved by the Animal Ethics Committee of the University of Sydney.
Mycobacterium avium infection
The M. avium isolate used is a virulent strain of serotype 8 which produces a fatal infection in C57BL/6 mice,15 and was kindly provided by C. Cheers (University of Melbourne, Victoria, Australia). The M. avium was grown in Middlebrook 7H9 broth with supplement (Difco Laboratories, Detroit, MI) and stored at −70°. Prior to use, the suspension was thawed at 37° and briefly sonicated (10 s) to disperse clumps. Six weeks after the last DNA boost and 12 weeks after the BCG immunization, the mice were infected with 1 × 106 c.f.u. M. avium. by intravenous injection (i.v.i). Mice were killed at 4 weeks after the infection and the number of bacteria in homogenates of the spleen and liver were determined by culture of serial dilutions on Middlebrook 7H11 Bacto agar for 10 days.
Immunological responses
Mice were bled 2 weeks following the final DNA immunization and the presence of antigen-specific immunoglobulin G (IgG) antibodies determined by a solid-phase enzyme-linked immunosorbent assay (ELISA) using r35 000 MW protein, as previously described.10 The spleens were collected either 2 weeks following the third DNA immunization or 4 weeks following M. avium challenge and single cell suspensions prepared. The cells were cultured in complete RPMI medium supplemented with 2 mm glutamate, 50 µmβ-mercaptoethanol and 10% fetal calf serum (CSL Bioscience, Melbourne, Australia) and stimulated with M. avium 35 000 MW antigen (10 µg/ml) or whole M. avium sonicate (10 µg/ml) or medium alone for 16 hr. The number of IFN-γ-secreting cells was measured by enzyme-linked spot (ELISpot) assay using the monoclonal antibodies (mAbs) R46A2 for capture and XMG1.2 (Endogen, Woburn, MA) for recognition as previously described.8 The amount of IFN-γ released was quantified by ELISA using the mAbs R46A2 and biotinylated-XMG1.2 (Endogen) and recombinant murine IFN-γ as standard. The limit of detection was 0·4 U/ml with 1 U equivalent to 197 pg/ml.
Statistical analysis
The significance of the differences between groups were analysed using Fisher's Protected Least Significant Difference analysis of variance (anova) post hoc test for pair-wise comparison of multigrouped data sets, with log transformation of the bacterial counts. Differences with P < 0·05 were considered significant.
Results
Effect of p2AIL12 coimmunization on DNA vaccine induced immune responses
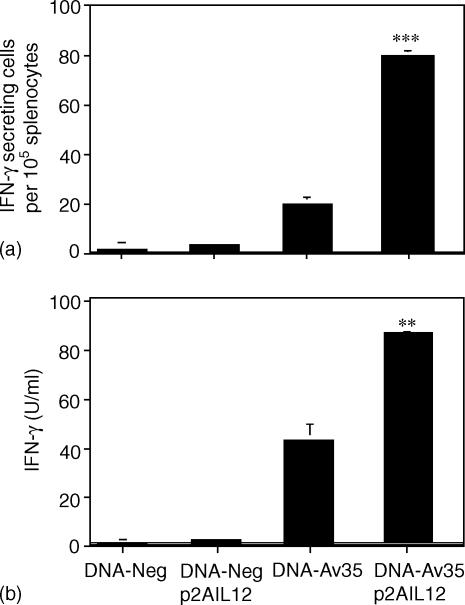
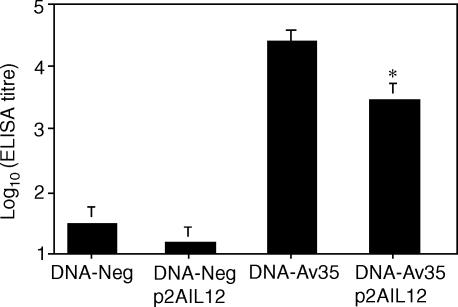
The p2AIL12 vector permits the coordinate expression of both the p35 and p40 chains of IL-12, resulting in the secretion biologically active IL-12.23 The effect of immunization with DNA-Av35, with and without p2AIL12, on the T-cell response to the 35 000 MW protein was assessed. Co-immunization with p2AIL12 significantly increased the frequency of antigen-specific IFN-γ secreting cells by fourfold (Fig. 1a) and the levels of IFN-γ released (Fig. 1b). Immunization with DNA-Av35 induces a strong IgG antibody response to conformational determinants on the protein.10 Co-delivery of p2AIL12 with DNA-Av35 resulted in a significant reduction in the anti-35 000 MW protein IgG titre (Fig. 2).
Figure 1.
Co-immunization with plasmid IL-12 increases the antigen-specific IFN-γ T-cell responses following DNA immunization. Two weeks following the third immunization with DNA-Av35 or the empty vector, DNA-Neg, with and without p2AIL12, splenocytes were stimulated overnight with M. avium 35 000 MW protein and the frequency of IFN-γ secreting cells (a) or amount of IFN-γ secreted (b) analysed. The data represent the means (± SEM) for five mice and are representative of three separate experiments. The significance of the differences between DNA-Av35 alone or with p2AIL12 were ***, P = 0·001 (a) and **, P =0·005 (b).
Figure 2.
Co-immunization with plasmid IL-12 reduces antigen-specific antibody following DNA immunization. Mice (n = 5) were immunized with DNA-Av35 or the empty vector, DNA-Neg, with and without p2AIL12. Two weeks following the third immunization the serum geometric mean titre (± SEM) of IgG anti-35 000 MW antibodies was determined by ELISA. The significance of the difference between immunization with DNA-Av35 alone and with pIL-12 was *, P = 0·05. The results are representative of three separate experiments.
Co-immunization with p2AIL12 improves protection by DNA-35 vaccines against challenge with M. avium
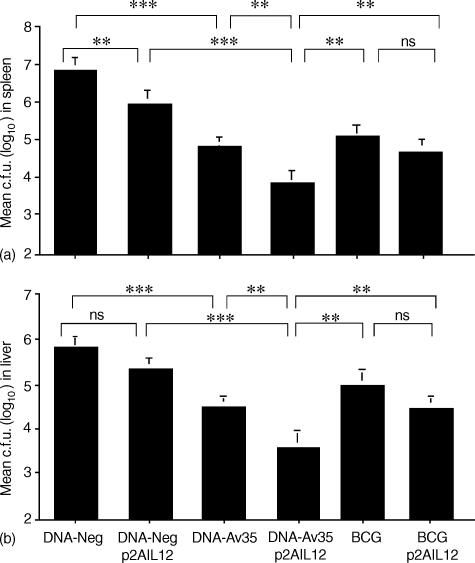
The effect of p2AIL12 on DNA vaccine-induced protection against intravenous challenge with M. avium was investigated 6 weeks after the last DNA immunization, when the protective effects of DNA-Av35 are maximal.10 Immunization with DNA-Av35 resulted in significant protective immunity against M. avium infection with a 2-log10 reduction in the M. avium load in the spleen (P < 0·001). The addition of p2AIL12 to the DNA-Av35 vaccine significantly increased the level of protection in both the spleen (P < 0·005) (Fig. 3a) and liver (P < 0·01) (Fig. 3b), with a further reduction in the bacterial load of about 1-log10. Immunization with p2AIL12 alone led to a small reduction in the bacterial load, which was significant in the spleen (Fig. 3b). When plasmid IL-12 was combined with a single BCG immunization, there was no significant decrease in mycobacterial load when tested 12 weeks later (Fig. 3a, b).
Figure 3.
Plasmid IL-12 enhances the protection against systemic M. avium infection in the spleen and liver following DNA immunization. Mice (n = 5) were immunized i.m. with DNA-Av35 or the empty vector, DNA-Neg, with and without p2AIL12, or by a single s.c. injection with BCG, with or without pIL-12 i.m. Six weeks following the last DNA immunization and 12 weeks after the BCG immunization, mice were challenged with 1 × 106 M. avium by the intravenous route. Four weeks later, the bacterial loads (c.f.u. ± SEM) were determined in the spleen (a) and liver (b). The significance of the differences between individual groups were determined by anova and were ***, P < 0·001; **, P < 0·01, and ns, not significant. The results are representative of three separate experiments.
Effect of coadministration of p2AIL12 on protective IFN-γ T-cell responses
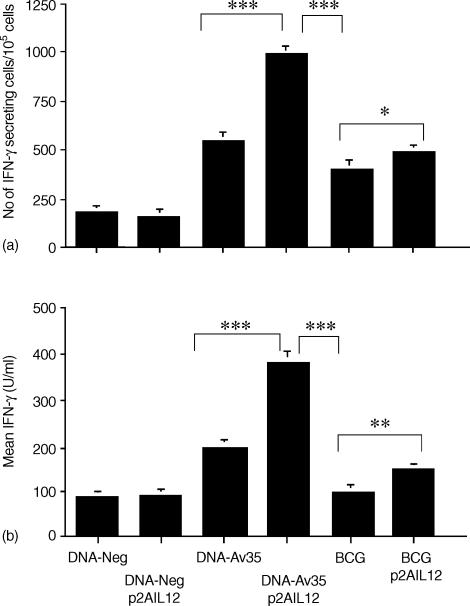
The 35 000 MW protein is a dominant antigen in the murine response to M. avium infection. Control mice showed a moderate IFN-γ T-cell response to the protein following infection with M. avium, and this was increased in mice previously immunized with DNA-Av35 (Fig. 4a, b). Mice immunized with DNA-Av35 and p2AIL12 demonstrated significantly greater IFN-γ responses to 35 000 MW protein alone (Fig. 4), as well as to M. avium sonicate (data not shown), when compared to recipients of DNA-Av35 alone. There was a doubling in the number of IFN-γ secreting cells and the amount of IFN-γ released. Immunization with BCG and p2AIL12 also resulted in significantly increased responses to M. avium sonicate (data not shown) and the 35 000 MW antigen, however, immunization with DNA-Av35 and pIL-12 was more effective (Fig. 4). Therefore priming with the DNA-Av35 and p2AIL12 was the most efficient strategy to induce protective IFN-γ-secreting T-cell responses.
Figure 4.
Co-administration of plasmid IL-12 with the DNA-Av35 vaccine enhances the 35 000 MW protein-specific IFN-γ T-cell response following M. avium challenge. Mice (n = 5) were immunized as described in Fig. 3. Following M. avium challenge the number of splenocytes secreting IFN-γ (mean ± SEM) (a) and the amount of IFN-γ secreted (mean ± SEM) (b) in response to M. avium 35 000 MW antigen were determined. The significance of differences between groups immunized with DNA-35 alone or with p2AIL12 were ***, P < 0. 0·001; **, P < 0·01; and *, P < 0·05. The results are representative of three separate experiments.
Discussion
Subunit vaccines against mycobacterial infection have considerable potential advantages. As non-replicating vaccines they should be safe in immunodeficient subjects, particularly in areas of high human immunodeficiency virus endemicity, and could be used for repeat immunization to boost flagging memory T-cell responses. In addition, because they induce cellular immunity to only a small subset of mycobacterial antigens, it would be possible to detect infection with virulent mycobacteria, such as M. tuberculosis, by the development of T-cell responses to other mycobacterial proteins. As yet, however, no single mycobacterial antigen delivered as a DNA or protein vaccines is reproducibly better than BCG at inducing protective immunity against M. tuberculosis, M. avium or M. leprae infection in murine models.12 Further, subunit vaccines appear less effective than BCG at controlling replication of M. tuberculosis in the more susceptible guinea pigs, although some single antigen vaccines do reduce immunopathology and prolong guinea pig survival.26 Therefore a variety of approaches are being pursued to increase their immunogenicity, particularly for DNA vaccines.22 This study demonstrates that coadministration of plasmid-encoded IL-12 with DNA expressing the dominant M. avium 35 000 MW protein enhances the immunogenicity of the DNA vaccine and is significantly more protective than BCG against a strain of M. avium which causes progressive infection (Fig. 3). The 35 000 MW protein was first identified as the homologue of the M. leprae major membrane protein I,27 which elicits either a strong CD4 T cell and/or antibody response in > 90% of leprosy patients across the leprosy spectrum.25 The M. avium 35 000 MW protein stimulates a strong T-cell response during infection with M. avium in mice (Fig. 4). The 3-log10 reduction in bacterial load in the spleen following immunization with DNA-Av35 and plasmid IL-12 was significantly higher than the level of protection observed in separate experiments with DNA vaccines expressing the M. avium 65 000 MW heat-shock protein or M. bovis antigen 85A.11 These induced a four- and eightfold reduction in M. avium load in the spleen, respectively.11 The apparent enhanced effect with DNA-Av35 and p2AIL12 may be due to both the immunodominance of the 35 000 MW protein and the adjuvant effect of IL-12.
The Th1 pattern of cellular responses induced during mycobacterial infections is largely a result of the secretion of IL-12 by infected dendritic cells. The level of IL-12 production from BCG-infected DCs can be increased in vitro by stimulation of cell surface CD40 and the inhibition of endogenous IL-10.28,29 This results in increased primary activation of Th1-like T cells both in vitro and in vivo. In addition, DNA induces a Th1-bias via the immunostimulatory effects of CpG oligonucleotide sequences in bacterial DNA, which stimulate Toll-like receptor 9 on DCs to initiate IL-12 secretion through the nuclear factor-κB-dependent pathway.30 Despite this effect of DNA alone, in this study maximum Th1-like response was induced when additional IL-12 was expressed by plasmid IL-12 leading to an increase in the number of M. avium- specific IFN-γ-secreting T cells by fourfold compared to DNA-35 alone (Fig. 1). One advantage of this p2AIL12 vector is that equivalent amounts of the p40 and p35 chains of IL-12 are produced within the transfected cells, and this prevents an excess of p40 chain with the possible inhibitory effects of p40 homodimers. Co-administration of p2AIL12 with DNA vaccines expressing M. tuberculosis secreted proteins increased the frequency of both CD4 and CD8 IFN-γ secreting specific T cells and increased the protective effect of DNA-85B in both the lungs and spleen.23 Intriguingly, codelivery of p2AIL12 with DNA-35 reduced the titre of the IgG antibody response to the protein (Fig. 2), and a similar inhibition of specific antibody occurred when p2AIL12 was combined with DNA-85B or DNA-MPT64.23 This is consistent with the known role that IL-12 plays in promoting a Th1-like response whilst inhibiting Th2-like responses.14 By contrast, the combination of a plasmid secreting GM-CSF with DNA-85B or DNA-MPT64 increased both the antibody and IFN-γ secreting T-cell responses and this effect was not associated with increased protection against M. tuberculosis.31
Plasmid IL-12 alone given prior to M. avium challenge resulted in a small, but significant, reduction in the M. avium load in the spleen, but not in the liver (Fig. 3). There is conflicting evidence on the effects of rIL-12 therapy during M. avium infection. Initial reports indicated that rIL-12 therapy of mice reduced the mycobacterial load during established M. avium infection.32 By contrast, treatment of M. avium-infected beige mice had no effect on the multiplication of the bacteria, although rIL-12 therapy appeared to reduce the early growth of M. tuberculosis in normal mice.33 These differences may be caused by the variation in the virulence of the M. avium strains tested. Silva and colleagues19 found that rIL-12 treatment reduced the growth of low, but not high, virulence strains of M. avium. Further, rIL-12 increased the activation of IFN-γ-secreting CD4 T cells, which were able to transfer increased clearance of M. avium to irradiated recipients.16 When used as immunotherapy, IL-12 may increase the clearance of M. avium through the activation of natural killer cells,19 as well by enhancing specific T-cell immunity. The cytokine IL-18, which is secreted by both macrophages and DCs, is also involved in the deviation of naïve CD4 T cells to the Th1-like phenotype. Therapy with plasmid IL-18 during intranasal infection with M. avium reduced the bacterial load in the lung during the treatment period and this was associated with increased IFN-γ production in the lung.34 However the addition of plasmid IL-18 to IL-12 as a further adjuvant during immunization with DNA-85B did not increase the protective effect against M. tuberculosis infection.35
No significant increase in protective effect was achieved when p2AIL-12 was delivered i.m. at the same time as BCG immunization, although this produced a small increase in the postchallenge IFN-γ response (Fig. 4b). The addition of CpG oligonucleotides or rIL-1236 or p2AIL1223 did improve the efficacy of BCG against pulmonary M. tuberculosis infection. These differences may relate to the timing and route of delivery of the BCG and the adjuvant IL-12 or oligonucleotides. In the case of plasmid delivery, it is uncertain if the effect of the IL-12 is caused by expression at the site of injection, in this case i.m., or in the draining lymph node. Antigen presentation following DNA immunization probably occurs through transfected DCs which rapidly migrate from the site of immunization to the draining lymph nodes37 and cotransfection of the same DCs with pIL-12 may produce the optimal effect. In addition the prolonged production of bioactive IL-12 by transfected muscle cells may continue to the sustained adjuvant effect of pIL-12. Co-administration of the pIL-12 vector by the intradermal or s.c. route at the same site and time as the BCG injection may help to decipher if the effect of IL-12 is a localized one.
The addition of rIL-12 to M. avium culture filtrate proteins as an adjuvant resulted in an increase in the protective effect against infection with M. avium (strain 2477) from 0·4- to . The efficacy of this subunit vaccine lasted for 6 months and waned by 12 months. Although the addition of rIL-12 increased the protection at six months, rIL-12 accelerated the loss of protective efficacy at 12 months.38 There may be advantages in administering both the cytokine and antigen as DNA vectors. Immunization with a leishmania protein with either recombinant IL-12 or IL-12-expressing DNA protected mice challenged with Leishmania major 2 weeks later, but the protective immunity was only sustained for 12 weeks when the IL-12 and antigen were delivered as DNA vectors.39 Recombinant IL-12 has a short half-life in vivo, but production of IL-12 from the plasmid may sustain and induce a more prolonged protective immune response. The adjuvant effects of CpG motifs in the DNA vaccines may also contribute to sustained IL-12 production induced by DNA itself. Thus DNA expressing the leishmanial antigen39 or protein antigen immunized with CpG motifs40 stimulated long-term protective immunity against Leishmania major. Therefore, the initial burst of IL-12, either as recombinant protein or expressed from the DNA vector, is sufficient to initiate the development of a strong Th1 T-cell response to the accompanied antigen, but the continued secretion of plasmid-derived IL-12 or the presence of the CpG motifs may be necessary to sustain the protective Th1-like immune responses.
In summary, the addition of plasmid IL-12 to the DNA-Av35 vaccine enhanced protective immunity against M. avium infection. DNA vaccine expressing the M. leprae homologue of the 35 000 MW protein induced moderate protection against mouse footpad infection with M. leprae in outbred Swiss mice.12 It will be of interest to assess this adjuvant strategy with plasmid IL-12 in the M. leprae infection model. Infection with M. leprae in humans results in a spectrum of immune responses from strong Th1-like T-cell immunity to Th2-associated antibody responses. BCG and other live mycobacterial vaccines have been tested as therapeutic vaccines in lepromatous leprosy patients to deviate the immune response to the tuberculoid pattern of potent Th1-like T cell reactivity to M. leprae antigens. rIL-12 is capable of generating an antigen-specific Th1-type response in the presence of an ongoing infection-driven Th2-type response.14 It is possible that a therapeutic vaccine consisting of IL-12 with appropriate mycobacterial antigens may promote Th1 T-cell responses in subjects with established lepromatous leprosy and so assist in the clearance of the infection
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and the NSW Health Department through its research and development infrastructure grants programme. E. Martin and A. Kamath were recipients of Australian Postgraduate Awards, and E. Martin was also supported by the Cooperative Research Centre for Vaccine Technology. We thank Dr P. Chaplin for the provision of the original FMDV 2 A vector and Dr A. Bean and Dr C. Feng for helpful discussions.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. Who global surveillance and monitoring project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Benson CA. Disease due to the Mycobacterium avium complex in patients with AIDS. Epidemiology and clinical syndrome. Clin Infect Dis. 1994;18:S218–S22. doi: 10.1093/clinids/18.supplement_3.s218. [DOI] [PubMed] [Google Scholar]
- 3.Collins FM. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin Microbiol Rev. 1989;2:360–7. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine PEM. Variation in protection by BCG. Implications of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–44. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tascon RE, Colston MJ, Ragno S, Stavropoulos E, Gregory D, Lowrie DB. Vaccination against tuberculosis by DNA injection. Nature Med. 1996;2:888–92. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 7.Huygen K, Content J, Olivier D, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–8. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 8.Kamath AT, Feng CG, Macdonald M, Briscoe H, Britton WJ. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–7. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin E, Roche PW, Triccas JA, Britton WJ. DNA encoding a single mycobacterial antigen protects against leprosy infection. Vaccine. 2001;19:1391–6. doi: 10.1016/s0264-410x(00)00374-1. [DOI] [PubMed] [Google Scholar]
- 10.Martin E, Kamath AT, Triccas JA, Britton WJ. Protection against virulent Mycobacterium avium infection following DNA vaccination with the 35 kDa protein is accompanied by the induction of IFN-γ secreting CD4+ T cells. Infect Immun. 2000;68:3090–6. doi: 10.1128/iai.68.6.3090-3096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velaz-Faircloth M, Cobb AJ, Horstman AL, Henry SC, Frothingham R. Protection against Mycobacterium avium by DNA vaccines expressing mycobacterial antigens as fusion proteins with green fluorescent protein. Infect Immun. 1999;67:4243–50. doi: 10.1128/iai.67.8.4243-4250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britton WJ, Palendira U. Improving vaccines against tuberculosis. Immunol Cell Biol. 2002;81:34–45. doi: 10.1046/j.0818-9641.2002.01143.x. [DOI] [PubMed] [Google Scholar]
- 13.Castro AG, Silva RA, Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J Immunol. 1995;155:2013–9. [PubMed] [Google Scholar]
- 14.Schopf LR, Bliss JL, Lavigne LM, Chung CL, Wolf SF, Sypek JP. Interleukin-12 is capable of generating and antigen-specific Th1-type response in the presence of an ongoing infection-driven Th2-type response. Infect Immun. 1999;67:2166–71. doi: 10.1128/iai.67.5.2166-2171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders BM, Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance of mice to Mycobacterium avium complex infection. Infect Immun. 1995;63:4011–5. doi: 10.1128/iai.63.10.4011-4015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva RA, Florido M, Appelberg R. Interleukin-12 primes CD4 T cells for interferon-gamma production and protective immunity during Mycobacterium avium infection. Immunology. 2001;103:368–74. doi: 10.1046/j.1365-2567.2001.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casanova J-L, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 18.Mohagheghpour N, van Vollenhoven A, Goodman J, Bermudez L. Interaction of Mycobacterium avium with human monocyte-derived macrophages. Infect Immun. 2000;68:5824–9. doi: 10.1128/iai.68.10.5824-5829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva RA, Pais TF, Appelberg R. Evaluation of IL−12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J Immunol. 1998;161:5578–85. [PubMed] [Google Scholar]
- 20.Alfonso LCC, Scharton TM, Vieria LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1993;263:235–7. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 21.Sin JI, Kim JJ, Boyer JD, Ciccarelli RB, Higgins TJ, Weiner DB. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J Virol. 1999;73:501–9. doi: 10.1128/jvi.73.1.501-509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guranathan S, Klinman D, Seder RA. DNA vaccines: Immunology, applications and optimisation. Annu Rev Immunol. 2000;18:927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 23.Palendira U, Kamath AT, Feng CG, Martin E, Chaplin PJ, Triccas JA, Britton WJ. Coexpression of interleukin-12 chains by a self-splicing vector increases the protective cellular immune response of DNA and Mycobacterium bovis BCG vaccines against Mycobacterium tuberculosis. Infect Immun. 2002;70:1949–56. doi: 10.1128/IAI.70.4.1949-1956.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan MD, Drew J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 1994;13:928–33. doi: 10.1002/j.1460-2075.1994.tb06337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Triccas JA, Roche PW, Winter N, Feng CG, Butlin R, Britton WJ. A 35 kDa protein is a major target of the human immune response to Mycobacterium leprae. Infect Immun. 1996;64:5171–7. doi: 10.1128/iai.64.12.5171-5177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin SL, D'Souza C, Roberts AD, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 2001;66:2951–9. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triccas JA, Winter N, Roche PW, Gilpin A, Kendrick KE, Britton WJ. Molecular and immunological analysis of the Mycobacterium avium homologue of the immunodominant Mycobacterium leprae 35 kDa protein. Infect Immun. 1998;66:2684–90. doi: 10.1128/iai.66.6.2684-2690.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demangel C, Palendira U, Feng CG, Heath AW, Bean AGD, Britton WJ. Stimulation of dendritic cells via CD40 enhances the immune responses to Mycobacterium tuberculosis infection. Infect Immun. 2001;69:2456–61. doi: 10.1128/IAI.69.4.2456-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demangel C, Bean AGD, Feng CG, Britton WJ. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur J Immunol. 2002;32:994–1002. doi: 10.1002/1521-4141(200204)32:4<994::AID-IMMU994>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;8:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 31.Kamath AT, Hanke T, Briscoe H, Britton WJ. Co-immunisation with DNA vaccines expressing granulocyte–macrophage colony stimulating factor and mycobacterial secreted proteins enhances T-cell immunity, but not protective efficacy against tuberculosis. Immunology. 1999;96:511–6. doi: 10.1046/j.1365-2567.1999.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyabishi K, Yamazaki J, Kasama T, Katsura T, Kasahara K, Wolf SF, Shinamura T. Interleukin (IL)-12 in susceptible mice infected with Mycobacterium avium and amelioration of established infection by IL−12 replacement therapy. J Infect Dis. 1996;174:564–9. doi: 10.1093/infdis/174.3.564. [DOI] [PubMed] [Google Scholar]
- 33.Lounis N, Truffot-Pernot C, Ridley RG, Alber G, Grosset JH. Impacts of interleukin-12 on multiplication of Mycobacterium tuberculosis and Mycobacterium avium complex in mice. Clin Microb Infect. 1999;5:331–8. doi: 10.1111/j.1469-0691.1999.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Cho D, Kim TS. Induction of in vivo resistance to Mycobacterium avium infection by intramuscular injection with DNA encoding interleukin-18. Immunology. 2001;102:234–41. doi: 10.1046/j.1365-2567.2001.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triccas JA, Sun L, Palendira U, Britton WJ. Comparative affects of plasmid-encoded interleukin-12 and interleukin-18 on the protective efficacy of DNA vaccination against Mycobacterium tuberculosis. Immunol Cell Biol. 2002;80:358–63. doi: 10.1046/j.1440-1711.2002.01087.x. [DOI] [PubMed] [Google Scholar]
- 36.Freidag BL, Melton GB, Collins F, Klinman DM, Cheever A, Stobie L, Suen W, Seder RA. CpG oligodeoxynucelotides and interleukin-12 improve the efficacy of Mycobacterium bovis BCG vaccination in mice challenged with M. tuberculosis. Infect Immun. 2000;68:2948–53. doi: 10.1128/iai.68.5.2948-2953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA vaccination: Transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169–78. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva RA, Pais TF, Appelberg R. Effects of interleukin-12 in the long-term protection conferred by a Mycobacterium avium subunit vaccine. Scand J Immunol. 2000;52:531–3. doi: 10.1046/j.1365-3083.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 39.Gurunathan S, Prussin C, Sacks DL, Seder RA. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat Med. 1998;4:1409–15. doi: 10.1038/4000. [DOI] [PubMed] [Google Scholar]
- 40.Stacey KJ, Blackwell JM. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect Immun. 1999;67:3719–26. doi: 10.1128/iai.67.8.3719-3726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]